Abstract
Members of the HER (ERBB) receptor protein tyrosine kinase family play an important role in regulating cellular division, proliferation, differentiation, and migration and have prognostic significance in a number of cancers. Here, we sought to define their role in extrahepatic cholangiocarcinoma (EHCC). HER2 and HER3 protein expression was studied in 230 EHCC cases using a tissue microarray and compared with clinicopathological variables, including the survival of EHCC patients. HER3 was predominantly localized to the cytoplasm, whereas HER2 exhibited a membranous expression pattern. Overexpression of HER2 and HER3 was observed in 6 % (13/224) and 39 % (90/230) of EHCCs, respectively. Membranous HER2 overexpression occurred more frequently in intraductal papillary neoplasms with an associated invasive carcinoma than in tubular adenocarcinomas (P=0.02). HER3 protein was more commonly overexpressed in nodular and infiltrative than in papillary tumors (P=0.03). HER3 overexpression was associated with decreased survival in both univariate (P=0.01) and multivariate (P=0.008) analyses, whereas HER2 overexpression was not associated with survival. HER2 and HER3 are overexpressed in subsets of EHCC patients. Notably, HER3 overexpression is correlated with decreased patient survival, suggesting that HER3 constitutes a prognostic factor as well as a potential candidate for targeted therapy.
Keywords: Extrahepatic, Bile duct, Cholangiocarcinoma, HER2, HER3, Immunohistochemistry, Prognosis
Introduction
Extrahepatic cholangiocarcinoma (EHCC) is a malignant epithelial neoplasm of the biliary tract, from the hepatic hilum to the distal bile duct, that accounts for 70–90 % of all cholangiocarcinomas [1]. EHCC is a relatively uncommon cancer in Western countries but is more prevalent in Eastern Asian countries, including Korea [1–3]. Surgical resection of the tumor is the only curative therapeutic modality for patients with EHCC but can be applied only in a limited number of patients with localized or locally advanced disease [4]. The 5-year survival rate of EHCC patients following surgical resection is approximately 20 % [5]. Several neoadjuvant therapies, including chemotherapy, radiation therapy, and photodynamic therapy, have been extensively studied, but none have yielded significant survival advantages for EHCC patients [1, 6]. Therefore, identification of new biomarkers for early detection and/or development of new therapeutic regimens based on a better understanding of the biological mechanisms of this deadly disease are essential for decreasing mortality among EHCC patients.
Epidermal growth factor receptors (EGFRs) are members of a membrane receptor protein tyrosine kinase family that includes HER1 (ErbB1/EGFR), HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4). After binding ligands, EGFRs are activated by receptor homo- or heterodimerization, resulting in the phosphorylation of various downstream substrates, which mediate intracellular signal transduction. EGFR signaling pathways play an important role in regulating cell division, proliferation, differentiation, and migration [7]. The EGFR family member HER3 lacks intrinsic protein tyrosine kinase activity [8]. However, binding of neuregulin family ligands enables HER3 to form functional heterodimers with EGFR or HER2 that act through the cytoplasmic domain of HER3 to exert tyrosine phosphorylation activity [9]. Thus, by heterodimerizing with other EGFR proteins, HER3 can participate in diverse signal transduction cascades and regulate various cellular processes [7, 9]. The development of tyrosine kinase inhibitors of EGFR or HER2 was initially considered a promising strategy for blocking EGFR or HER2 pathways in several solid cancers, including breast and lung cancers. However, evidence suggests that HER3 may be responsible for the resistance to these therapeutic regimens, which often develops [10]. Overexpression of HER3 has been reported in several malignant neoplasms in gastrointestinal tract organs, including gastric [11–13], colorectal [14–19], and ampulla of Vater [18] cancers. Although a few studies have demonstrated HER2 expression in cholangiocarcinoma, showing that it is relatively infrequent in these tumors [20–23], there have been no comprehensive studies of HER2 and HER3 expression in EHCCs. In the current study, we analyzed HER2 and HER3 protein expression in 230 EHCC cases using tissue microarray immunohistochemistry. We demonstrate that HER3 expression in EHCC constitutes a prognostic factor.
Materials and methods
Patients and tumor samples
The study population consisted of 230 patients with surgically resected EHCCs treated at Asan Medical Center, University of Ulsan College of Medicine, in Seoul, South Korea, between 1991 and 2005. Carcinomas with an epicenter in the extrahepatic bile duct were included in this study, whereas carcinomas from the ampulla of Vater or pancreas were not. Carcinomas arising in the gallbladder or intrahepatic bile duct with extension to the extrahepatic bile duct were also excluded. Information regarding the age and gender of patients, surgical procedure, survival time, and survival status was obtained by reviewing EHCC patients’ medical records. Data pertinent to location, size, and growth pattern of the tumor were obtained from surgical pathology reports. Materials were obtained with appropriate human protection approval from the Institutional Review Board of the Asan Medical Center (Project Number 2011–0734). Information regarding post-operative radiation, chemotherapy, and performance status of patients was not analyzed in this study.
Tissue microarray construction
Tissue microarrays were constructed from archival formalin-fixed, paraffin-embedded tissue blocks as previously described [24]. Briefly, 230 extrahepatic cholangiocarcinoma cases and 12 normal extrahepatic bile ducts were included. A representative area was carefully selected for each tumor or normal biliary epithelia from a hematoxylin-and-eosin-stained section of a donor block. Each case was represented by two to four 1.5-mm-diameter cores.
Immunohistochemistry and scoring
Immunohistochemistry was performed on 4-μm-thick tissue microarray sections as previously described [25]. Briefly, tissue sections were deparaffinized and hydrated in xylene and serially diluted ethanol, respectively. Endogenous peroxidase was blocked by incubation in 3 % H2O2 for 10 min. Antigen retrieval was performed in a steam pressure cooker using preheated antigen retrieval buffer, pH 6 (Dako, Glostrup, Denmark), at 95 °C for 10 min. Non-specific binding of antibodies was minimized by incubating sections with Protein Block (Dako) for 15 min. Microarrays were incubated at room temperature for 30 min with rabbit polyclonal anti-HER2 (c-erbB2, A0485; 1:750 dilution; Dako) or overnight at 4 °C with mouse monoclonal anti-HER3 (RTJ.2, 1:500 dilution; Santa Cruz, CA, USA). Antigen–antibody reactions were detected with an LSAB+peroxidase kit (Dako) and 3,3´-diaminodbenzidine (Dako). Negative controls were composed of identically treated histologic sections with the omission of primary antibodies. Immunostained sections were lightly counterstained with hematoxylin, dehydrated in ethanol, and cleared in xylene. We did not perform immunohistochemistry for Her2 and Her3 on full sections. Tissue core representativity might be a concern, but certainly for 1.5-mm cores previous studies have demonstrated that two to four tissue cores are representative with 95–97 % concordance rate [62].
