Abstract
Context
In healthy individuals, glucose-dependent insulinotropic polypeptide (GIP) enhances insulin secretion and reduces bone resorption by up to 25% estimated by absolute placebo-corrected changes in carboxy-terminal type 1 collagen crosslinks (CTX) during GIP and glucose administration. In patients with type 2 diabetes (T2D), GIP’s insulinotropic effect is impaired and effects on bone may be reduced.
Objective
To investigate GIP’s effect on bone biomarkers in patients with T2D.
Design
Randomized, double-blinded, crossover study investigating 6 interventions.
Patients
Twelve male patients with T2D.
Interventions
A primed continuous 90-minute GIP infusion (2 pmol/kg/min) or matching placebo (saline) administered at 3 plasma glucose (PG) levels (i.e., paired days with “insulin-induced hypoglycemia” (PG lowered to 3 mmol/L), “fasting hyperglycemia” (mean PG ~8 mmol/L), or “aggravated hyperglycemia” (mean PG ~12 mmol/L).
Main Outcome Measures
Bone biomarkers: CTX, procollagen type 1 N-terminal propeptide (P1NP) and PTH.
Results
On days with insulin-induced hypoglycemia, CTX was suppressed by up to 40 ± 15% during GIP administration compared with 12 ± 11% during placebo infusion (P < 0.0001). On days with fasting hyperglycemia, CTX was suppressed by up to 36 ± 15% during GIP administration, compared with 0 ± 9% during placebo infusion (P < 0.0001). On days with aggravated hyperglycemia, CTX was suppressed by up to 47 ± 23% during GIP administration compared with 10 ± 9% during placebo infusion (P = 0.0005). At all glycemic levels, P1NP and PTH concentrations were similar between paired days after 90 minutes.
Conclusions
Short-term GIP infusions reduce bone resorption by more than one-third (estimated by absolute placebo-corrected CTX reductions) in patients with T2DM, suggesting preserved bone effects of GIP in these patients.
Précis
Short-term GIP infusions reduce the bone resorption marker CTX by one-third in patients with type 2 diabetes independent of glycemic levels.
Keywords: Gastric inhibitory polypeptide, glucose-dependent insulinotropic polypeptide (GIP), bone markers, procollagen type 1 N-terminal propeptide (P1NP), carboxy-terminal collagen type 1 crosslinks (CTX)
Bone is remodeled throughout the day in a coupled process resorption and formation regulated by several factors including mechanical stimuli, nutrients, and hormonal factors [1, 2]. Glucose-dependent insulinotropic polypeptide (GIP) is one such hormonal factor secreted from the small intestine into the bloodstream after a meal and linking food intake to bone homeostasis [3]. Anabolic effects of GIP on bone are substantiated by in vitro data from bone cells showing functional GIP receptors and various in vivo preclinical models indicating bone preserving and osteotrophic effects of GIP [3-7]. In short-term clinical studies, exogenous infusion of GIP reduces bone resorption estimated by plasma concentrations of carboxy-terminal collagen type 1 crosslinks (CTX), a biochemical marker reflecting the rate of osteoclastic bone resorption. In metabolically healthy fasting individuals, exogenous GIP in physiological amounts leads to a 25% acute reduction in CTX after 1 hour [8]. Glucose administration in itself leads to a 25% reduction in CTX. Importantly, GIP plus IV glucose administration together have been shown to result in a 50% reduction of CTX [8], explaining most of the reduction in CTX levels observed after oral glucose ingestion [9], where GIP and glucose levels are also normally raised [10]. A biomarker of bone formation (e.g., procollagen type 1 N-terminal propeptide [P1NP]) seem relatively unaffected or even slightly increased by acute GIP administration [11, 12]. In contrast, PTH, which is a modulator of bone turnover and calcium homeostasis [13], has previously been reported to be modestly suppressed by GIP in healthy individuals and in patients with type 1 diabetes [11, 12].
