Abstract
Sclerostin antibody (Scl-Ab) is an anabolic bone agent that has been shown to increase bone mass in clinical trials of adult diseases of low bone mass, such as osteoporosis and osteogenesis imperfecta (OI). Its use to decrease bone fragility in pediatric OI has shown efficacy in several growing mouse models, suggesting translational potential to pediatric disorders of low bone mass. However, the effects of pharmacologic inhibition of sclerostin during periods of rapid growth and development have not yet been described with respect to the cranium, where lifelong deficiency of functioning sclerostin leads to patterns of excessive bone growth, cranial compression, and facial palsy.
In the present study, we undertook dimensional and volumetric measurements in the skulls of growing Brtl/+ OI mice treated with Scl-Ab to examine whether therapy induced phenotypic changes similar to those observed clinically in patients with Sclerosteosis or Van Buchem disorder. Mice treated between 3–14 weeks of age with high doses of Scl-Ab show significant calvarial thickening capable of rescuing OI-induced deficiencies in skull thickness. Other changes in cranial morphology, such as lengths and distances between anatomic landmarks, intracranial volume, and suture interdigitation, showed minimal effects of Scl-Ab when compared to growth-induced differences over the treatment duration. Treatment-induced narrowing of foramina was limited to sites of vascular, but not neural passage, suggesting patterns of local regulation. Together, these findings reveal a site-specificity of Scl-Ab action in the calvaria with no measurable cranial nerve impingement or brainstem compression. This differentiation from the observed outcomes of lifelong sclerostin deficiency complements reports of Scl-Ab treatment efficacy at other skeletal sites with the prospect of minimal cranial secondary complications.
Keywords: Sclerostin antibody, Osteogenesis Imperfecta, cranial morphology, anabolic effect, vascularity
INTRODUCTION
Osteogenesis Imperfecta (OI) is a genetic disorder caused by collagen-related mutations, resulting in brittle bones, high fragility rates, and associated skeletal deformities.1 Pharmacologically, pediatric OI is primarily managed with bisphosphonates, which work as osteoclast inhibitors to reduce high bone turnover as well as modify bone size and shape due to disruptions in modeling-associated growth.2,3 Currently the only approved anabolic therapies for bone formation act by signaling through the parathyroid hormone receptor,4,5 which cannot be utilized to treat pediatric individuals due to potential side effects.6,7 Thus, there is room for development of anabolic treatment options to support bone formation for therapy of pediatric OI.
Sclerostin is an osteocyte-specific glycoprotein that negatively regulates bone formation by blocking canonical Wnt signaling.8 Sclerostin Antibody (Scl-Ab) therapy has emerged as a potential means to interfere with sclerostin and thus increase bone formation. It has been shown to increase bone mass in clinical trials of adult diseases of low bone mass, such as osteoporosis9 and OI.10 Its use to decrease bone fragility in pediatric OI has been evaluated in several growing mouse models by us and others, and significant gains in cortical and trabecular bone mass at non-cranial sites during periods of rapid bone growth have been reported.10,11,12,13,14 At cortical bone sites, we previously demonstrated that Scl-Ab is capable of changing quiescent and resorbing surfaces into bone formation surfaces, leading to decrements in whole bone fragility.15 Enhancing bone deposition could be beneficial to pediatric individuals with OI, however, loss of resorbing surfaces could also pose a risk that is unique to this population. Localized resorption is critical to reshaping bones during growth; in the skull such remodeling is critical for growth of the brain, nerves and blood vessels. Anomalies associated with lifelong sclerostin deficiency or loss of function, such as that observed in patients with Sclerosteosis or Van Buchem disease, may predict some of the potential risks of utilizing Scl-Ab mediated therapy in a developing pediatric population.
Patients with lifelong deficiency in sclerostin demonstrate patterns of excessive bone growth resulting in cranial nerve compression and facial palsy associated with nerve impingement.16,17 The most perilous morphological change observed in crania of patients with Van Buchem’s disease is narrowing of the foramen magnum, which can result in sudden death due to compression of the brain stem.18 Patients heterozygous for sclerostin deficiency or loss of function do not show similar deleterious outcomes,19 suggesting a gene dosing effect with potential translation to pharmacologic inhibition of sclerostin during growth and development.
Individuals with OI have unique skull morphology that may impact Scl-Ab associated changes. Due to OI, the cranium is often abnormally shaped,20 and, in some forms of OI, individuals demonstrate abnormally thin skulls.21 Although rare, prominent occipital regions or flattening of the cranial vault may occur.22 A common radiographic indicator of OI is the presence of Wormian bones, accessory skull bones completely surrounded by a suture line.23 These abnormal ossicles are frequently found in individuals with OI due to reduced skull ossification.24 Murine cranial development has been used extensively to evaluate genetic impact on human cranial bone development, and 3D microCT has been assessed to validate the mouse cranium as a relevant model for clinical features of OI25 and other genetic disorders.26 While Scl-Ab has been explored in many simulated disease models of low bone mass resulting from ovariectomy,27,28 orchidectomy,29 glucocorticoid exposure,30 disuse,31,32 spinal cord injury,33 rotator cuff healing,34 and fracture repair,35,36 these studies and others were typically performed in mature animals where most cranial developmental processes are complete. Similarly, in studies of young OI mice, the effects of pharmacologic sclerostin inhibition on the cranium during periods of rapid growth and development have not yet been described.10,12,13,14,15 These outcomes may have significant implications in determining the safety of Scl-Ab for treatment of low bone mass in pediatric populations.
The purpose of this study was to determine the morphological changes in the developing crania induced by Scl-Ab. To assess this, the Brtl/+ (HET) mouse model of OI37,38,39 was treated with Scl-Ab throughout a period of rapid growth. Cranial morphology was assessed by microCT at multiple sites to determine the influence of sclerostin inhibition on modulation of bone mass.
METHODS
Treatment Design and Functional Assessment
As part of a larger dose-response study, male Brtl/+ and wild-type (WT) mice were administered one of five doses of Scl-Ab VI (3,6,12.5, 25, 50 mg/kg, Amgen, Thousand Oaks, CA and UCB, Brussels, Belgium) and compared to saline injected controls (n=10/group). Doses were administered subcutaneously biweekly, from age 3 to 14 weeks. Previously, we have demonstrated that biweekly administration of 25 mg/kg Scl-Ab induced significant therapeutic benefits in femora of Brtl/+ between 3 and 8 weeks of age.10,40 In the present study, we chose Scl-Ab dose and durations to double our prior treatment exposure to better understand the outcomes of a maximal, sustained effect of treatment throughout growth. Animals were housed in specific pathogen free cages with standard 12-hour light/dark cycles with ad lib access to food and water. All studies were approved by the University of Michigan Institutional Animal Care and Use Committee (IACUC). At 8 and 13 weeks of age, mice were assessed for symptoms characteristic of Bell’s Palsy, a facial paralysis that could indicate facial nerve compression, by puffing air across the eyes to assess the blink reflex, and by assessing asymmetry in mouse whisker movement following previously described protocols.41 Following euthanasia by CO2, skulls were dissected and preserved for microCT by fixing in 10% NBF, and subsequently stored in 70% ethanol.