HER2 protein expression was scored on a scale of 0 to 3 using the following gastric cancer staging system [26]: 0, no reactivity or membranous reactivity in less than 10 % of cells; 1+, faint/barely perceptible membranous reactivity in ≥10 % of cells or reactivity in only part of the cell membrane; 2+, weak to moderate complete or basolateral membranous reactivity in ≥10 % of tumor cells; 3+, strong complete or basolateral membranous reactivity in ≥10 % of tumor cells. Cases receiving a HER2 score of 3+ were considered positive for HER2 expression.
The results of immunohistochemical staining for HER3 were scored based on the intensity of staining as 0 (negative), 1 (weak), or 2 (strong), and centage of positive epithelial cells as 0 (<5 %), 1 (6–25 %), 2 (26–50 %), 3 (51–75 %), or 4 (>76 %). A Histo-score was generated as the product of intensity and area. The Histo-score was then dichotomized into no/lower expression (Histo-score, 0–6) and overexpression (Histo-score, 8).
Statistical analysis
Statistical analyses were performed using SPSS version 17 (SPSS Inc., Chicago, IL, USA). Associations between categorical variables were examined using Pearson’s chi-square and Fisher’s exact tests. Survival curves were calculated by the Kaplan–Meier method, and statistical significance was evaluated using the log-rank test and the Cox proportional hazards regression model. P<0.05 was considered as statistically significant.
Results
Clinicopathological characteristics of cases
The clinicopathological characteristics of cases are summarized in Table 1. Patient age ranged from 30 to 84 years (mean, 60.9 years). Of the 230 patients, 164 were men and 66 were women. Tumor size ranged from 0.5 to 6 cm (mean, 2.5 cm). Thirty-one cases were pT1 tumors, 84 were pT2, 91 were pT3, and 24 were pT4. The length of patient follow-up time ranged from 1 to 127 months, and median survival time at last follow-up was 22 months.
Table 1.
Clinicopathologic characteristics of patients with EHCC
| Variables | No. of patients |
|---|---|
| Mean age | 230 |
| 60.9 years | |
| Gender | |
| Male | 164 |
| Female | 66 |
| Mean tumor size | 230 |
| 2.5 cm | |
| Location | |
| Perihilar | 106 |
| Distal | 118 |
| Diffuse | 6 |
| Histologic subtype | |
| Tubular adenocarcinoma | 193 |
| Intraductal papillary neoplasm with an associated invasive carcinoma | 15 |
| Intestinal type adenocarcinoma | 9 |
| Mucinous carcinoma | 4 |
| Adenosquamous carcinoma | 6 |
| Clear cell carcinoma | 1 |
| Signet ring cell carcinoma | 1 |
| Sarcomatoid carcinoma | 1 |
| pT classification | |
| pT1 | 31 |
| pT2 | 84 |
| pT3 | 91 |
| pT4 | 24 |
| Lymph node metastasis | |
| Present | 76 |
| Absent | 154 |
| Hepatic invasion | |
| Present | 10 |
| Absent | 220 |
| Pancreatic invasion | |
| Present | 104 |
| Absent | 126 |
| Duodenal invasion | |
| Present | 24 |
| Absent | 206 |
| Perineural invasion | |
| Present | 167 |
| Absent | 63 |
| Vascular invasion | |
| Present | 71 |
| Absent | 159 |
HER2 and HER3 expression
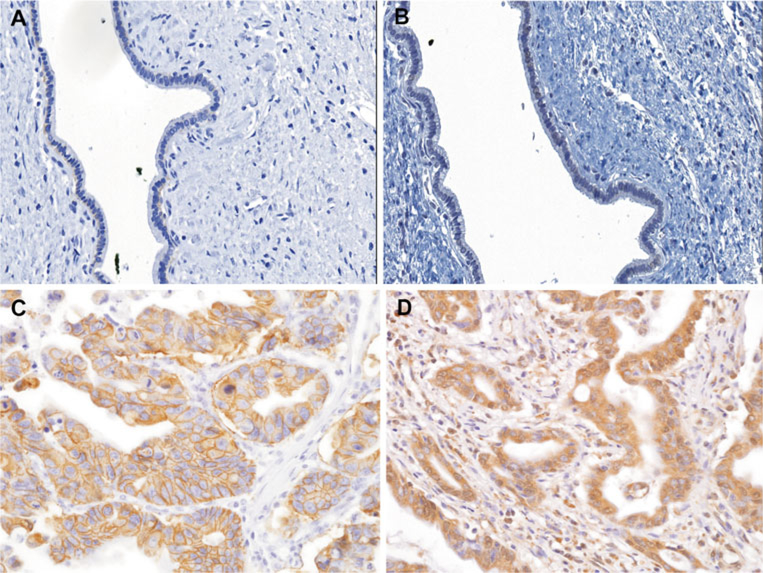
HER2 protein expression patterns were analyzed in tumors from a total of 224 EHCC patients using tissue microarray immunohistochemistry. Information regarding six patients was excluded from the HER2 analysis because their tissue microarray cores were either detached or folded during the HER2 immunohistochemical staining procedure. Representative images of the expression of HER2 and HER3 in EHCC tumors and normal biliary epithelial cells are shown in Fig. 1. All 12 samples of normal biliary epithelial cells did not show immunoreactivity for either HER2 or HER3. HER3 was predominantly distributed in a cytoplasmic pattern, whereas HER2 was mainly localized to the cell membrane in EHCC cases. HER2 and HER3 overexpression was observed in 6 % (13/224 cases) and 39 % (90/230 cases) of EHCCs, respectively. Only the histological subtype was significantly associated with HER2 overexpression (P= 0.02). Specifically, intraductal papillary neoplasms with associated invasive carcinoma cases showed significantly more HER2 overexpression than other histological subtypes, including tubular adenocarcinoma, adenosquamous carcinoma, intestinal type adenocarcinoma, and mucinous carcinoma, which did not express HER2 (Table 2). HER3 protein was more commonly overexpressed in nodular and infiltrative tumors than in papillary tumors (P=0.03). HER3 overexpression was more frequent in cases without than in cases with invasion of the pancreas (P=0.02).
