Abstract
Background:
Cabozantinib and gemcitabine improve tumor control in pancreatic ductal adenocarcinoma (PDAC) in vitro through c-Met inhibition. We sought to determine the maximum tolerated dose (MTD) of this combination.
Methods:
Patients with advanced PDAC with ≤ 1 prior treatment and adequate performance status were eligible. Cabozantinib was given orally once daily, beginning day (−) 7 and continued with gemcitabine given intravenously on days 1, 8, and 15 every 28 days. Dose level was assigned using Time to Event Continual Reassessment Method (TITE-CRM). Primary endpoint was MTD, defined as the highest dose level at which ≤ 25% of patients incurred a dose-limiting toxicity (DLT) in the first 35 days of therapy. Secondary endpoints included response rate, progression-free survival (PFS), overall survival (OS) and urinary biomarker assessment.
Results:
Twelve patients were treated, and an MTD was not determined. The probability of DLT was > 25% for all dose levels tested. DLTs included grade 3 ALT/AST elevations and thrombocytopenia. Three patients had partial responses, but each discontinued therapy due to toxicity. Median PFS and OS were 4.7 (95% CI: 1.4 – 9.7) and 10.1 months (95% CI: 3.6 – 20.6). Biomarker analysis, though limited in scope, showed changes in c-Met and VEGF that corresponded with response.
Conclusions:
An MTD for the combination was not established. Cabozantinib and gemcitabine appear impractical for further development due to DLT at low doses and continuing toxicities with ongoing therapy. Acknowledging the small sample size, responses were seen suggesting further investigation of c-Met inhibition in PDAC may be warranted.
Keywords: Cabozantinib, XL-184, Gemcitabine, Pancreatic Cancer
Introduction
The prognosis for patients with pancreatic ductal adenocarcinoma (PDAC) is poor, with a 5 year overall survival of 7.6% [1]. It is projected that pancreatic cancer-related deaths will increase and surpass those from breast, prostate, and colorectal cancers combined by 2030 [2]. Gemcitabine has been a standard therapy in metastatic PDAC [3]. As compared to gemcitabine alone, combination regimens of 5-flurouracil/leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) [4] and gemcitabine/nab-paclitaxel [5] have demonstrated improvement in overall survival. Disease control rates and survival remain far from satisfactory, however, and as such, novel treatments are critically needed in this patient population.
Hepatocyte growth factor (HGF) and its receptor, c-Met, regulate a number of normal biological functions [6–10] and in malignancy, promote growth, invasion, angiogenesis, and metastases [11–13]. Their expression is associated with a poor prognosis [14–16]. HGF and c-Met are known to be activated in PDAC [17–19], and phosphorylated c-Met expression is increased in gemcitabine-resistant pancreatic cell lines [20]. Inhibition of c-Met signaling in-vitro synergistically enhances the anti-angiogenic effects of VEGF blockade [21]. Furthermore, we and others have demonstrated a link between c-Met expression and pancreatic cancer stem cells, a reservoir of self-renewing cells promoting PDAC progression and resistance to conventional chemotherapies [22–25].
Cabozantinib is an orally bioavailable c-Met inhibitor, which targets multiple receptor tyrosine kinases (RTK’s), including primarily c-Met, VEGFR2, KIT, and RET [22,23,26]. In preclinical evaluation here, cabozantinib significantly prevented tumor growth in an orthotopic PDAC implantation model in NOD/SCID mice [22]. This inhibitory effect was further enhanced with the addition of gemcitabine, with tumor growth delay greater with the combination than either agent alone. Based on these considerations, we conducted a phase I study to determine the maximum tolerated dose (MTD) of the combination of cabozantinib and gemcitabine in patients with advanced PDAC.
Patients and Methods
Eligibility and Scheduled Assessments
Patients 18 years and older with pathologically confirmed, locally advanced or metastatic PDAC were eligible. Study participants were required to have good performance status (ECOG 0 or 1) and adequate organ function (absolute neutrophil count ≥1500/mm3, platelets ≥ 100,000/mm3, hemoglobin ≥ 9 g/dL, serum creatinine ≤ 1.5 x upper limit of normal (ULN) or calculated creatinine clearance ≥60 mL/min, total serum bilirubin ≤1.5, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤ 2.5 x ULN if no disease involvement of the liver, or ≤5 x ULN with liver involvement). Patients were excluded if they had received more than 1 prior systemic treatment regimen for PDAC. Prior gemcitabine was allowed only if received as neoadjuvant or adjuvant therapy and provided that at least 6 months had elapsed from the completion of that therapy to study treatment. Other exclusion criteria included brain metastases, major bleeding event within 3 months of study initiation, active treatment with therapeutic doses of anticoagulants, or uncontrolled significant illness including active cardiac disease or gastrointestinal disorders associated with high risk for perforation or fistula formation.
