Supplemental Digital Content is available in the text
Keywords: F-18 fluorodeoxyglucose positron emission tomography/computed tomography, pancreatic cancer, prognosis, texture analysis
Abstract
Imaging parameters including metabolic or textural parameters during F-18 fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) are being used for evaluation of malignancy. However, their utility for prognosis prediction has not been thoroughly investigated. Here, we evaluated the prognosis prediction ability of imaging parameters from preoperative FDGPET/CT in operable pancreatic cancer patients.
Sixty pancreatic cancer patients (male:female = 36:24, age = 67.2 ± 10.5 years) who had undergone FDGPET/CT before the curative intent surgery were enrolled. Clinico-pathologic parameters, metabolic parameters from FDGPET/CT; maximal standard uptake value (SUVmax), glucose-incorporated SUVmax (GI-SUVmax), metabolic tumor volume, total-lesion glycolysis, and 53 textural parameters derived from imaging analysis software (MaZda version 4.6) were compared with overall survival.
All the patients underwent curative resection. Mean and standard deviation of overall follow-up duration was 16.12 ± 9.81months. Among them, 39 patients had died at 13.46 ± 8.82 months after operation, whereas 21 patients survived with the follow-up duration of 18.56 ± 9.97 months. In the univariate analysis, Tumor diameter ≥4 cm (P = .003), Preoperative Carbohydrate antigen 19-9 ≥37 U/mL (P = .034), number of metastatic lymph node (P = .048) and GI-SUVmax (P = .004) were significant parameters for decreased overall survival. Among the textural parameters, kurtosis3D (P = .052), and skewness3D (P = .064) were potentially significant predictors in the univariate analysis. However, in multivariate analysis only GI-SUVmax (P = .026) and combined operation (P = .001) were significant independent predictors of overall survival.
The current research result indicates that metabolic parameter (GI-SUVmax) from FDGPET/CT, and combined operation could predict the overall survival of surgically resected pancreatic cancer patients. Other metabolic or textural imaging parameters were not significant predictors for overall survival of localized pancreatic cancer.
1. Introduction
Pancreatic adenocarcinoma is one of the deadliest cancers worldwide with a 5-year survival rate of less than 9%.[1,2] Because of its poor prognosis, a variety of treatment has been emerging but only surgical resection is still considered the mainstay for long-term survival of pancreatic cancer patients.[3,4] However, even after curative intent surgery without remnant tumor (R0 resection), the median overall survival was only 21.3-37.7 months.[5,6] Several prognostic factors for surgically resected pancreatic cancer have previously been reported including lymph node (LN) metastasis, differentiation of tumors, lympho-vascular invasion, surgical margin involvement, and serum carbohydrate antigen 19-9 (CA19-9) level.[5,7]
F-18 Fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) is a proven imaging modality for a variety of cancers.[8,9] The pancreatic cancer has been effectively evaluated by FDG PET/CT in the diagnosis, staging, and treatment response to chemotherapy.[10,11] Regarding the prognosis prediction of pancreatic cancer, several metabolic parameters from FDG PET/CT have been suggested in the literature.[12,13] They are primarily derived from the quantitative assessment of FDG uptake in the tumor mass. The standardized uptake value (SUV) is the mostly well-known metabolic parameter, and there are several derivatives of SUV according to the target volume for SUV measurement such as maximum SUV, mean SUV, and peak SUV. Among the SUV derivatives, maximum SUV (SUVmax) is most advocated because it is easy to measure in a very reproducible way and there are abundance of proven evidence of usefulness of SUVmax in many clinical circumstances.[14,15] Recently, volume-based parameters such as metabolic tumor volume (MTV) or total lesion glycolysis (TLG = mean SUV × MTV) have been successfully utilized for prognosis prediction of pancreatic cancer, especially for locally advanced pancreatic cancer under chemo-radiation therapy.[10,16]
In addition, FDG uptake in a tumor mass has been often adjusted to the blood level of glucose (ie, glucose-incorporated SUVmax, GI-SUVmax), because FDG competes with glucose for tumor uptake through the common transporter in the tumor cell membrane.[17] The normalization of glucose level is particularly important in case of pancreatic cancer that is closely associated with diabetes mellitus (DM).[18–21] Furthermore, thanks to the development of analytic tool for imaging data, interrelations of individual pixels can be interrogated in a mathematical way, generating the unique texture pattern of given imaging data (=texture analysis). This kind of texture analysis firstly has been applied to the high resolution images such as histopathology or magnetic resonance imaging[22] and being expanded to nuclear imaging data such as PET.[23,24]
Numerous images and metabolic parameters have been applied to the FDG PET/CT for predicting the prognosis of pancreatic cancer.[15,16,25,26] However, the most useful determinant for prognosis prediction of pancreatic cancer has never been thoroughly investigated, to our best knowledge. Here, we aimed to investigate the imaging parameters (metabolic or textural) obtainable from the FDG PET/CT in terms of prognosis prediction of pancreatic cancer patients who underwent a curative intent surgical resection.
