Abstract
H-type hypertension, defined as a combination of hypertension and hyperhomocysteinemia (Hhcy), is associated with atherosclerosis and, therefore, increased stroke risk. However, the role of hypertension and Hhcy in high-risk stroke populations has not been studied. The present study investigated the prevalence of H-type hypertension in a high-risk stroke population of Hainan Province, China and to assess possible joint effects between hypertension and Hhcy for increased carotid intima-media thickness (CIMT). In this community-based cross-sectional study, 959 high-risk stroke subjects (age, 65.8 ± 10.8 years; 46.6% men) were recruited from Hainan Province, China. The demographic and clinical characteristics were collected, and blood samples were obtained. Analysis of variance or chi-square tests were performed to compare variates among groups based on both homocysteine levels and blood pressure status. The associations of hypertension and Hhcy with increased CIMT were evaluated through logistic regression. The prevalence of H-type hypertension was 34.8% in this population, with a higher ratio of H-type hypertension in men than in women. Compared with the normotension and normal homocysteine subgroup, the risk of increased CIMT was significantly higher in the subgroup with hypertension and Hhcy (odds ratio [OR] = 2.639; 95% confidence interval [CI], 1.690–4.091) after adjusting for age and sex. Increased CIMT was affected by an additive synergetic interaction between Hhcy and hypertension (synergy index = 1.105). It emphasized the clinical importance of anti-hypertension and lowering Hhcy in the high-risk stroke population.
Keywords: carotid intima-media thickness, high-risk stroke population, hyperhomocysteinemia, hypertension
1. Introduction
Stroke has been shown to result in high morbidity and mortality worldwide[1] as well as in China.[2,3] From 1990 to 2017, the age-standardized global stroke incidence rate has decreased, but the stroke prevalence rate has increased.[4] A similar trend was found in China, stroke remains the most leading cause of disability-adjusted life-years.[3] Although we have rapid methods for diagnosing stroke, primary stroke prevention is still challenging, especially for high-risk stroke populations. Helping people to pay more attention to the control of stroke risk factors and reducing stroke burden is urgent. Hence, identifying stroke risk factors and understanding their relationships and interactions are critical to alleviating the burden of stroke among high-risk stroke population.
It is universally acknowledged that hypertension is one of the main risk factors for stroke. Hyperhomocysteinemia (Hhcy) is another independent risk factor for stroke.[5] Experimental studies have reported that excessive accumulation of Hhcy could aggravate oxidation reactions and inflammation in vascular endothelial cells, thereby leading to atherosclerosis. The combination of hypertension and Hhcy, termed H-type hypertension,[6] is strongly associated with atherosclerotic cerebrovascular diseases.[7,8] The risk of stroke is significantly higher in hypertensive patients with Hhcy than in patients with either condition alone.[6,9] In addition to anti-hypertensive treatments, lowering homocysteine levels can also benefit H-type hypertension subjects.[10,11] In 2009, the prevalence of hypertension in China was 29.6%, and approximately 75% of hypertensive patients had Hhcy.[9,12] A cross-sectional study comprising a general population aged ≥60 years in Beijing, China, reported that 50.2% of elderly people have H-type hypertension.[13] The prevalence of H-type hypertension in the high-risk stroke population in China, however, is unknown.
Combined with Hhcy, hypertension increase the effect on carotid artery atherosclerosis, which is the major cause of stroke. Carotid ultrasonography to assess the carotid intima-media thickness (CIMT), a simple, noninvasive, valuable, and reproducible method, is a well-established diagnostic marker for the carotid atherosclerotic process.[14] Zhang et al[15] reported that patients with both hypertension and Hhcy had a 1.67-fold (95% confidence interval [CI], 1.15–2.42) higher risk of increased CIMT than healthy controls, and higher than that in patients with only hypertension or Hhcy. To date, no literature has reported the relationship between H-type hypertension and increased CIMT in a high-risk stroke population.