For at least 2 reasons, it is of interest to examine to what extent biochemical bone markers in patients with type 2 diabetes (T2D) are affected by GIP. First, patients with T2D have a higher risk of bone fractures compared with healthy individuals [14] and a reduction in bone turnover may contribute to this heightened fracture risk in these patients [15]. Second, it is well established that the insulin-releasing property of GIP is severely impaired in patients with T2D (i.e., they have a reduced incretin effect) [16, 17]. The reduced insulinotropic effect of GIP develops secondary to the diabetic state [18], and may be due to the chronic hyperglycemia and subsequent GIP-receptor downregulation on pancreatic beta cells [19, 20]. Furthermore, the insulinotropic effect of GIP is highly glucose-dependent (i.e., almost absent during fasting and hypoglycemia) [17, 21]. It is currently unknown whether the “GIP defect” also applies to the effects of GIP on bone turnover [22]. For these reasons, we investigated the effects of GIP during three distinct glycemic levels, respectively, on selected markers of bone homeostasis (CTX, P1NP, and PTH) in patients with T2D.
Material and Methods
Experimental procedures
This study is based on additional analyses of plasma from a previously published randomized, double-blind, crossover study, which included 12 patients with T2D [17]. Additional analyses were approved by the scientific ethics committee protocol number: H-D-2009-0078 amendment no. 29347. We refer to a previous publication and ClinicalTrials.gov (Identifier: NCT01414556) for details on materials and methods [17]. Briefly stated, we included male patients (N = 12) with T2D (age: 62 ± 5 years [mean ± SD]; body mass index: 29 ± 4 kg/m2; HbA1c: 6.5 ± 0.4% [48 ± 5 mmol/L]; fasting plasma glucose 7.9 ± 1.0 mmol/L; diabetes duration: 51 ± 11 months). Each patient was studied on 6 different days performed in randomized order: 2 days with “insulin-induced hypoglycemia”; 2 days with fasting hyperglycemia; and 2 days with “aggravated hyperglycemia.” During insulin-induced hypoglycemia, exogenous glucose was administered to keep plasma glucose in the range of 3 to 3.5 mmol/L, whereas insulin (Actrapid, Novo Nordisk, Bagsværd, Denmark) mixed with 1% human albumin was infused at a rate of 1 mU × kg-1 × min-1 from time -25 minutes until end of the study period. During days with “fasting hyperglycemia,” no insulin or glucose was administered. During aggravated hyperglycemia, the plasma glucose was raised to 1.5 × fasting values (resulting in a mean value of 12 mmol/L). On these matched days with similar glycemia, patients received an IV infusion of either GIP (4 pmol × kg-1 × min-1 for 15 minutes followed by 2 pmol × kg-1 × min-1 for the remaining 75 minutes to mimic endogenous postprandial plasma GIP excursions) or a matched volume of saline. Patients were investigated in the fasting state semirecumbently positioned in a hospital bed with cannulas inserted into contralateral cubital veins for infusions and blood samples, respectively. The arm used for sampling blood was wrapped in a heating pad (~50°C) for arterialization of the blood. Plasma glucose was measured bedside every 5 minutes, allowing the plasma glucose level to be clamped by an adjustable continuous infusion of 20% dextrose (w/v).
Ethics
Oral and written informed consent was obtained from all participants before inclusion. The study complied with the Declaration of Helsinki (fifth revision, Edinburgh, 2000). The original study was registered with clinicaltrials.gov (clinical trial reg. no. NCT01414556), and the research protocol was approved by the Scientific-Ethical Committee of the Capital Region of Denmark (reg. no. H-D-2009-0078 with amendment no. 29347).
