Abstract
PURPOSE:
KRAS mutations and tumor location have been associated with response to targeted therapy among stage IV colorectal cancer patients in various trials. We aimed to conduct the first population-based examination of associations between KRAS mutations, tumor location and survival, and assess factors associated with documented KRAS testing.
METHODS:
Patients of stage IV adenocarcinoma of the colon/rectum diagnosed from 2010–2013 were extracted from SEER data. Analyses of patient characteristics, KRAS testing, and tumor location were conducted using logistic regression. Cox proportional hazards models assessed relationships of KRAS mutations, tumor location and risk of all-cause death.
RESULTS:
Of 22,542 patients, 30% received KRAS testing; 44% of those had mutations. Those tested tended to be younger, married, metropolitan area residents, and have private insurance or Medicare. Rates of KRAS testing also varied by Registry (range: 20–46%). Those with right-sided colon cancer compared (versus left-sided) tended to be older, female, black, have mucinous, KRAS-mutant tumors, and greater risk of death (HR: 1.27, 95% CI: 1.21, 1.30). KRAS mutations were not associated with greater risk of death in the overall population. However, they were associated with greater risk of death among left-sided patients (HR: 1.19; 95% CI: 1.05, 1.34).
CONCLUSION:
This large population-based study demonstrated that among patients initially diagnosed with stage IV colorectal cancer, right-sided colon cancer was associated with greater risk of death compared to left-sided cancer, and KRAS mutations were only associated with risk of death in left-sided colon cancer. An unexpected finding was that among stage IV patients, blacks were more strongly associated with right-sided cancer compared to whites. Future studies should further explore these associations and determine the role of biology vs. treatment differences. In addition, KRAS testing is increasing, but there is wide geographic variation where disparities related to insurance coverage and rurality may warrant further study.
BACKGROUND
Approximately 134,490 patients of colorectal cancer (CRC) were diagnosed in the U.S. in 2016.1 Of those, 21% were stage IV with only 13.5% five-year relative survival.2 While a small proportion of these patients can be cured with surgery and neoadjuvant or adjuvant treatments, most are incurable and treatment focuses on prolonging life and improving quality of life. Targeted agents such as epidermal growth factor receptor (EGFR) inhibitors, including cetuximab, can inhibit tumor growth, but studies have shown tumors with mutations in the KRAS gene (KRAS-mutant) respond poorly to anti-EGFR therapy (ClinicalTrials.gov identifier: NCT00113763, NCT00113776).3–9 In 2009, the National Comprehensive Cancer Network (NCCN) recommended KRAS testing for patients with stage IV CRC at time of diagnosis, and only patients with tumors of wild-type KRAS gene (KRAS-WT) be treated with anti-EGFR therapy.10 Subsequently, KRAS testing was included as a CRC site-specific factor (SSF 9) to be collected by National Cancer Institute (NCI) Surveillance, Epidemiology and End Results (SEER) registries beginning with patients diagnosed in 2010 as part of the Collaborative Stage (CS) Data Collection System, version 2.
In our previous study of the SEER KRAS variable, only 23% of patients diagnosed in 2010 with stage IV CRC were KRAS tested, and there was variation in testing rates among registries. Of those tested, there were no survival differences between the 40% of KRAS-mutant patients and the 60% KRAS-WT patients.11 This was unexpected because KRAS-WT patients could receive anti-EGFR to lengthen survival, however only one year of follow-up was assessed.
Recent studies suggest KRAS mutation status is not the only factor that should be considered when selecting chemotherapeutic interventions in stage IV CRC. CRC is a molecularly heterogeneous entity, resulting in differing mechanisms of carcinogenesis between sporadic right-sided colon cancers and left-sided colon cancers.12,13 Right-sided cancers are more commonly associated with female gender, older age, hypermutation of KRAS, PI3KCA (Phosphatidylinositol 3-kinase, catalytic, alpha polypeptide), increased frequency of microsatellite instability (MSI) and wild-type BRAF tumors.14 They are also more frequently associated with advanced disease, poorly-differentiated tumors, and mucinous histology compared to left-sided cancers).15 Left-sided cancers demonstrate increased expression of EGFR and higher frequency of chromosomal instability.15–17 Clinical outcomes based on tumor location alone have been debated, though many trials have shown KRAS-WT left-sided cancers may have better overall survival, progression-free survival, and improved response to anti-EGFR therapy compared to right-sided cancers. Two trials have demonstrated KRAS-WT left-sided cancers patients survived longer if their treatment included cetuximab, whereas KRAS-WT right-sided cancer patients survived longer if their treatment included bevacizumab (antibody against vascular endothelial growth factor A) (ClinicalTrials.gov identifier: NCT00265850).18
In light of these recent studies highlighting the importance of sidedness on survival, our primary objective was to examine both KRAS status and sidedness in stage IV CRC patients using population-based SEER data. Our second objective was to assess trends and factors associated with KRAS testing captured by SEER registries.
METHODS
Patients
Microscopically confirmed stage IV adenocarcinomas of the colon or rectum diagnosed from 2010–2013 were extracted from SEER Registries with at least 100 CRC patients using SEER*Stat (version 8.3.2).19 These registries included California, Georgia, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle-Puget Sound, Utah, Kentucky, Louisiana, and New Jersey. Patients were excluded if diagnosed on autopsy or death certificate only and had a non-specific ICD-O-3 site code (i.e., Colon, NOS). Access to SSF-9 (KRAS status) was granted by the National Cancer Institute. SEER guidelines for coding SSF-9 stated registrars should use all information gathered through completion of surgery(ies) in first course of treatment, or all information available within four months of the diagnosis date in the absence of disease progression, whichever is longer.20 The Human Subjects Office did not consider this study to be human subjects’ research.
Study Variables
Outcome variables included documentation of KRAS testing, KRAS results and survival as of December 31, 2013. Testing was considered performed if SSF 9 contained values of ‘abnormal (mutated)’ or ‘normal (wild-type)’; otherwise KRAS testing was considered not done if SSF-9 values contained ‘test ordered, results not in chart,’ ‘not done,’ or ‘unknown.’