Fig. 1.
Representative immunohistochemical staining of HER2 and HER3 in EHCC. Normal bile duct epithelia show negativity for HER2 (a) and HER3 (b). Cholangiocarcinoma show strong and complete membranous expression of HER2 (c) and diffuse and strong cytoplasmic HER3 immunopositivity (d)
Table 2.
Comparison between HER2 and HER3 immunohistochemical staining results and clinicopathologic factors in EHCC
| Variable | Total no. of cases | HER2 expression (%) | P-value | Total no. of cases | HER3 expression (%) | P-value |
|---|---|---|---|---|---|---|
| Gender | 0.36 | 0.46 | ||||
| Male | 161 | 11 (6.8) | 164 | 67 (40.9) | ||
| Female | 63 | 2 (3.2) | 66 | 23 (34.8) | ||
| Location | 0.18 | 0.21 | ||||
| Perihilar | 101 | 9 (8.9) | 106 | 48 (45.3) | ||
| Distal | 117 | 4 (3.4) | 118 | 40 (33.9) | ||
| Diffuse | 6 | 0 (0.0) | 6 | 2 (33.3) | ||
| Growth pattern | 0.39 | 0.03* | ||||
| Papillary | 22 | 1 (4.5) | 23 | 4 (17.4) | ||
| Nodular | 14 | 2 (14.3) | 14 | 8 (57.1) | ||
| Infiltrative | 188 | 10 (5.3) | 193 | 78 (40.4) | ||
| Differentiation | 1 | 0.13 | ||||
| Well differentiated | 63 | 4 (6.3) | 66 | 32 (48.5) | ||
| Moderately differentiated | 123 | 7 (5.7) | 125 | 46 (36.8) | ||
| Poorly differentiated | 38 | 2 (5.3) | 39 | 12 (30.8) | ||
| Histologic subtype | 0.02* | 0.64 | ||||
| Tubular adenocarcinoma | 188 | 8 (4.3) | 193 | 73 (37.8) | ||
| Intraductal papillary neoplasm with an associated invasive carcinoma | 14 | 5 (35.7) | 15 | 7 (46.7) | ||
| Intestinal type adenocarcinoma | 9 | 0 (0.0) | 9 | 5 (55.6) | ||
| Mucinous carcinoma | 4 | 0 (0.0) | 4 | 1 (25.0) | ||
| Adenosquamous carcinoma | 6 | 0 (0.0) | 6 | 2 (33.3) | ||
| Clear cell carcinoma | 1 | 0 (0.0) | 1 | 1 (100.0) | ||
| Signet ring cell carcinoma | 1 | 0 (0.0) | 1 | 0 (0.0) | ||
| Sarcomatoid carcinoma | 1 | 0 (0.0) | 1 | 1 (100.0) | ||
| pT classification | 0.35 | 0.08 | ||||
| pT1 | 29 | 3 (10.3) | 31 | 16 (51.6) | ||
| pT2 | 82 | 6 (7.3) | 84 | 38 (45.2) | ||
| pT3 | 89 | 4 (4.5) | 91 | 27 (29.7) | ||
| pT4 | 24 | 0 (0.0) | 24 | 9 (37.5) | ||
| Duodenal invasion | 0.37 | 1 | ||||
| Absent | 200 | 13 (6.5) | 206 | 81 (39.3) | ||
| Present | 24 | 0 (0.0) | 24 | 9 (37.5) | ||
| Hepatic invasion | 1 | 0.74 | ||||
| Absent | 215 | 12 (5.6) | 220 | 87 (39.5) | ||
| Present | 9 | 1 (11.1) | 10 | 3 (30.0) | ||
| Pancreatic invasion | 0.15 | 0.02* | ||||
| Absent | 121 | 10 (8.3) | 126 | 58 (46.0) | ||
| Present | 103 | 3 (2.9) | 104 | 32 (30.8) | ||
| Perineural invasion | 0.2 | 0.13 | ||||
| Absent | 62 | 6 (9.7) | 63 | 30 (47.6) | ||
| Present | 162 | 7 (4.3) | 167 | 60 (35.9) | ||
| Vascular invasion | 0.56 | 0.56 | ||||
| Absent | 154 | 10 (6.5) | 159 | 60 (37.7) | ||
| Present | 70 | 3 (4.3) | 71 | 30 (42.3) | ||
| Lymph node metastasis | 1 | 0.67 | ||||
| Absent | 153 | 9 (5.9) | 154 | 62 (40.3) | ||
| Present | 71 | 4 (5.6) | 76 | 28 (36.8) | ||
| Resection marginal status | 1 | 0.15 | ||||
| Negative | 173 | 10 (5.8) | 176 | 64 (36.4) | ||
| Positive | 51 | 3 (5.9) | 54 | 26 (48.1) | ||
| Stage grouping | 0.46 | 0.4 | ||||
| Stage IA | 24 | 3 (12.5) | 25 | 12 (48.0) | ||
| Stage IB | 60 | 3 (5.0) | 60 | 28 (46.7) | ||
| Stage IIA | 58 | 3 (5.2) | 58 | 18 (31.0) | ||
| Stage IIB | 58 | 4 (6.9) | 63 | 23 (36.5) | ||
| Stage III | 24 | 0 (0.0) | 24 | 9 (37.5) |
P<0.05 (significant)
Among 13 HER2-overexpressing EHCCs, seven (54 %) showed HER3 overexpression. There was a significant correlation between cases with HER2 overexpression and those with HER3 overexpression (R=0.24, P<0.001) but not between HER2 and HER3 co-overexpression and clinicopathological parameters (Table 3).
Table 3.