Hence, the present study aimed to investigate the prevalence of H-type hypertension in a high-risk stroke population and to demonstrate whether hypertension and Hhcy have a possible synergistic interaction toward the risk of increased CIMT.
2. Subjects and methods
2.1. Subjects
The study protocol was conducted according to the guidelines of the Helsinki Declaration and was approved by the Ethics Committee of the Affiliated Haikou Hospital, Central South University Xiangya School of Medicine (No. 2015–016). All the study subjects provided written informed consent. The data and study materials are available from the corresponding author upon request.
The high-risk population screening and intervention project for stroke was conducted by the National Health Development Planning Commission Stroke Screening and Prevention Engineering since 2012. For the retrospective cross-sectional analysis, we used data from this project which investigated in Hainan province, China. In brief, individuals were enrolled from the North (Haikou), Central (Wenchang), and South (Ledong) Hainan Province. One urban and one rural location were selected from each of the 3 screening sites by randomization. The baseline survey was conducted between January 2015 and December 2016. Finally, the study collected 959 high-risk stroke subjects.
2.2. Inclusion and exclusion criteria
This study relied on the high-risk population screening and intervention project for stroke. In that project, high-risk stroke population was defined as subjects with age ≥40 years and have ≥3 risk factors in the next 8 items: hypertension, hyperlipidemia, diabetes, smoking, lack physical exercise, atrial fibrillation, family history of stroke, body mass index (BMI) ≥26 kg/m2. In this study, participants were included if they met the following eligibility criteria: original residents aged ≥40 years and lived in the location ≥1 year; and the medical records reporting at least 3 risk factors. Accordingly, participants were excluded if they had a history of stroke or mental health conditions.
2.3. Questionnaire and covariate definitions
The questionnaire was used to conduct face-to-face screening, and including the following characteristics. Demographic, including name, sex, age, height, and weight. Behaviors related to health, including smoking status and daily physical exercise (current smokers were defined as subjects who smoked >1 cigarette per day for >1 year; a lack of exercise was defined as physical exercise <3 times per week for <30 minutes per session of moderate to intense exercise). Total intake of vegetables and fruits per week. Medical history, including hypertension, diabetes, hyperlipidemia, atrial fibrillation, history of stroke, and family history of stroke. Hypertension was defined as systolic blood pressure (SBP) ≥140 mm Hg and/or diastolic blood pressure (DBP) ≥90 mm Hg or self-reported use of antihypertensive drugs. Diabetes was defined as fasting blood glucose (FBG) ≥7.0 mmol/L or glycosylated hemoglobin A1c (HbA1c) ≥6.5% or self-reported antidiabetic treatment. Hyperlipidemia was defined as total cholesterol (TC) ≥5.2 mmol/L, triglycerides (TG) ≥1.7 mmol/L, high-density lipoprotein-cholesterol (HDL-C) <1.0 mmol/L, low-density lipoprotein-cholesterol (LDL-C) ≥3.4 mmol/L or self-reported lipid-lowering treatment.[16] Atrial fibrillation was defined as absolute arrhythmia or current medical treatment. A family history of stroke was defined as the patient's parents or siblings have suffered a stroke. Medication use, defined as taking either an antihypertensive treatment, lipid-lowering therapy, or an antidiabetic agent. Besides, Hhcy was defined as plasma homocysteine ≥15 μmol/L.[17]
2.4. Carotid B-mode ultrasonography
CIMT was measured using a Philips Color Doppler Ultrasound System (iU22, Philips, Amsterdam, the Netherlands) equipped with a multifrequency 3 to 12 MHz high-resolution linear transducer. Two well-trained and certified ultrasound technicians performed all carotid imaging and CIMT measurements. Under the Mannheim consensus, CIMT was defined as the distance between the leading edge of the lumen interface and the leading edge of the collagenous upper layer of the adventitia in common carotid arteries from both sides of the neck[18,19]: 1.2 mm ≥ CIMT > 1 mm was considered an increased value.[19]
2.5. Blood biochemical parameters
Blood samples were collected from each participant after overnight fasting and immediately stored in an ice container and then sent to the central laboratory of Affiliated Haikou Hospital, Central South University Xiangya School of Medicine for testing, including FBG, HbA1c, TG, TC, HDL-C, LDL-C, creatinine, and homocysteine levels. All laboratory measurements were performed using an AU5800 automated biochemistry analyzer (Beckman, Tokyo, Japan).