Patient demographic variables at time of diagnosis included age, registry, sex, race/ethnicity, diagnosis year, insurance status, marital status, and residence in metropolitan vs. non-metropolitan/rural area. Tumor characteristics included location (right-sided = cecum, ascending colon, hepatic flexure, transverse vs. left-sided = splenic flexure, descending colon, sigmoid, rectosigmoid junction), histology (adenocarcinoma vs. mucinous adenocarcinoma), grade, T-stage, N-stage, M-stage, which was further broken down by metastatic site when sample size allowed, and single cancer vs multiple cancers (separate primary cancer before, during or after diagnosis of stage IV CRC). Treatment variables included surgery and radiation therapy (yes/no).
Statistical Analysis
Logistic regression was used to determine characteristics associated with receipt of KRAS testing and with right-sided cancer (vs. left). All variables listed above were considered for inclusion into the KRAS testing model, and age, sex, race, year of diagnosis, grade, TNM stage and single vs. multiple cancers were considered for inclusion into the right-sided CRC model because of their potential plausible relationship to tumor sidedness. Four Cox proportional hazards models were constructed to evaluate associations between KRAS testing, tumor location, and survival while controlling for patient, tumor, and treatment characteristics: 1) CRC patients regardless of KRAS testing status or tumor site (left vs right vs rectum); 2) CRC patients who received KRAS testing; 3) Left-sided patients who received KRAS testing; 4) Right-sided patients who received KRAS testing. Only patients diagnosed from 2010–2012 were included in survival analyses to allow for sufficient follow-up time. The following variables were included into all survival models regardless of significance: KRAS results, age, race, diagnosis year, insurance, marital status, stage group, T-stage, N-stage, and M-stage/metastatic sites. Analyses were conducted using SAS software (version 9.4, SAS Institute, Cary, NC).
RESULTS
There were 27,231 stage IV CRC patients identified and 22,542 (83%) met inclusion criteria (2,598 excluded because tumor site was non-specific, 29 were death certificate only, 1,272 were not microscopically confirmed, 731 had non-adenocarcinoma histology, and 59 were from Alaska). The overall KRAS testing rate was 30% (Table 1). Of the 6,794 tested, 44% were KRAS-mutant and 56% were KRAS-WT.
Table 1.
KRAS test results among Stage IV CRC patients by tumor site, 2010–2013 (N=22,542).
| Total | Rectum | |
|---|---|---|
| N=22,542 | N=6,699 | |
| N (%) | N (%) | |
| 010: Abnormal (mutated) | 3005 (13.3) | 790 (11.8) |
| 020: Normal (wild type) | 3789 (16.8) | 1107 (16.5) |
| 997: Test ordered, results not in chart | 268 (1.2) | 68 (1.0) |
| 998: Test not done | 8125 (36.0) | 2436 (36.4) |
| 999: Unknown | 7355 (32.6) | 2298 (34.3) |
| Yes | 6794 (30.1) | 1897 (28.3) |
| No | 15748 (69.9) | 4802 (71.7) |
KRAS Testing
Table 2 displays frequencies and row percentages for patient/tumor characteristics by KRAS testing status, as well as odds ratios (OR) and 95% confidence intervals (CI) from a logistic model in which the dependent variable was KRAS testing and the independent variables included all those listed in Table 2. Univariate analysis demonstrated that KRAS testing rates most substantially varied by age (43% for those <30 years vs. 15% for 80+), registry (46% in Seattle vs. 20% in Louisiana), diagnosis year (25% in 2010 vs. 35% in 2013), and marital status (33% for married vs. 21% for widowed). The following variables were significantly associated with KRAS testing in the logistic model (p<0.05): younger age, registry (California was referent because it had largest number of patients), later year of diagnosis, covered by private insurance or Medicare (vs. Medicaid or uninsured), married, metropolitan area residence, colon cancer (vs. rectal), well-differentiated grade (vs. unknown), N-stage ≥ N1a, and metastasis to liver or lung only, or to multiple organs (vs. other single organ/site or metastases not otherwise specified) (Table 2).