Measurements
We refer to a previous publication for a description of glucose and GIP measurements [17]. CTX was measured using a commercially available sandwich ELISA kit according to the manufacturer’s instruction (serumCrossLaps ELISA, Immunodiagnostic Systems Nordic A/S, Copenhagen, Denmark). The ELISA uses highly specific monoclonal antibodies directed against the amino acid sequence EKAHD-β-GGR derived from the c-terminal telopeptide region of collagen 1. Plasma P1NP was measured using the IDS-iSYS intact P1NP assay (Immunodiagnostic Systems). Plasma PTH was measured using the IDS-iSYS Intact PTH assay. The P1NP and the PTH assays are chemiluminescence immunoassays and were carried out on a dedicated automated analyzer, iSYS (Immunodiagnostic Systems) according to the manufacturer’s instructions.
Statistical analysis
Results are reported as mean ± SD and in the figures as mean ± SEM. Baseline was the mean of 2 samples (time points -15 and 0 minutes). Potential differences in plasma concentrations of glucose, hormones, and biomarkers of bone turnover over time were explored with repeated-measures ANOVA reporting P values for differences over time (A), between interventions (i.e., GIP or placebo) (B), and for the interaction of intervention with time (AB). If a significant difference regarding treatment (GIP administration) or a significant interaction of treatment effects with time was identified, results at single time points were compared with Sidak-corrected multiple comparisons. P values (adjusted for multiple comparisons) < 0.05 were considered significant. Statistical evaluation and graphic presentation were performed using in GraphPad Prism 8 (La Jolla, CA).
Results
Glucose and GIP
Plasma insulin, glucagon, and glucose and GIP concentrations have previously been published [17]. Plasma glucose at baseline was similar on study days with an overall mean of 7.8 ± 1.0 mmol/L. During insulin-induced hypoglycemia, plasma glucose levels was lowered to 3.4 ± 0.3 mmol/L by 60 minutes and kept at that level until 90 minutes. During the 2 matched days with aggravated hyperglycemia, the mean plasma glucose was immediately raised and maintained at a mean of 12 ± 1.2 mmol/L until 90 minutes. Baseline values of GIP were similar with an overall mean of 20 ± 4.0 pmol/L. During days with GIP infusion, similar mean steady-state concentrations of intact GIP were reached (overall mean: 70 ± 10 pmol/L), whereas GIP levels remained stable at the basal level during saline infusion.
CTX
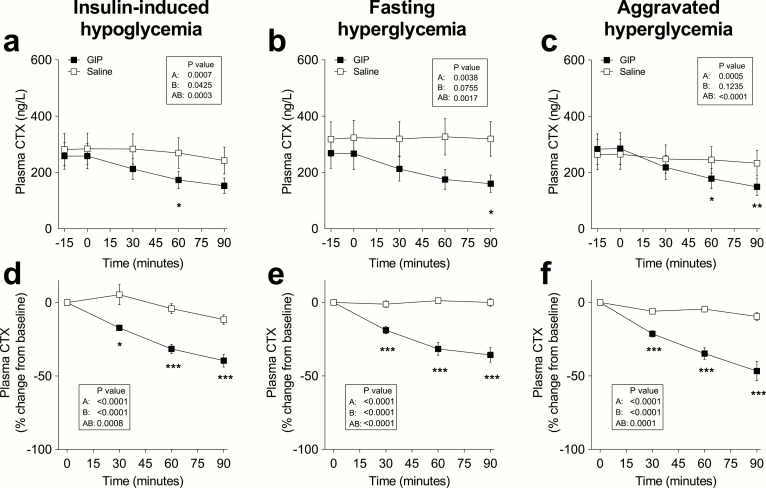
Plasma CTX concentrations at baseline were similar on all study days (overall mean: 0.280 ± 0.183 µg/L) (Fig. 1a). On days with insulin-induced hypoglycemia, CTX was increasingly suppressed by up to 40 ± 15% after 90 minutes of GIP administration compared with 12 ± 11% during placebo infusion (P < 0.0001). On days with fasting hyperglycemia, CTX was suppressed by up to 36 ± 15% during GIP administration, compared with 0 ± 9% during placebo infusion (P < 0.0001) (Fig. 1b). On days with aggravated hyperglycemia, CTX was suppressed by up to 47 ± 23% during GIP administration compared with 10 ± 9% during placebo infusion (P = 0.0005) (Fig. 1c).