The Institutional Review Board of the University of Michigan approved this trial, and written informed consent was obtained from all individual participants included in the study.
Pre-treatment evaluation included complete blood count with differential, coagulation profile, chemistry panel, urine protein to creatinine ratio, CA19–9, pregnancy test, thyroid function tests, and electrocardiogram (ECG). Safety evaluations were performed weekly during the first 35 days of therapy and then every two weeks thereafter and included complete blood count with differential and complete chemistry panels. In addition, prior to each cycle of therapy, amylase, lipase, magnesium, phosphorous, LDH, urinalysis with urine protein to creatinine ratio, and for women, a pregnancy screening test were performed. TSH was re-checked prior to cycle 3 and then as clinically indicated, and ECG performed on cycle 1, day 15 and cycle 2, day 1, then as clinically indicated.
Treatment and Toxicity Evaluation
Cabozantinib was taken orally once daily in a fasted state, beginning on day −7 and continued with gemcitabine administered intravenously over 30 minutes on days 1, 8, and 15 every 28 days. Study design intended to deliver gemcitabine at usual dose and schedule and escalate cabozantinib from a starting dose level of 20 mg daily, to doses tolerable as a single agent (60–80 mg). Dose levels investigated are depicted in Table 2. The starting dose level (1) was cabozantinib 20 mg orally daily and gemcitabine 1000 mg/m2. Doses were assigned in accordance with a Time to Event Continual Reassessment Method (TITE-CRM) [27,28]. Dose level escalation could not occur until at least two patients had been observed for the entire DLT interval at a dose level. Exelixis, Inc provided cabozantinib in 20 mg tablets.
Table 2:
Assigned Dose Levels and Rates of Dose Limiting Toxicities (n=10 evaluable patients)
| Dose Level | Dose | Number Treated | Number with DLT | DLT | Prior Probability of DLT | Posterior Probability of DLT | 95% HPD Credible Interval |
|---|---|---|---|---|---|---|---|
| −1 | 20C, 800G | 4 | 1 | Grade 3 thrombocytopenia (Day 15) |
0.05 | 0.27 | 0.06 – 0.46 |
| 1 | 20C, 1000G | 5 | 2 | Grade 3 ALT elevation (Day 8) Grade 3 AST/ALT elevation (Day 15) |
0.10 | 0.36 | 0.14 – 0.57 |
| 2 | 40C, 1000G | 1 | 1 | Grade 3 ALT elevation and Grade 3 thrombocytopenia (Day 22) |
0.15 | 0.43 | 0.21 – 0.65 |
| 3 | 60C, 1000G | 0 | 0 | 0.20 | 0.49 | 0.29 – 0.70 | |
| 4 | 80C, 1000G | 0 | 0 | 0.25 | 0.54 | 0.35 – 0.73 |
C: Cabozantinib, dose in mg; G: Gemcitabine, dose in mg/m2; HPD: Highest Posterior Density
Toxicities were scored using the Common Toxicity Criteria for Adverse Events (CTCAE, version 4.0). Cabozantinib was interrupted for ≥ grade 2 non-hematologic toxicity attributable to this agent and ≥grade 3 thrombocytopenia or complicated neutropenia without regard to attribution. When toxicities resolved to ≤ grade 1, cabozantinib was restarted without dose reduction in cases of hematological toxicity or first episode of grade 2 non-hematologic toxicity. One dose level reduction of cabozantinib was required in the event of grade 3 non-hematologic toxicity or recurrent grade 2 non-hematologic toxicity. The lowest protocol dose of cabozantinib was 20 mg orally once daily. If toxicity occurred at this lowest dose level, judgement as to the risk benefit ratio of continuing therapy was made by the primary investigator in regard to treatment interruption only or removal from the study. Gemcitabine dose was held in cases of ≥ grade 3 non-hematologic toxicity, ≥ grade 3 thrombocytopenia or grade 4 neutropenia. In these cases, dose was held until toxicity resolved to ≤grade 1 and then resumed at 25% of the original dose. On days gemcitabine was due, if ANC was between 500–999/microL or platelets were 50,000–99,000/microL, gemcitabine was reduced by 50% that day, with full dosing resumed at next infusion dependent upon count recovery.