Table 2.
Patient, tumor, and treatment characteristics by KRAS testing, and odds of receiving testing (N=22,542).
| KRAS Testing | Adjusted OR* (95% CI) | |
|---|---|---|
| N=6,794 | ||
| N (%) | ||
| 0–29 | 77 (42.5) | 4.96 (3.59, 6.84) |
| 30–39 | 329 (47.8) | 5.26 (4.37, 6.34) |
| 40–49 | 996 (40.8) | 3.93 (3.44, 4.49) |
| 50–59 | 1766 (35.5) | 3.2 (2.84, 3.61) |
| 60–69 | 1854 (31.4) | 2.58 (2.31, 2.89) |
| 70–79 | 1196 (26.1) | 1.94 (1.73, 2.18) |
| 80+ | 576 (15.3) | 1.00 [Referent] |
| Female | 2972 (29.4) | 0.99 (0.93, 1.06) |
| Male | 3822 (30.8) | 1.00 [Referent] |
| California | 2473 (29.1) | 1.00 [Referent] |
| Connecticut | 397 (38.7) | 1.64 (1.42, 1.88) |
| Detroit | 417 (33.0) | 1.23 (1.07, 1.40) |
| Georgia | 848 (32.2) | 1.11 (1.00, 1.23) |
| Hawaii | 148 (39.8) | 1.81 (1.43, 2.28) |
| Iowa | 286 (30.5) | 1.15 (0.98, 1.35) |
| Kentucky | 394 (27.0) | 0.90 (0.79, 1.03) |
| Louisiana | 320 (20.0) | 0.59 (0.51, 0.67) |
| New Jersey | 637 (24.2) | 0.84 (0.76, 0.94) |
| New Mexico | 222 (43.2) | 2.17 (1.79, 2.63) |
| Seattle | 527 (45.7) | 2.13 (1.87, 2.43) |
| Utah | 125 (28.8) | 0.91 (0.73, 1.14) |
| White | 5193 (30.2) | 1.00 [Referent] |
| Black | 969 (28.6) | 0.97 (0.88, 1.06) |
| Other | 617 (32.2) | 0.95 (0.85, 1.06) |
| Unknown | 15 (23.4) | 0.59 (0.32, 1.07) |
| 2010 | 1386 (24.6) | 1.00 [Referent] |
| 2011 | 1670 (29.5) | 1.31 (1.20, 1.42) |
| 2012 | 1798 (31.8) | 1.46 (1.34, 1.59) |
| 2013 | 1940 (34.7) | 1.65 (1.52, 1.80) |
| Private/Medicare | 5301 (30.6) | 1.00 [Referent] |
| Medicaid | 1009 (28.6) | 0.82 (0.76, 0.90) |
| Uninsured | 370 (30.1) | 0.79 (0.69, 0.90) |
| Unknown | 114 (24.8) | 0.88 (0.70, 1.11) |
| Married | 3759 (33.4) | 1.00 [Referent] |
| Divorced/separated | 826 (30.8) | 0.87 (0.79, 0.96) |
| Widowed | 654 (20.8) | 0.85 (0.76, 0.94) |
| Single/never married | 1300 (30.2) | 0.79 (0.73, 0.86) |
| Unknown | 255 (21.9) | 0.59 (0.50, 0.68) |
| Metro area | 5983 (30.5) | 1.00 [Referent] |
| Non-metro area/rural | 810 (27.9) | 0.85 (0.77, 0.94) |
| Right side Colon | 2701 (29.6) | 1.00 (0.94, 1.07) |
| Left side Colon | 2849 (31.9) | 1.00 [Referent] |
| Rectum | 1244 (27.8) | 0.86 (0.79, 0.94) |
| Adenocarcinoma | 6150 (30.1) | 1.00 [Referent] |
| Mucinous adenocarcinoma | 644 (30.1) | 0.98 (0.88, 1.09) |
| Well (I) differentiated | 277 (29.9) | 1.00 [Referent] |
| Moderately (II) differentiated | 3817 (31.0) | 0.99 (0.85, 1.15) |
| Poorly (III) differentiated | 1431 (31.6) | 0.99 (0.84, 1.17) |
| Undifferentiated (IV) | 252 (34.4) | 1.06 (0.85, 1.32) |
| Unknown | 1017 (25.1) | 0.83 (0.70, 0.98) |
| ≤ T3 | 3347 (30.7) | 1.00 [Referent] |
| ≥ T4a | 2124 (32.7) | 1.04 (0.97, 1.12) |
| TX (Unknown) | 1323 (25.7) | 0.95 (0.87, 1.05) |
| N0 | 1840 (26.8) | 1.00 [Referent] |
| ≥ N1a | 4404 (33.5) | 1.16 (1.07, 1.24) |
| NX (Unknown) | 550 (21.8) | 0.77 (0.69, 0.87) |
| M1a: Liver or lung only | 2998 (30.7) | 1.00 [Referent] |
| M1a: Bone or brain only | 33 (20.5) | 0.68 (0.45, 1.01) |
| M1a: Other single organ/site | 374 (25.0) | 0.74 (0.65, 0.85) |
| M1b: Multiple organs/sites | 3139 (31.7) | 1.05 (0.98, 1.12) |
| M1NOS | 250 (20.9) | 0.67 (0.57, 0.78) |
| Yes | 5601 (31.3) | 1.08 (1.00, 1.17) |
| No | 1193 (25.8) | 1.00 [Referent] |
| Yes | 4221 (25.8) | 1.05 (0.96, 1.14) |
| No or Unknown | 2573 (32.6) | 1.00 [Referent] |
| Yes or recommended | 814 (26.8) | 0.90 (0.82, 1.00) |
| No/unknown/refused | 5980 (28.7) | 1.00 [Referent] |
Odds ratios are adjusted for all variables in table.
KRAS testing variation by registry and diagnosis year were examined and results are provided in Figure 1. Rates increased each year among most registries; Connecticut experienced the largest increase (103%) with 23% tested in 2010 and 46% in 2013, followed by Iowa (95%) with 21% tested in 2010 and 41% in 2013. A few registries showed some decreases in testing between years including Utah, Hawaii and Kentucky.
Figure 1.
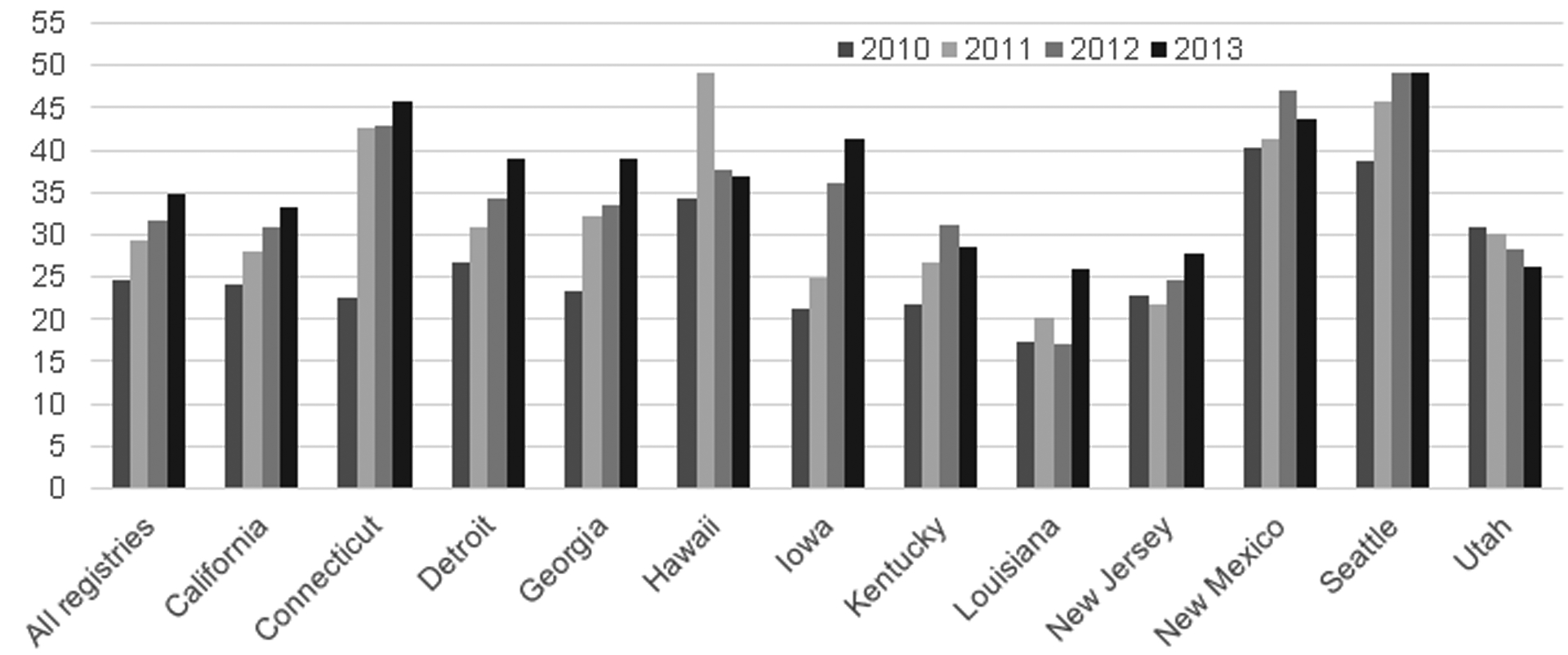
KRAS testing rates by SEER Registry and year, 2010–2013.
