ABSTRACT
Background:
The assessment of renal function in clinical practice remains challenging. Using creatinine to assess the glomerular filtration rate (GFR) is notoriously inaccurate, and determination of the true GFR, e.g., using inulin or iohexol, is laborious and not feasible in daily practice. Proenkephalin (PENK) is a novel candidate biomarker for kidney function that is filtrated in the glomerulus, has shown to represent steady-state GFR in patients with different severities of renal insufficiency. In this pilot study in non-steady-state critically ill patients, we compared plasma PENK concentrations with creatinine-based GFR assessments and validated both against the “true GFR” measured using a gold standard method: iohexol plasma clearance.
Methods:
Twenty-three critically ill patients with septic shock were included. Kidney function was determined using the Modification of Diet in Renal Disease formula (eGFRMDRD), Endogenous Creatinine Clearance (GFRECC), and iohexol plasma clearance (GFRiohexol) during a 6-h window. Plasma PENK concentrations were measured using the penKid immunoassay.
Results:
The eGFRMDRD and GFRECC correlated with the GFRiohexol (R2 = 0.82, P < 0.0001 and R2 = 0.82, P < 0.0001 respectively); however, bias and variability were considerable: the eGFRMDRD overestimated the true GFR with 31 ± 35% (95% limits of agreement: −37% to 100%) and the GFRECC with 37 ± 49% (95% limits of agreement: −59% to 133%). Plasma PENK concentrations showed a very strong inverse correlation with the GFRiohexol (R2 = 0.90, P < 0.0001) which tended to be better compared with the correlation of eGFRMDRD (P = 0.06) and GFRECC (P = 0.08) with the GFRiohexol.
Conclusions:
In this pilot study in non-steady-state critically ill sepsis patients, GFR appears to be more accurately reflected by plasma PENK concentrations compared to conventional creatinine-based methods. Therefore, PENK holds promise as an accurate and feasible biomarker to determine kidney function during non-steady-state conditions in the critically ill.
Keywords: Acute kidney injury, endogenous creatinine clearance, glomeral filtration rate, iohexol plasma clearance, proenkephalin, renal function, sepsis
Abbreviations: AKI, acute kidney injury, BSA, body surface area, DOR, delta opioid receptor, ECC, endogenous creatinine clearance, EDTA, ethylenediaminetetraacetic acid, GFR, glomerular filtration rate, HPLC, high performance liquid chromatography, ICU, intensive care unit, IQR, interquartile range, KIM-1, Kidney Injury Molecule-1, MDRD, Modification of Diet in Renal Disease, NAG, N-acteyl-ß-D-glucosaminidase, NGAL, neutrophil gelatinase-associated lipocalin, PENK, proenkephalin, RRT, renal replacement therapy, sCr, serum creatinine, SIRS, systemic inflammatory response syndrome
INTRODUCTION
Acute kidney injury (AKI) is a growing global medical concern with unresolved issues related to diagnosis, prevention, as well as treatment. In critically ill patients, AKI has an incidence rate of 20% to 75% (1) and is a significant contributor to morbidity and mortality (2). Hitherto, the inability to properly and timely diagnose impaired renal function has hampered both clinical research and patient care in this field.
Kidney function is defined as glomerular filtration rate (GFR), and serum creatinine (sCr) is by far the most widely used surrogate marker to estimate the GFR. In terms of estimating GFR, the Modification of Diet in Renal Disease (MDRD), which is based on sCr, is most frequently used in clinical practice. Calculation of endogenous creatinine clearance (ECC), making use of both serum and urinary creatinine values, is only sparsely employed. In any case, creatinine-based methods have evident shortcomings. Apart from the lag-time and limited sensitivity of serum creatinine, reliability is limited due to the influence of fluid therapy, feeding, and muscle mass (3). In addition, risk of urine collection errors and active renal secretion and reabsorption of creatinine influence urinary creatinine concentrations. Combined, these issues severely limit the accuracy of creatinine-based methods, especially in hospitalized patients.