MicroCT Imaging
To assess the extreme Scl-Ab-induced conditions that might lead to morphological changes in the skull, crania from mice treated with the maximum dose of 50 mg/kg were compared to the control group. In order to verify whether Scl-Ab dose impacts the extent of morphological changes, locations that exhibited significant morphological changes were also analyzed in mice treated with intermediate doses of 25 mg/kg. To compare the extent of dimensional changes caused by Scl-Ab in relation to changes observed during growth, additional crania of untreated mice at 3 weeks of age were dissected, scanned and analyzed.
Crania were imaged using micro-computed tomography (Bruker, Skyscan 1176) at 9-micron voxel size. Scan parameters included a 0.3-degree increment angle, 2 frames averaged, 65-kVp and 385-μA X-ray source with a 1-mm Al filter to reduce beam hardening artifacts.
Dimensional Shape Analysis
To test for deformations in the mouse crania similar to those found in individuals with OI, spatial coordinates of the nasale, nasion, bregma, lambda, the intersection of interparietal and occipital bones (IP/OP) at the midline, and the intersection of parietal, temporal, and interparietal bone (PIT) were found using commercially available software (Dragonfly 3.0 Object Research Systems) (Figure 1 AB). Using these coordinates, distances between anatomic landmarks and the angles between those segments were computed (Table 1) using protocols adapted from Richtsmeier et al.42 Distances and angles such as these are highly inter-correlated with each other, making individual statistical testing difficult.43 To better control for this we used geometric morphometric methods44,45 implemented in the R package Geomorph46 to assess variation in shape (i.e., the relative positions of the landmarks, which encompasses both the relative lengths of all inter-landmark distances and the angles between those segments). By treating each set of landmarks as a single configuration, with a single shape, a measure of the magnitude of shape distance (Proscrutes distance) was created. Differences between shapes can be illustrated visually by deformation grids47 while trajectory analysis48 can then be used to determine overall statistical relevance. Trajectory analysis was used to compare vectors connecting selected group means, in this case, untreated WT vs. untreated Brtl/+ for genotypic assessment, and untreated WT to treated Brtl/+ for rescue effect.
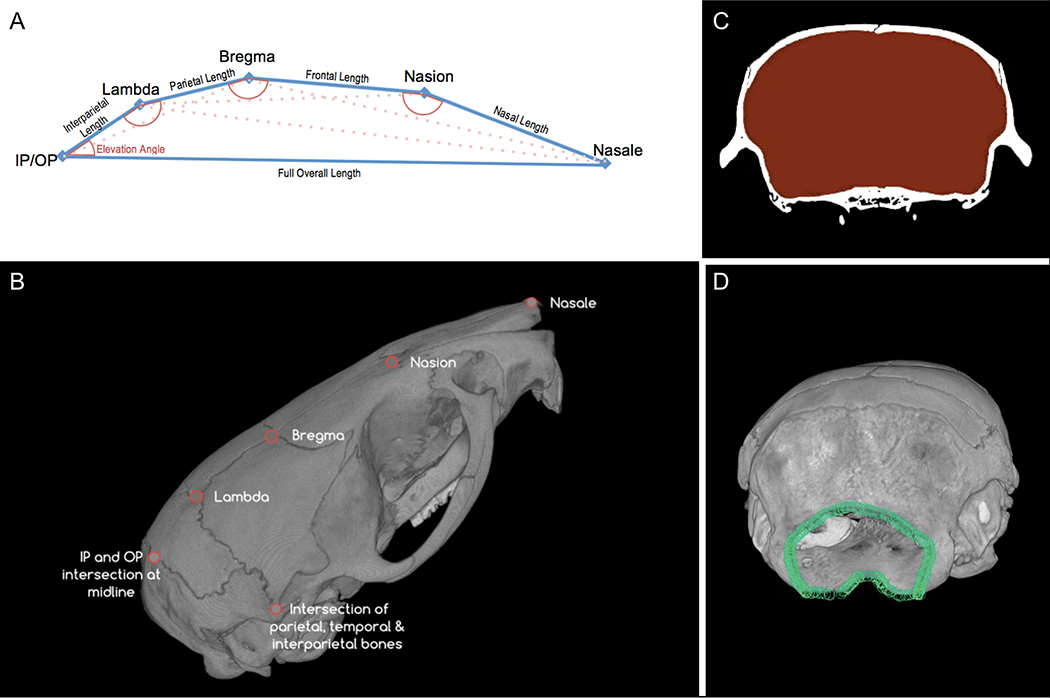
Figure 1:
A) Landmark locations and calculated values. B) Landmark locations measured on exertior of crania. C) Sample region of interest obtained for intracranial volume. D) Sample Foramen Magnum outline.
Table 1.
Measured values and landmarks used
| Measurement | Landmarks Used |
|---|---|
| Length | |
| Mean Cranial Length | IP/OP Nasale |
| Mean Interparietal Length | IP/OP Lambda |
| Parietal Length | Lambda Bregma |
| Frontal Length | Bregma Nasion |
| Nasal Length | Nasion Nasale |
| Angle | |
| Mean Interior Angle at Lambda | IP/OP, Lambda, Bregma |
| Mean Interior Angle at Bregma | Lambda, Bregma, Nasion |
| Mean Interior Angle at Nasion | Bregma, Nasion, Nasale |
| Mean Interior Lateral Angle | IP/OP, PTI, Perpendicular |
| Mean Interior Elevation Angle | Lambda, IP/OP, Nasale |
Suture Morphology
Suture interdigitation plays a critical role in developing a connection between calvarial bones, resisting mechanical tension49,50. Wnt signaling plays an important role in suture development,51 and suture morphology and interdigitation that evolves during growth and development.52 To quantify the effects of genotype and on the complexity of cranial sutures, the parietal suture was traced from the bregma to lambda at 45-micron intervals. Coordinates were analyzed based on the sum of deviation of distance from a normalized midline.
Calvarial Thickness Analysis
To evaluate the effect of Scl-Ab on skull thickness, the thicknesses of the parietal bone was calculated using measurement and visualization software (Bruker, CTAn), by creating a region of interest through transverse slices around the entire parietal bone from the lambda to bregma. A region of interest was created within the cranium for every transverse slice from the nasion to the foramen magnum (Figure 1C); total intracranial volume was also calculated using the same software.