Figure 1.
C-terminal telopeptide of type I collagen (CTX) levels during IV infusion of GIP (black squares) or saline (white squares) and expressed (a-c) as absolute plasma concentration or (d-f) percent of baseline during 6 separate days with either (a, d) insulin-induced hypoglycemia, (b, e) fasting hyperglycemia, or (c, f) aggravated hyperglycemia, respectively. Data are means ± SEM. Statistical analyses were done with repeated-measures ANOVA reporting P values for (A) differences over time, (B) between interventions (i.e., GIP or placebo), and (AB) for the interaction of intervention with time. Significant differences are indicated by asterisks according to Sidak’s multiple comparisons test: *P < 0.05; **P = 0.001-0.01; ***P < 0.001. GIP, glucose-dependent insulinotropic polypeptide.
P1NP
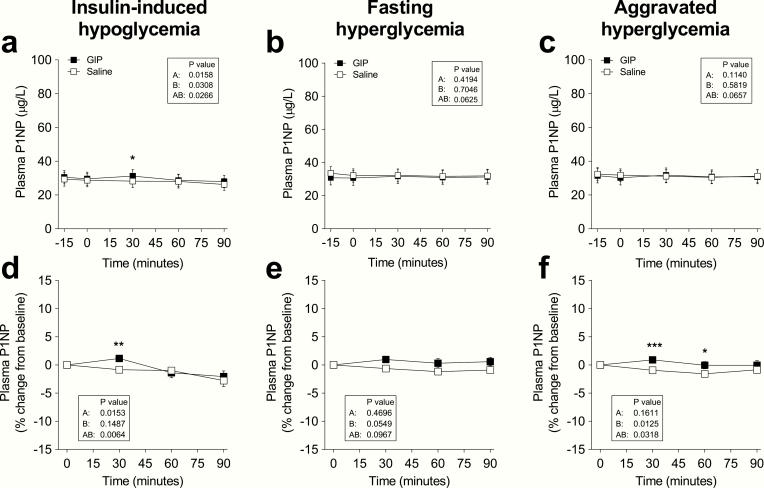
Plasma P1NP concentrations at baseline were similar on all study days (overall mean: 31 ± 14 µg/L) (Fig. 2a-c). On days with insulin-induced hypoglycemia, GIP increased P1NP concentrations after 30 minutes (P = 0.025). After 90 minutes, there were no statistically significant differences between P1NP changes during GIP and saline administration at all glycemic levels (Fig. 2d-f). The percentage change from baseline to 90 minutes during insulin-induced hypoglycemia was -5 ± 15% (GIP) vs. -9 ± 10% (saline), (P = 0.72); during fasting hyperglycemia, it was 2 ± 8% (GIP) vs. -3 ± 5% (saline), (P = 0.22); and during aggravated hyperglycemia, it was 1 ± 11% (GIP) vs. -3 ± 5% (saline), (P = 0.68).
Figure 2.
Procollagen type 1 N-terminal propeptide (P1NP) levels during IV infusion of GIP (black squares) or saline (white squares) and expressed (a-c) as absolute plasma concentration or (d-f) percent of baseline during 6 separate days with either (a, d) insulin-induced hypoglycemia, (b, e) fasting hyperglycemia, or (c, f) aggravated hyperglycemia, respectively. Data are means ± SEM. Statistical analyses were done with repeated-measures ANOVA reporting P values for differences (A) over time, (B) between interventions (i.e., GIP or placebo), and (AB) for the interaction of intervention with time. Significant differences are indicated by asterisks according to Sidak’s multiple comparisons test: *P < 0.05; **P = 0.001-0.01; ***P < 0.001. GIP, glucose-dependent insulinotropic polypeptide.