A more accurate estimation of GFR will facilitate secondary prevention of AKI (4) and dosing adjustments of renally excreted medications. This is possible using administration and determination of the renal clearance of exogenous compounds, such as the gold standard inulin (5) and the iodine contrast agent iohexol (6), or the isotopes 51Cr-EDTA (7) and 125I-iothalamate (8). In critically ill patients, plasma clearance of iohexol (GFRiohexol) has a very strong correlation with the method using inulin (R2 = 0.96) (6). Because of the widespread availability and the safety of this iodine-containing contrast medium, it represents an excellent and feasible method to determine the GFR for research purposes (9, 10). However, following administration of these compounds, multiple blood and/or urine samples have to be obtained in the period afterward to calculate their clearance. As such, these methods are labor-intensive, prone to sampling or determination errors, and hardly feasible in daily clinical practice. Consequently, creatinine-based methods are still widely used signifying a clear unmet need for a new biomarker to accurately and efficiently assess kidney function in critically ill patients, as emphasized in the Intensive Care research agenda on AKI (11).
Proenkephalin (PENK) is a potential new functional biomarker for kidney function. PENK is a stable opioid peptide cleaved from the same precursor of endogenous opioids as enkephalins (12). Previous work has revealed that plasma concentrations of enkephalins are inversely correlated to the GFR (13), and PENK has been shown to correlate well with creatinine-based GFR estimations in cardiac disease (14, 15), chronic kidney disease (16), and sepsis (17). Also, PENK correlates well with measured GFR using iothalamate in patients with stable kidney function (18–20). Although the accuracy of creatinine-based methods may be acceptable during stable kidney function, a new method to assess GFR is warranted, especially during non-steady-state conditions, but prospective studies are lacking.
In the current pilot study, we investigated the relationship between plasma PENK concentrations and the “true GFR” determined by iohexol plasma clearance in a cohort of critically ill sepsis patients and compared its accuracy to creatinine-based methods to determine GFR.
PATIENTS AND METHODS
Study design and patient selection
In this prospective pilot study, 23 adult patients admitted to the intensive care unit (ICU) of the Radboud University Medical Center in Nijmegen, the Netherlands were included if they fulfilled the sepsis or septic shock diagnosis criteria (sepsis-3 criteria) (21). Patients were excluded in case of a known allergy for iodine-containing compounds, if they underwent recent medical imaging using iodine contrast substances, if they required renal replacement therapy, or in case of a history of thyroid dysfunction, myasthenia gravis, or pheochromocytoma. Iohexol was administered via a venous cannula already in place and blood was sampled from an already present arterial cannula. The study was reviewed by the local ethics committee (CMO Arnhem-Nijmegen) and the need for consent was waived. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Iohexol plasma clearance
After collection of a baseline blood sample, patients received an intravenous bolus of 1 mL iohexol (Omnipaque 240 mg/mL). The syringe used for iohexol administration was weighed before and after injection to calculate the exact amount of iohexol administered. To prevent contamination with iohexol, subsequent blood samples were collected from a different venous catheter. Ethylenediaminetetraacetic acid (EDTA) anticoagulated blood was obtained before (baseline) and 10, 45, 90, 135, 240, and 360 min following iohexol administration. An overview of the sampling time points is depicted in Figure 1. Samples were centrifuged immediately after withdrawal at 2000 g at 4°C for 10 min, and plasma was stored at −80°C until analysis. Iohexol concentrations were determined using High Performance Liquid Chromatography (HPLC) at the Department of Pharmacology and Toxicology, Radboudumc Nijmegen. The GFRiohexol was calculated using the area under the disappearance curve of the slow phase using the slope-intercept method and the last four time points of the “slow phase.” GFRiohexol calculations were corrected for body surface area (BSA), after which the Brøchner-Mortensen correction was applied to account for the fast/redistribution phase (22, 23).
Fig. 1.
Study procedures.
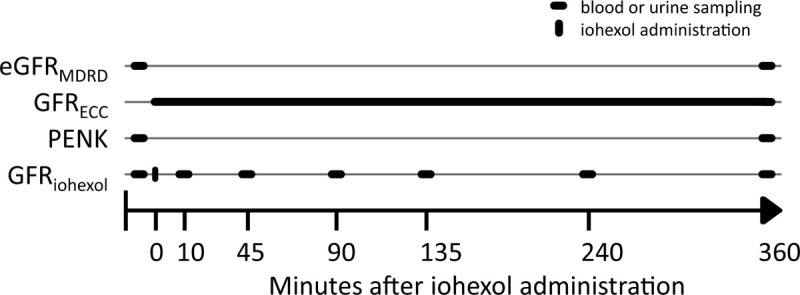
Timeline of study procedures after inclusion of a patient. eGFRMDRD indicates GFR estimated using the MDRD formula; GFRECC, GFR calculated using endogenous creatinine clearance; PENK, proenkephalin; GFRiohexol, GFR calculated using plasma clearance of iohexol.