2. Methods and materials
2.1. Patients
Eighty consecutive patients with resectable or borderline resectable pancreatic cancer, (locally advanced tumors without systemic spread)[27] who underwent curative intent surgeries between July 2009 and September 2012 were retrospectively investigated. Among these patients, 60 patients with pancreatic cancer (male:female, 36:24; age, 67.2 ± 10.5 years) without remnant residual disease (R0 resection) were included in this study. Patients without a pre-surgical FDG PET/CT scan, those who had remnant disease (R1, R2 resection), those who undergone neoadjuvant therapy before the surgery, and those who had double primary malignancy were excluded from this study. The clinical and surgical parameters were recorded by an experienced surgical oncologist (Y.S.Y, 20 years of experience in hepato-pancreato-biliary surgery). The pathologic data were confirmed by the dedicated pathologist of the institute (Seoul National University Bundang Hospital). The institutional review board approved the study design and exempted the acquisition of patients’ consent. Combined resection was performed in each procedure when tumor abutted to other organs or encasing the portal vein or superior mesenteric vein (SMV).
2.2. FDG PET/CT acquisition and image analysis
FDG PET/CT was performed in mean 9 ± 11.8 days before the surgery in all the patients (range, 1–71 days). Blood glucose level checked using a glucometer (ACCU-CHEK Performa, Roche, Mannheim, Germany) was mean 122.0 ± 37.8 mg/dL at the time of FDG injection. PET/CT was performed using a 64-channel multi-detector CT integrated PET scanner (DVCT, GE Healthcare). Fifty minutes after injection of FDG (5.18 MBq/kg body weight), spiral CT for attenuation correction and image co-registration was acquired using the following parameters: tube potential of 120 kVp, tube current of 60 to 210 mA with AutoMa current modulation function, beam collimation of 40 mm (0.625 × 64), pitch factor of 0.516:1, coverage speed of 41.24 mm/s, and tube rotation time of 0.5 seconds. Then, emission PET was done from skull base to upper thigh level with 2.5 minutes acquisition time per bed. PET images were 3D reconstructed with a vendor-supplied image reconstruction algorithm (VUE point, GE Healthcare).
FDG uptake was quantitated using the SUV, which was calculated like followings:
SUV = [Decay-corrected radioactivity in a lesion (kBq/mL)]/[Injected radioactivity normalized by lean body weight (kBq/g)]
Lean body weight was used for SUV calculation to limit the fatty tissue effect that is resistant to FDG uptake in fasting conditions.[17,28] The equations for the lean body weight were: 1.1 × weight(kg) − (128 × {weight2/[100 × height (m)]2}) for men, and 1.07 × weight(kg) − (148 × {weight2/[100 × height(m)]2}) for women.[29]
MTV and TLG were measured using the vendor-provided software (PETVCAR, GE healthcare) on a workstation (AW VolumeShare5, GE healthcare) (Fig. 1A and B). Thresholds of 50% or 70% of the peak SUV were applied to each MTV and TLG.
Figure 1.