PTH
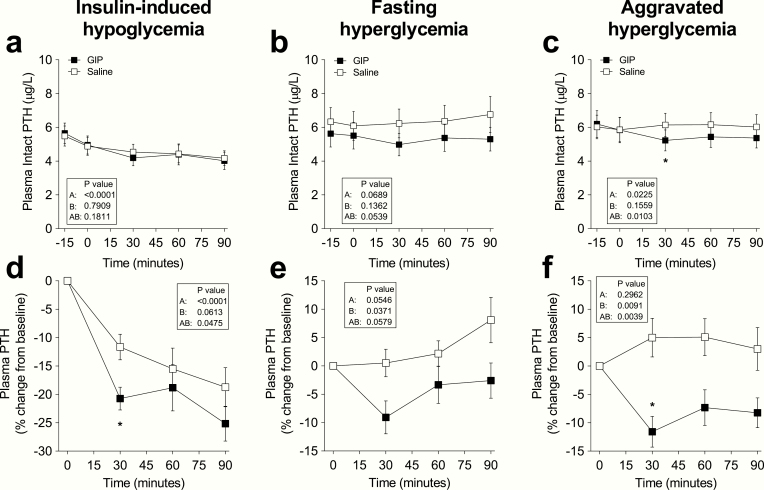
Plasma PTH concentrations at baseline were similar on all study days (overall mean 5.7 ± 2.4 pmol/L) (Fig. 3a-c). On days with aggravated hyperglycemia, PTH was suppressed by 11 ± 9.5% during GIP administration compared with a 5 ± 12% increase during saline infusion (P < 0.0001). Absolute PTH values were also reduced by the GIP infusion during insulin-induced hypoglycemia and fasting hyperglycemia, but these differences were statistically nonsignificant when compared with saline (P = 0.18 and P = 0.054, respectively). After 90 minutes, there were no statistically significant differences in PTH changes during GIP and saline administration at all glycemic levels (Fig. 3d-f). The percentage change from baseline to 90 minutes during insulin-induced hypoglycemia was -25 ± 11% (GIP) vs. -19 ± 12% (saline), (P = 0.07); during fasting hyperglycemia, it was -3 ± 11% (GIP) vs. 8 ± 14% (saline), (P = 0.09); and during aggravated hyperglycemia, it was -8 ± 9% (GIP) vs. 3 ± 13% (saline), (P = 0.08).
Figure 3.
Plasma concentrations of PTH levels during iv infusion of GIP (black squares) or saline (white squares) and expressed as (a-c) absolute plasma concentration or (d-f) percent of baseline during 6 separate days with either (a, d) insulin-induced hypoglycemia, (b, e) fasting hyperglycemia, or (c, f) aggravated hyperglycemia, respectively. Data are means ± SEM. Statistical analyses were done with repeated-measures ANOVA reporting P values for (A) differences over time, (B) between interventions (i.e., GIP or placebo), and (AB) for the interaction of intervention with time. Significant differences are indicated by asterisks according to Sidak’s multiple comparisons test: *P < 0.05. GIP, glucose-dependent insulinotropic polypeptide.
Discussion
Based on analyses of plasma from a randomized clinical crossover trial in patients with T2D, we demonstrate that GIP strongly suppresses the bone resorption biomarker CTX across various levels of plasma glucose levels (ranging from 3 to 12 mmol/L). With combined administration of glucose and GIP, we observed a GIP-induced 30% CTX reduction (as absolute and placebo-corrected values) after 1 hour. We did not include a control group in this study, but the magnitude of the saline-placebo-adjusted responses to GIP seem comparable to the 25% reduction observed after 1 hour in metabolically healthy individuals under similar research conditions [8]. The slightly greater CTX reduction in the present experiments occurs despite the fact that GIP only leads to a 1.4-fold increase in insulin secretion rate in this cohort of patients T2D [17], which is much lower than the 2.4-fold increase observed in metabolically healthy young individuals [18]. Thus, our findings seem to refute that the effects of GIP on bone resorption is impaired in patients with T2D, unlike the insulinotropic effect of GIP [17].