Estimated GFR and endogenous creatinine clearance
The GFR was estimated using the MDRD equation (eGFRMDRD) using the average of the plasma creatinine concentration at baseline and 6 h after iohexol administration, to overcome the possible change of kidney function in this time period (see Fig. 1). For calculation of endogenous creatinine clearance (GFRECC) urine was collected for 6 h, starting directly following iohexol administration. Plasma creatinine, as well as urinary creatinine, urea, and sodium, were determined at the Department of Laboratory Medicine of the Radboud University Medical Center using routine clinical methods (24).
Plasma proenkephalin concentrations
PENK concentrations were determined in the EDTA plasma samples obtained at baseline and 6 h following iohexol administration (see Fig. 1) using the sphingotest penKid immunoassay (SphingoTec GmbH, Hennigsdorf Germany). Plasma PENK concentrations of both time points were averaged to reflect the PENK concentration during the 6-h period of the GFRiohexol and GFRECC determinations.
Categories of renal function for correct medication dosing
Patients were categorized based on their GFRiohexol, eGFRMDRD, and the GFRECC according to frequently used cut-off values of the GFR to adjust medication dose according to the GFR (30, 60, and 90 mL/min/1.73m2). The number of patients that would have been misclassified using the eGFRMDRD or the GFRECC compared with the GFRiohexol were reported.
Statistical analysis
Data were tested for normality using the D’agostino & Pearson omnibus test. Data are provided as mean ± SD or median [interquartile range], depending on their distribution. Following log-transformation of not normally distributed data, we used Pearson's correlation to investigate the relationship between different methods. The level of agreement between GFRiohexol, eGFRMDRD, and GFRECC was assessed using the Bland-Altman method, whereby data are shown as percentage differences (25). Bland-Altman agreement analysis requires that methods have the same unit of measurement and therefore, the relationship between GFRiohexol and PENK was only assessed using correlation analysis. Correlation coefficients were reciprocally tested using the method described by Steiger (26, 27). A two-sided P value <0.05 was considered statistically significant. Statistical analyses were conducted using GraphPad Prism version 5.03 (GraphPad Software, San Diego, Calif).
RESULTS
Twenty-three patients with sepsis, of whom 20 had septic shock, were enrolled. Their age was 63 [47–74] years, APACHE II score was 22 ± 7 at ICU admission, and 30-day mortality rate was 22%. Median [IQR] baseline plasma creatinine concentration was 112 [71–232] μmol/L. Patient demographic characteristics are listed in Table 1.
Table 1.
Patient characteristics
GFRiohexol and creatinine-based methods to assess kidney function
Patients had a median [IQR] measured GFRiohexol of 36 [15–68] mL/min/1.73m2. eGFRMDRD and GFRECC were 57 [23–96] and 58 [18–150] mL/min/ 1.73m2, respectively. eGFRMDRD and GFRECC were correlated with the GFRiohexol (R2 = 0.82, P < 0.0001 and R2 = 0.82, P < 0.0001, respectively). In view of the lag-time of creatinine, we also correlated the GFRiohexol with GFRMDRD estimated using the serum creatinine value the next day and found a similar correlation (R2 = 0.78, P < 0.0001).
In a Bland-Altman comparison, eGFRMDRD overestimated the measured GFRiohexol with a bias of 31 ± 35% (95% limits of agreement: −37% to 100%) (Fig. 2). The use of GFRECC resulted in an overestimation of 37 ± 49% (95% limits of agreement: −59% to 133%) (Fig. 3).
Fig. 2.
Bland-Altman plot of GFRMDRD and GFRiohexol.

Mean bias and 95% limits of agreement (%) are shown. GFRMDRD shows an inaccurate (mean bias), overestimation of the “true GFR” of 31% and imprecise (wide limits of agreement) estimation of the glomerular filtration rate. MDRD indicates modification of diet in renal disease.
Fig. 3.
Bland-Altman plot of GFRECC and GFRiohexol.