Sidedness
Rectal cancers were excluded from sidedness analyses, leaving 18,060 patients with colon cancer: 49% left-sided and 51% right-sided. Of those KRAS tested, 37% of left-sided patients were KRAS-mutant compared to 52% of right-sided patients. Table 3 displays data in a manner analogous to Table 2 by right- vs. left-sided, in which the dependent variable was right-sided cancer and the independent variables included patient and tumor characteristics in Table 3. The proportions of those with right-sided cancer most substantially varied by age (31% of those <30 years vs. 64% of 80+), histology (64% mucinous vs. 49% non-mucinous), grade (47% of well-differentiated vs. 63% of undifferentiated), and radiation (32% yes vs. 52% no). In the logistic model, variables associated with right-sided cancer were older age, female gender, black race, , mucinous histology, poorly or un-differentiated grade, N-stage > N1a, metastasis to other single site (not liver, lung, bone or brain) or to multiple organs, and history of multiple cancers. (Table 3).
Table 3.
Patient, tumor and treatment characteristics by right- or left-sided colon cancer, and odds of having right-sided versus left-sided colon cancer (N=18,060; excludes rectal cancer).
| Left-sided | Adjusted OR* (95% CI) | |
|---|---|---|
| N = 8,926 | ||
| N (%) | ||
| 0–29 | 93 (68.9) | 0.22 (0.15, 0.32) |
| 30–39 | 348 (67.3) | 0.24 (0.19, 0.29) |
| 40–49 | 1187 (64.1) | 0.29 (0.25, 0.33) |
| 50–59 | 2116 (56.1) | 0.42 (0.38, 0.46) |
| 60–69 | 2394 (50.5) | 0.53 (0.48, 0.59) |
| 70–79 | 1623 (42.7) | 0.74 (0.67, 0.81) |
| 80+ | 1165 (36.0) | 1.00 [Referent] |
| Female | 3798 (44.8) | 1.35 (1.27, 1.44) |
| Male | 5128 (53.5) | 1.00 [Referent] |
| White | 6682 (49.1) | 1.00 [Referent] |
| Black | 1275 (44.2) | 1.45 (1.33, 1.58) |
| Other | 936 (62.4) | 0.62 (0.55, 0.69) |
| Unknown | 33 (62.3) | 0.73 (0.41, 1.29) |
| 2010 | 2243 (48.9) | 1.00 [Referent] |
| 2011 | 2256 (49.7) | 0.99 (0.90, 1.07) |
| 2012 | 2268 (49.8) | 1.00 (0.92, 1.09) |
| 2013 | 2159 (49.3) | 1.04 (0.96, 1.14) |
| Adenocarcinoma | 8256 (51.0) | 1.00 [Referent] |
| Mucinous adenocarcinoma | 670 (36.0) | 1.66 (1.49, 1.84) |
| Well (I) differentiated | 390 (53.1) | 1.00 [Referent] |
| Moderately (II) differentiated | 5137 (52.8) | 1.06 (0.90, 1.24) |
| Poorly (III) differentiated | 1473 (38.4) | 1.75 (1.48, 2.07) |
| Undifferentiated (IV) | 249 (36.8) | 1.74 (1.39, 2.17) |
| Unknown | 1677 (54.4) | 1.05 (0.89, 1.25) |
| ≤ T3 | 4211 (50.1) | 1.00 [Referent] |
| ≥ T4a | 2631 (45.6) | 1.03 (0.95, 1.10) |
| TX (Unknown) | 2084 (53.8) | 1.00 (0.91, 1.10) |
| N0 | 2940 (56.3) | 1.00 [Referent] |
| ≥ N1a | 4956 (45.3) | 1.55 (1.44, 1.67) |
| NX (Unknown) | 1030 (54.3) | 1.10 (0.98, 1.23) |
| M1a: Liver or lung only | 4057 (51.9) | 1.00 [Referent] |
| M1a: Bone or brain only | 50 (51.6) | 0.94 (0.62, 1.43) |
| M1a: Other single organ/site | 536 (44.3) | 1.15 (1.01, 1.31) |
| 4262 (52.8) | 3816 (47.2) | 1.13 (1.06, 1.21) |
| M1NOS | 467 (54.1) | 0.86 (0.74, 0.99) |
| Yes | 7225 (50.7) | 0.91 (0.85, 0.99) |
| No | 1701 (44.5) | 1.00 [Referent] |
| Yes | 5335 (46.6) | Not included in model |
| No or Unknown | 3591 (54.4) | |
| Yes or recommended | 683 (68.5) | Not included in model |
| No/unknown/refused | 8243 (48.3) | |
| Normal | 1790 (57.9) | |
| Mutated | 1059 (43.1) | Not included in model |
| No Testing Done | 6077 (48.6) |
Odds ratios adjusted for all variables in table unless otherwise indicated.
Survival
There were 16,952 CRC patients diagnosed from 2010–2012 and included in survival analyses. Of those, 46% (n=7,743) died within 12 months of diagnosis, 50% (n=8,438) survived more than 12 months, and 5% (n=771) had less than 12 months of follow-up but were last known as alive. KRAS testing rates across these groups differed significantly: 21% vs. 35% vs. 33% respectively (p<0.0001). The proportion of right-sided cancer across groups also differed significantly: 57% vs. 44% vs. 51% respectively (p<0.0001).