Interestingly, after glucose administration alone during aggravated hyperglycemia (i.e., when mean plasma glucose was raised from 8 to 12 mmol/L), the reduction in CTX was on average only 5% after 1 hour in these patients. In the aforementioned healthy individuals, CTX suppression induced by hyperglycemia alone (evaluated during a hyperglycemic clamp elevating the mean plasma glucose from 5 to 12 mmol/L) was approximately 25% after 1 hour [8]. Thus, based on such comparison, CTX suppression at aggravated hyperglycemia was lower in patients with T2D. However, during simultaneous GIP administration, the mean absolute CTX reduction of 47% after 90 minutes (at study end) compares quite well to the reductions in CTX in healthy individuals of around 50% during GIP administration at elevated glucose [8]. Thus, it seems as GIP partly compensated for the lack of glucose-induced CTX suppression in patients with T2D, a notion that requires further substantiation.
A reduction in CTX of 50% could be considered of a pharmacologically relevant magnitude if sustained over longer periods, as is the case with pharmacological antiresorptive treatments (e.g., bisphosphonate, RANKL inhibitors) used for fracture prevention in osteoporotic individuals [2]. Interestingly, the CTX reductions following antiresorptive pharmacological interventions are always coupled to a P1NP suppression [2]. In our experiments, GIP administration led to unaltered or even slightly increased bone formation depending on the prevailing glycemia (placebo-subtracted difference on P1NP of ~5%). The finding of a minor and short-lived (peaking after 30 minutes) stimulating effect on P1NP is similar to what has been observed in other studies [11, 23, 24]. Such slight increases in P1NP may be quite important because these occur at the same time with large decreases in CTX, thus reflecting a clear osteoanabolic effect. We also report slight suppressive effect of GIP on PTH, which was only statistically significant at 30 minutes during aggravated hyperglycemia. The clinical relevance of these modest changes in PTH and the potential contribution to the observed changes in CTX or P1NP is uncertain.
In a clinical context, our study has important limitations to consider. Particularly, the study setup, in which exogenous GIP was infused in high postprandial plasma concentrations after an overnight fast and without a control group limits the translatability to the normal postprandial state. Effects of GIP in the postprandial state may be better investigated by using a GIP receptor antagonist. Recently, a study using a novel GIP antagonist evaluated the effects of endogenous GIP on CTX excursions after a meal in patients with T2D and showed that the endogenous GIP response accounted for a placebo-adjusted CTX suppression of 20% [25]. Another limitation is the lack of measurement of other biochemical parameters relevant for the interpretation of bone homeostasis including among others calcitriol, calcium, phosphate, and alkaline phosphatase. The latter 2 were unaffected by a GIP infusion in a recent study [12].
The clinical implications of chronically activating the GIP axis is at present unknown, but novel long-acting GIP receptor agonists are in clinical development and may turn out to show clinically relevant bone-preserving effects. In support of a pharmacologically relevant effect, the available data suggest that the effect of GIP on CTX is dose dependent. Hence, a double dose of GIP (plasma GIP steady state 130 pmol/L) reduced CTX by 40% (placebo-subtracted at a similar level of hyperglycemia and after 1 hour) in patients with type 1 diabetes [11]. The long-term and dose-dependent effects of GIP merits further investigation. Based on the accumulating evidence, including the present study, postprandial GIP responses likely explain most, but not all, of the reduction in bone resorption occurring postprandially in healthy individuals as well as patients with T2D.