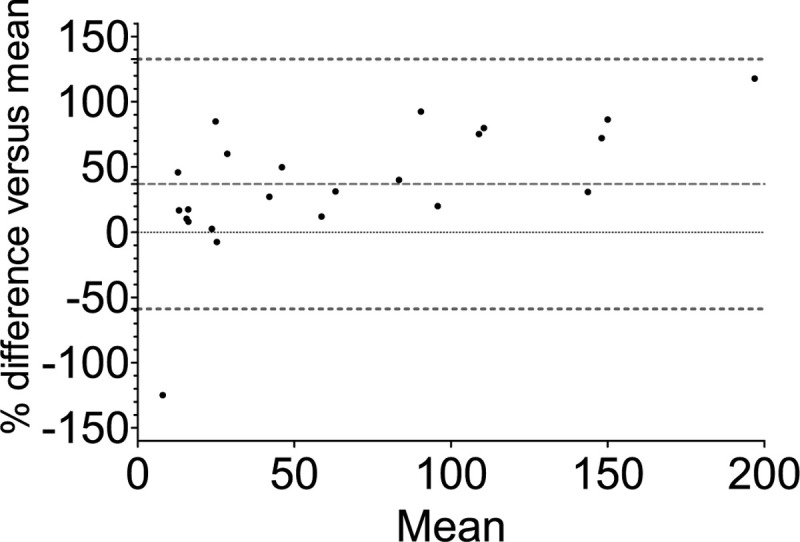
Mean bias and 95% limits of agreement (%) are shown. GFRECC shows an inaccurate (mean bias), overestimation of the “true GFR” of 37% and imprecise (wide limits of agreement) assessment of the glomerular filtration rate. ECC indicates endogenous creatinine clearance.
Plasma PENK concentration
Baseline and T = 6 h PENK plasma concentrations were 72 [37–118] and 61 [33–123] pmol/L, respectively. PENK plasma concentrations were inversely correlated with the GFRiohexol (R2 = 0.90, P < 0.001, Fig. 4). The correlation of PENK with GFRiohexol tended to be stronger than the correlation of eGFRMDRD and GFRECC with GFRiohexol: P = 0.06 and P = 0.08, respectively.
Fig. 4.
Correlation of plasma PENK concentrations with GFRiohexol.
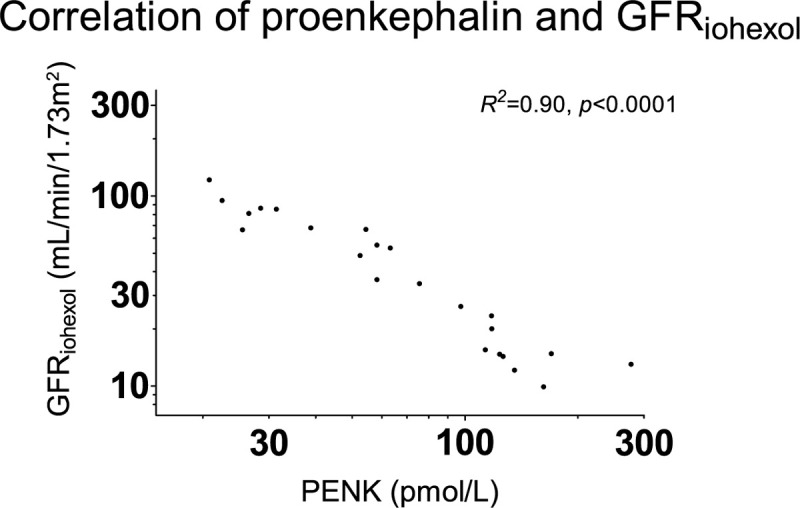
PENK plasma concentrations correlate strongly with the true GFR. PENK indicates proenkephalin; GFRiohexol, glomerular filtration rate calculated using plasma clearance of iohexol.
An overview of all kidney function parameters and the clinical outcome of the patients is listed in Table 2.
Table 2.