Foramen Analysis
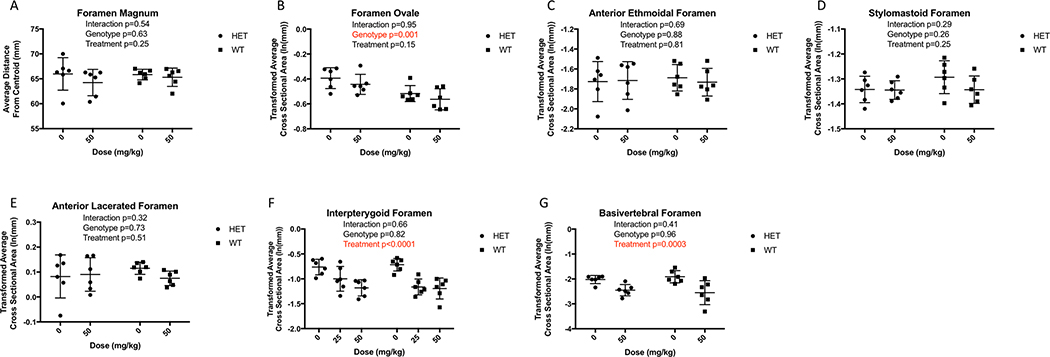
The effects of treatment on size were evaluated for the interpterygoid foramen, foramen ovale, anterior ethmoidal foramen, anterior lacerated foramen, stylomastoid foramen, and anterior semicircular auditory canal. Such locations were selected due to their consistent cylindrical structure, diverse physiological purpose, and variation in cross-sectional diameter and involvement in Sclerosteosis. To determine whether changes in vascular foramina are unique to the skull, the basivertebral foramen in the 5th lumbar vertebra was included in the study. Automatic thresholding through CTAn was applied to each region of interest and average cross-sectional areas orthogonal to foramina orientation were obtained for each site, utilizing local landmark locations to assure consistency. A similar technique was applied to the apex of the semicircular canal.
Because the foramen magnum has a complex, three-dimensional shape, a single planar projection would not suffice to capture changes in the magnitude of this opening. Instead, the three-dimensional outline of the foramen magnum was constructed using the outlining coordinates at a 9-micron interval for each specimen (Figure 1D). The three-dimensional centroid of this outline was calculated, and the size of the foramen was calculated as the square root of the sum of coordinates’ distances from the centroid.
Statistical Interpretation
Prior to statistical analysis, data was linearized. This transformation included taking the natural log of the square and cube root of area and volumetric measurements respectively. Statistical analysis of effects of genotype and treatment on shape was performed on the full set of foramina. A two-way multivariate ANOVA was used to test for differences between average cross-sectional areas of foramina.
Statistical comparisons among the genotype, control, and treated groups were made using a multiple regression two-way ANOVA, considering p < 0.05 as significant. Post-hoc analysis was conducted utilizing a Welch two-sample t-test when interaction was present or to evaluate for Type-1 error. Further analysis also included a comparison of Brtl/+ Scl-Ab to the WT vehicle group, evaluating Scl-Ab’s ability to provide restorative anabolic gains.
RESULTS
Genotype has modest influence on skull shape
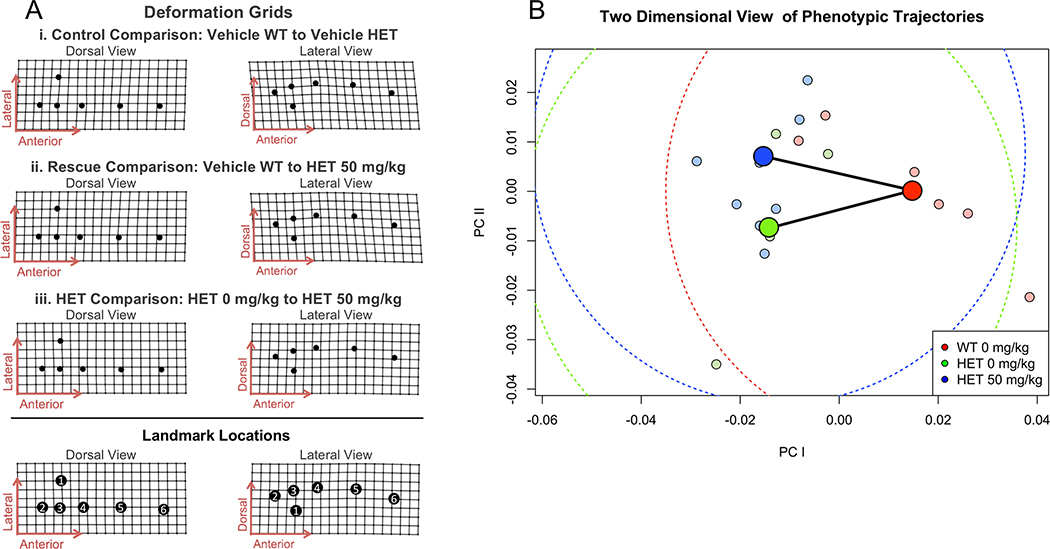
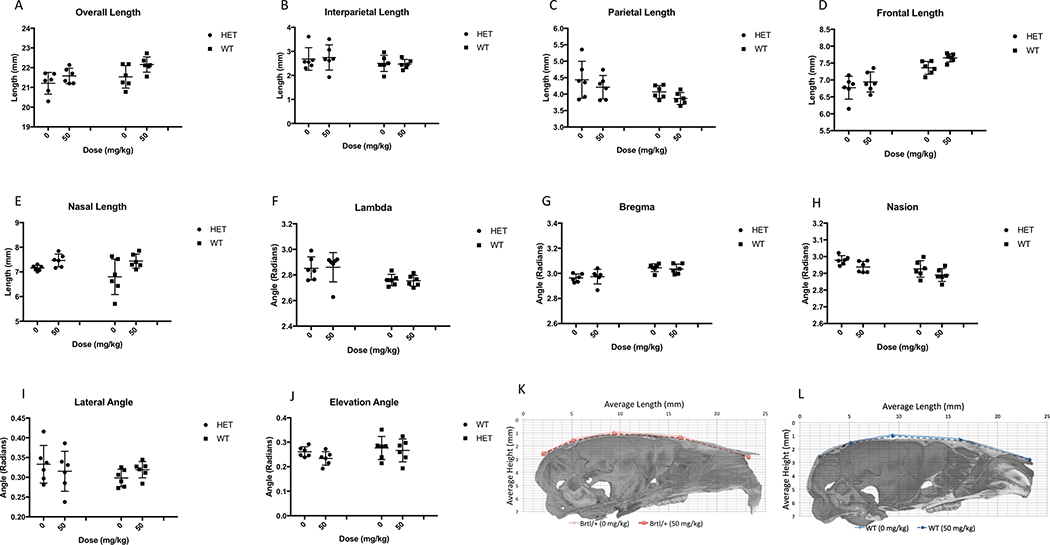
Deformed grid illustrations of the skull at 14 weeks suggest that untreated Brt/+ tend to have a more domed skull than WT, with parietal and nasal regions expanded relative to the rest of the skull (Figure 2Ai). To confirm the visual impressions obtained from examination of the deformed grids, we quantified changes in the lengths and angles that appeared to be most strongly affected (Table 2, Figure 3). These analyses confirm that the frontal bone is shorter in untreated Brtl/+ than in WT, while the other cranial bones are longer in untreated Brtl/+ than in WT (Table 2, Figure 3B–C,E). A larger, straighter lambda angle and smaller, sharper bregma angle in Brtl/+ contribute to the phenotype of a higher crowned, more domed appearance (Table 2, Figure 3FG). The net effect of these individual changes is to maintain a stable total length for the whole skull and for the calvarium despite differences in the individual components comprising those measurements. Statistical assessment of vectors to untreated Brtl/+ from untreated WT confirm that the effect of OI on overall cranial shape in these mice is quite small (Figure 2B).
Figure 2:
A) Dorsal and lateral views of deformations for three pairwise group differences. Landmark locations are identified in reference view. 1. PIT; 2. IP/OP; 3. Lambda; 4. Bregma; 5. Nasion; 6. Nasale. B) Trajectory analysis demonstrates a statistically insignificant difference between veh WT (red), veh HET (green), and treated HET (blue) mean shapes. Lightly colored points represent individual measurements; darker points are means of each group. Dashed curves represent radii of statistical difference between groups.