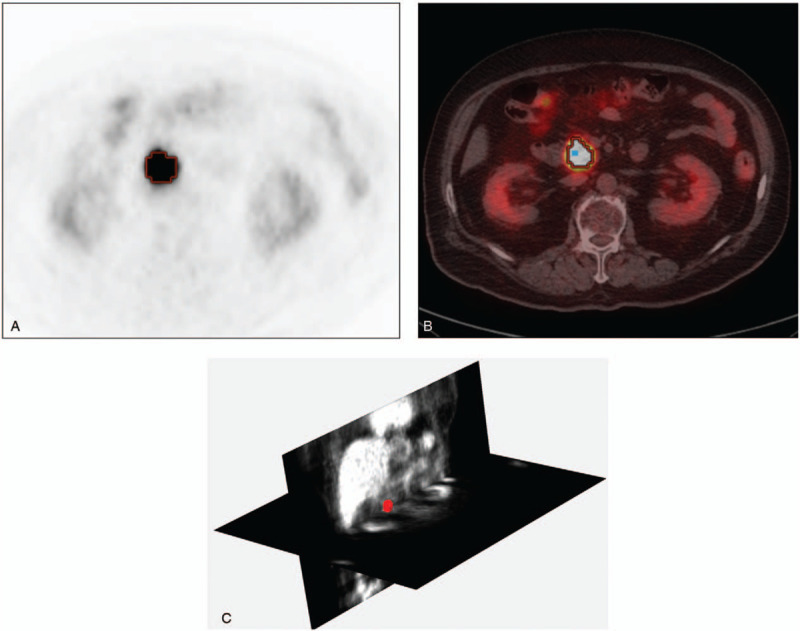
How to measure metabolic or textural parameters on FDG PET/CT. (A) PET transaxial image of a patient with pancreatic head cancer. (B) PET/CT fusion transaxial image showing the contour of 50% threshold from a pixel with peak SUV (blue dot within the contour). (C) 3-D textural analysis of the tumor mass over the PET images. Coronal and trans-axial plane PET images are shown and tumor mass is highlighted in red color. FDG PET/CT = F-18 fluorodeoxyglucose positron emission tomography/compute tomography, SUV = standardized uptake value.
2.3. Texture analyses
Three-dimensional texture analyses were done using PET image and software (MaZda, version 4.6) (Fig. 1C). Image files were first transferred to the workstation with the software. After drawing plane-by-plane 2-dimensional region-of-interests on trans-axial images, we could obtain the summed volume-of-interest. Then, the software provided 53 textural parameters, which were grouped to histogram-based parameters (mean, variance, skewness3D, kurtosis3D, percentile from 1% to 99%), gradient-based parameters (Gr-mean, Gr-variance, Gr-skewness, Gr-Kurtosis, percentage of pixels), run length matrix-based parameters (run length nonuniformity, grey level nonuniformity, long run emphasis, short run emphasis, fraction of image in runs) with 4 computations for vertical, horizontal, 45-degree, and 135-degree directions, and co-occurrence matrix-derived parameters (angular second moment, contrast, correlation, sum of squares, inverse difference moment, sum average, sum variance, sum entropy, entropy, difference variance, difference entropy) (Supplement 1).
2.4. Parameters for prognosis prediction
The tested clinic-surgico-pathologic parameters were age, sex, tumor size, tumor location, LN staging, preoperative CA19-9 level, type of surgery, histologic grades, peri-neural invasion, portal vein invasion, angiolymphatic invasion, combined resection of the adjacent organ, intra-operative (Intra-OP) transfusion, history of post-operative (post-OP) complication, post-OP adjuvant chemotherapy, post-OP adjuvant radiotherapy, and DM. The used PET parameters were SUVmax, glucose-incorporated SUVmax (GI-SUVmax with cutoff of 600), MTV with threshold of 50% or 70% of peak SUV, and TLG with threshold of 50% or 70% of peak SUV. Finally, the 53 textural parameters were included for the relation with survival. All the clinic-pathologic, metabolic, and textural parameters were tested regarding the ability of prognosis prediction for the univariate analyses. Among the tested parameters in the univariate analyses, statistically significant parameters were selected and included in the multivariate analyses.
2.5. Statistical analysis
Overall survival was the primary end-point of the current study. Univariate and multivariate Cox regression analyses were performed for the clinic-pathologic factors and metabolic parameters. Survival curves were investigated by using the Kaplan–Meier methods in univariate analysis of metabolic and clinicopathological factors. All the statistical analyses were done using IBM SPSS version 22 (IBM Corp, Armonk, NY) and MedCalc software (version 12.4.0.0. MedCalc software bvba). Otherwise, P-value of less than .05 was the cutoff for the statistical significance.