Disease was assessed using computed tomography (CT) or magnetic resonance imaging (MRI) at baseline and then every 8 weeks while on study treatment. Objective tumor response was determined per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1). CA19–9 level was measured at baseline and then prior to each cycle of therapy. Study treatment continued until the progressive disease (PD) per RECIST criteria, unacceptable toxicity, or patient desire to withdraw from the study.
Study Endpoints
The primary study endpoint was the MTD of the combination of cabozantinib and gemcitabine, defined as the highest dose level at which ≤ 25% of patients incurred a dose limiting toxicity (DLT) within the first 35 days of therapy. Hematologic DLT was defined by any of the following 1.) any documented ≥ grade 3 thrombocytopenia 2.) any documented grade 4 neutropenia lasting more than 7 days or 3.) any ≥ grade 3 neutropenic fever. Non-hematologic DLT was defined by any of the following: 1.) documented ≥ grade 2 non-hematologic toxicity attributable to cabozantinib and/or gemcitabine, with toxicity intolerable to the patient and requiring a dose interruption for ≥7 days or 2.) ≥ grade 3 non-hematologic toxicities that were possibly, probably, or definitely related to cabozantinib and/or gemcitabine except for easily managed adverse events (e.g. electrolytes). Additionally, isolated ≥ grade 3 ALT/or AST elevations did not constitute DLT unless elevations persisted off therapy for ≥48 hours or recurred with re-introduction of study treatment. Secondary endpoints included progression free survival (PFS), overall survival (OS), response rate, safety of the two drug regimen, and assessment of correlative biomarkers.
Correlative Molecular Biomarker Assessment
Urine samples were obtained to assess for HGF, c-Met, and VEGF levels, at baseline, following 7 days of cabozantinib monotherapy, prior to cycle 2 (day 35) and then prior to each odd numbered cycle. Thirty mL of urine was obtained at each time point and frozen at −20 degrees C for subsequent analysis. Urine samples were processed and analyzed at the National Cancer Institute (KC, JS). Briefly, commercial ELISA assays were performed to assess urine HGF (Sigma-Aldrich, Lot 328655) and VEGF levels (R&D Systems, Lot 328655) with measurements obtained on Bayer DCA 2000+. Samples were analyzed in triplicate, randomized in two batches so all samples from the same patient were on the same plate. For urine VEGF, measurements were analyzed to a representative concentration curve. However, for urine HGF, due to small variations of measurements among plates, each plate was analyzed to its own curve. All results for urine HGF and VEGF levels were normalized to urine creatinine levels. c-Met levels were obtained via an electrochemiluminescence assay as previously described.[29]
Statistical Considerations/Analyses
For the primary MTD endpoint, an intent-to-treat (ITT) analysis was performed to include all patients who received at least 7 days of cabozantinib and the first dose of gemcitabine. Under the TITE-CRM paradigm, the relationship between dose and toxicity is summarized by a single-parameter (α) logistic model that represents the assumed relationship prior to the collection of any patient data and determines the prior probability rates. The distribution of α was updated based on data collected as patients were treated on the trial and then used to calculate a posterior distribution of toxicity, which represents the probability a future patient will experience toxicity at a given dose. Enrollment of 24 patients was planned for estimation of the dose-toxicity function, expecting to complete accrual in 1.5 years.
For the secondary endpoints, PFS and OS were calculated from first day of study treatment, until documented disease progression or death (PFS) or death (OS). There was no loss to follow-up. The product-limit method was used to estimate both PFS and OS, and the median along with 95% confidence intervals is reported.
Results
Sixteen patients consented to study, with 4 failing screening evaluation due to biochemical pancreatitis, LFT abnormalities, anemia and stable metastatic disease on therapy. Characteristics of 12 treated patients are detailed in Table 1. Median age was 61 (range 41–74). All patients had metastatic disease at the time of enrollment with the majority treatment naïve (n=7). Two of the 12 patients had recurrent disease after pancreatoduodenectomy and adjuvant therapy. The most common sites of metastasis included liver (n=5), lung (n=3), and peritoneum (3).