Table 2.
Mean length and angle measurements. Genotype percent changes compares WT to HET, Scl-Ab Treatment percent change compares 0 mg/kg to 50 mg/kg.
| HET 0mg/kg |
HET 50mg/kg |
WT 0mg/kg |
WT 50mg/kg |
Genotype | Scl-Ab Treatment | |
|---|---|---|---|---|---|---|
| Length (mm) | ||||||
| Mean Cranial Length | 21.21±0.55 | 21.58±0.39 | 21.53±0.57 | 22.16±0.39 | −2.06% | 2.34% |
| Mean Interparietal Length | 2.68±0.47 | 2.75±0.53 | 2.50±0.34 | 2.47±0.20 | 9.26% | 0.77% |
| Parietal Length | 4.44±0.56 | 4.21±0.36 | 4.07±0.19 | 3.87±0.18 | 8.94% | −5.05% |
| Frontal Length | 6.77±0.34 | 6.94±0.30 | 7.36±0.20 | 7.66±0.12 | −8.72% | 3.33% |
| Nasal Length | 7.16±0.11 | 7.47±0.26 | 6.80±0.72 | 7.44±0.29 | 2.74% | 6.81% |
| Angle (radian) | ||||||
| Mean Interior Angle at Lambda | 2.85±0.09 | 2.86±0.12 | 2.76±0.04 | 2.75±0.04 | 3.63% | 0.00% |
| Mean Interior Angle at Bregma | 2.96±0.03 | 2.97±0.06 | 3.05±0.03 | 3.03±0.04 | −2.47% | −0.17% |
| Mean Interior Angle at Nasion | 2.98±0.03 | 2.94±0.03 | 2.93±0.05 | 2.89±0.04 | 1.72% | −1.35% |
| Mean Interior Lateral Angle | 0.33±0.05 | 0.32±0.05 | 0.30±0.02 | 0.32±0.02 | 4.84% | 1.59% |
| Mean Interior Elevation Angle | 0.26±0.02 | 0.23±0.03 | 0.28±0.05 | 0.27±0.05 | −10.91% | −7.41% |
Figure 3.
Micro-CT data from cranial landmark measurements demonstrate genotype and Scl-Ab effect on cranial lengths (A –E ) and (F –J ) on cranial angles. Superimposed mean lengths and angles of crania for (K ) Brtl/+ and (L ) WT mice. Data are shown as mean ± SEM of measurements.
Scl-Ab influences cranial shape
Small differences between average cranial shapes suggest that Scl-Ab may have a weak effect on the way that OI alters skull growth (Figure 2). Treated Brtl/+ also differed from untreated WT in having a more domed skull, with similar relative elongation of the parietal and nasal (Figure 2Aii), but with more flexion of the nasal than in untreated Brtl/+ (Figure 2Aiii). As shown in the plane of the first two PCs of the data (Figure 2B), the distributions of the three groups overlap broadly, and vectors to treated and untreated Brtl/+ from WT are not significantly different, indicating the differences between overall skull shapes are small relative to within-group variation.
Parietal thickness increases with Scl-Ab treatment, rescuing the genotypic deficit
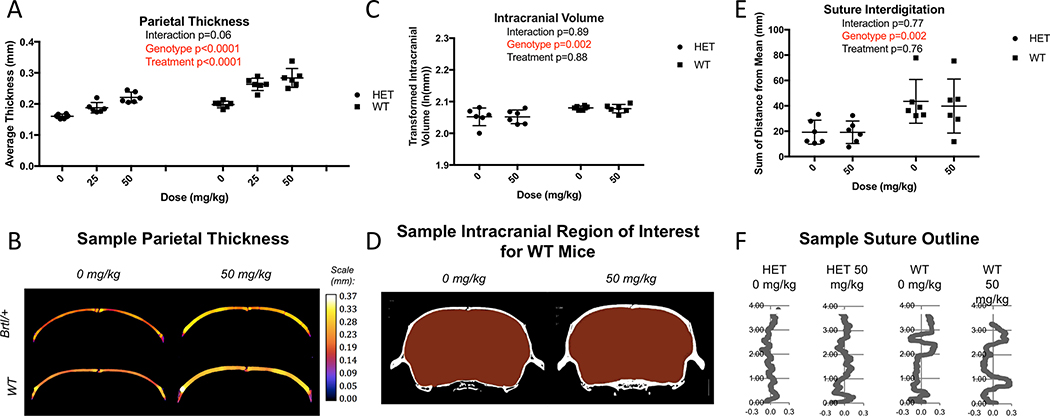
Parietal thickness is strongly influenced by both genotype (p<0.0001) and treatment (p<0.0001) (Figure 4A). Brtl/+ have 23.6% thinner parietal bones than WT. Treatment with 25 mg/kg Scl-Ab eliminated that difference (p=0.99). Representative calvarial cross sections from both Brtl/+ and WT mice are shown in Figure 4B.
Figure 4:
A) MicroCT data of the parietal bone reveals average parietal thickness is impacted by genotype and treatment. B) Sample parietal thickness regions of interest. Color look-up scale represents the measured thickness at each location in mm using local thickness computation. C, D) MicroCT imaging reveals intracranial volume is impacted by genotype, but not SclAb treatment. E) MicroCT data of the parietal suture reveals interdigitation is impacted by phenotype, but not Scl-Ab treatment. F) Sample suture outlines demonstrate interdigitation differs between genotype, but is not impacted by Scl-Ab. Data is shown as mean ± SEM of measurements.
Intracranial volume is not impacted by Scl-Ab despite genotypic differences
These more domed, thinner calvarial bones found in Brtl/+ resulted in a 7.6% smaller intracranial volume compared to WT mice (p=0.002) (Figure 4A). While Scl-Ab induced significant calvarial thickening, intracranial volume was more strongly influenced by overall patterns of skull shape. Accordingly, there were no significant differences in intracranial volume between the control and treatment groups for either Brtl/+ and WT mice (p=0.88).
Scl-Ab does not influence suture interdigitation
The midline parietal suture of Brtl/+ mice has 53.9% less interdigitation (smaller mean squared deviation from the midline) than in WT mice (p=0.002) (Figure 4E). Scl-Ab treatment had no effect on suture interdigitation in both Brtl/+ and WT mice (p=0.76). Representative images of the morphologic tracings of sutures from each experimental group are shown in Figure 4E. No Wormian bones were observed on any of the sutures.
Scl-Ab induces few changes in cross-sectional areas of passages through cranial bones
Multivariate analysis of all 6 cranial foramina that were examined indicated a significant effect of treatment but only a marginal effect of genotype (Table 3). There was not a significant interaction, thus the effect of treatment was not influenced by genotype. In post-hoc analyses of the individual foramina, only the foramen ovale differed significantly between genotypes, being larger in Brtl/+ (Figure 5). With respect to Scl-Ab treatment, the interpterygoid foramen, which is a venous passage,53 showed significant, dose dependent decrease in size in both genotypes (Figure 5F). However, there was no indication that cranial foramina with nerve functions had similar narrowing effects with treatment. The foramen ovale, anterior lacerated foramen, and stylomastoid foramen all function as nerve passages54 while the anterior ethmoidal foramen serves as a passageway for the internal ethmoidal artery, vein, and nerve55 and neither these, nor the foramen magnum were affected by genotype (p=0.63) or treatment (p=0.25) (Figure 5). Consistent with these results, no mice exhibited changes in blink or whisker responses that would indicate facial nerve compression.