2.6. Statistical analyses
All statistical analyses were conducted using SPSS 22.0 (IBM, Chicago, IL). The data are presented as means ± standard deviations or medians (interquartile range) for continuous variables and as proportions for categorical variables. Comparisons of the continuous variables were performed by analysis of variance or nonparametric tests where appropriate. Differences in categorical variables were analyzed using chi-square tests. The Bonferroni method was used to for multiple comparisons between the normal homocysteine and normotension (N-N) group, the simple hypertension (N-H) group, the simple Hhcy (H-N) group, and the Hhcy and hypertension (H-H) group. Multivariate logistic regression was used to analyze the association of homocysteine level and blood pressure with increased CIMT.
Since synergistic interaction can be multiplicative or additive, the interaction between hypertension and Hhcy on the risk of increased CIMT was investigated on both the multiplicative and additive scales. Multiplicative interaction was evaluated by multivariate logistic regression models. If there was no multiplicative interaction between Hhcy and hypertension, the regression coefficients and a covariance matrix for the logistic model were calculated. Subsequently, the Excel spreadsheet established by Andersson et al[20] was used to calculate the interaction indices on an additive scale and the corresponding confidence intervals. All values were two-tailed, and a P < .05 was considered statistically significant.
3. Results
3.1. Clinical characteristics and the prevalence of H-type hypertension
In total, 959 participants were enrolled in the present study. The average age was 65.8 ± 10.9 years, 46.6% were men, 19.9% were current smokers, 67.8% follow a low-salt diet and take the right amount of vegetables, and 18.1% of subjects did not participate in physical exercise. The average value of homocysteine levels was 15.9 ± 8.5 μmol/L. Of the participants, 70.7% were considered to have hypertension, 34.8% have H-type hypertension, 47.3% have diabetes, 91.3% have hyperlipidemia, 6.0% have atrial fibrillation, and 14.3% have a history of stroke.
The study subjects were divided into 4 groups (Table 1). Subjects in the H-H group or the H-N group were older than those in the N-N group. Subjects in the H-H group had higher rates of smoking, increased CIMTs, and higher levels of homocysteine, TC, and LDL-C than those in the N-N group (P < .05). More subjects were men in the H-H group than that in the H-N group. Compared with the H-N group, subjects in the H-H group had a higher prevalence of a history of hypertension and lower HDL-C levels (P < .05). Subjects in the H-H group had a higher prevalence of increased CIMT values and had lower HDL-C levels than patients in the N-H group (P < .05). The percentage taking medications, such as antihypertension, lipid-lowering therapy, or an antidiabetic agent, did not show statistical differences among the 4 groups (P > .05).
Table 1.
Demographic and clinical characteristics of the study population.