Comparison of results of immunohistochemical staining for HER2 and HER3 co-expression with clinicopathologic factors in EHCC
| Variable | Total no. of cases | HER2 and HER3 co-overexpression (%) | P-value |
|---|---|---|---|
| Gender | 0.2 | ||
| Male | 161 | 7 (4.3) | |
| Female | 63 | 0 (0.0) | |
| Location | 0.09 | ||
| Perihilar | 101 | 6 (5.9) | |
| Distal | 117 | 1 (0.9) | |
| Diffuse | 6 | 0 (0.0) | |
| Growth pattern | 0.36 | ||
| Papillary | 22 | 0 (0.0) | |
| Nodular | 14 | 1 (7.1) | |
| Infiltrative | 188 | 6 (3.2) | |
| Differentiation | 1 | ||
| Well differentiated | 63 | 2 (3.2) | |
| Moderately differentiated | 123 | 4 (3.3) | |
| Poorly differentiated | 38 | 1 (2.6) | |
| Histologic subtype | 0.12 | ||
| Tubular adenocarcinoma | 188 | 4 (2.1) | |
| Intraductal papillary neoplasm with an associated invasive carcinoma | 14 | 3 (21.4) | |
| Intestinal type adenocarcinoma | 9 | 0 (0.0) | |
| Mucinous carcinoma | 4 | 0 (0.0) | |
| Adenosquamous carcinoma | 6 | 0 (0.0) | |
| Clear cell carcinoma | 1 | 0 (0.0) | |
| Signet ring cell carcinoma | 1 | 0 (0.0) | |
| Sarcomatoid carcinoma | 1 | 0 (0.0) | |
| pT classification | 0.66 | ||
| pT1 | 29 | 1 (3.4) | |
| pT2 | 82 | 4 (4.9) | |
| pT3 | 89 | 2 (2.2) | |
| pT4 | 24 | 0 (0.0) | |
| Duodenal invasion | 0.61 | ||
| Absent | 200 | 7 (3.5) | |
| Present | 24 | 0 (0.0) | |
| Hepatic invasion | 1 | ||
| Absent | 215 | 7 (3.3) | |
| Present | 9 | 0 (0.0) | |
| Pancreatic invasion | 0.46 | ||
| Absent | 121 | 5 (4.1) | |
| Present | 103 | 2 (1.9) | |
| Perineural invasion | 0.4 | ||
| Absent | 62 | 3 (4.8) | |
| Present | 162 | 4 (2.5) | |
| Vascular invasion | 0.44 | ||
| Absent | 154 | 6 (3.9) | |
| Present | 70 | 1 (1.4) | |
| Lymph node metastasis | 1 | ||
| Absent | 153 | 5 (3.3) | |
| Present | 71 | 2 (2.8) | |
| Resection marginal status | 1 | ||
| Absent | 173 | 5 (2.9) | |
| Present | 51 | 2 (3.9) | |
| Stage grouping | 0.94 | ||
| Stage IA | 24 | 1 (4.2) | |
| Stage IB | 60 | 2 (3.3) | |
| Stage IIA | 58 | 2 (3.4) | |
| Stage IIB | 58 | 2 (3.4) | |
| Stage III | 24 | 0 (0.0) |
Survival analysis
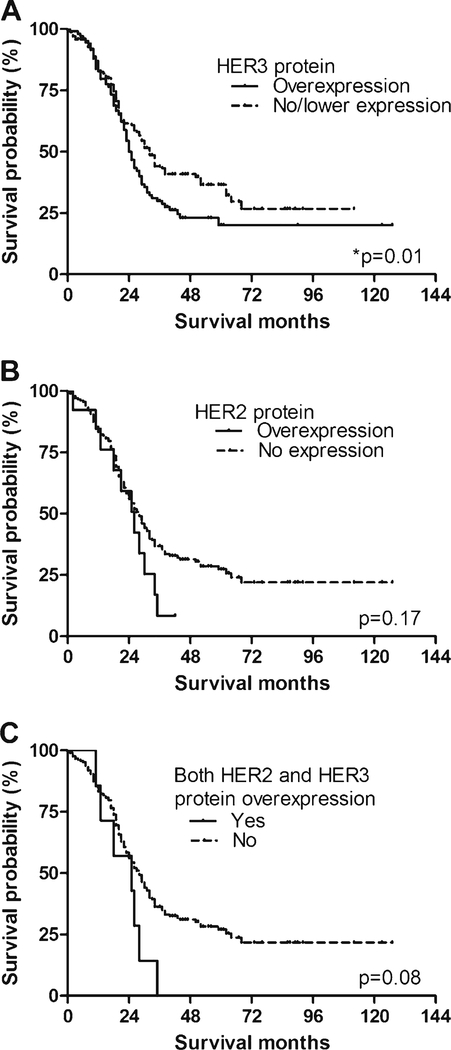
Median survival of patients with HER3 overexpression (24.0 months) was significantly worse than that of patients with no/lower HER3 expression (31.9 months; P=0.01, log-rank test; Fig. 2a). The 1-, 3-, and 5-year survival rates in the HER3-overexpression group were 82.2, 22.6, and 13.9 %, respectively, whereas the corresponding rates in the no/lower HER3-expression group were 82.6, 40.5, and 31.2 %.
Fig. 2.
Survival curves stratified by HER2 and HER3 expression in EHCC patients. a Median survival in patients in the HER3-overexpression group (24.0 months) was significantly shorter than that for patients in the no/ lower HER3-expression group (31.9 months; P=0.01, log-rank test). b Survival was not significantly different between patients in the HER2-overexpression group (25.9 months) and the no/lower HER2-expression group (27.8 months; P=0.17, log-rank test). c Median survival in EHCC patients with both HER2 and HER3 co-overexpression (24.8 months) was marginally different from that in patients without HER2 and HER3 co-overexpression (28.0 months; P=0.08)
There was no significant difference in survival between patients with HER2 overexpression (median survival, 25.9 months) and those without HER2 expression (median survival, 27.8 months; P=0.17, log-rank test; Fig. 2b).
Median survival of EHCC patients with both HER2 and HER3 overexpression (24.8 months) tended to be lower than that in patients without HER2 and HER3 co-overexpression (28.0 months; P=0.08; Fig. 2c), but this did not reach statistical significance.
Association between survival and other clinicopathological factors
The relationships between other clinicopathological variables and survival are summarized in Table 4. Of these additional clinicopathological variables, tumor differentiation status (P<0.001), pT classification (P=0.01), lymph node metastasis (P<0.001), liver invasion (P=0.01), and vascular invasion (P=0.02) were also significantly associated with survival. In contrast, survival was not associated with gender (P=0.38), tumor location (P=0.47), growth pattern (P=0.09), pancreatic invasion (P=0.24), duodenal invasion (P=0.07), perineural invasion (P=0.36), or resection margin status (P=0.10).