Table 3.
Foramen multivariate analysis of variance (MANOVA) results
| Factor | Pillai’s Trace | Approximate F Value | P-Value |
|---|---|---|---|
| Treatment | 0.865 | 12.761 | P<0.001 |
| Genotype | 0.533 | 2.284 | 0.089 |
| Treatment and Genotype | 0.254 | 0.681 | 0.686 |
Figure 5.
Micro-CT average cross-sectional measurements of foramen reveal the interpterygoid is the only foramen impacted by Scl-Ab (A –F ), despite genotypic differences (G ). Micro-CT of basivertebral foramen reveal Scl-Ab induces changes in average cross-sectional area. Data are shown as mean ± SEM of measurements.
Similar to the interpterygoid foramen, the basivertibral foramen, an extracranial postnatal foramen with strictly vascular function56 exhibited substantial constriction in treated mice (61.0% smaller, p=0.0003).
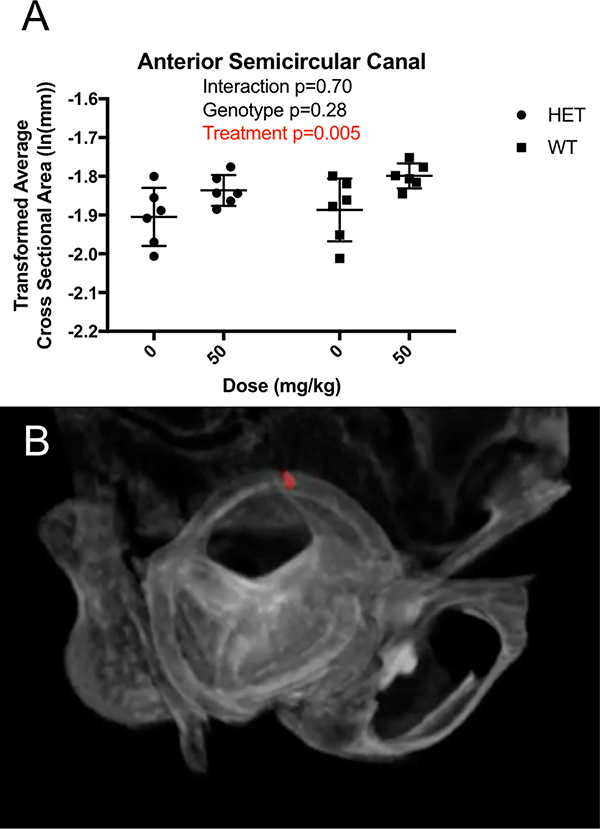
Interestingly, the cross-sectional area of the anterior semicircular canal, a fluid filled segment of the inner ear that is critical for coordinating eye movement with head movement and which not differ between genotypes (p=0.28), was 16.1% larger in treated than in untreated mice (p=0.005) (Figure 6A).
Figure 6:
MicroCT average cross-sectional measurements of the anterior semicircular canal reveals A) Scl-Ab causing an increase in cross-section, with no impact by phenotype. B) Sample region of interest measured. Data is shown as mean ± SEM of measurements
Behavioral analyses confirmed no effect of Scl-Ab
Assessment of blink and whisker reflexes after 5 and 10 weeks of treatment (8 and 13 weeks of age) were unremarkable across genotypes. No changes were observed over time or bilaterally, with no overt functional deficiencies that would be suggestive of significant nerve impingement as a consequence of bone overgrowth or resulting from genotypic differences between mice.
Twenty-One day old crania demonstrate the extent of growth-related dimensional changes observed during treatment
To compare the extent of dimensional changes caused by Scl-Ab treatment in relation to the changes observed during growth over the treatment period, twenty-one day old (21D) cranial morphology was measured in a manner consistent with aged samples (Figure S1). To calculate and compare growth percent, each group at 14 weeks (i.e. Scl-Ab and Veh for both genotypes) was compared within genotype to corresponding 21D values.
The alterations in growth prompted by Scl-Ab treatment are minor in comparison to the overall growth that occurs naturally during the treatment period (Figure S1). When compared to three-week controls, overall cranial lengths of Brtl/+ and WT Veh expanded by 14.9% and 13.5% respectively over 21D values. Scl-Ab-treated animals showed a growth of 16.9% (Brtl/+) and 16.7% (WT) over 21D values, reflecting minimal differences in growth patterns with the addition of Scl-Ab. Similarly, Scl-Ab induced minor changes in growth from 21D compared to Veh-treated growth patterns over the same period in the frontal (Brtl/+ Veh: 11.0% vs. Scl-Ab: 13.8%; WT Veh: 13.8% vs. Scl-Ab: 18.4%), and nasal (Brtl/+ Veh: 42.1% vs. Scl-Ab: 48.2%; WT Veh: 8.4% vs. Scl-Ab: 16.5%) lengths. Treatment effects in relation to growth effects are further summarized in Figure S1. Similar modest effects of Scl-Ab on growth with respect to angular changes in the crania are summarized in Figure S2.
Due to the early developmental stage, we were unable to isolate individual foramina in 21D crania at the resolution used for microCT imaging.
There is a significant difference between the parietal thickness of all control and treatment groups and the 21D crania (p<0.001) (Figure S3). Unlike the relatively minor changes in skull lengths and angles, the increase in parietal thickness due to treatment with Scl-Ab is significantly modified to levels closer to the increase in thickness that occurs during normal development alone. Compared to the corresponding 21D genotype, at 14 weeks the parietal thickness of Brtl/+ and WT Veh increased by 117.5% and 145.2% respectively; whereas treatment with 25 mg/kg of Scl-Ab provoked growth by 154.6% (Brtl/+) and 225.3% (WT). Similarly, 50 mg/kg of Scl-Ab induced a growth of 199.8% (Brtl/+) and 250.6% (WT) vs. 21D thicknesses.
DISCUSSION
The purpose of this study was to determine whether Scl-Ab administration induces morphological changes in the developing crania. Through this study, several phenotypic differences between Brtl/+ and WT cranial attributes were observed. Brtl/+ mice showed a modestly domed appearance with significant reductions in calvarial thickness vs. WT, consistent with clinical observations of abnormally thin skulls.21 Calvaria were sensitive to the treatment effects of Scl-Ab which was capable of rescuing this deficit. Gains in calvarial thickness were consistent with our previously described gains in femora cortical thickness in Brtl/+, confirming Scl-Ab treatment effect occurs as expected in the cranium as well as long bone sites.10 Differences in cranial lengths and angles showed trends towards minor effects with Scl-Ab, however, these did not impact overall morphology in comparison to the magnitude of response observed in parietal thickness, and effects due to growth. With treatment, cranial volume was preserved, suggesting that Scl-Ab does not induce intracranial compression to the extent observed clinically with homozygous genetic deficiencies of sclerostin. Expansion of the anterior semicircular canal due to Scl-Ab was unexpected and may be an indication that other morphological changes occurred in the inner ear that might affect balance or hearing.