Table 1:
Patient Demographic and Disease Characteristics
| Characteristic | Number |
|---|---|
| Age, years | |
| Median (range) | 61 (41–74) |
| Sex | |
| Men | 6 |
| Women | 6 |
| Stage at Enrollment | |
| Metastatic | 12 |
| ECOG Performance Status | |
| 0 | 2 |
| 1 | 10 |
| Prior Therapy | |
| None | 7 |
| Surgery + Adjuvant Therapy | 2 |
| Chemotherapy | 3 |
| Radiation | 2 |
| CA19–9 Level at Enrollment | |
| Median (range), U/mL | 1319 (9–93,533) |
| Sites of Disease | |
| Liver | 5 |
| Lung | 3 |
| Peritoneum | 3 |
| Retroperitoneal lymph nodes | 4 |
| Pelvic mass | 1 |
The starting dose level (1) was cabozantinib 20 mg administered orally once daily and gemcitabine 1000 mg/m2 given intravenously over 30 minutes. Two of the 12 treated patients were non-evaluable for DLT: one patient elected hospice and withdrew from the study prior to first gemcitabine infusion and the other patient developed prolonged cholangitis preventing further therapy (not neutropenic, event deemed related to endobiliary stent dysfunction). Dose level assignments in the remaining 10 evaluable patients are shown in Table 2. DLTs were experienced by 4 patients: two at dose level (1), one at dose level (−1), and one at dose level (2). DLTs included grade 3 thrombocytopenia (n=2) and grade 3 AST/ALT elevations (n=3). The earliest DLT was a grade 3 ALT elevation occurring on day 8 of the first cycle of therapy. With these results, the posterior probability for DLT was >25% in all dose levels tested. As experience with the regimen increased, we concluded that our prior expectations for the probability of DLT were too low and that, in fact, the toxicity profile for this regimen was higher than anticipated. As we were not enthusiastic in continuing development of the combination at dose level (−1) or lower, the trial was closed to accrual, and the MTD was not determined.
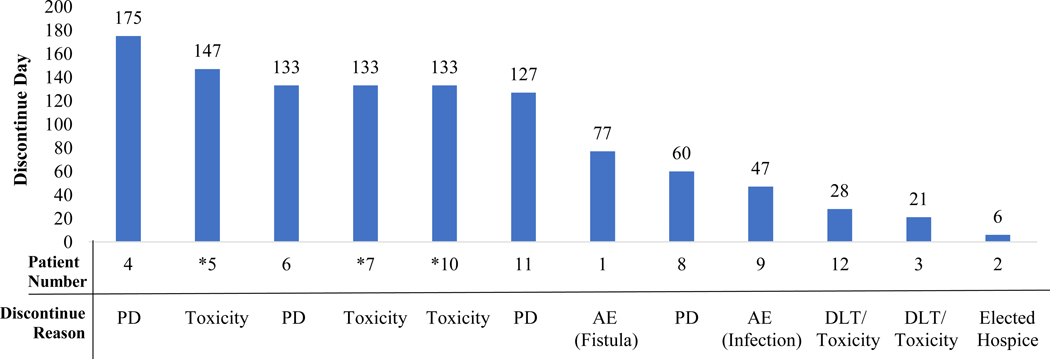
The time each patient remained on study treatment and reason(s) for discontinuation are outlined in Figure 1. Seven of 12 patients (64%) discontinued therapy due to toxicity. The median number of cycles of treatment administered in the cohort was 3 (range 1–6). Aside from DLT, all 11 evaluable patients experienced at least one grade 3 toxicity (Table 3). The most common grade 3 adverse events (AEs) included neutropenia (n=5) and AST/ALT elevations (n=5). Other common AEs included fatigue, nausea, hypertension, and diarrhea. Of note, one patient developed a fistula between her pancreatic body/tail mass and transverse colon during the course of treatment, possibly secondary to previous diagnostic percutaneous biopsy.
Figure 1:
Time to and Reason(s) for Discontinuation of Therapy.
*Denotes a patient who had a partial response to treatment
PD: Progressive disease; AE: Adverse Event; DLT: Dose Limiting Toxicity
Table 3:
Treatment Related Toxicities of Cabozantinib and Gemcitabine (n=11 evaluable patients).
| Adverse Event | Grade 1 (n) | Grade 2 (n) | Grade 3 (n) | Grade 4 (n) |
|---|---|---|---|---|
| Hematologic | ||||
| Anemia | 5 | 3 | - | - |
| Neutropenia | 1 | 1 | 5 | - |
| Thrombocytopenia | 2 | 2 | 2 | - |
| Non-Hematologic | ||||
| Fatigue | 5 | 1 | - | - |
| Flu-like symptoms | - | - | 1 | - |
| Diarrhea | 2 | 1 | - | - |
| Nausea | 6 | - | - | - |
| Mucositis | 1 | - | - | - |
| AST/ALT Elevations | 2 | 3 | 5 | - |
| Hypertension | - | 3 | 1 | - |
| Proteinuria | - | 1 | - | - |
| Alopecia | 2 | - | - | - |
| Myalgia | 1 | 1 | - | - |
Grading of severity of adverse events based on NCI CTCAE v4.0.