Table 4.
Univariate analysis of pathologic features affecting survival in EHCC patients
| Variable | Characteristics | Median survival time (month) | P-value | 95 % confidence interval |
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
| HER2 expression | Low | 27.8 | 0.17 | 24.2 | 31.4 |
| High | 25.9 | 18.6 | 33.2 | ||
| HER3 expression | Low | 31.9 | 0.01* | 27.3 | 36.5 |
| High | 24.0 | 22.0 | 26.0 | ||
| Gender | Male | 28.4 | 0.38 | 24.8 | 32.0 |
| Female | 21.8 | 15.0 | 28.6 | ||
| Location | Perihilar | 24.8 | 0.47 | 21.1 | 28.4 |
| Distal | 30.0 | 25.5 | 34.5 | ||
| Diffuse | 12.0 | 0 | 42.7 | ||
| Growth pattern | Papillary | 50.0 | 0.09 | ||
| Nodular | 30.0 | 10.8 | 49.2 | ||
| Infiltrative | 25.9 | 22.2 | 29.6 | ||
| Differentiation | Well | 34.0 | <0.001* | 24.0 | 44.0 |
| Moderate | 26.8 | 22.5 | 31.2 | ||
| Poor | 17.5 | 11.0 | 24.0 | ||
| pT classification | pT1 | 44.0 | 0.01* | ||
| pT2 | 24.9 | 22.2 | 27.6 | ||
| pT3 | 25.9 | 19.0 | 32.9 | ||
| pT4 | 27.0 | 14.5 | 39.5 | ||
| Perineural invasion | Absent | 28.9 | 0.36 | 21.8 | 36.0 |
| Present | 26.1 | 21.8 | 30.4 | ||
| Vascular invasion | Absent | 28.9 | 0.02* | 22.9 | 34.9 |
| Present | 26.1 | 20.9 | 31.2 | ||
| Duodenal invasion | Absent | 26.9 | 0.07 | 23.6 | 30.2 |
| Present | 27.0 | 14.5 | 39.5 | ||
| Liver invasion | Absent | 28.0 | 0.01* | 24.9 | 31.1 |
| Present | 17.5 | 0.0 | 35.7 | ||
| Pancreas invasion | Absent | 26.1 | 0.24 | 21.6 | 30.6 |
| Present | 27.0 | 22.2 | 31.8 | ||
| Resection margin status | Negative | 28.8 | 0.1 | 24.9 | 32.7 |
| Positive | 23.1 | 17.5 | 28.7 | ||
| Lymph node metastasis | Absent | 30.0 | <0.001* | 27.0 | 33.0 |
| Present | 18.2 | 15.5 | 20.9 | ||
P<0.05 (significant)
Multivariate analysis of clinicopathological factors
The independent prognostic significance of HER3 expression as well as of other clinicopathological parameters was determined by applying the Cox proportional hazards model (Table 5). HER3 overexpression (P= 0.007), differentiation (P=0.002), liver invasion (P= 0.03), and lymph node metastasis (P=0.002) remained as prognostic factors.
Table 5.
Multivariate analysis for prognosis in EHCC
| Variable | P-value | Relative risk | 95 % confidence interval |
|---|---|---|---|
| HER3 expression | 0.007* | 1.6 | 1.14–2.26 |
| Differentiation | 0.002* | 1.52 | 1.16–2.00 |
| pT classification | 0.066 | 1.21 | 0.99–1.48 |
| Vascular invasion | 0.646 | 1.1 | 0.75–1.62 |
| Liver invasion | 0.025* | 2.47 | 1.12–5.45 |
| Lymph node metastasis | 0.002* | 1.81 | 1.25–2.65 |
Duodenal invasion was not included because it was a covariate with pT classification
P<0.05 (significant)
Discussion
In this study, we observed overexpression of HER2 and HER3 protein in a subset of EHCCs, with about 6 % of EHCC cases overexpressing HER2 (13/224) and 39 % (90/ 230) overexpressing HER3. In addition, we found HER3 overexpression to be an independent prognostic factor in EHCC patients. HER3 is unique among members of the HER receptor tyrosine kinase family in that it lacks intrinsic tyrosine kinase activity but it does contain six consensus phosphotyrosine sites, which bind the SH2 domain of the three regulatory subunits of phosphoinositide-3-kinase (PI3K) [27, 28]. Binding of HER3 can activate the PI3K/Akt signaling pathway, which is a critical regulator of many cellular processes. HER2-mediated transformation of epithelial cells is associated with the activation of the PI3K/Akt signaling pathway in breast cancer. Several targeted therapeutics, including trastuzumab, a monoclonal antibody against the extracellular domain of HER2, and lapatinib, an EGFR/HER2 tyrosine kinase inhibitor, have been approved for use in the treatment of HER2-overexpressing breast cancers. It has been proposed that inhibition of HER3 phosphorylation and blockade of the PI3K/Akt signaling pathway are required in order to obtain a tumor suppression effect despite differences in the operating mechanisms of these therapeutic agents [29]. HER2–HER3 heterodimerization plays a key role in HER2-mediated transformation, tumor progression, and drug resistance [29]. Previous studies on loss of HER3 expression in HER2-dependent cells have demonstrated reduced PI3K signaling and decreased cell proliferation, suggesting that HER3 is essential for HER2-driven carcinogenesis [30, 31]. Treatment of HER2-amplified tumors with tyrosine kinase inhibitors results in a compensatory increase in HER3 expression, membranous HER3 localization, and decreased HER3 dephosphorylation [32]. MET-dependent phosphorylation of HER3 has been identified as one mechanism of resistance to EGFR inhibitors in lung cancer [33]. A recent study demonstrated that blocking both HER2 and HER3 inhibits the PI3K/Akt pathway more effectively than blocking HER2 or HER3 alone, suggesting that also HER3 should be inhibited in patients with HER2- and PI3K-dependent cancers in order to completely block the PI3K/Akt pathway [29]. Our group previously demonstrated that the Akt pathway is activated in a large proportion of EHCCs (84 %) [24]. In the present study, we demonstrate that HER3 is overexpressed in 39 % of EHCC cases. Considering the current study and the results of previous studies of breast cancers and EHCCs, we hypothesize that application of a therapeutic regimen for blocking HER3, such as AMG888, together with HER2 inhibitors and PI3K inhibitors might be effective in a subset of EHCC patients with activated Akt and HER3.