Narrowing of foramina occurred in two locations that function as vascular passageways, but did not occur in important neural foramina, including the foramen magnum, foramen ovale, anterior ethmoidal foramen, stylomastoid foramen, and anterior lacerated foramen. The narrowing of the basivertebral foramen extended this observation to a non-cranial site but the absence of similar responses in other foramina that included both nerve and vasculature (anterior ethmoid foramen) clouds possible explanations. One possible factor may be osteocyte proximity to affected sites. Since osteocytes are predominantly responsible for synthesizing sclerostin, the proximity or quantity of osteocytes in defined regions could restrict the effect of treatment to sites of substantial sclerostin secretion. Alternatively, a neural self-protection effect may limit Scl-Ab effects at these sites; similar self-protection effects may maintain critical vascular passages, but not the foramina that pass small and variable members of venous plexuses. While not characterized directly in these studies, local mechanical differences between foramen which permit vascular perfusion versus those dedicated to nervous innervation may induce local differences in sclerostin expression, which may influence the local anabolic response. The anabolic actions of Scl-Ab have been shown to be heightened in normally loaded bones compared to those with reduced mechanical loading in some31,32 but not all studies.57
The implications of these findings of narrowing in some, but not other foramina are unclear. A leading concern for pharmacologic inhibition of sclerostin during growth and development is that treatment may phenocopy intercranial pressure, nerve entrapment and facial deformities present in patients with lifelong sclerostin deficiency.16,17,18 Functional nervous changes were not observed, and foramen functioning exclusively as nervous passages were not narrowed. In patients with sclerosteosis, increased intracranial pressure can occur from reduced intracranial diameter, calvarial thickening, and jugular vein occlusion.19 In the present study, these findings were limited to some vascular foramen occlusion, while intracranial volumes and shapes remained unchanged. The present findings demonstrate outcomes following a high dose for an extreme duration and suggest need for additional studies to better understand how this finding could be mitigated through a less severe treatment regimen, or when Scl-Ab is combined with an anti-resorptive,58,59 possibly facilitating reduced drug exposure from both medications.
This study has several limitations. Accuracy of our measurements is dependent on our 9-micron scanning resolution. To account for this restraint, we investigated a variety of foramina that spanned multiple orders of magnitude in size. Larger foramina provided us with a reference point that was less sensitive to scanning resolution. Facial palsies resulting from nerve compression are observed in patients with both osteoblastic16,60,61 and osteoclastic17 defects, and Scl-Ab has known anabolic and anti-resorptive effects.9,10 MicroCT imaging revealed site-specific structural changes in response to Scl-Ab but cannot differentiate the cellular mechanisms involved. Due to technical limitations that prevented us from matching histologic sectioning planes to microCT locations across the skull, we were unable to differentiate anti-resorptive from anabolic actions at these sites and acknowledge the need for future studies to derive these parameters at sites that were both responsive, and resistant to Scl-Ab. We were also limited to the phenotype of our Brtl/+ mouse model. Untreated Brtl/+ deviated greatest from WT with reduced calvarial thickness and intracranial volume, while differences in overall size and shape of the skull were more modest, reflecting some, but not all craniofacial features of OI. OI is a highly variable disease; different types of collagen mutation can lead to a range of symptoms and responses to treatment. Therefore, the preliminary results from this study may not be fully reflective of all OI conditions, and do not fully elucidate a direct cellular mechanism for our findings. The present study suggests a similarity of action independent of genetic mutation in the most prominent phenotypic features- calvarial thickness and intracranial volume. Whether similar treatment responses would occur across multiple OI models remains unknown and contributes to the need for the field to better understand diverging outcomes in this disorder.62
Our study was initially designed to examine the effect of Scl-Ab over a period of rapid long-bone growth.10 However, approximately 80% of overall cranial growth in mice occurs during 7 to 14 days of age.63 We began treatment of mice at 21 days of age, which developmentally corresponds to a human age of approximately 2 years based on overall central nervous system and reproductive development.64 The majority of the cranial development occurs prior to this period,65 we may have missed earlier phases of cranial growth regulated differentially by Scl-Ab. The present findings should be considered in the context of these developmental milestones.
Scl-Ab has been shown to increase bone mass in adult individuals with OI and based on promising pre-clinical mouse models of the disorder8,10,11,14,15, is proposed as a novel therapy for pediatric use. Lifelong genetic depletion of functional sclerostin can result in craniofacial abnormalities, and reports to-date have not described whether sclerostin inhibition phenocopies these traits when administered during periods of growth and development. In the present study, phenotypic calvarial thinning was rescued, while abnormalities reminiscent of Sclerosteosis or Van Buchem disorder including cranial compression and nerve impingement were not observed in this study. These findings likely reflect the difference between the temporary action of Scl-Ab therapy initiated at 3 weeks of age in mice versus patients with a lifelong deficiency of functional sclerostin during cranial bone growth and development. This differentiation from the observed outcomes of lifelong sclerostin deficiency complements reports of Scl-Ab treatment efficacy with the prospect of minimal cranial secondary complications.
Supplementary Material
Supplementary Figure 1: MicroCT of 21 day cranium reveal Scl-Ab elicits minor changes in A) overall length and B) interparietal length, and more significant changes in C) parietal, D) frontal, and E) nasal length. Data is shown as mean ± SEM of measurements
Supplementary Figure 2: MicroCT of 21 day cranium reveal the angular changes due to Scl-Ab are small compared to changes that occur during natural growth (a-e). Data is shown as mean ± SEM of measurements
Supplementary Figure 3: MicroCT of 21 day cranium reveal the anabolic changes due to Scl-Ab are similar in magnitude to those observed in natural growth. Data is shown as mean ± SEM of measurements
ACKNOWLEDGEMENTS
The authors gratefully thank Dr. David Kohn, Basma Khoury, Carol Whitinger, Rob Goulet, and Rebecca Falzon, for technical assistance and thoughtful discussion. Scl-Ab was provided by Amgen (Thousand Oaks, CA) and UCB (Brussels, Belgium). Research funding provided by NIH (RO1AR062522, P30AR069620, S10 OD017979-01A1).