3. Results
3.1. Overall survival of the patients
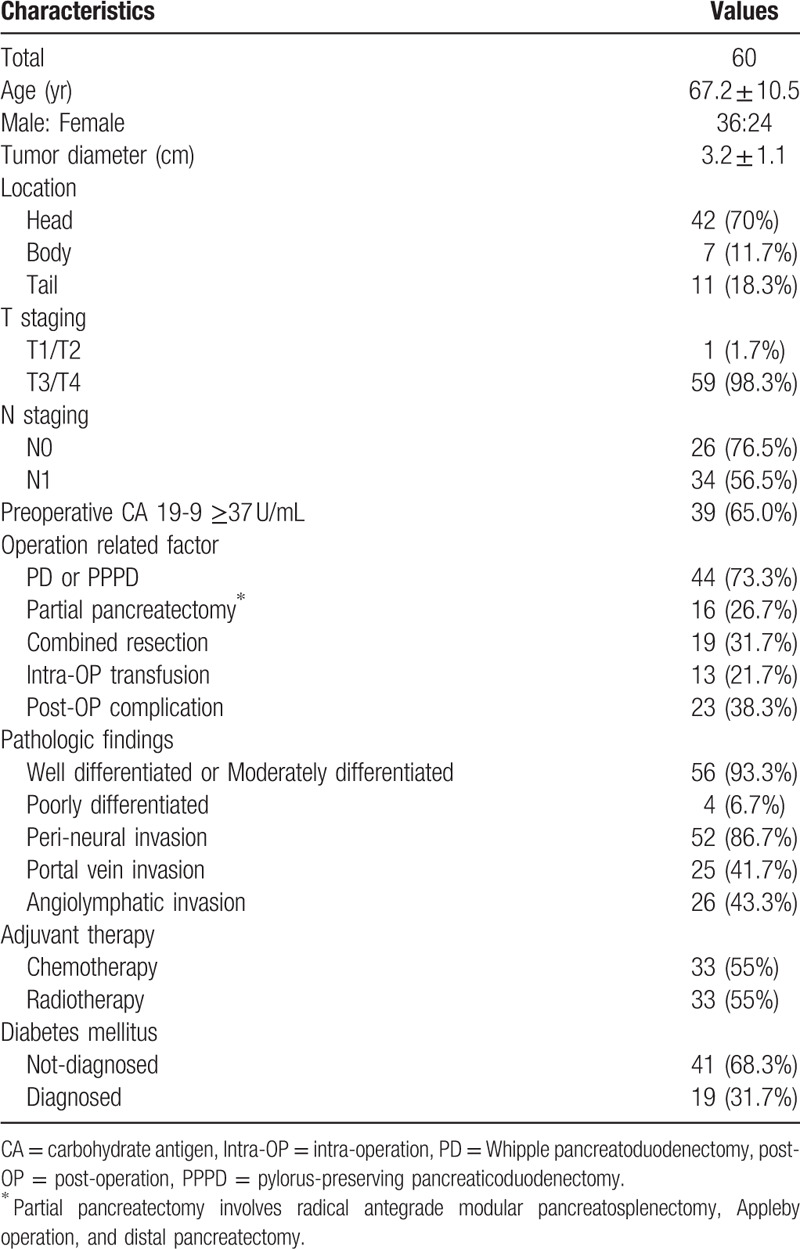
The end of follow up date for whole cohort was August 2013. Mean and standard deviation of overall follow-up duration was 16.12 ± 9.81 months (range, 3.2–47.9 months). Among them, 39 patients had died at 13.46 ± 8.82 months after operation, whereas 21 patients survived with the follow-up duration of 18.56 ± 9.97 months. The overall patient clinicopathological characteristics are summarized in Table 1. Forty-four patients underwent Whipple pancreatoduodenectomy or pylorus-preserving pancreaticoduodenectomy and 16 patients underwent partial pancreatectomy including radical antegrade modular pancreatosplenectomy, Appleby, and distal pancreatectomy. 19 patients had been diagnosed DM before the surgery whereas 41 patients had not.
Table 1.
Patients characteristics.