HER3 overexpression has been identified in malignant neoplasms from several organs, including melanoma, and prostate, colorectal, lung, ovarian, and gastric cancers [17, 30,34–38]. In addition, HER3 overexpression has been reported to be associated with outcome in melanomas and lung and gastric cancers [34, 38, 39]. Although HER3 is specifically required for HER2-driven tumorigenesis and has been extensively studied in the HER2-amplified subtype of breast cancers, other types of cancer also show higher HER3 expression without accompanying HER2 overexpression [30, 40]. In these tumors, HER3 may function as an allosteric activator of other members of the HER receptor tyrosine kinase family, such as EGFR [41–43]. EGFR expression has been reported in 8–57 % of cholangiocarcinomas, and patients with EGFR-positive tumors showed worse clinical outcome than those with EGFR-negative cancers [20, 23, 44, 45]. The absence of a survival difference in patients with HER2 overexpression and the significantly shorter survival in patients with HER3 overexpression, as demonstrated in this study, suggest a possible link between shorter survival time in the HER3-overexpression group and EGFR–HER3 heterodimerization in a subset of these patients. These patients may benefit from a therapeutic regimen that includes other types of HER inhibitors, including erlotinib, cetuximab, and lapatinib, which have been investigated recently in cholangiocarcinomas [46–50].
HER2 overexpression in cholangiocarcinomas has been explored in various studies and has shown to vary widely (between 5 and 76 %) [20, 51–57]. These discrepancies might be explained by the lack of a standardized methodology, different standards of interpretation, or differences in tumor location. In recent reports, the HER2 expression rate in resected EHCCs was reported to be 5 to 10 % [20, 23, 45, 52]. The approximately 6 % HER2 expression rate identified in the current study is in agreement with these previous studies. Consistent with some previous reports, we found a higher HER2 overexpression rate in intraductal papillary neoplasm with an associated invasive carcinoma than in other histological types [20, 45, 54]. Although the HER2 overexpression rate is not exceptionally high in EHCCs, a subset of patients with HER2 overexpression might benefit from HER2-targeted monoclonal therapy.
The recently updated (7th edition) American Joint Committee on Cancer Staging Manual divided EHCCs into perihilar and distal bile duct cancers [58]. The term “distal bile duct cancers” was applied to cancers distal to the insertion of the cystic duct. This division reflects the growing evidence for distinct biological patterns in perihilar and distal EHCCs [59–61]. However, in our study, the proportions of HER2 and HER3 overexpression were similar in perihilar and distal bile duct cancer groups.
In conclusion, HER2 and HER3 proteins are overexpressed in subsets of EHCCs. HER3 overexpression is correlated with decreased patient survival and therefore constitutes a prognostic factor and a potential therapeutic target in patients with EHCCs.
Acknowledgments
We thank Ylaya Kris for technical assistance.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Hee Jin Lee, Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 388-1, Pungnap-dong, Songpa-gu, Seoul 138-736, Korea.
Joon-Yong Chung, Applied Molecular Pathology Laboratory and Tissue Array Research Program, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, Bethesda MD, USA.
Stephen M. Hewitt, Applied Molecular Pathology Laboratory and Tissue Array Research Program, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, Bethesda MD, USA
Eunsil Yu, Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 388-1, Pungnap-dong, Songpa-gu, Seoul 138-736, Korea.
Seung-Mo Hong, Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 388-1, Pungnap-dong, Songpa-gu, Seoul 138-736, Korea.
References
- 1.Malhi H, Gores GJ (2006) Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol 45:856–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong SM, Kim MJ, Pi DY, Jo D, Cho HJ, Yu E, Ro JY (2005) Analysis of extrahepatic bile duct carcinomas according to the New American Joint Committee on Cancer staging system focused on tumor classification problems in 222 patients. Cancer 104:802–810 [DOI] [PubMed] [Google Scholar]
- 3.Nagakawa T, Kayahara M, Ikeda S, Futakawa S, Kakita A, Kawarada H, Matsuno M, Takada T, Takasaki K, Tanimura H, Tashiro S, Yamaoka Y (2002) Biliary tract cancer treatment: results from the Biliary Tract Cancer Statistics Registry in Japan. J Hepatobiliary Pancreat Surg 9:569–575 [DOI] [PubMed] [Google Scholar]
- 4.Seyama Y, Makuuchi M (2007) Current surgical treatment for bile duct cancer. World J Gastroenterol 13:1505–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan H, Pawlik TM, Wolfgang CL, Choti MA, Cameron JL, Schulick RD (2007) Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg 11:1488–1496, discussion 1496–1487 [DOI] [PubMed] [Google Scholar]
- 6.Thomas MB (2007) Biological characteristics of cancers in the gallbladder and biliary tract and targeted therapy. Crit Rev Oncol Hematol 61:44–51 [DOI] [PubMed] [Google Scholar]
- 7.Sithanandam G, Anderson LM (2008) The ERBB3 receptor in cancer and cancer gene therapy. Cancer Gene Ther 15:413–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL 3rd (1994) Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci U S A 91:8132–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Citri A, Yarden Y (2006) EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 7:505–516 [DOI] [PubMed] [Google Scholar]
- 10.Hsieh AC, Moasser MM (2007) Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br J Cancer 97:453–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poller DN, Spendlove I, Baker C, Church R, Ellis IO, Plowman GD, Mayer RJ (1992) Production and characterization of a polyclonal antibody to the c-erbB-3 protein: examination of c-erbB-3 protein expression in adenocarcinomas. J Pathol 168:275–280 [DOI] [PubMed] [Google Scholar]