Eight patients were evaluable for response, and the best response to therapy included 3 patients with partial response (PR), 3 with stable disease (SD), and 2 with PD. Each patient who experienced PR, however, discontinued treatment during continuing response due to AEs. The median PFS and OS for treated patients was 4.7 months (95% CI: 1.4–9.7) and 10.1 months (95% CI: 3.6–20.6), respectively.
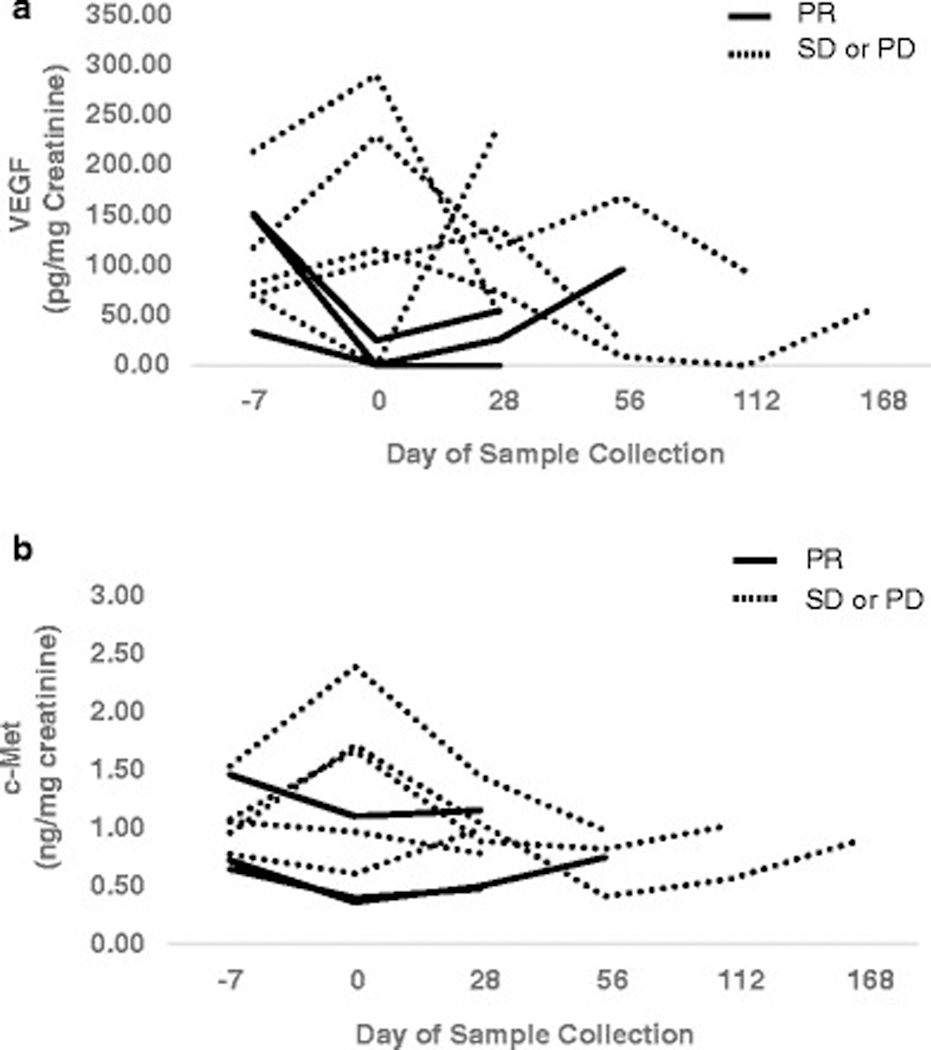
Due to the small number of patients on this study, the biomarker analysis must be viewed as hypothesis generating. As shown in Figure 2, urinary VEGF, c-met and HGF could be measured in every evaluable patient. Moreover, those patients who had a reduction of VEGF levels >75% from their baseline value to their measurement after 1 week of cabozantinib treatment achieved a PR (3 of 3 patients). Similarly, those patients who had a reduction of at least 25% in c-Met levels achieved a PR (3 of 4 patients). Though additional studies are needed to confirm these findings, the possibility exists that these urinary biomarkers may be useful for monitoring disease status.
Figure 2:
Urinary Levels of VEGF a and c-Met b measured serially with first measurement obtained prior to initiation of therapy (day–7). Data presented include all patients with at least two measurements. Each line represents a unique patient. Values were normalized to urinary creatinine levels. PR: Partial Response; SD: Stable Disease; PD: Progressive Disease.