Among all 16,952 CRC patients, Cox models showed the following variables were associated with increased hazard of death: older age, male gender, black race, earlier diagnosis year, Medicaid or no insurance, marital status of single, divorced or widowed, poorly or undifferentiated grade, T-stage ≥T4a or unknown, N-stage ≥N1a or unknown, metastasis to bone or brain only, multiple organs, or NOS, no surgery, no radiation and no KRAS testing (Table 4). Compared to left-sided cancer, those with right-sided cancer had greater risk of death (HR: 1.27, CI: 1.22, 1.32) and those with rectal cancer had lower risk (HR: 0.90, CI: 0.85, 0.96). There was no significant difference in risk between those with KRAS-mutant vs. KRAS-WT.
Table 4.
Hazard ratios for stage IV patients of colorectal cancer: all patients and those with KRAS testing, 2010–12.
| All Patients | KRAS Tested Patients Only | ||
|---|---|---|---|
| (N=16,952) | (N=4,854) | ||
| Adjusted HR* (95% CI) | Adjusted HR* (95% CI) | ||
| Normal | 1.00 [Referent] | 1.00 [Referent] | |
| Mutated | 1.04 (0.97, 1.12) | 1.04 (0.96, 1.12) | |
| No Testing Done | 1.31 (1.25, 1.39) | - | |
| 0–29 | 1.00 [Referent] | 1.00 [Referent] | |
| 30–39 | 0.98 (0.77, 1.26) | 1.13 (0.77, 1.67) | |
| 40–49 | 1.01 (0.80, 1.26) | 1.10 (0.77, 1.58) | |
| 50–59 | 1.16 (0.93, 1.45) | 1.28 (0.90, 1.83) | |
| 60–69 | 1.39 (1.11, 1.73) | 1.51 (1.06, 2.15) | |
| 70–79 | 1.83 (1.46, 2.28) | 1.80 (1.25, 2.57) | |
| 80+ | 2.83 (2.26, 3.54) | 2.73 (1.89, 3.96) | |
| White | 1.00 [Referent] | 1.00 [Referent] | |
| Black | 1.11 (1.06, 1.17) | 1.18 (1.06, 1.30) | |
| Other | 0.92 (0.86, 0.99) | 0.94 (0.85, 1.03) | |
| 2010 | 1.00 [Referent] | 1.00 [Referent] | |
| 2011 | 0.99 (0.95, 1.03) | 0.95 (0.87, 1.04) | |
| 2012 | 0.94 (0.89, 0.98) | 0.93 (0.84, 1.03) | |
| Private/Medicare | 1.00 [Referent] | 1.00 [Referent] | |
| Medicaid | 1.24 (1.18, 1.31) | 1.19 (1.07, 1.32) | |
| Uninsured | 1.32 (1.21, 1.43) | 1.25 (1.06, 1.47) | |
| Unknown | 1.10 (0.97, 1.25) | 1.23 (0.92, 1.64) | |
| Married | 1.00 [Referent] | 1.00 [Referent] | |
| Divorced/separated | 1.10 (1.04, 1.17) | 1.11 (0.98, 1.23) | |
| Widowed | 1.19 (1.12, 1.26) | 1.27 (1.12, 1.43) | |
| Single/never married | 1.20 (1.14, 1.27) | 1.23 (1.12, 1.36) | |
| Unknown | 1.00 (0.92, 1.09) | 0.85 (0.69, 1.05) | |
| Right side Colon | 1.27 (1.22, 1.32) | 1.39 (1.28, 1.51) | |
| Left side Colon | 1.00 [Referent] | 1.00 [Referent] | |
| Rectum | 0.90 (0.85, 0.96) | 0.90 (0.81, 1.01) | |
| Well (I) differentiated | 1.00 [Referent] | 1.00 [Referent] | |
| Moderately (II) differentiated | 0.97 (0.89, 1.07) | 1.05 (0.87, 1.27) | |
| Poorly (III) differentiated | 1.43 (1.30, 1.58) | 1.68 (1.38, 2.05) | |
| Undifferentiated (IV) | 1.56 (1.37, 1.78) | 1.85 (1.44, 2.39) | |
| Unknown | 1.13 (1.02, 1.24) | 1.25 (1.02, 1.53) | |
| ≤ T3 | 1.00 [Referent] | 1.00 [Referent] | |
| ≥ T4a | 1.26 (1.20, 1.32) | 1.23 (1.13, 1.34) | |
| TX (Unknown) | 1.14 (1.07, 1.20) | 1.09 (0.97, 1.22) | |
| N0 | 1.00 [Referent] | 1.00 [Referent] | |
| ≥ N1a | 1.10 (1.05, 1.15) | 1.07 (0.97, 1.17) | |
| NX (Unknown) | 1.17 (1.10, 1.25) | 1.15 (1.00, 1.32) | |
| M1a: Liver or lung only | 1.00 [Referent] | - | |
| M1a: Bone or brain only | 1.77 (1.45, 2.18) | - | |
| M1a: Other single organ/site | 0.78 (0.72, 0.85) | **1.00 [Referent] | |
| M1b: Multiple organs/sites | 1.37 (1.32, 1.43) | 1.42 (1.31, 1.53) | |
| M1NOS | 1.28 (1.18, 1.39) | 1.21 (0.98, 1.49) | |
| Yes | 0.48 (0.46, 0.51) | 0.52 (0.47, 0.58) | |
| No or Unknown | 1.00 [Referent] | 1.00 [Referent] | |
| Yes or recommended | 0.75 (0.70, 0.80) | NS | |
| No/unknown/refused | 1.00 [Referent] | NS | |
| Female | 0.91 (0.87, 0.94) | NS | |
| Male | 1.00 [Referent] | NS | |
Hazard ratios determined using manual backward selection to retain only those variables significant at p <0.05, except the following variables which were forced into each model: KRAS results, age, race, diagnosis year, insurance, marital status, T stage, N stage, and M Stage/Metastatic sites.
Due to small numbers, metastatic sites were not included in the KRAS tested patients only model; overall M stage was used.