3.2. Univariate analysis for metabolic and textural parameters
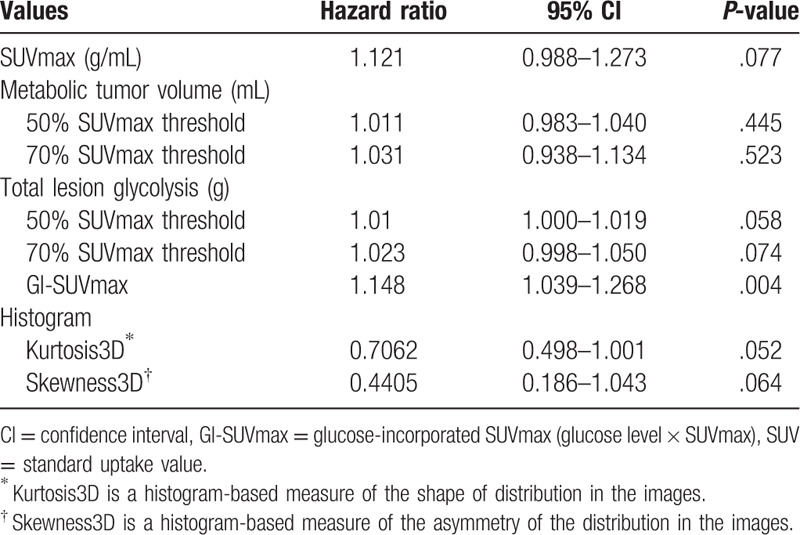
The univariate analysis of metabolic parameters from FDG PET/CT is displayed in Table 2. The data for textural parameters depend on the survival status are provided as the supplemental information (Supplement 2). In the univariate analysis of metabolic and textural parameters, only GI-SUVmax was significant parameter for overall survival (hazard ratio 1.148, 95% confidence interval 1.039–1.268, P = .004) whereas SUVmax was not significant. SUVmax, 50% SUVmax threshold, and 70% SUVmax threshold TLG showed possibility as prognostic factors (P-value = .077, .058, and .074, respectively), but were not statistically significant. In textural analysis, no one showed the statistical significance, but histogram-based kurtosis and skewness showed possibility (P-value = .052, and .064, respectively).
Table 2.
Univariate Cox regression analysis for the overall survival according to the metabolic and textural parameters.
3.3. Survival analysis for clinico-pathologic factors and metabolic analysis
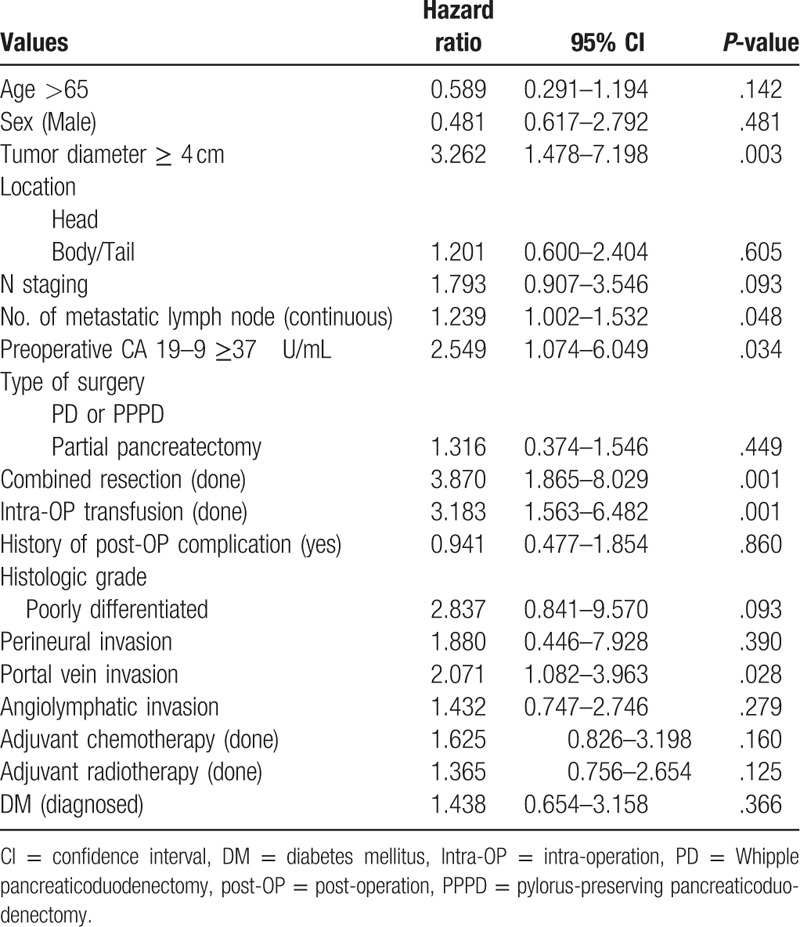
In univariate analysis of the clinico-pathologic factors, Tumor diameter ≥4 cm (mean + 1 standard deviation) (P = .003), preoperative CA19-9 ≥37 U/mL (P = .034), combined resection (P = .001), intra-OP transfusion (P = .001), and portal vein invasion (P = .028) were significant prognostic factor for overall survival (Table 3). N staging was not significant prognostic factor, but number of metastatic LN was significant prognostic factor (P = .048) Type of surgery, adjuvant chemotherapy, adjuvant radiotherapy, and DM failed to show statistical significance.