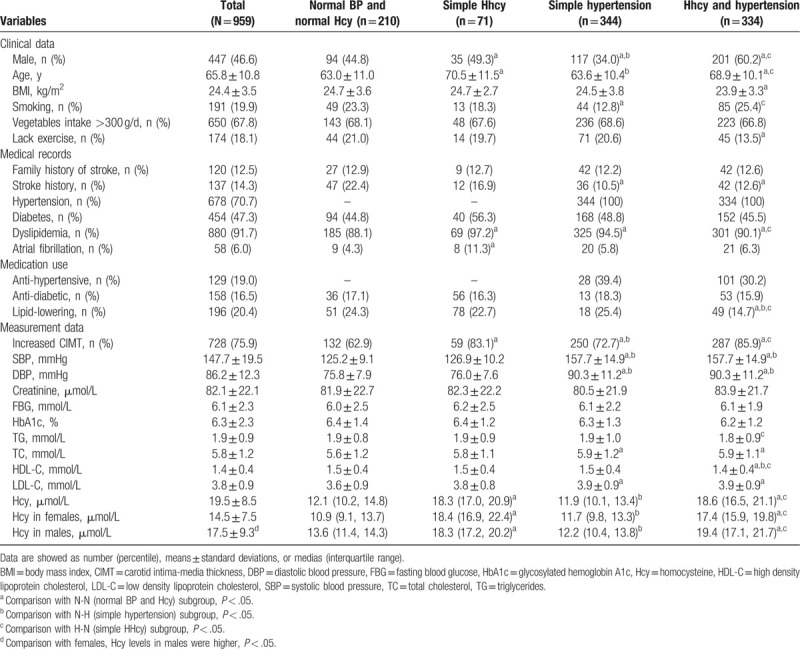
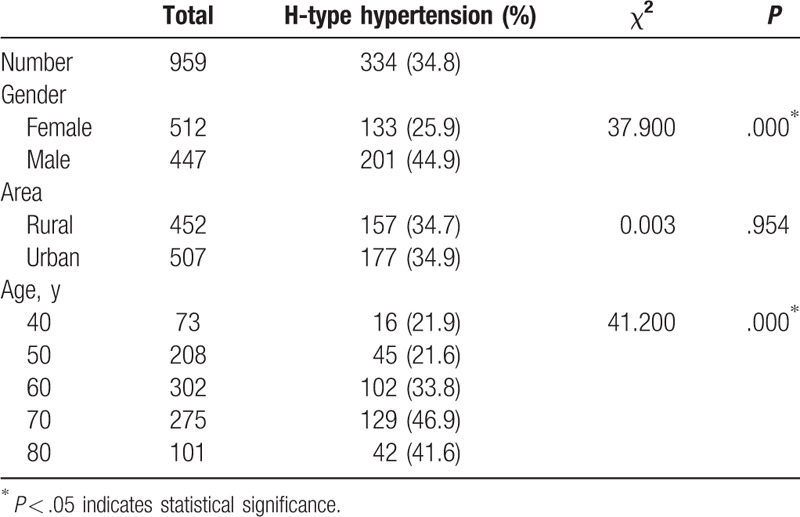
Both the H-N group and the H-H group had higher levels of homocysteine than the N-N group. The H-N group and the H-H group had higher levels of homocysteine than the N-H group. Both homocysteine levels (14.51 ± 7.47 vs 17.51 ± 9.31; Table 1) and the proportion with H-type hypertension (25.9% vs 44.9%; Table 2) were higher in the male subgroup than in the female subgroup.
Table 2.
The prevalence of H-type hypertension in the high-risk stroke population.
3.2. Risk factors for increased CIMT
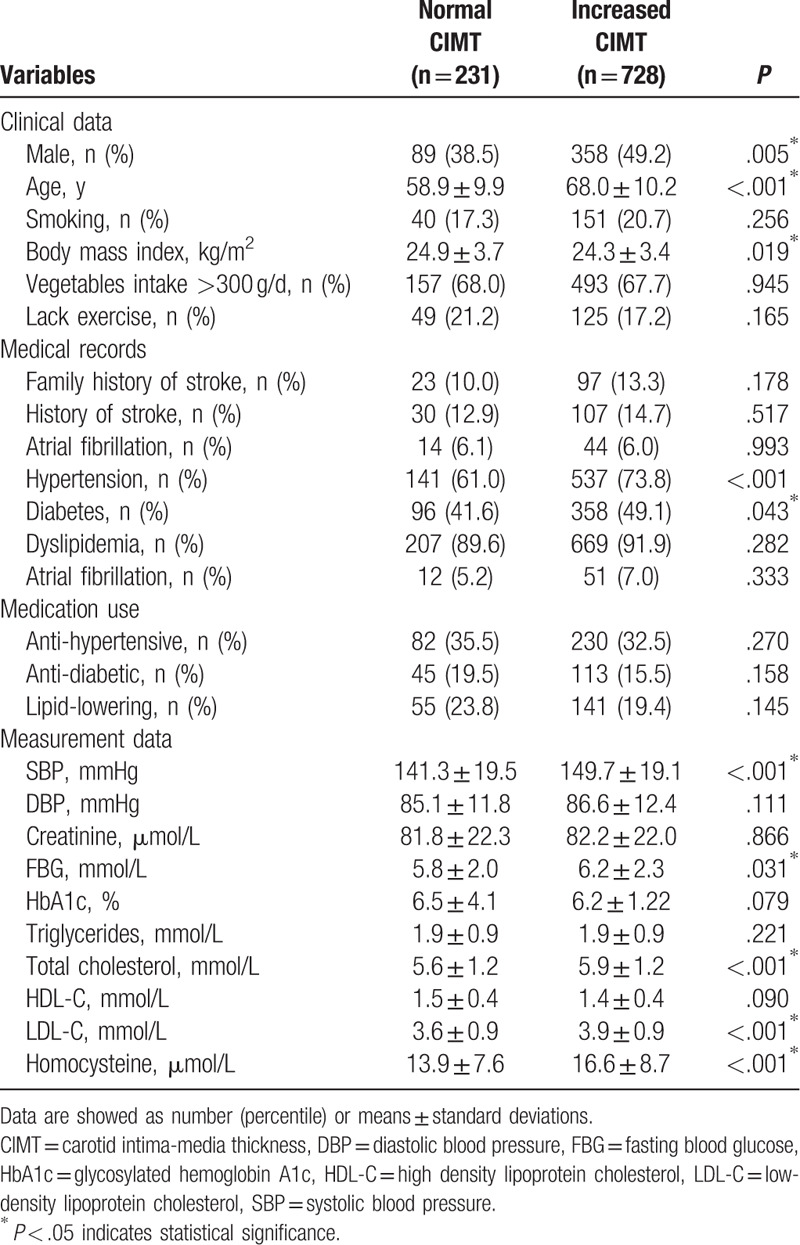
The 959 participants were divided into 2 subgroups based on their measured CIMT values (normal vs increased), and the P values for sex, age, BMI, SBP, FBG, HbA1c, TC, LDL-C, homocysteine, and percentage of hypertension indicated statistically significant differences (Table 3).
Table 3.
Comparisons between subgroups with normal and increased CIMT.
3.3. H-type hypertension is positively associated with increased CIMT
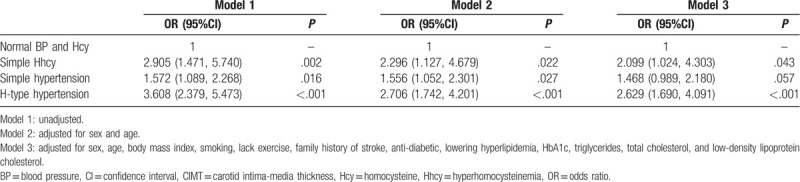
To investigate the effect of coexistent hypertension and Hhcy on the risk of increased CIMT, we divided the subjects into 4 categories (subjects with normotension and Hhcy, those with simple hypertension, those with simple Hhcy, and those with H-type hypertension). The associations of increased CIMT risk and H-type hypertension and its components are shown in Table 4. The regression analysis showed that hypertension (odds ratio [OR], 1.556; 95% confidence interval [CI], 1.052–2.301), Hhcy (OR, 2.296; 95% CI, 1.127–4.679), and H-type hypertension (OR, 2.706; 95% CI, 1.742–4.201) were significantly associated with the risk of increased CIMT after adjusting for sex and age. Adjusted for all the covariates, the risk of increased CIMT was significantly higher in the H-H group than in the N-H group (OR, 2.629; 95% CI, 1.690–4.091). The results indicated that subjects with H-type hypertension had the highest risk for increased CIMT while compared with subjects with Hhcy or subjects with hypertension.
Table 4.
Associations of increased CIMT risk with hypertension and Hhcy in the high-risk stroke population.