Cox models were run on the subset of patients who had KRAS testing (n=4,854) and similar relationships emerged as in the model with the 16,952 patients. KRAS mutations were not associated with risk of death, while right-sided cancer was associated with a greater risk of death compared to left-sided and rectal cancer (Table 4). However, when separate models were run for the 2,064 left-sided patients and the 1,930 right-sided patients, having a KRAS mutation was associated with greater risk of death among left-sided patients (HR: 1.18, CI: 1.05, 1.33) but not right-sided (HR: 0.93, CI: 0.83, 1.03). In both left- and right-sided models, advanced age, single/never married, higher grade, T-stage ≥ 4a, M-stage 1b, and no surgical treatment were associated with increased hazard of death (Table 5). For right-sided only, those diagnosed in earlier years had greater risk of death. For left-sided only, black race, Medicaid or no insurance, widowed, unknown N-stage, and metastases NOS were associated with greater risk of death.
Table 5.
Hazard ratios for stage IV colon cancer patients who received KRAS testing: right vs. left, diagnosis years 2010–12. (Excluded rectal cancer patients)
| Right-sided (N=1,930) | Left-sided (N=2,064) | |
|---|---|---|
| Adjusted HR* (95% CI) | Adjusted HR* (95% CI) | |
| Normal | 1.00 [Referent] | 1.00 [Referent] |
| Mutated | 0.93 (0.83, 1.03) | 1.18 (1.05, 1.33) |
| 0–29 | 1.00 [Referent] | 1.00 [Referent] |
| 30–39 | 0.88 (0.41, 1.89) | 1.09 (0.64, 1.87) |
| 40–49 | 0.91 (0.44, 1.87) | 1.14 (0.69, 1.90) |
| 50–59 | 1.06 (0.52, 2.15) | 1.40 (0.85, 2.30) |
| 60–69 | 1.29 (0.63, 2.62) | 1.51 (0.92, 2.49) |
| 70–79 | 1.44 (0.71, 2.93) | 1.93 (1.16, 3.22) |
| 80+ | 2.17 (1.05, 4.45) | 2.94 (1.72, 5.01) |
| White | 1.00 [Referent] | 1.00 [Referent] |
| Black | 0.98 (0.85, 1.15) | 1.39 (1.18, 1.63) |
| Other | 0.81 (0.64, 1.03) | 0.88 (0.73, 1.06) |
| 2010 | 1.00 [Referent] | 1.00 [Referent] |
| 2011 | 0.89 (0.79, 1.02) | 1.03 (0.89, 1.18) |
| 2012 | 0.84 (0.73, 0.97) | 1.01 (0.86, 1.18) |
| Private/Medicare | 1.00 [Referent] | 1.00 [Referent] |
| Medicaid | 1.18 (0.99, 1.40) | 1.26 (1.07, 1.47) |
| Uninsured | 1.32 (0.99, 1.75) | 1.34 (1.04, 1.73) |
| Unknown | 1.11 (0.66, 1.87) | 1.21 (0.79, 1.86) |
| Married | 1.00 [Referent] | 1.00 [Referent] |
| Divorced/separated | 1.05 (0.88, 1.25) | 1.05 (0.87, 1.26) |
| Widowed | 1.18 (1.00, 1.39) | 1.24 (1.00, 1.54) |
| Single/never married | 1.23 (1.05, 1.45) | 1.24 (1.06, 1.44) |
| Unknown | 0.93 (0.68, 1.29) | 0.87 (0.62, 1.22) |
| Well (I) differentiated | 1.00 [Referent] | 1.00 [Referent] |
| Moderately (II) differentiated | 0.96 (0.70, 1.32) | 1.26 (0.95, 1.68) |
| Poorly (III) differentiated | 1.40 (1.02, 1.93) | 2.05 (1.51, 2.78) |
| Undifferentiated (IV) | 1.51 (1.03, 2.21) | 2.14 (1.42, 3.23) |
| Unknown | 1.21 (0.86, 1.70) | 1.31 (0.96, 1.79) |
| ≤ T3 | 1.00 [Referent] | 1.00 [Referent] |
| ≥ T4a | 1.24 (1.10, 1.41) | 1.22 (1.07, 1.40) |
| TX (Unknown) | 1.05 (0.86, 1.28) | 1.10 (0.92, 1.32) |
| N0 | 1.00 [Referent] | 1.00 [Referent] |
| ≥ N1a | 1.14 (0.98, 1.32) | 1.08 (0.93, 1.25) |
| NX (Unknown) | 1.24 (0.98, 1.58) | 1.25 (1.01, 1.55) |
| M1a | 1.00 [Referent] | 1.00 [Referent] |
| M1b | 1.28 (1.14, 1.44) | 1.53 (1.35, 1.74) |
| M1NOS | 0.99 (0.69, 1.42) | 1.66 (1.21, 2.29) |
| Yes | 0.53 (0.45, 0.64) | 0.49 (0.42, 0.58) |
| No or Unknown | 1.00 [Referent] | 1.00 [Referent] |
Manual backward selection was used to retain variables significant at p <0.05, except the following which were forced into all models: KRAS results, age, registry, race, diagnosis year, insurance, marital status, T stage, N stage, & M stage.
Due to small numbers, metastatic sites were not included in Right- and Left-sided models; overall M stage was used instead.
DISCUSSION
Despite NCCN recommending all stage IV CRC patients be tested for KRAS mutations at time of diagnosis, only 30% of patients diagnosed in 2010–2013 were tested according to SEER data. Overall testing rates increased from 25% in 2010 to 35% in 2013, and there was variation by registry with almost half of 2013 patients having documentation of testing in Seattle, compared to only one-quarter of patients in Louisiana. Similar to our findings from 2010 patients, those recorded as having testing tended to be younger, married and living in metro areas.11 However, the current analysis also showed those with private insurance or Medicare were more likely to receive testing than those with Medicaid or no insurance. Given the high cost of anti-EGFR therapy—approximately $25,000 for an 8-week cycle—there may be disparities in who is offered KRAS testing based on ability to pay for anti-EGFR therapy.21 Patients recorded as not having KRAS testing had greater risk of death after controlling for other factors. This could potentially reflect that those tested received higher quality care, and/or were offered EGFR inhibitors which extended their lives. It is also possible oncologists were less likely to recommend KRAS testing for patients who had poor prognoses, or patients with poor prognoses may have declined to receive targeted therapy and therefore were not tested.