Kidney function parameters and outcome of the patients
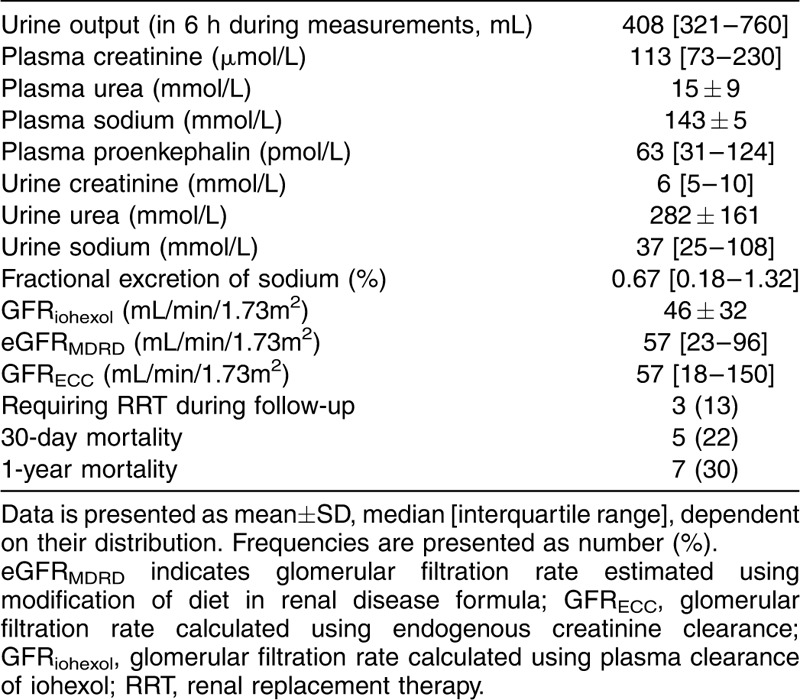
Performance of creatinine-based methods to categorize patients for medication dosing
Patients were categorized according to the GFRiohexol to frequently used cut-off values of the GFR for medication dosing (Table 1). When using the eGFRMDRD to adjust medication dosages, 30% of the patients are misclassified. When using GFRECC, 48% of the patients are misclassified.
DISCUSSION
In the current pilot study, we demonstrate that the MDRD and ECC, two widely used creatinine-based methods to estimate kidney function in daily clinical practice, result in large variation and significant overestimation of the true GFR in non-steady-state critically ill sepsis patients. A clinical consequence is that a significant proportion of these patients are misclassified for GFR category, e.g., for purposes of adjusting medication dosages according to their renal function. In contrast, plasma PENK concentrations showed a stronger correlation with the true GFR than both conventional measures. These pilot data indicate that PENK may represent a feasible and accurate marker of renal function in critically ill sepsis patients that are not in renal steady-state.
The medical need of an accurate and feasible technique to assess kidney function in non-steady-state critically ill patients is still unmet. Most novel biomarkers relate to kidney tubular stress or injury (28), and to a smaller extent to kidney function, and have not yet succeeded in conquering a place in daily clinical practice. Several possible explanations can be put forward. First, the proposed biomarkers may be judged not to be specific enough. For example, injury biomarker neutrophil gelatinase-associated lipocalin (NGAL) levels are also increased in patients suffering from systemic inflammatory response syndrome (SIRS) in the absence of AKI (17, 29). The same holds true for the functional biomarker cystatin C, levels of which are influenced by inflammation, as well as other conditions, including use of steroids and thyroid dysfunction (30, 31). A second, more practical, issue that impedes the use of several novel biomarkers of renal damage or renal stress is the fact that they have to be determined in urine. Compared with blood, obtaining urine may be less feasible. Finally, implementation of novel biomarkers in clinical practice is difficult. Ideally, an equation to estimate GFR using PENK should be developed. Assisting physicians to provide a known parameter (a GFR) instead of a concentration of PENK with which they are not familiar will probably facilitate implementation in clinical practice.
In most cases, validation of novel biomarkers for kidney function is performed by comparison to creatinine-based methods (32). This represents an important intrinsic shortcoming, because creatinine is not the gold-standard and does not accurately reflect current kidney function, which is once again exemplified by the present study. New biomarkers could show a limited correlation with creatinine-based methodology as a consequence of the limited accuracy of the creatinine-based methodology, and therefore it is not suitable to validate new markers of renal function against creatinine-based methodologies. In addition, the use of such a creatinine-based system for the diagnosis of AKI, for instance the KDIGO criteria, could thus also result in misclassification of renal function impairment of patients. In this respect, the use of an iohexol-based method of measuring GFR represents an important strength of the present work. A disadvantage of methods such as iohexol plasma clearance is related to the fact that there is no consensus on when to consider a decrease in GFR an episode of AKI. However, there are several studies that describe the effects on outcome of small rise in sCr. In a study in patients admitted to an oncology ICU, it was found that even small increases of sCr of 0 μmol/L to 17 μmol/L are associated with prolonged hospital stay and mortality (33). The results of two large cardiac surgery studies show a higher mortality in patients with a minimal increase of sCr (34), as well as an association with a composite endpoint of myocardial infarction, heart failure, stroke, or long-term all-cause mortality (35). These studies illustrate that small increases in plasma creatinine may reflect large decreases of GFR and thereby contribute to an impaired general outcome.