REFERENCES
- 1.Marini JC, Forlino A, Bächinger HP, et al. 2017. Osteogenesis imperfecta. Nat. Rev. Dis. Primers 3:17052. [DOI] [PubMed] [Google Scholar]
- 2.Zeitlin L, Fassier F, Glorieux FH. 2003. Modern approach to children with osteogenesis imperfecta. J Pediatr Orthop; 12:77–87. [DOI] [PubMed] [Google Scholar]
- 3.Rauch F, Glorieux FH. 2004. Osteogenesis imperfecta. Lancet 363(9418)1377–85. [DOI] [PubMed] [Google Scholar]
- 4.Miller PD, Hattersley G, Riis BJ et al. 2016. Effect of abaloparatide vs. Placebo on new vertebral fractures in postmenopausal women with osteoporosis : A randomized clinical trial. JAMA, 316(7) :722–33. [DOI] [PubMed] [Google Scholar]
- 5.Neer RM, Arnaud CD, Zanchetta JR et al. 2001. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. NEJM 344(19)1434–41. [DOI] [PubMed] [Google Scholar]
- 6.Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, Westmore MS, Linda Y, Nold JB. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol. May-Jun 2002;30(3):312–21. Epub 2002/06/08. [DOI] [PubMed] [Google Scholar]
- 7.Jolette J, Attalla B, Varela A, Long GG, Mellal N, Trimm S, Smith SY, Ominsky MS, Hattersley G. Comparing the incidence of bone tumors in rats chronically exposed to the selective PTH type 1 receptor agonist abaloparatide or PTH(1–34). Regul Toxicol Pharmacol. June 2017;86:356–65. Epub 2017/04/09. [DOI] [PubMed] [Google Scholar]
- 8.Moester MJC, Papapoulos SE, Löwik CWGM, & van Bezooijen RL 2010. Sclerostin: Current Knowledge and Future Perspectives. Calcif Tissue Int 87(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosman FF, Crittenden DB, Adachi JD, et al. 2016. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med 375(16): 1532–1543. [DOI] [PubMed] [Google Scholar]
- 10.Sinder BP, Salemi JD, Ominsky MS, Caird MS, Marini JC, & Kozloff KM 2015. Rapidly growing Brtl/ mouse model of osteogenesis imperfecta improves bone mass and strength with sclerostin antibody treatment. Bone 71: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roschger A, Roschger P, Keplingter P, et al. 2014. Effect of sclerostin antibody treatment in a mouse model of severe osteogenesis imperfecta. Bone 66:182–188. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsen CM, Barber LA, Ayturk UM, et al. 2014. Targeting the LRP5 Pathway Improves Bone Properties in a Mouse Model of Osteogenesis Imperfecta. J Bone Miner Res 29: 2297–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grafe I, Alexander S, Yang T, et al. 2015. Sclerostin antibody treatment improves the bone phenotype of Crtap mice, a model of recessive osteogenesis imperfecta. J Bone Miner Res 31(5):1030–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardinal M, Tys J, Roels T, et al. 2019. Sclerostin antibody reduces long bone fractures in the oim/oim model of osteogenesis imperfecta. Bone 124:137–147. [DOI] [PubMed] [Google Scholar]
- 15.Sinder BP, White LE, Salemi JD, et al. 2014. Adult Brtl/+ mouse model of osteogenesis imperfecta demonstrates anabolic response to sclerostin antibody treatment with increased bone mass and strength. Osteoporos Int 25: 2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner J,VanBezooijen R, Mervis B, et al. 2005. Bone mineral density in sclerosteosis; affected individuals and gene carriers. J Clin Endocrinol Metab. 90:6392–5. [DOI] [PubMed] [Google Scholar]
- 17.Sharifi M, Ereifej L & Lewiecki EM 2015. Sclerostin and Skeletal health. Rev Endocr Metab Disord 16:149. [DOI] [PubMed] [Google Scholar]
- 18.Wengenroth M, Vasvari G, Federspil PA, Mair J, Schneider P, & Stippich C 2009. Case 150: Van Buchem Disease (Hyperostosis Corticalis Generalisata). Radiology 253(1): 272–276. [DOI] [PubMed] [Google Scholar]
- 19.van Lierop AH, Appelman-Dijkstra NM, & Papapoulos SE 2017. Sclerostin deficiency in humans. Bone, 96: 51–62. [DOI] [PubMed] [Google Scholar]
- 20.Kovero O, Pynnönen S, Kuurila-Svahn K, Kaitila I, & Waltimo-Sirén J 2006. Skull base abnormalities in osteogenesis imperfecta: a cephalometric evaluation of 54 patients and 108 control volunteers. J Neurosurg 105(3): 361–370. [DOI] [PubMed] [Google Scholar]
- 21.Davies MW, Inglis GD, Jardine LA, & Koorts PJ 2013. Antenatal consults: a guide for neonatologists and paediatricians, 1st ed Sydney: Churchill Livingstone Elsevier. [Google Scholar]
- 22.Renaud A, Aucourt J, Weill J, et al. 2013. Radiographic features of osteogenesis imperfecta. Insights Imaging 4(4): 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semler O, Cheung MS, Glorieux FH and Rauch F 2010, Wormian bones in osteogenesis imperfecta: Correlation to clinical findings and genotype. Am J Med Genet 152A(7): 1681–1687. [DOI] [PubMed] [Google Scholar]
- 24.Bellary S, Steinberg A, Mirzayan N, Shirak M, et al. 2013. Wormian Bones: A review. Clin Anat 26:922–927. [DOI] [PubMed] [Google Scholar]
- 25.Eimar H, Tamimi F, Retrouvey J-M, Rauch F, Aubin JE, McKee MD 2016. Craniofacial and dental defects in the Col1a1Jrt/+ mouse model of osteogenesis imperfecta. J Dent Res, 95(7): 761–8. [DOI] [PubMed] [Google Scholar]
- 26.De Carlos F, Varela I, Germana A, et al. 2008. Microcephalia with mandibular and dental dysplasia in adult Zmpste24-deficient mice. J Anat, 213(5):509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Ominsky MS, Warmington KS, Morony S, et al. 2009. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res, 24:578. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Niu QT, Warmington KS, Asuncion FJ, et al. 2014. Progressive increases in bone mass and bone strength in an ovariectomized rat model of osteoporosis after 26 weeks of treatment with a sclerostin antibody. Endocrinology 155:4785. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Ominsky MS, Villasensor KS, et al. 2018. Sclerostin antibody reverses bone loss by increasing bone formation and decreasing bone resorption in a rat model of male osteoporosis. Endocrinology 159:260–271. [DOI] [PubMed] [Google Scholar]
- 30.Yao W, Dai W, Jiang L, Lay EY, et al. 2016. Sclerostin-antibody treatment of glucocorticoid-induced osteoporosis maintained bone mass and strength. Osteoporos Int, 27:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian X, Jee WS, Li X, Paszty C, Ke HZ 2011. Sclerostin antibody increases bone mass by stimulating bone formation and inhibiting bone resorption in a hindlimb-immobilization rat model. Bone, 48:197. [DOI] [PubMed] [Google Scholar]