Table 3.
Univariate Cox regression analysis for the overall survival according to the clinic-pathologic parameters.
Among the tested parameters, tumor diameter ≥4 cm, number of metastatic LN, preoperative CA 19-9 ≥37 U/mL, combined resection, intra-OP transfusion, and portal vein invasion were involved for multivariate analysis. Only GI-SUVmax was included from metabolic parameters. In multivariate analysis of overall patients, Combined resection (odds ratio 4.881, 95% confidence interval 1.963–12.138, P = .001) and GI-SUVmax (odds ratio 1.265, 95% confidence interval 1.028–1.556, P = .026) were significant independent factor for survival (Table 4). Tumor diameter ≥4 cm and the number of metastatic LN were not statistically significant, but showed comparable results (P = .061 and P = .074) (Table 4). Kaplan–Meier plots showed significant difference in 2 groups divided by GI-SUVmax with median cut-off value 551, whereas SUVmax did not with median cut-off value 4.6 (Fig. 2A and B). Kaplan–Meier plots also showed the significant difference in overall survivals depending on the combined surgery and comparable result depending on N staging (Fig. 3A and B).
Table 4.
Multivariate Cox regression analysis for the overall survival according to the clinic-pathologic and metabolic parameters.
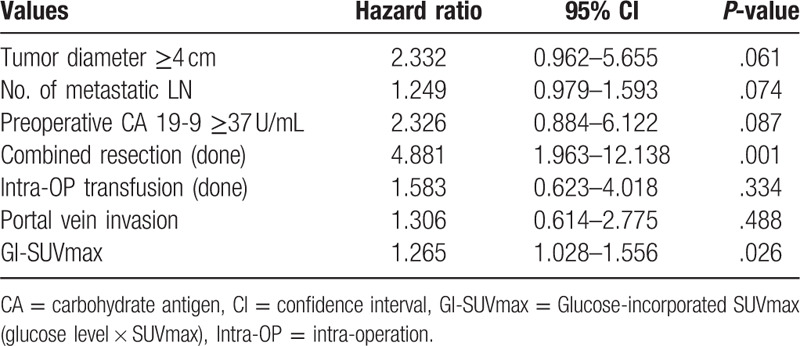
Figure 2.
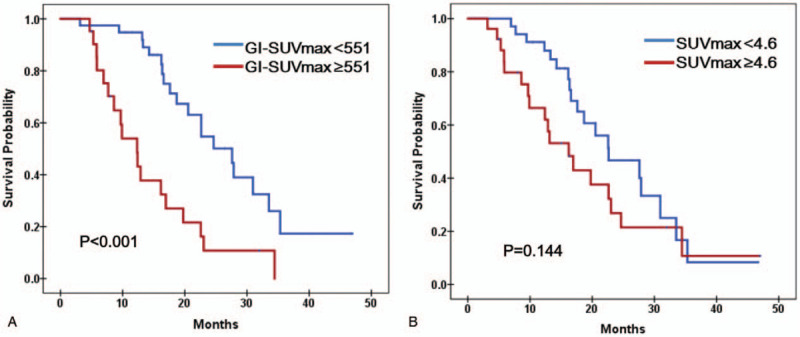
Survival analysis according to the GI-SUVmax and SUVmax with cutoff of 551 and 4.6. (A) Patients with GI-SUVmax more than 551 had a significantly poor overall survival than patients with GI-SUVmax less than 551 (P < .001 by Log rank test of Kaplan–Meier survival curves). (B) There is no significant survival difference between 2 groups with higher or lower SUVmax than 4.6. GI-SUVmax = glucose-incorporated SUVmax, SUVmax = maximal standard uptake value.
Figure 3.