Among those who received KRAS testing according to SEER data, KRAS mutations occurred more frequently in right-sided cancer patients, which is consistent with previous findings.14,22 Similar to an evaluation of European clinical trial patients by Missiaglia (2014), we found right-sided cancer was significantly associated with poorly-differentiated tumors of mucinous histology penetrating to the surface of the visceral peritoneum or beyond (≥T4a), lymph node metastasis (≥N1a), and metastasis to more than one organ/site or the peritoneum (stage IVB).15 In addition, among those diagnosed with stage IV colon cancer, blacks were more likely to have right-sided cancer. Other studies examining all stages of colon cancer have detected an association between black race and right-sided cancer.23,24
In Cox models including patients with left- or right-sided cancers, right-sided cancer was associated with greater risk of death after controlling for patient/tumor characteristics, which is consistent with findings from a recent meta-analysis.25 One possible explanation is that right-sided cancer is associated with increased BRAF mutations, and BRAF mutations are associated with worse prognosis and poorer response to cetuximab.26 BRAF mutation status was not available in SEER data so we could not explore this as a potential driver of poorer survival among right-sided patients.
In the overall survival analysis of KRAS-tested patients, we did not find a survival advantage for patients with KRAS-WT tumors compared to KRAS-mutant tumors. We did, however, find a survival advantage for KRAS-WT tumors within the population of patients with left-sided tumors. One explanation for not finding a survival advantage in the overall study population (not stratified by tumor location) may be that EGFR-inhibitors are only effective in left-sided tumors and not right-sided tumors. This explanation is supported by a retrospective analysis of the Phase III CALGB/SWOG 80405 clinical trial (ClinicalTrials.gov identifier: NCT00265850)27 by Venook et al, in which the researchers found that among patients who received cetuximab, those with left-sided tumors lived 37.5 months, whereas those with right-sided tumors survived only 16.4 months. Furthermore, they found among those with right-sided tumors, treatment with bevacizumab was associated with longer survival compared to cetuximab (24.5 months vs. 16.4 months), and conversely, among patients with left-sided tumors, treatment with cetuximab was associated with longer survival compared to bevacizumab (37.5 months vs. 32.1 months).28 Our results are consistent with the Venook study findings and support the concept that left-sided KRAS-WT primary tumors are more likely to benefit from the anti-EGFR targeted therapy; therefore, these patients’ outcomes are better than those with left-sided KRAS-mutant primary tumors. In addition, our results suggest that patients with right-sided KRAS-WT primary tumors do not benefit from the anti-EGFR targeted therapy; therefore, these patients’ outcomes are not better than those with right-sided KRAS-mutant primary tumors. This may be due to more frequent active signaling via the EGFR pathway in left-sided compared to right-sided cancers, which may in turn make left-sided cancer more responsive to anti-EGFR therapy.15,29 Also, as previously mentioned, right-sided patients likely had more BRAF mutations which are more resistant to anti-EGFR therapy.30–32 In contrast to anti-EGFR therapy, the effect of bevacizumab has been shown to be independent of tumor location.34 The nearly significant hazard ratio for KRAS-mutant right-sided tumors was 0.92 (CI: 0.83, 1.03) compared to KRAS-WT right-sided tumors, which could potentially suggest worse survival for those who received cetuximab instead of bevacizumab. This could explain why models containing both right- and left-sided patients did not show significant survival advantage of KRAS-WT status, and highlights the importance of stratification by tumor location.
Our study has several limitations. As previously mentioned, we did not have information about BRAF mutations. Also, the SEER program does not release data on chemotherapy in the Public Use Dataset due to concerns about the completeness of this information, and we cannot assume that all patients who received KRAS testing with a WT result received anti-EGFR therapy. As recommended by NCCN, KRAS testing may have occurred at the time of diagnosis for planning purposes,35,36 but then traditional chemotherapy may have still been given as the first line of treatment due to physician or patient preference, or due to financial barriers associated with the very high cost of anti-EGFR therapy.
In addition, the KRAS variable is still relatively new, and increasing testing rates may be driven in part by more complete capture of KRAS information by registrars as they gain more experience in collecting this variable. Furthermore, a quality control study of the KRAS variable in Iowa and Louisiana patients found coding guidelines were being applied somewhat inconsistently related to timing of the testing. Some included instances of KRAS testing occurring several months post-diagnosis in determining second-course treatment, whereas others only counted testing within the first 4 months post-diagnosis per coding guidelines. Also, the primary source of information for cancer registries is hospital records. Given that KRAS mutation analysis is a referral test in most institutions, and often ordered by oncologists, some test results may only be available in physician office records and not always available to registrars.6 While these data issues may have some impact on the accuracy of the KRAS data, there is currently no other population-based source that can provide a more accurate assessment of KRAS testing across the nation.
Despite limitations, the study has important strengths. We were able to assess KRAS testing rates across patient, tumor, and treatment characteristics, and examine relationships between KRAS status, sidedness and survival using population-based data that avoided the selection bias inherent in the clinical trial setting. The SEER population includes an estimated 28% of cancers in the U.S.37
Our results have several implications for future research. First, examining health disparities related to KRAS testing and subsequent treatment with anti-EGFR therapy with respect to race, insurance status and rurality is warranted. Similarly, exploring associations between right-sided cancer and black race among those with stage IV cancer is critical given the poorer prognosis associated with right-sided cancer. Also, assessing other variables that could alter prognosis, such as micro-satellite instability (MSI) and BRAF is important, but difficult to do on a population basis since MSI and BRAF are not required elements for SEER registries. By better understanding how sidedness and molecular characteristics of right-sided vs left-sided tumors affect response to therapy, patient response to treatment can be enhanced. . Clinical trials in stage IV CRC should incorporate as much information as possible regarding tumor sidedness and various molecular tumor markers in order to determine optimal targeted therapy regimens.
Grant Support:
This work was supported in part under NIH/NCI contract number HHSN261201000030C with Louisiana State University Health Sciences Center (VWC); NIH/NCI contract number HHSN261201300020I with University of Iowa (MEC, AK, CFL).