- 12.Sanidas EE, Filipe MI, Linehan J, Lemoine NR, Gullick WJ, Rajkumar T, Levison DA (1993) Expression of the c-erbB-3 gene product in gastric cancer. Int J Cancer 54:935–940 [DOI] [PubMed] [Google Scholar]
- 13.Slesak B, Harlozinska A, Porebska I, Bojarowski T, Lapinska J, Rzeszutko M, Wojnar A (1998) Expression of epidermal growth factor receptor family proteins (EGFR, c-erbB-2 and c-erbB-3) in gastric cancer and chronic gastritis. Anticancer Res 18:2727–2732 [PubMed] [Google Scholar]
- 14.Ciardiello F, Kim N, Saeki T, Dono R, Persico MG, Plowman GD, Garrigues J, Radke S, Todaro GJ, Salomon DS (1991) Differential expression of epidermal growth factor-related proteins in human colorectal tumors. Proc Natl Acad Sci U S A 88:7792–7796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurer CA, Friess H, Kretschmann B, Zimmermann A, Stauffer A, Baer HU, Korc M, Buchler MW (1998) Increased expression of erbB3 in colorectal cancer is associated with concomitant increase in the level of erbB2. Hum Pathol 29:771–777 [DOI] [PubMed] [Google Scholar]
- 16.Kapitanovic S, Radosevic S, Slade N, Kapitanovic M, Andelinovic S, Ferencic Z, Tavassoli M, Spaventi S, Pavelic K, Spaventi R (2000) Expression of erbB-3 protein in colorectal adenocarcinoma: correlation with poor survival. J Cancer Res Clin Oncol 126:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kountourakis P, Pavlakis K, Psyrri A, Rontogianni D, Xiros N, Patsouris E, Pectasides D, Economopoulos T (2006) Prognostic significance of HER3 and HER4 protein expression in colorectal adenocarcinomas. BMC Cancer 6:46. doi:1471-2407-6-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friess H, Wang L, Zhu Z, Gerber R, Schroder M, Fukuda A, Zimmermann A, Korc M, Buchler MW (1999) Growth factor receptors are differentially expressed in cancers of the papilla of Vater and pancreas. Ann Surg 230:767–774, discussion 774–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friess H, Yamanaka Y, Kobrin MS, Do DA, Buchler MW, Korc M (1995) Enhanced erbB-3 expression in human pancreatic cancer correlates with tumor progression. Clin Cancer Res 1:1413–1420 [PubMed] [Google Scholar]
- 20.Yoshikawa D, Ojima FI, Iwasaki M, Hiraoka N, Kosuge T, Kasai S, Hirohashi S, Shibata T (2008) Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer 98:418–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su WC, Shiesh SC, Liu HS, Chen CY, Chow NH, Lin XZ (2001) Expression of oncogene products HER2/Neu and Ras and fibrosisrelated growth factors bFGF, TGF-beta, and PDGF in bile from biliary malignancies and inflammatory disorders. Dig Dis Sci 46:1387–1392 [DOI] [PubMed] [Google Scholar]
- 22.Harder J, Waiz O, Otto F, Geissler M, Olschewski M, Weinhold B, Blum HE, Schmitt-Graeff A, Opitz OG (2009) EGFR and HER2 expression in advanced biliary tract cancer. World J Gastroenterol 15:4511–4517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shafizadeh N, Grenert JP, Sahai V, Kakar S (2010) Epidermal growth factor receptor and HER-2/neu status by immunohistochemistry and fluorescence in situ hybridization in adenocarcinomas of the biliary tree and gallbladder. Hum Pathol 41:485–492 [DOI] [PubMed] [Google Scholar]
- 24.Chung JY, Hong SM, Choi BY, Cho H, Yu E, Hewitt SM (2009) The expression of phospho-AKT, phospho-mTOR, and PTEN in extrahepatic cholangiocarcinoma. Clin Cancer Res 15:660–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takikita M, Xie R, Chung JY, Cho H, Ylaya K, Hong SM, Moskaluk CA, Hewitt SM (2011) Membranous expression of Her3 is associated with a decreased survival in head and neck squamous cell carcinoma. J Transl Med 9:126 doi:1479-5876-9-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hofmann M, Stoss O, Shi D, Buttner R, van de Vijver M, Kim W, Ochiai A, Ruschoff J, Henkel T (2008) Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 52:797–805 [DOI] [PubMed] [Google Scholar]
- 27.Soltoff SP, Carraway KL 3rd, Prigent SA, Gullick WG, Cantley LC (1994) ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol Cell Biol 14:3550–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prigent SA, Gullick WJ (1994) Identification of c-erbB-3 binding sites for phosphatidylinositol 3'-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J 13:2831–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, Dave B, Cook RS, Pao W, McKinely E, Manning HC, Chang J, Arteaga CL (2011) Transcriptional and posttranslational up-regulation of HER3 (ErbB3 ) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci USA 108:5021–5026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stem HM (2008) A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res 68:5878–5887 [DOI] [PubMed] [Google Scholar]
- 31.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF 3rd, Hynes NE (2003) The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A 100:8933–8938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM (2007) Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 445:437–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Janne PA (2007) MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316:1039–1043 [DOI] [PubMed] [Google Scholar]
- 34.Reschke M, Mihic-Probst D, van der Horst EH, Knyazev P, Wild PJ, Hutterer M, Meyer S, Dummer R, Moch H, Ullrich A (2008) HER3 is a determinant for poor prognosis in melanoma. Clin Cancer Res 14:5188–5197 [DOI] [PubMed] [Google Scholar]
- 35.Lozano JJ, Soler M, Bermudo R, Abia D, Fernandez PL, Thomson TM, Ortiz AR (2005) Dual activation of pathways regulated by steroid receptors and peptide growth factors in primary prostate cancer revealed by factor analysis of microarray data. BMC Genomics 6:109 doi:1471-2164-6-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun M, Behrens C, Feng L, Ozburn N, Tang X, Yin G, Komaki R, Varella-Garcia M, Hong WK, Aldape KD, Wistuba II (2009) HER family receptor abnormalities in lung cancer brain metastases and corresponding primary tumors. Clin Cancer Res 15:4829–4837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson BJ, Weatherill J, Miller EP, Lessells AM, Langdon SP, Miller WR (1995) c-erbB-3 protein expression in ovarian tumours. Br J Cancer 71:758–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Begnami MD, Fukuda E, Fregnani JH, Nonogaki S, Montagnini AL, da Costa WL, Soares FA Jr (2011) Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol 29:3030–3036 [DOI] [PubMed] [Google Scholar]