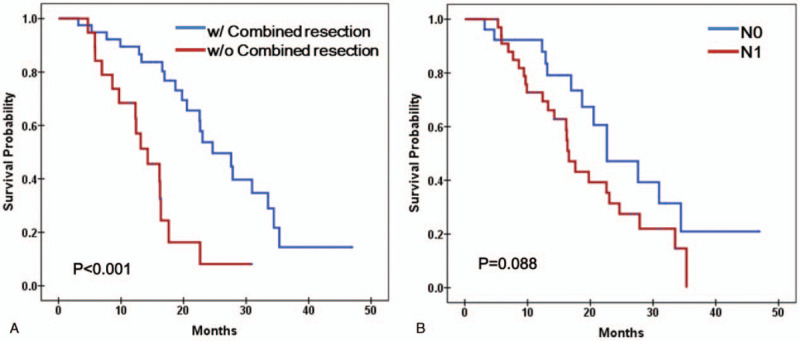
Survival analysis according to the combined resection and LN metastasis. (A) Patients underwent combined resection had a significantly poor overall survival than patients without combined (P < .001 by log rank test of Kaplan-Meier survival curve). (B) Patient with LN metastasis did not showed statistically significant difference in overall survival, but showed comparable result (P = .088 by Log rank test of Kaplan–Meier survival curves). LN = lymph node.
4. Discussion
Diabetes or pre-diabetes have been suggested to be risk factors for pancreatic cancer[19–21] or to be early manifestation of pancreatic cancer.[18] Although the exact underlying pathogenetic mechanism has not been elucidated yet, impaired glucose control, compensatory hyperinsulinemia, and accompanying tumorigenic effects of glucose/insulin with inflammation might play crucial roles for development of pancreatic cancer in diabetic patients.[20] Recently, high level of fasting serum glucose itself was reported to be a risk factor of pancreatic cancer. In fact, high level of fasting plasma glucose reduced the detection rate of pancreatic cancer during FDG PET/CT.[30] Therefore, incorporation of glucose level to the SUVmax might reflect both the tumorigenic environment (glucose level) and the tumor aggressiveness, uncovering true FDG uptake, which had been suppressed by the glucose. Because FDG and glucose enter into tumor cells via the same transporters, FDG uptake would be overwhelmingly reduced by the high glucose concentration compared with the tracer dose range concentration of FDG.[31–33] It is of note that the opposite is not true. In the clinical scenario of FDG injection, the concentration of FDG would be so minimal compared to the glucose concentration that the variation of FDG dose would not tremendously affect the glucose intake to the tumor cells.
Combined resections of the PV, SMV, and adjacent organs have shown survival benefits for patients with borderline resectable pancreatic cancer.[34–36] However, most of the patients undergoing combined resections require complex surgeries involving vascular resection and reconstruction compared to patients with localized pancreatic cancer.[37] They are at a higher risk of perioperative morbidity and occult distant metastasis.[37] Therefore, this result can be interpreted as patients with borderline resectable pancreatic cancer undergoing combined surgery have an independent risk factor for prognosis compared to the patients with localized pancreatic cancer.
With respect to the imaging parameters for FDG PET/CT, our study results are somewhat advanced than the results of previous studies.[16,38,39] SUVmax was reported to be a significant predictor of early recurrence of resectable pancreatic cancer.[38,39] MTV and TLG were the significant predictors of prognosis in non-resectable locally-advanced pancreatic cancer.[16] However, the glucose level has not been adjusted to SUV or other metabolic parameters in those studies even though the glucose level can affect the SUV level and has some important prognostic implications in pancreatic cancer. In this regard, the current study result with corrected SUV using GI-SUVmax has clinical impact that has not been investigated before. Not only the metabolic activity of the tumor, but also the metabolic environment should be considered together for the comprehensive evaluation of the patient with pancreatic cancer.
Texture analysis is a novel computational technique for the analysis of tumor heterogeneity.[24] Tumor heterogeneity is an important characteristic reflecting the diversity of tumor microenvironment. Histopathologic or imaging data have been actively applied to the tumor heterogeneity analysis, and nuclear imaging data are recently being used for the same purpose.[23] However, the clinical usefulness of tumor heterogeneity is not completely established. Furthermore, there are contradictory results about the effectiveness of tumor heterogeneity analysis using texture analysis for FDG PET/CT.[23,40] In the current research, the texture analysis was performed using 3D volume data rather than 2D plane data, and a number of textural parameters from the 3D data of PET/CT were comprehensively compared with known clinic-pathologic parameters, surgical parameters, and metabolic parameters from FDG PET/CT. As a result, none of the textural parameters remained to be significant predictor of prognosis. This result may indicate the limitation of tumor heterogeneity analysis of an imaging study or just failure of textural parameters from FDG PET/CT for prognosis prediction of surgically resectable pancreatic cancer. In any case, no further study is warranted for this kind of complex analyses dealing within numerous data sets.