3.4. Synergetic interaction between hypertension and Hhcy toward increased CIMT
To demonstrate whether hypertension and Hhcy have a synergistic interaction for the risk of increased CIMT, we calculated the association on both the multiplicative and additive scales and adjusted for all possible covariates. There was no multiplicative interaction between hypertension and Hhcy for the risk of increased CIMT (P = .405). For the additive scale, the relative excess risk due to interaction (RERI) was 0.238 (95% CI, 0.189–0.295), which means that 0.238 times the risk was caused by the interaction between hypertension and Hhcy. The attributable proportion due to interaction (AP) was 0.068 (95% CI, 0.020–0.115), which indicates that the interaction caused 6.8% of the total impact in the H-H group. The synergy index (S) was 1.105 (95% CI, 1.011–1.203), which also confirms the synergetic interaction for increased CIMT between hypertension and Hhcy.
4. Discussion
In this community-based cross-sectional study among a high-risk stroke population in Hainan Province, the prevalence of H-type hypertension was 34.8%. H-type hypertension is positively associated with increased CIMT, by the additive synergistic interaction between Hhcy and hypertension.
It is deduced that the prevalence of H-type hypertension in Hainan Province was lower than the average value in China. The pooled prevalence of Hhcy in South China (16.0%) was considerably lower than that in Central (21.0%) and North China (34.3%).[21] The prevalence of hypertension showed the same trend with geographic differences in China.[22] One study involving 1460 elderly Chinese individuals reported that the prevalence of H-type hypertension was 51.4% in Beijing, China.[13] To date, data on H-type hypertension prevalence among high-risk stroke populations remain lacking. A series of factors may influence the formation of H-type hypertension, such as dietary habits, cooking habits, vegetable and fruit intake, and plasma vitamin B concentrations.[23] Additionally, other factors, such as smoking, physical inactivity, poor renal function, and other serious systemic diseases, may be responsible. Importantly, poor renal function can decrease the renal metabolic extraction of homocysteine and promote an elevation in plasma homocysteine levels.[24] Genetic background may be another essential factor. Most of the gene variants associated with folic acid metabolism and methylation could increase homocysteine levels and subsequently influence the development of H-type hypertension.[25]
A sex difference in homocysteine concentrations, the prevalence of H-type hypertension, and the prevalence of increased CIMT were noted in the present study. Substantial evidence suggests that homocysteine concentrations were higher in men than in women. Clifford et al[25] demonstrated that both being male and having single nucleotide polymorphisms of methionine metabolism-related genes are associated with elevated homocysteine concentrations in healthy Caucasian adults. In the current study, homocysteine levels were higher in men than in women. Furthermore, a close connection between men and the odds of H-type hypertension was observed. Taking these findings together, sex was associated with the presence of H-type hypertension, suggesting a role of homocysteine in the process of developing H-type hypertension, which is prominent in male high-risk stroke patients. This difference might be affected by female hormones, which have been demonstrated to have antioxidant effects.
The present study revealed that hypertension and Hhcy were associated with the risk of increased CIMT in a Chinese high-risk stroke population. A previous study indicated that elderly men with H-type hypertension have a greater proportion of increased CIMT compared with normotensive and normal homocysteine controls.[26] The third National Health and Nutrition Examination Survey study reported that a 5 μmol/L increase in plasma homocysteine levels was associated with a mean increase of 3.5 mm Hg in SBP. Hhcy may contribute to atherosclerotic lesion by promoting leukocyte recruitment and inducing the expression of interleukin-8,[27] reducing the synthesis of nitric oxide and subsequently leading to oxidative stress, inducing inflammation, and exacerbating vascular endothelial dysfunction,[28] thus increasing arterial stiffness and inducing the development of hypertension.[15] Furthermore, hypertension can trigger endothelial dysfunction, induce an Hhcy-mediated toxic effect, and potentiate atherosclerosis. In the current study, Hhcy and hypertension were considered independent risk factors for increased CIMT. After adjusting for confounding, the risks for increased CIMT were significantly higher in the H-H group and the N-H group than in the N-N group. The interaction analysis demonstrated that Hhcy displayed additive synergistic effects in combination with hypertension on increased CIMT. Special attention should be paid to individuals with hypertension and Hhcy because of the close association with increased CIMT.