Discussion:
In this study, we sought to determine the MTD of the combination of cabozantinib and gemcitabine in patients with advanced pancreatic cancer. Gemcitabine is a tolerable drug as a single agent, with brief, predictable and uncomplicated myelosuppression as its most common adverse event. The overall incidence of grade 3 ALT/AST elevation or severe fatigue with gemcitabine is reported as ≤10% (Gemcitabine drug package insert; Eli Lilly). The most frequently reported adverse events with single agent cabozantinib are non-hematologic toxicities including fatigue, gastrointestinal and cardiovascular toxicities (Cabozantinib drug package insert; Exelixis). Cabozantinib is dosed as a single agent between 40 and 140 mg daily, and there does appear to be a relationship between daily dose and tolerance. An open-label trial comparing 40 to 100 mg daily in patients with prostate cancer demonstrated that fewer patients in the high dose group were able to receive intended dose (90% vs 55%, respectively), and dose reductions were more frequent with the higher dose group (median dose administered: 55 mg) [30]. This trial suggested a cabozatinib dose of 60 mg orally once daily in subsequent phase III trials [31,32].
As toxicities of cabozantinib and gemcitabine are generally non-overlapping, our trial design intended and expected to escalate cabozantinib from a starting dose of 20 mg to 80 mg daily, using gemcitabine at its standard dose and schedule. Instead, DLTs were observed at each dose level tested, including dose level (−)1. The probability for DLT approached or exceeded our target rate of 25%. Additionally, treatment beyond the DLT interval (35 days) was associated with persistent, comfort limiting ≥ grade 2 fatigue and continuing grade 3 toxicities, despite cabozantinib interruption and gemcitabine dose reduction and schedule changes (i.e., gemcitabine every other week). As even lower doses of cabozantinib or reduced dose intensity of gemcitabine was judged as not clinically desirable, a decision to close the trial to further accrual without establishing an MTD was made.
The adverse events noted in our study were similar to those noted in published trials utilizing cabozantinib, except for an increase in hematological toxicities [30,33–35]. Specifically, neutropenia and thrombocytopenia, presumably secondary to the combination were noted, similar to an experience with cabozantinib in combination with temozolomide in a phase I trial in advanced gliomas [35]. Perhaps the resultant poor tolerance of cabozantinib and gemcitabine might have been anticipated, as the precedent of well tolerated oral targeted therapies, such as imatinib, have been difficult to combine with cytotoxic treatments [36].
In this study, dose level (1) utilized the lowest available dose of cabozantinib, based on findings from a prior discontinuation trial using the drug as a single agent in advanced malignancies, including patients with PDAC (ClinicalTrials.gov #: NCT00940225; unpublished data). Despite using this lowest available dose of cabozantinib and appreciating the small sample size, we were struck by the poor tolerance of the regimen, with 64% of patients discontinuing therapy due to adverse events. Patients were fit at the time of enrollment (ECOG 0 or 1), and therapy was provided by investigators familiar with pancreatic cancer treatment suggesting the combination as too difficult to tolerate. Furthermore, patients continued to experience persistent toxicities despite treatment interruptions of cabozantinib and dose/schedule modifications of gemcitabine. While cabozantinib is better tolerated as a single agent, it appears unlikely that it can be combined with cytotoxic chemotherapy agents, at least on a daily dosing schedule. Investigation of intermittent cabozantinib dosing, with demonstration of in-vivo on-target effect might be considered as a strategy for further development of this drug in combination with gemcitabine or other agents. In that regard, again acknowledging small sample size, 3 PR in 8 evaluable patients was not expected. While this may have been due to chance, this observation might also suggest a benefit of targeting c-Met in PDAC. Of interest, reduction in urinary VEGF and c-Met levels appeared to correlate with response. This finding needs to be further prospectively evaluated in a larger cohort.
In conclusion, the combination of continuous daily dosing of cabozantinib and weekly gemcitabine is impractical for further development due to DLTs and ongoing treatment limiting toxicities. Nevertheless, despite the small sample size, clinical activity was noted with three PR, suggesting that further investigation of c-Met inhibition may be warranted in PDAC. Finally, prospective evaluation of urinary biomarkers for c-MET activity and inhibition as prognostic and predictive indicators in patients with PDAC is indicated.
Acknowledgements:
The authors acknowledge Bill Reisdorph for management of the study specific IND 114,716.