Several studies in other patient groups investigated the correlation of PENK plasma concentrations with measured GFR using iothalamate. In a cohort of chronic heart failure, PENK is correlated with the measured GFR (R2 = 0.62, P = 0.002). Of interest, no correlation could be demonstrated between PENK and the measured kidney damage markers: N-acteyl-ß-D-glucosaminidase (NAG), Kidney Injury Molecule-1 (KIM-1), and NGAL (19), but as these are injury markers and not functional markers, it is not unexpected that their correlation is limited. In healthy altruistic kidney donors, PENK correlated inversely with measured GFR (R2 = 0.55 P = 0.001) (20). Finally, in a mixed cohort of kidney donors and transplant recipients an inverse correlation between PENK and measured GFR was found that was not further specified (18). In a predictive model developed using PENK, age, sex, and transplant status, a moderate precision (percentage of predicted GFR within 30% of measured GFR) of 49% was found (95% CI: 46%–52%) (18). These studies used iothalamate as an exogenous compound for GFR calculations. However, iothalamate is known to overestimate the true GFR with 10% to 15%, as it is actively secreted in the tubules (36, 37), which is not the case for iohexol (37). In the current study, we found a high correlation between PENK and iohexol-determined GFR. Possibly, the described lower correlations found in the mentioned studies may therefore partially be explained by the use of iothalamate instead of iohexol. Moreover, it is important to study PENK in patients with rapid changes in kidney function as creatinine is particularly unreliable in these patients. The above-mentioned studies used cohorts of patients in a relatively stable (renal) situation. Our finding that the PENK concentration reflects the current GFR in critically ill non-steady-state patients may suggest that PENK is able to detect a change in GFR prior to creatinine. Future studies should investigate if indeed PENK has an advantage over creatinine in the swiftness to detect changes in renal function in critically ill patients.
Several aspects related to PENK require further study. For instance, as enkephalins are opioids, the corresponding expression of delta opioid receptor (DOR) is of interest. The kidneys display one of the highest densities of DORs, only second to neural tissue (38). More information is needed on renal effects of delta opioid receptor-agonism and antagonism (39). While PENK itself, being a by-product in the maturation of enkephalins from the precursor peptide, does not have a described signaling function, the possibility of feedback regulation by opioids has to be considered. Nevertheless, preliminary unpublished clinical investigations of our group have not revealed a systematic influence of exogenous opioids on plasma levels of PENK. To date, no interactions between medication and PENK have been reported. However, the possibility that other diseases influence PENK plasma concentrations independent of renal function should be evaluated in future cohorts.
Finally, this pilot study is limited by the use of a relatively small study population, necessitating confirmation in a larger cohort, and focuses on critically ill patients with sepsis. Data from other (non-sepsis) critically ill patients is needed to determine the generalizability of our findings.
CONCLUSIONS
In sepsis patients, plasma PENK concentrations strongly correlate with iohexol plasma clearance, a method to measure the “true GFR.” Creatinine-based methods to assess renal function show limited accuracy and precision and consequently misclassify the GFR category of a significant proportion of patients. This is important in clinical practice, for instance when adjusting drug dosing based on renal function. PENK appears to reflect renal function more precisely compared with conventional creatinine-based methods, indicating that PENK holds promise as an accurate and feasible biomarker to determine kidney function in non-steady-state critically ill sepsis patients.
ACKNOWLEDGMENTS
The authors thank everyone who contributed to this study, in particular Jelle Gerretsen as the laboratory technician of the Intensive Care Research.
Footnotes
PP received travel and consultancy reimbursements from SphingoTec.
This project was financed by the Department of Intensive Care Medicine of the Radboud University Medical Center, Nijmegen, the Netherlands.