Although our study provides new insight into the association between hypertension, Hhcy, and the risk of increased CIMT, it has several limitations that deserve comment. First, the study subjects lived in Southern China, the prevalence of H-type hypertension cannot be extrapolated to other regions. Second, the level of B vitamins in plasma could affect the concentration of homocysteine. We just collected the daily consumption of vegetables and fruits, data on the precise serum concentrations of folate and vitamin B12 were not collected. Third, we did not evaluate the association between H-type hypertension and carotid plaque or stenosis due to their sparsity in the present study. Finally, inflammation and oxidative stress involvement are blamed in many diseases, including obesity, hypertension, diabetes, and hyperlipidemia.[29] The contribution of inflammation and oxidative stress to atherosclerosis should be acknowledged, however, this cross-sectional design cannot establish causal relationships.
In conclusion, a community-based cross-sectional study in southern China was conducted, in which we found that the prevalence of H-type hypertension in a high-risk stroke population was 34.8%. Increased CIMT was affected by an additive synergetic interaction between hypertension and Hhcy. Considering the strong association of H-type hypertension with increased CIMT, our findings are of clinical importance for primary stroke prevention.
Acknowledgments
The authors thank all subjects for their participation in the study.
Author contributions
Conceptualization: Feng Zhou, Dan Yu.
Data curation: Dan Yu.
Formal analysis: Feng Zhou, Dan Hou, Dan Yu.
Funding acquisition: Dan Yu, Feng Zhou.
Investigation: Feng Zhou, Dan Hou, Dan Yu.
Methodology: Feng Zhou.
Writing – original draft: Feng Zhou, Dan Yu.
Writing – review & editing: Yukai Wang, Dan Yu.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, CIMT = carotid intima-media thickness, DBP = diastolic blood pressure, FBG = fasting blood glucose, HbA1c = glycosylated hemoglobin A1c, HDL-C = high-density lipoprotein-cholesterol, Hhcy = hyperhomocysteinemia, LDL-C = low-density lipoprotein-cholesterol, OR = odds ratio, SBP = systolic blood pressure, TC = total cholesterol, TG = triglycerides.
How to cite this article: Zhou F, Hou D, Wang Y, Yu D. Evaluation of H-type hypertension prevalence and its influence on the risk of increased carotid intima-media thickness among a high-risk stroke population in Hainan Province, China. Medicine. 2020;99:35(e21953).
This research was supported by the Key Scientific and Technological Project of Hainan Province, China (No. ZDXM20130066), and the Medical Scientific Research Foundation of Guangdong Province, China (No. B2019062).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151–210.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the global burden of disease study 2013. Lancet 2016;387:251–72.. [DOI] [PubMed] [Google Scholar]
- [3].Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145–58.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Avan A, Digaleh H, Di Napoli M, et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the Global Burden of Disease Study 2017. BMC Med 2019;17:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–236.. [DOI] [PubMed] [Google Scholar]
- [6].Hu DY, Xu XP. Prevention of stroke relies on valid control “H” type hypertension. Zhonghua Nei Ke Za Zhi 2008;47:976–7.. [PubMed] [Google Scholar]
- [7].Casas JP, Bautista LE, Smeeth L, et al. Homocysteine and stroke: evidence on a causal link from Mendelian randomization. Lancet 2005;365:224–32.. [DOI] [PubMed] [Google Scholar]
- [8].Qin X, Huo Y. H-type hypertension, stroke and diabetes in China: opportunities for primary prevention. J Diabetes 2016;8:38–40.. [DOI] [PubMed] [Google Scholar]