This work was funded in part by the intramural program of the NCI.
Funding: Exelixs, Inc provided cabozantinib for this study.
References
- 1.Howlander NNA, Krapcho M, et al. (2015) SEER Cancer Statistics Review, 1975–2012. Posted to SEER web site [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74 (11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155 [DOI] [PubMed] [Google Scholar]
- 3.Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15 (6):2403–2413 [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, Groupe Tumeurs Digestives of U, Intergroup P (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364 (19):1817–1825. doi: 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369 (18):1691–1703. doi: 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM (1992) Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol 119 (3):629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takayama H, La Rochelle WJ, Anver M, Bockman DE, Merlino G (1996) Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc Natl Acad Sci U S A 93 (12):5866–5871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trusolino L, Bertotti A, Comoglio PM (2010) MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol 11 (12):834–848. doi: 10.1038/nrm3012 [DOI] [PubMed] [Google Scholar]
- 9.Comoglio PM (1993) Structure, biosynthesis and biochemical properties of the HGF receptor in normal and malignant cells. EXS 65:131–165 [PubMed] [Google Scholar]
- 10.Di Renzo MF, Narsimhan RP, Olivero M, Bretti S, Giordano S, Medico E, Gaglia P, Zara P, Comoglio PM (1991) Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene 6 (11):1997–2003 [PubMed] [Google Scholar]
- 11.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4 (12):915–925. doi: 10.1038/nrm1261 [DOI] [PubMed] [Google Scholar]
- 12.Benvenuti S, Comoglio PM (2007) The MET receptor tyrosine kinase in invasion and metastasis. J Cell Physiol 213 (2):316–325. doi: 10.1002/jcp.21183 [DOI] [PubMed] [Google Scholar]
- 13.Graveel CR, DeGroot JD, Su Y, Koeman J, Dykema K, Leung S, Snider J, Davies SR, Swiatek PJ, Cottingham S, Watson MA, Ellis MJ, Sigler RE, Furge KA, Vande Woude GF (2009) Met induces diverse mammary carcinomas in mice and is associated with human basal breast cancer. Proc Natl Acad Sci U S A 106 (31):12909–12914. doi: 10.1073/pnas.0810403106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponzo MG, Lesurf R, Petkiewicz S, O’Malley FP, Pinnaduwage D, Andrulis IL, Bull SB, Chughtai N, Zuo D, Souleimanova M, Germain D, Omeroglu A, Cardiff RD, Hallett M, Park M (2009) Met induces mammary tumors with diverse histologies and is associated with poor outcome and human basal breast cancer. Proc Natl Acad Sci U S A 106 (31):12903–12908. doi: 10.1073/pnas.0810402106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghoussoub RA, Dillon DA, D’Aquila T, Rimm EB, Fearon ER, Rimm DL (1998) Expression of c-met is a strong independent prognostic factor in breast carcinoma. Cancer 82 (8):1513–1520 [DOI] [PubMed] [Google Scholar]
- 16.Kong DS, Song SY, Kim DH, Joo KM, Yoo JS, Koh JS, Dong SM, Suh YL, Lee JI, Park K, Kim JH, Nam DH (2009) Prognostic significance of c-Met expression in glioblastomas. Cancer 115 (1):140–148. doi: 10.1002/cncr.23972 [DOI] [PubMed] [Google Scholar]
- 17.Di Renzo MF, Poulsom R, Olivero M, Comoglio PM, Lemoine NR (1995) Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res 55 (5):1129–1138 [PubMed] [Google Scholar]
- 18.Ebert M, Yokoyama M, Friess H, Buchler MW, Korc M (1994) Coexpression of the c-met proto-oncogene and hepatocyte growth factor in human pancreatic cancer. Cancer Res 54 (22):5775–5778 [PubMed] [Google Scholar]
- 19.Furukawa T, Duguid WP, Kobari M, Matsuno S, Tsao MS (1995) Hepatocyte growth factor and Met receptor expression in human pancreatic carcinogenesis. Am J Pathol 147 (4):889–895 [PMC free article] [PubMed] [Google Scholar]
- 20.Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE (2007) Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol 14 (12):3629–3637. doi: 10.1245/s10434-007-9583-5 [DOI] [PubMed] [Google Scholar]