Footnotes
This study has not been presented at any conferences or meetings.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review 1975 – 2010 In: National Cancer Institute ed. Bethesda, MD; 2013. [Google Scholar]
- 3.Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27(12):2091–2096. [DOI] [PubMed] [Google Scholar]
- 4.De Roock W, Jonker DJ, Di Nicolantonio F, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304(16):1812–1820. [DOI] [PubMed] [Google Scholar]
- 5.Lievre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26(3):374–379. [DOI] [PubMed] [Google Scholar]
- 6.Morton RF, Hammond EH. ASCO Provisional Clinical Opinion: KRAS, Cetuximab, and Panitumumab-Clinical Implications in Colorectal Cancer. J Oncol Pract. 2009;5(2):71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vale CL, Tierney JF, Fisher D, et al. Does anti-EGFR therapy improve outcome in advanced colorectal cancer? A systematic review and meta-analysis. Cancer Treat Rev. 2012;38(6):618–625. [DOI] [PubMed] [Google Scholar]
- 8.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757–1765. [DOI] [PubMed] [Google Scholar]
- 9.Tejpar S, Peeters M, Humblet Y, et al. Relationship of efficacy with KRAS status (wild type versus mutant) in patients with irinotecan-refractory metastatic colorectal cancer, treated with irinotecan and escalating doses of cetuximab: the EVEREST experience (preliminary data). Journal of Clinical Oncology. 2008;26(Suppl 1: Abstract 4001). [Google Scholar]
- 10.Engstrom PF, Arnoletti JP, Benson AB 3rd, et al. NCCN Clinical Practice Guidelines in Oncology: colon cancer. J Natl Compr Canc Netw. 2017;7(8):778–831. [DOI] [PubMed] [Google Scholar]
- 11.Charlton ME, Karlitz JJ, Schlichting JA, Chen VW, Lynch CF. Factors Associated With Guideline-recommended KRAS Testing in Colorectal Cancer Patients: A Population-based Study. Am J Clin Oncol. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen H, Yang J, Huang Q, et al. Different treatment strategies and molecular features between right-sided and left-sided colon cancers. World J Gastroenterol. 2015;21(21):6470–6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee GH, Malietzis G, Askari A, Bernardo D, Al-Hassi HO, Clark SK. Is right-sided colon cancer different to left-sided colorectal cancer? - a systematic review. Eur J Surg Oncol. 2015;41(3):300–308. [DOI] [PubMed] [Google Scholar]
- 14.Brule SY, Jonker DJ, Karapetis CS, et al. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer. 2015;51(11):1405–1414. [DOI] [PubMed] [Google Scholar]
- 15.Missiaglia E, Jacobs B, D’Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25(10):1995–2001. [DOI] [PubMed] [Google Scholar]
- 16.Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol. 2008;15(9):2388–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nawa T, Kato J, Kawamoto H, et al. Differences between right- and left-sided colon cancer in patient characteristics, cancer morphology and histology. J Gastroenterol Hepatol. 2008;23(3):418–423. [DOI] [PubMed] [Google Scholar]
- 18.Heinemann V vWL, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S, et al. Gender and tumor location as predictors for efficacy: Influence on endpoints in first-line treatment with FOLFIRI in combination with cetuximab or bevacizumab in the AIO KRK 0306 (FIRE3) trial. J Clin Oncol 2014;32:5s(suppl; abstr 3600). [Google Scholar]
- 19.American Joint Committee on Cancer. CS Collaborative Stage Data Collection System.
- 20.National Cancer Institute. SEER Training Modules: General Guidelines for Collaborative Stage. In: National Institutes of Health ed; 2016. [Google Scholar]
- 21.Shankaran V, Ortendahl JD, Purdum AG, et al. Cost-Effectiveness of Cetuximab as First-line Treatment for Metastatic Colorectal Cancer in the United States. Am J Clin Oncol. 2015. [DOI] [PubMed] [Google Scholar]
- 22.Tong JH, Lung RW, Sin FM, et al. Characterization of rare transforming KRAS mutations in sporadic colorectal cancer. Cancer Biol Ther. 2014;15(6):768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carethers JM, Murali B, Yang B, et al. Influence of race on microsatellite instability and CD8+ T cell infiltration in colon cancer. PLoS One. 2014;9(6):e100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troisi RJ, Freedman AN, Devesa SS. Incidence of colorectal carcinoma in the U.S.: an update of trends by gender, race, age, subsite, and stage, 1975–1994. Cancer. 1999;85(8):1670–1676. [PubMed] [Google Scholar]
- 25.Petrelli F, Tomasello G, Borgonovo K, et al. Prognostic Survival Associated With Left-Sided vs Right-Sided Colon Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016. [DOI] [PubMed] [Google Scholar]
- 26.De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11(8):753–762. [DOI] [PubMed] [Google Scholar]
- 27.Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. Jama. 2017;317(23):2392–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venook AP. Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). Journal Clinical Oncology. 2016;34(suppl; abstr 3504). [Google Scholar]
- 29.Kuramochi H, Nakamura A, Nakajima G, et al. PTEN mRNA expression is less pronounced in left- than right-sided colon cancer: a retrospective observational study. Bmc Cancer. 2016;16:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu HC, Thiam TK, Lu YJ, et al. Mutations of KRAS/NRAS/BRAF predict cetuximab resistance in metastatic colorectal cancer patients. Oncotarget. 2016;7(16):22257–22270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loupakis F, Ruzzo A, Cremolini C, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer. 2009;101(4):715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Therkildsen C, Bergmann TK, Henrichsen-Schnack T, Ladelund S, Nilbert M. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: A systematic review and meta-analysis. Acta Oncol. 2014;53(7):852–864. [DOI] [PubMed] [Google Scholar]
- 33.Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loupakis F, Yang D, Yau L, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer. 2017;Version 3.2017. [DOI] [PubMed] [Google Scholar]
- 36.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. 2017;Version 1.2017. [DOI] [PubMed] [Google Scholar]
- 37.U.S. Department of Health and Human Services. SEER Surveillance, Epidemiology, and End Results. In: National Institutes of Health ed; 2012. [Google Scholar]