In conclusion, the incretion hormone GIP robustly suppresses bone resorption in patients with T2D. In contrast, exogenous glucose administration is relatively inefficient in suppressing bone resorption in these patients with T2D. Altogether, the effects of GIP on bone appear uncoupled from the glucoregulatory effects and our results lend further support to the already established role for GIP as a postprandial modulator of bone turnover in humans.
Acknowledgments
The authors are grateful to the participating patients and for laboratory assistance from J. Purtoft, N. Kjeldsen, and S. M. Schmidt from the Center for Diabetes Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark, and Nadia Quardon at the Department of Clinical Biochemistry, Rigshospitalet, Glostrup, University of Copenhagen, Copenhagen, Denmark.
Financial Support: The study was supported by an unrestricted grant from the Novo Nordisk Foundation.
Author Contributions: M.C. contributed to study design, the clinical experiments, and the data analysis as well as drafted the manuscript. A.L. contributed to data analysis. S.C. contributed to the clinical experiments. N.R.J. and J.J.H. contributed with biochemical measurements. F.K.K. contributed to study design and to data analysis. All authors revised and approved the final manuscript.
Glossary
Abbreviations
- CTX
carboxy-terminal collagen crosslinks
- GIP
glucose-dependent insulinotropic polypeptide
- P1NP
procollagen type 1 N-terminal propeptide
- T2D
type 2 diabetes
Additional Information
Disclosure Summary: M.C. is a minority shareholder of Antag Therapeutics. A.L. has received lecture fees from Novo Nordisk and Astra Zeneca. F.K.K. has served on scientific advisory panels and/or been part of speaker’s bureaus for, served as a consultant to, and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, MedImmune, MSD/Merck, Mundipharma, Norgine, Novo Nordisk, Sanofi, and Zealand Pharma; and is a minority shareholder of Antag Therapeutics. J.J.H. has served on scientific advisory panel and/or been part of speaker’s bureau for Novo Nordisk; and is a board memner and minority shareholder of Antag Therapeutics. T.V. has served on scientific advisory panels and/or speakers’ bureaus or has served as a consultant to and/or received research support from Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Eli Lilly, MSD/Merck, Mundipharma, Novo Nordisk, Sanofi. and SunPharma. N.R.J. has nothing to declare.
Data Availability: The datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References and Notes
- 1. Zaidi M, Yuen T, Sun L, Rosen CJ. Regulation of skeletal homeostasis. Endocr Rev. 2018;39(5):701-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eastell R, Szulc P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017;5(11):908-923. [DOI] [PubMed] [Google Scholar]
- 3. Bollag RJ, Zhong Q, Ding KH, et al. . Glucose-dependent insulinotropic peptide is an integrative hormone with osteotropic effects. Mol Cell Endocrinol. 2001;177(1-2):35-41. [DOI] [PubMed] [Google Scholar]
- 4. Bollag RJ, Zhong Q, Phillips P, et al. . Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology. 2000;141(3):1228-1235. [DOI] [PubMed] [Google Scholar]
- 5. Pacheco-Pantoja EL, Ranganath LR, Gallagher JA, Wilson PJ, Fraser WD. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol. 2011;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xie D, Cheng H, Hamrick M, et al. . Glucose-dependent insulinotropic polypeptide receptor knockout mice have altered bone turnover. Bone. 2005;37(6):759-769. [DOI] [PubMed] [Google Scholar]
- 7. Zhong Q, Itokawa T, Sridhar S, et al. . Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am J Physiol Endocrinol Metab. 2007;292(2):E543-E548. [DOI] [PubMed] [Google Scholar]