- 32.Spatz JM, Ellman R, Cloutier AM, Louis L, et al. 2013. Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J Bone Miner Res, 28:865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beggs LA, Ye F, Ghosh P, Beck DT, et al. Sclerostin inhibition prevents spinal cord injury-induced cancellous bone loss. JBMR, 2015. 30:681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah SA, Kormpakis I, Havlioglu N, et al. 2017. Sclerostin antibody treatment enhances rotator cuff tendon-to-bone healing in an animal model. J Bone Joint Surg Am 99:855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald MM, Morse A, Mikulec K, Peacookm L, et al. 2012. Inhibition of sclerostin by systemic treatment with sclerostin antibody enhances healing of proximal tibial defects in ovariectomized rats. J Orthop Res, 30:1541. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Rui Y, Cheng TY, Huang S, et al. 2016. Effects of Sclerostin Antibody on the Healing of Femoral Fractures in Ovariectomised Rats. Calcif Tissue Int, 98:263. [DOI] [PubMed] [Google Scholar]
- 37.Kozloff KM, Carden A, Bergwitz C, et al. 2004. Brittle IV Mouse Model for Osteogenesis Imperfecta IV Demonstrates Postpubertal Adaptations to Improve Whole Bone Strength. J Bone Miner Res, 19: 614–622. [DOI] [PubMed] [Google Scholar]
- 38.Uveges TE, Collin-Osdoby P, Cabral WA, et al. 2008. Cellular Mechanism of Decreased Bone in Brtl Mouse Model of OI: Imbalance of Decreased Osteoblast Function and Increased Osteoclasts and Their Precursors. J Bone Miner Res 23: 1983–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forlino A, Marini JC 2000. Osteogenesis imperfecta: prospects for molecular therapeutics. Mol Genet Metab 71(1–2):225–32. [DOI] [PubMed] [Google Scholar]
- 40.Perosky JE, Khoury B, Jenks T, Ward F, et al. 2016. Single dose of bisphosphonate preserves gains in bone mass following cessation of sclerostin antibody in Brtl/+ osteogenesis imperfecta model. Bone, 93:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi H, Hitsumoto Y, Honda N, et al. 2001. Mouse Model of Bells palsy Induced by Reactivation of Herpes Simplex Virus Type 1. J Neuropathol Exp Neurol 60(6): 621–627. [DOI] [PubMed] [Google Scholar]
- 42.Richtsmeier JT, Baxter LL and Reeves RH 2000. Parallels of craniofacial maldevelopment in down syndrome and Ts65Dn mice. Dev Dyn 217: 137–145. [DOI] [PubMed] [Google Scholar]
- 43.Bookstein FL, Chernoff B, Elder RL, Humphries J, Smith G, Strauss R 1985. Morphometrics in Evolutionary Biology: The Geometry of Size and Shape Change, with Examples from Fishes. Academy of Natural Sciences of Philadelphia, Special Publication No. 15. [Google Scholar]
- 44.Bookstein FL. 1991. Morphometric Tools for Landmark Data Geometry and Biology. Cambridge Univ. Press. [Google Scholar]
- 45.Bookstein FL. 1996. Standard formula for the uniform shape component in landmark data Pp. 153–168 in LF Marcus, M Corti, A Loy, GJP Naylor, DE. Slice, eds. Advances in Morphometrics. NATO Advanced Science Institutes Series, Series A, Life Sciences, Vol. 284 Plenum Press. [Google Scholar]
- 46.Adams DC, Collyer ML, Kaliontzopolou A, and Sherrat E. 2017. Geomorph : Software for geometric morphometric analysis. R package version 3.0.5. http://cran.r-project.org/package=geomorph. [Google Scholar]
- 47.Thompson DAW, 1942. (reprinted 1992) On Growth and Form The complete revised edition. (2nd ed.) Dover. [Google Scholar]
- 48.Adams DC and Collyer ML. (2007). Analysis of character divergence along environmental gradients and other covariates. Evolution, 61 (3): 510–515. [DOI] [PubMed] [Google Scholar]
- 49.Yoshimura K, Kobayashi R, Ohmura T, Kajimoto Y, Miura T 2016. A new mathematical model for pattern formation by cranial sutures, J Theo Biol 408:66–74. [DOI] [PubMed] [Google Scholar]
- 50.Byron CD, Borke J, Yu J, et al. 2004. Effects of increased muscle mass on mouse sagittal suture morphology and mechanics. Anat Rec A Discov Mol Cell Evol Biol 279:676–684. [DOI] [PubMed] [Google Scholar]
- 51.Behr B, Longaker MT, Quarto N 2010. Differential activation of canonical Wnt signaling determines cranial sutures fate: a novel mechanism for sagittal suture craniosynostosis. Dev Biol 344(2):922–40. [DOI] [PubMed] [Google Scholar]
- 52.Miura T, Perlyn CA, Kinboshi M, Ogihara N, et al. 2009. Mechanism of skull suture maintenance and interdigitation. J Anat 215(6):642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paxinos G 2015. The Rat Nervous System Amsterdam: Academic Press. [Google Scholar]
- 54.Greene EC (n.d.). Anatomy of the rat Braintree, MA: Braintree Scientific. [Google Scholar]
- 55.Gray H, Carter H Anatomy of the human body 20th ed Philadelphia: Lea & Febiger, 1918. [Google Scholar]
- 56.Clinical Anatomy Associates, Inc. (n.d). Retrieved November 26, 2017, from https://clinanat.com/100-mtd/142-basivertebral-foramen.
- 57.Agholme F, Isaksson H, Li X, Ke HZ, Aspenberg P 2011. Anti-sclerostin antibody and mechanical loading appear to influence metaphyseal bone independently in rats. Acta Orthop, 82:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olvera D, Stolzenfeld R, Marini JC, Caird MS, Kozloff KM 2018. Low Dose of Bisphosphonate Enhances Sclerostin Antibody-Induced Trabecular Bone Mass Gains in Brtl/+ Osteogenesis Imperfecta Mouse Model. J Bone Miner Res, 33(7):1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Little DG, Peacock L, Mikulec K, Kneissel M, et al. 2017. Combination sclerostin antibody and zoledronic acid treatment outperforms either treatment alone in a mouse model of osteogenesis imperfecta. Bone, 101:96. [DOI] [PubMed] [Google Scholar]
- 60.Beneke JE. 1993. Facial nerve dysfunction in osteopetrosis. Laryngoscope 103(5):494–7. [DOI] [PubMed] [Google Scholar]
- 61.Benichou OD, Laredo JD, de Vernejoul MC. 2000. Type II autosomal dominant osteopetrosis (Albers-Schonberg disease): clinical and radiological manifestations in 42 patients. Bone 26(1):87–93. [DOI] [PubMed] [Google Scholar]
- 62.Kozloff. 2019. Osteogenesis Imperfecta: A Need to Understand Divergent Treatment Outcomes in a Disorder Rich in Heterogeneity. JBMR 34(2):205. [DOI] [PubMed] [Google Scholar]
- 63.Vora SR, Camci ED, Cox TC 2015. Postnatal Ontogeny of the Cranial Base and Craniofacial Skeleton in Male C57BL/6J Mice: A Reference Standard for Quantitative Analysis . Front. Physiol 6:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baldrick P 2004. Developing drugs for pediatric use: a role of juvenile animal studies? Regul Toxicol Pharmacol 39: 381–389. [DOI] [PubMed] [Google Scholar]
- 65.Jin S, Sim K, Kim S 2016. Development and Growth of the Normal Cranial Vault: An Embryologic Review. J Korean Neurosurg Soc. 59(3): 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: MicroCT of 21 day cranium reveal Scl-Ab elicits minor changes in A) overall length and B) interparietal length, and more significant changes in C) parietal, D) frontal, and E) nasal length. Data is shown as mean ± SEM of measurements
Supplementary Figure 2: MicroCT of 21 day cranium reveal the angular changes due to Scl-Ab are small compared to changes that occur during natural growth (a-e). Data is shown as mean ± SEM of measurements
Supplementary Figure 3: MicroCT of 21 day cranium reveal the anabolic changes due to Scl-Ab are similar in magnitude to those observed in natural growth. Data is shown as mean ± SEM of measurements