- 39.Yi ES, Harclerode D, Gondo M, Stephenson M, Brown R Younes M, Cagle PT (1997) High c-erbB-3 protein expression is associated with shorter survival in advanced non-small cell lung carcinomas. Mod Pathol 10:142–148 [PubMed] [Google Scholar]
- 40.Kluger HM, DiVito K, Berger AJ, Halaban R, Ariyan S, Camp RL, Rimm DL (2004) Her2/neu is not a commonly expressed therapeutic target in melanoma—a large cohort tissue microarray study. Melanoma Res 14:207–210 [DOI] [PubMed] [Google Scholar]
- 41.Ueno Y, Sakurai H, Tsunoda S, Choo MK, Matsuo M, Koizumi K, Saiki I (2008) Heregulin-induced activation of ErbB3 by EGFR tyrosine kinase activity promotes tumor growth and metastasis in melanoma cells. Int J Cancer 123:340–347 [DOI] [PubMed] [Google Scholar]
- 42.Engelman JA, Janne PA, Mermel C, Pearlberg J, Mukohara T, Fleet C, Cichowski K, Johnson BE, Cantley LC (2005) ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc Natl Acad Sci U S A 102:3788–3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soler M, Mancini F, Meca-Cortes O, Sanchez-Cid L, Rubio N, Lopez-Fernandez S, Lozano JJ, Blanco J, Fernandez PL, Thomson TM (2009) HER3 is required for the maintenance of neuregulin-dependent and -independent attributes of malignant progression in prostate cancer cells. Int J Cancer 125:2565–2575 [DOI] [PubMed] [Google Scholar]
- 44.Jan YY, Yeh TS, Yeh JN, Yang HR, Chen MF (2004) Expression of epidermal growth factor receptor, apomucins, matrix metalloproteinases, and p53 in rat and human cholangiocarcinoma: appraisal of an animal model of cholangiocarcinoma. Ann Surg 240:89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakazawa K, Dobashi Y, Suzuki S, Fujii H, Takeda Y, Ooi A (2005) Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. J Pathol 206:356–365 [DOI] [PubMed] [Google Scholar]
- 46.Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, Donehower RC, Fitch T, Picus J, Erlichman C (2006) Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol 24:3069–3074 [DOI] [PubMed] [Google Scholar]
- 47.Dragovich T, Huberman M, Von Hoff DD, Rowinsky EK, Nadler P, Wood D, Hamilton M, Hage G, Wolf J, Patnaik A (2007) Erlotinib plus gemcitabine in patients with unresectable pancreatic cancer and other solid tumors: phase IB trial. Cancer Chemother Pharmacol 60:295–303 [DOI] [PubMed] [Google Scholar]
- 48.Paule B, Herelle MO, Rage E, Ducreux M, Adam R, Guettier C, Bralet MP (2007) Cetuximab plus gemcitabine–oxaliplatin (GEMOX) in patients with refractory advanced intrahepatic cholangiocarcinomas. Oncology 72:105–110 [DOI] [PubMed] [Google Scholar]
- 49.Andre T, Reyes-Vidal JM, Fartoux L, Ross P, Leslie M, Rosmorduc O, Clemens MR, Louvet C, Perez N, Mehmud F, Scheithauer W (2008) Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer 99:862–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Safran H, Miner T, Resnick M, Dipetrillo T, McNulty B, Evans D, Joseph P, Plette A, Millis R, Sears D, Gutman N, Kennedy T (2008) Lapatinib/gemcitabine and lapatinib/gemcitabine/oxaliplatin: a phase I study for advanced pancreaticobiliary cancer. Am J Clin Oncol 31:140–144 [DOI] [PubMed] [Google Scholar]
- 51.Aishima SI, Taguchi KI, Sugimachi K, Shimada M, Tsuneyoshi M (2002) c-erbB-2 and c-Met expression relates to cholangiocarcinogenesis and progression of intrahepatic cholangiocarcinoma. Histopathology 40:269–278 [DOI] [PubMed] [Google Scholar]
- 52.Altimari A, Fiorentino M, Gabusi E, Gruppioni E, Corti B, D'Errico A, Grigioni WF (2003) Investigation of ErbB1 and ErbB2 expression for therapeutic targeting in primary liver tumours. Dig Liver Dis 35:332–338 [DOI] [PubMed] [Google Scholar]
- 53.Chow NH, Huang SM, Chan SH, Mo LR, Hwang MH, Su WC (1995) Significance of c-erbB-2 expression in normal and neoplastic epithelium of biliary tract. Anticancer Res 15:1055–1059 [PubMed] [Google Scholar]
- 54.Endo K, Yoon BI, Pairojkul C, Demetris AJ, Sirica AE (2002) ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology 36:439–450 [DOI] [PubMed] [Google Scholar]
- 55.Terada T, Ashida K, Endo K, Horie S, Maeta H, Matsunaga Y, Takashima K, Ohta T, Kitamura Y (1998) c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology 33:325–331 [DOI] [PubMed] [Google Scholar]
- 56.Ukita Y, Kato M, Terada T (2002) Gene amplification and mRNA and protein overexpression of c-erbB-2 (HER-2/neu) in human intrahepatic cholangiocarcinoma as detected by fluorescence in situ hybridization, in situ hybridization, and immunohistochemistry. J Hepatol 36:780–785 [DOI] [PubMed] [Google Scholar]
- 57.Voravud N, Foster CS, Gilbertson JA, Sikora K, Waxman J (1989) Oncogene expression in cholangiocarcinoma and in normal hepatic development. Hum Pathol 20:1163–1168 [DOI] [PubMed] [Google Scholar]
- 58.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti AI (2010) AJCC cancer staging manual. Springer, New York [Google Scholar]
- 59.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL (1996) Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 224:463–473, discussion 473–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD (2007) Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 245:755–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Argani P, Shaukat A, Kaushal M, Wilentz RE, Su GH, Sohn TA, Yeo CJ, Cameron JL, Kern SE, Hruban RH (2001) Differing rates of loss of DPC4 expression and of p53 overexpression among carcinomas of the proximal and distal bile ducts. Cancer 91:1332–1341 [PubMed] [Google Scholar]
- 62.Watanabe A, Cornelison R, Hostetter G (2005) Tissue microarrays: applications in genomic research. Expert Rev Mol Diagn 5:171–181 [DOI] [PubMed] [Google Scholar]