- 21.Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V, Tabruyn SP, You WK, Chapman HA, Christensen JG, Aftab DT, McDonald DM (2012) Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov 2 (3):270–287. doi: 10.1158/2159-8290.CD-11-0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, Simeone DM (2011) c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology 141 (6):2218–2227 e2215. doi: 10.1053/j.gastro.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 23.Hage C, Rausch V, Giese N, Giese T, Schonsiegel F, Labsch S, Nwaeburu C, Mattern J, Gladkich J, Herr I (2013) The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis 4:e627. doi: 10.1038/cddis.2013.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki A, Nakauchi H, Taniguchi H (2004) Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes 53 (8):2143–2152 [DOI] [PubMed] [Google Scholar]
- 25.Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N, Taniguchi H (2007) Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology 132 (2):720–732. doi: 10.1053/j.gastro.2006.11.027 [DOI] [PubMed] [Google Scholar]
- 26.You WK, Sennino B, Williamson CW, Falcon B, Hashizume H, Yao LC, Aftab DT, McDonald DM (2011) VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res 71 (14):4758–4768. doi: 10.1158/0008-5472.CAN-10-2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Quigley J, Pepe M, Fisher L (1990) Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics 46 (1):33–48 [PubMed] [Google Scholar]
- 28.Cheung YK, Chappell R (2000) Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics 56 (4):1177–1182 [DOI] [PubMed] [Google Scholar]
- 29.Athauda G, Giubellino A, Coleman JA, Horak C, Steeg PS, Lee MJ, Trepel J, Wimberly J, Sun J, Coxon A, Burgess TL, Bottaro DP (2006) c-Met ectodomain shedding rate correlates with malignant potential. Clin Cancer Res 12 (14 Pt 1):4154–4162. doi: 10.1158/1078-0432.CCR-06-0250 [DOI] [PubMed] [Google Scholar]
- 30.Smith MR, Sweeney CJ, Corn PG, Rathkopf DE, Smith DC, Hussain M, George DJ, Higano CS, Harzstark AL, Sartor AO, Vogelzang NJ, Gordon MS, de Bono JS, Haas NB, Logothetis CJ, Elfiky A, Scheffold C, Laird AD, Schimmoller F, Basch EM, Scher HI (2014) Cabozantinib in chemotherapy-pretreated metastatic castration-resistant prostate cancer: results of a phase II nonrandomized expansion study. J Clin Oncol 32 (30):3391–3399. doi: 10.1200/JCO.2013.54.5954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith MR DBJ, Sternberg CN, et al. (2015) Final analysis of COMET-1: Cabozantinib (Cabo) versus prednisone (Pred) in metastatic castration-resistant prostate cancer (mCRPC) patients (pts) previously treated with docetaxel (D) and abireterone (A) and or enzalutamide (E). J Clin Oncol Suppl 7; abstr 139 [Google Scholar]
- 32.Basch EM SM, De Bono JS, et al. (2015) Final analyiss of COMET-2: Cabozantinib (Cabo) versus mitoxantrone/prednisone (MP) in metastatic castration resistant prostate cancer (mCRPC) patients (pts) with moderate to severe pain who were previously treated with docetaxel (D) and abireterone (A) and/or enzalutamide. J Cell Oncol suppl 7; abstr 141 [Google Scholar]
- 33.Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC, Niederle B, Cohen EE, Wirth LJ, Ali H, Hessel C, Yaron Y, Ball D, Nelkin B, Sherman SI (2013) Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 31 (29):3639–3646. doi: 10.1200/JCO.2012.48.4659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, Hammers H, Hutson TE, Lee JL, Peltola K, Roth BJ, Bjarnason GA, Geczi L, Keam B, Maroto P, Heng DY, Schmidinger M, Kantoff PW, Borgman-Hagey A, Hessel C, Scheffold C, Schwab GM, Tannir NM, Motzer RJ, Investigators M (2015) Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 373 (19):1814–1823. doi: 10.1056/NEJMoa1510016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiff D, Desjardins A, Cloughesy T, Mikkelsen T, Glantz M, Chamberlain MC, Reardon DA, Wen PY (2016) Phase 1 dose escalation trial of the safety and pharmacokinetics of cabozantinib concurrent with temozolomide and radiotherapy or temozolomide after radiotherapy in newly diagnosed patients with high-grade gliomas. Cancer 122 (4):582–587. doi: 10.1002/cncr.29798 [DOI] [PubMed] [Google Scholar]
- 36.Moss RA, Moore D, Mulcahy MF, Nahum K, Saraiya B, Eddy S, Kleber M, Poplin EA (2012) A Multi-institutional Phase 2 Study of Imatinib Mesylate and Gemcitabine for First-Line Treatment of Advanced Pancreatic Cancer. Gastrointest Cancer Res 5 (3):77–83 [PMC free article] [PubMed] [Google Scholar]