- 8. Nissen A, Christensen M, Knop FK, Vilsbøll T, Holst JJ, Hartmann B. Glucose-dependent insulinotropic polypeptide inhibits bone resorption in humans. J Clin Endocrinol Metab. 2014;99(11):E2325-E2329. [DOI] [PubMed] [Google Scholar]
- 9. Bjarnason NH, Henriksen EE, Alexandersen P, Christgau S, Henriksen DB, Christiansen C. Mechanism of circadian variation in bone resorption. Bone. 2002;30(1):307-313. [DOI] [PubMed] [Google Scholar]
- 10. Calanna S, Christensen M, Holst JJ, et al. . Secretion of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes: systematic review and meta-analysis of clinical studies. Diabetes Care. 2013;36(10):3346-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christensen MB, Lund A, Calanna S, et al. . Glucose-dependent insulinotropic polypeptide (GIP) inhibits bone resorption independently of insulin and glycemia. J Clin Endocrinol Metab. 2018;103(1):288-294. [DOI] [PubMed] [Google Scholar]
- 12. Gasbjerg LS, Hartmann B, Christensen MB, et al. . GIP’s effect on bone metabolism is reduced by the selective GIP receptor antagonist GIP(3-30)NH2. Bone. 2020;130:115079. [DOI] [PubMed] [Google Scholar]
- 13. Bellido T, Saini V, Pajevic PD. Effects of PTH on osteocyte function. Bone. 2013;54(2):250-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moayeri A, Mohamadpour M, Mousavi SF, Shirzadpour E, Mohamadpour S, Amraei M. Fracture risk in patients with type 2 diabetes mellitus and possible risk factors: a systematic review and meta-analysis. Ther Clin Risk Manag. 2017;13:455-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hygum K, Starup-Linde J, Harsløf T, Vestergaard P, Langdahl BL. Mechanisms in endocrinology: diabetes mellitus, a state of low bone turnover - a systematic review and meta-analysis. Eur J Endocrinol. 2017;176(3):R137-R157. [DOI] [PubMed] [Google Scholar]
- 16. Vilsbøll T, Knop FK, Krarup T, et al. . The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide-regardless of etiology and phenotype. J Clin Endocrinol Metab. 2003;88(10):4897-4903. [DOI] [PubMed] [Google Scholar]
- 17. Christensen MB, Calanna S, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide: blood glucose stabilizing effects in patients with type 2 diabetes. J Clin Endocrinol Metab. 2014;99(3):E418-E426. [DOI] [PubMed] [Google Scholar]
- 18. Christensen MB, Gasbjerg LS, Heimbürger SM, Stensen S, Vilsbøll T, Knop FK. GIP’s involvement in the pathophysiology of type 2 diabetes. Peptides. 2020;125:170178. [DOI] [PubMed] [Google Scholar]
- 19. Xu G, Kaneto H, Laybutt DR, et al. . Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes. 2007;56(6):1551-1558. [DOI] [PubMed] [Google Scholar]
- 20. Højberg PV, Vilsbøll T, Rabøl R, et al. . Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52(2):199-207. [DOI] [PubMed] [Google Scholar]
- 21. Christensen M, Vedtofte L, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes. 2011;60(12):3103-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stensen S, Gasbjerg LS, Helsted MM, Hartmann B, Christensen MB, Knop FK. GIP and the gut-bone axis - physiological, pathophysiological and potential therapeutic implications. Peptides. 2020;125:170197. [DOI] [PubMed] [Google Scholar]
- 23. Bergmann NC, Lund A, Gasbjerg LS, et al. . Separate and combined effects of GIP and GLP-1 infusions on bone metabolism in overweight men without diabetes. J Clin Endocrinol Metab. 2019;104(7):2953-2960. [DOI] [PubMed] [Google Scholar]
- 24. Skov-Jeppesen K, Svane MS, Martinussen C, et al. . GLP-2 and GIP exert separate effects on bone turnover: a randomized, placebo-controlled, crossover study in healthy young men. Bone. 2019;125:178-185. [DOI] [PubMed] [Google Scholar]
- 25. Stensen S, Gasbjerg LS, Krogh LS, et al. . 64-OR: postprandial effects of endogenous glucose-dependent insulinotropic polypeptide in type 2 diabetes. Diabetes. 2019;68(Supplement 1):64-OR. [Google Scholar]