The study was reviewed by the local ethics committee (CMO Arnhem-Nijmegen) and the need for consent was waived.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
PP conceived the study and received travel and consultancy reimbursements. RB drafted the manuscript. GL, MK, and PP revised the manuscript. RvG and GL conducted the study. RB, GL, RvG, and PP analyzed the data.
The authors report no conflicts of interest.
REFERENCES
- 1.Bellomo R, Ronco C, Mehta RL, Asfar P, Boisrame-Helms J, Darmon M, Diehl JL, Duranteau J, Hoste EAJ, Olivier JB, et al. Acute kidney injury in the ICU: from injury to recovery: reports from the 5th Paris International Conference. Ann Intensive Care 7 (1):49, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pannu N, James M, Hemmelgarn B, Klarenbach S. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 8 (2):194–202, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delanaye P, Cavalier E, Pottel H. Serum creatinine: not so simple!. Nephron 136 (4):302–308, 2017. [DOI] [PubMed] [Google Scholar]
- 4.(KDIGO) KDIGO: Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2 (1):1–138, 2012. [Google Scholar]
- 5.Sterner G, Frennby B, Mansson S, Nyman U, Van Westen D, Almen T. Determining ‘true’ glomerular filtration rate in healthy adults using infusion of inulin and comparing it with values obtained using other clearance techniques or prediction equations. Scand J Urol Nephrol 42 (3):278–285, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Erley CM, Bader BD, Berger ED, Vochazer A, Jorzik JJ, Dietz K, Risler T. Plasma clearance of iodine contrast media as a measure of glomerular filtration rate in critically ill patients. Crit Care Med 29 (8):1544–1550, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Brochner-Mortensen J, Giese J, Rossing N. Renal inulin clearance versus total plasma clearance of 51Cr-EDTA. Scand J Clin Lab Invest 23 (4):301–305, 1969. [DOI] [PubMed] [Google Scholar]
- 8.Skov PE. Glomerular filtration rate in patients with severe and very severe renal insufficiency. Determined by simultaneous inulin, creatinine and 125 iothalamate clearance. Acta Med Scand 187 (5):419–428, 1970. [DOI] [PubMed] [Google Scholar]
- 9.Delanaye P, Ebert N, Melsom T, Gaspari F, Mariat C, Cavalier E, Bjork J, Christensson A, Nyman U, Porrini E, et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 1: How to measure glomerular filtration rate with iohexol? Clin Kidney J 9 (5):682–699, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delanaye P, Melsom T, Ebert N, Back SE, Mariat C, Cavalier E, Bjork J, Christensson A, Nyman U, Porrini E, et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 2: why to measure glomerular filtration rate with iohexol? Clin Kidney J 9 (5):700–704, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickkers P, Ostermann M, Joannidis M, Zarbock A, Hoste E, Bellomo R, Prowle J, Darmon M, Bonventre JV, Forni L, et al. The intensive care medicine agenda on acute kidney injury. Intensive Care Med 43 (9):1198–1209, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ernst A, Kohrle J, Bergmann A. Proenkephalin A 119-159, a stable proenkephalin A precursor fragment identified in human circulation. Peptides 27 (7):1835–1840, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Smith R, Grossman A, Gaillard R, Clement-Jones V, Ratter S, Mallinson J, Lowry PJ, Besser GM, Rees LH. Studies on circulating met-enkephalin and beta-endorphin: normal subjects and patients with renal and adrenal disease. Clin Endocrinol (Oxf) 15 (3):291–300, 1981. [DOI] [PubMed] [Google Scholar]
- 14.Arbit B, Marston N, Shah K, Lee EL, Aramin H, Clopton P, Maisel AS. Prognostic usefulness of proenkephalin in stable ambulatory patients with heart failure. Am J Cardiol 117 (8):1310–1314, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Ng LL, Squire IB, Jones DJ, Cao TH, Chan DC, Sandhu JK, Quinn PA, Davies JE, Struck J, Hartmann O, et al. Proenkephalin, renal dysfunction, and prognosis in patients with acute heart failure: a GREAT Network Study. J Am Coll Cardiol 69 (1):56–69, 2017. [DOI] [PubMed] [Google Scholar]