- [9].Towfighi A, Markovic D, Ovbiagele B. Pronounced association of elevated serum homocysteine with stroke in subgroups of individuals: a nationwide study. J Neurol Sci 2010;298:153–7.. [DOI] [PubMed] [Google Scholar]
- [10].Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA 2015;313:1325–35.. [DOI] [PubMed] [Google Scholar]
- [11].Han L, Wu Q, Wang C, et al. Homocysteine, ischemic stroke, and coronary heart disease in hypertensive patients: a population-based, prospective cohort study. Stroke 2015;46:1777–86.. [DOI] [PubMed] [Google Scholar]
- [12].Li JP, Huo Y, Liu P. Efficacy and safety of Enalapril-folate acid tablets in lowering blood pressure and plasma homocysteine. Beijing Da Xue Xue Bao Yi Xue Ban 2007;39:614–8.. [PubMed] [Google Scholar]
- [13].Ma L, Li L, Tang Z. Epidemiological characteristics of hyperhomocysteinemia and H-type hypertension in the elderly in Beijing, China. Clin Exp Hypertens 2017;39:640–4.. [DOI] [PubMed] [Google Scholar]
- [14].Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007;115:459–67.. [DOI] [PubMed] [Google Scholar]
- [15].Zhang Z, Fang X, Hua Y, et al. Combined effect of hyperhomocysteinemia and hypertension on the presence of early carotid artery atherosclerosis. J Stroke Cerebrovasc Dis 2016;25:1254–62.. [DOI] [PubMed] [Google Scholar]
- [16].Revised Joint Committee of guidelines for Prevention Treatment of Dyslipidemia in Chinese Adults. Guidelines for prevention and treatment of dyslipidemia in Chinese adults (Revised edition 2016). Chin Circ J 2016;31:937–52.. [Google Scholar]
- [17].Simons PC, Algra A, Bots ML, et al. Common carotid intima-media thickness and arterial stiffness. Circulation 1999;100:951–7.. [DOI] [PubMed] [Google Scholar]
- [18].Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). Cerebrovasc Dis 2012;34:290–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Simon A, Megnien JL, Chironi G. The value of carotid intima-media thickness for predicting cardiovascular risk. Arterioscler Thromb Vasc Biol 2010;30:182–5.. [DOI] [PubMed] [Google Scholar]
- [20].Andersson T, Alfredsson L, Källberg H, et al. Calculating measures of biological interaction. Eur J Epidemiol 2005;20:575–9.. [DOI] [PubMed] [Google Scholar]
- [21].Yang B, Fan S, Zhi X, et al. Prevalence of hyperhomocysteinemia in China: a systematic review and meta-analysis. Nutrients 2014;7:74–90.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hao L, Ma J, Stampfer MJ, et al. Geographical, seasonal and gender differences in folate status among Chinese adults. J Nutr 2003;133:3630–5.. [DOI] [PubMed] [Google Scholar]
- [23].Wang X, Bots ML, Yang F, et al. Prevalence of hypertension in China: a systematic review and meta-regression analysis of trends and regional differences. J Hypertens 2014;32:1919–27.. [DOI] [PubMed] [Google Scholar]
- [24].Ye Z, Wang C, Zhang Q, et al. Prevalence of homocysteine-related hypertension in patients with chronic kidney disease. J Clin Hypertens (Greenwich) 2017;19:151–60.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clifford AJ, Chen K, McWade L, et al. Gender and single nucleotide polymorphisms in MTHFR, BHMT, SPTLC1, CRBP2, CETP, and SCARB1 are significant predictors of plasma homocysteine normalized by RBC folate in healthy adults. J Nutr 2012;142:1764–71.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Okura T, Miyoshi K, Irita J, et al. Hyperhomocysteinemia is one of the risk factors associated with cerebrovascular stiffness in hypertensive patients, especially elderly males. Sci Rep 2014;4:5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Poddar R, Sivasubramanian N, DiBello PM, et al. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation 2001;103:2717–23.. [DOI] [PubMed] [Google Scholar]
- [28].Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J 2015;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Epingeac ME, Gaman MA, Diaconu CC, et al. The evaluation of oxidative stress in obesity. Rev Chim 2019;70:2241–4.. [Google Scholar]