- 16.Schulz CA, Christensson A, Ericson U, Almgren P, Hindy G, Nilsson PM, Struck J, Bergmann A, Melander O, Orho-Melander M. High level of fasting plasma proenkephalin: a predicts deterioration of kidney function and incidence of CKD. J Am Soc Nephrol 28 (1):291–303, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H, Hur M, Lee S, Marino R, Magrini L, Cardelli P, Struck J, Bergmann A, Hartmann O, Di Somma S, et al. Proenkephalin, neutrophil gelatinase-associated lipocalin, and estimated glomerular filtration rates in patients with sepsis. Ann Lab Med 37 (5):388–397, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donato LJ, Meeusen JW, Lieske JC, Bergmann D, Sparwasser A, Jaffe AS. Analytical performance of an immunoassay to measure proenkephalin. Clin Biochem 58:72–77, 2018. [DOI] [PubMed] [Google Scholar]
- 19.Matsue Y, Ter Maaten JM, Struck J, Metra M, O’Connor CM, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JG, et al. Clinical correlates and prognostic value of proenkephalin in acute and chronic heart failure. J Card Fail 23 (3):231–239, 2017. [DOI] [PubMed] [Google Scholar]
- 20.Kieneker LM, Hartmann O, Struck J, Bergmann A, Gansevoort RT, Joosten MM, van den Berg E, de Boer RA, Bakker SJL. Plasma proenkephalin and poor long-term outcome in renal transplant recipients. Transplant Direct 3 (8):e190, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315 (8):801–810, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brochner-Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest 30 (3):271–274, 1972. [DOI] [PubMed] [Google Scholar]
- 23.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 317 (17):1098, 1987. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53 (4):766–772, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Bland JM, Altman DG. Measuring agreement in method comparison studies. Statist Methods Med Res 8 (2):135–160, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull 87 (2):245–251, 1980. [Google Scholar]
- 27. Lee IA and Preacher KJ. Calculation for the test of the difference between two dependent correlations with one variable in common. Available at; http://quantpsy.org/corrtest/corrtest2.htm, 2013, September. [Google Scholar]
- 28.Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt-Ott KM. Biomarkers in acute kidney injury - pathophysiological basis and clinical performance. Acta Physiol (Oxf) 219 (3):554–572, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martensson J, Bellomo R. The rise and fall of NGAL in acute kidney injury. Blood Purif 37 (4):304–310, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Fricker M, Wiesli P, Brändle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int 63 (5):1944–1947, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Johnston N, Jernberg T, Lindahl B, Lindback J, Stridsberg M, Larsson A, Venge P, Wallentin L. Biochemical indicators of cardiac and renal function in a healthy elderly population. Clin Biochem 37 (3):210–216, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Murray PT, Mehta RL, Shaw A, Ronco C, Endre Z, Kellum JA, Chawla LS, Cruz D, Ince C, Okusa MD. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int 85 (3):513–521, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuels J, Ng CS, Nates J, Price K, Finkel K, Salahudeen A, Shaw A. Small increases in serum creatinine are associated with prolonged ICU stay and increased hospital mortality in critically ill patients with cancer. Support Care Cancer 19 (10):1527–1532, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lassnigg A, Schmid ER, Hiesmayr M, Falk C, Druml W, Bauer P, Schmidlin D. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med 36 (4):1129–1137, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Liotta M, Olsson D, Sartipy U, Holzmann MJ. Minimal changes in postoperative creatinine values and early and late mortality and cardiovascular events after coronary artery bypass grafting. Am J Cardiol 113 (1):70–75, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Seegmiller JC, Burns BE, Schinstock CA, Lieske JC, Larson TS. Discordance between iothalamate and iohexol urinary clearances. Am J Kidney Dis 67 (1):49–55, 2016. [DOI] [PubMed] [Google Scholar]
- 37.Delanaye P, Jouret F, Le Goff C, Cavalier E. Concordance between iothalamate and iohexol plasma clearance. Am J Kidney Dis 68 (2):329–330, 2016. [DOI] [PubMed] [Google Scholar]
- 38.Denning GM, Ackermann LW, Barna TJ, Armstrong JG, Stoll LL, Weintraub NL, Dickson EW. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides 29 (1):83–92, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Kapusta DR. Opioid mechanisms controlling renal function. Clin Exp Pharmacol Physiol 22 (12):891–902, 1995. [DOI] [PubMed] [Google Scholar]