Our study had several limitations. First, the retrospective study design was a limitation of our study. Second, the follow-up period was relatively short. However, our study showed the prognostic significance of GI-SUVmax and combined resection even in these limited settings; whereas, the other metabolic and clinical parameters were not significant. In addition, multi-visceral resection, PV, and SMV resection were not distinguished and were considered as an additional combined resection altogether. Further larger-size prospective studies are needed to identify the prognostic values of the metabolic parameters adjusted to the plasma glucose level in patients with pancreatic cancer.
5. Conclusion
The survival of patients with completely resected pancreatic cancer depends on the tumor metabolic activity adjusted to the serum glucose level (GI-SUVmax = glucose-incorporated maximum SUV), and the concomitant operation, such as the portal vein, SMV, and multi-visceral resection. Other imaging parameters such as textural parameters did not play an important role for prognosis prediction of pancreatic cancer.
Author contributions
Conceptualization: Won Woo Lee, Min Young Yoo.
Data curation: Min Young Yoo, Yoo-Seok Yoon, Min Seok Suh.
Formal analysis: Min Young Yoo, Min Seok Suh, Yoo-Seok Yoon.
Supervision: Jai Young Cho, Ho-Seong Han, Won Woo Lee.
Visualization: Min Young Yoo, Min Seok Suh.
Writing – original draft: Min Young Yoo.
Writing – review & editing: Won Woo Lee.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CA19-9 = carbohydrate antigen 19-9, DM = diabetes mellitus, FDG PET/CT = F-18 fluorodeoxyglucose positron emission tomography/compute tomography, GI-SUVmax = glucose-incorporated maximal standard uptake value, Intra-OP = intra-operative, LN = lymph node, MTV = metabolic tumor volume, Post-OP = postoperative, SMV = superior mesenteric vein, SUVmax = maximal standard uptake value, TLG = total lesion glycolysis.
How to cite this article: Yoo MY, Yoon YS, Seok Suh M, Cho JY, Han HS, Woo Lee W. Prognosis prediction of pancreatic cancer after curative intent surgery using imaging parameters derived from F-18 fluorodeoxyglucose positron emission tomography/computed tomography. Medicine. 2020;99:35(e21829).
The authors of this work have nothing to disclose.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent: This retrospective study was approved by institutional review board. The requirement for written informed consent was waived due to the retrospective design.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 2009;6:699–708.. [DOI] [PubMed] [Google Scholar]
- [2].Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29.. [DOI] [PubMed] [Google Scholar]
- [3].Paez D, Labonte MJ, Lenz HJ. Pancreatic cancer: medical management (novel chemotherapeutics). Gastroenterol Clin North Am 2012;41:189–209.. [DOI] [PubMed] [Google Scholar]
- [4].Okano K, Suzuki Y. Strategies for early detection of resectable pancreatic cancer. World J Gastroenterol 2014;20:11230–40.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lewis R, Drebin JA, Callery MP, et al. A contemporary analysis of survival for resected pancreatic ductal adenocarcinoma. HPB 2013;151:49–60.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Strobel O, Hank T, Hinz U, et al. Pancreatic cancer surgery. Ann Surg 2017;265:565–73.. [DOI] [PubMed] [Google Scholar]
- [7].Hata S, Sakamoto Y, Yamamoto Y, et al. Prognostic impact of postoperative serum CA 19-9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol 2012;19:636–41.. [DOI] [PubMed] [Google Scholar]
- [8].Chang KJ, Lim I, Park JY, et al. The role of (18)F-FDG PET/CT as a prognostic factor in patients with synovial sarcoma. Nucl Med Mol Imaging 2015;49:33–41.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].J Jo I, Zeon SK, Kim SH, et al. Correlation of primary tumor FDG uptake with clinicopathologic prognostic factors in invasive ductal carcinoma of the breast. Nucl Med Mol Imaging 2015;49:19–25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chang JS, Choi SH, Lee Y, et al. Clinical usefulness of (1)(8)F-fluorodeoxyglucose-positron emission tomography in patients with locally advanced pancreatic cancer planned to undergo concurrent chemoradiation therapy. Int J Radiat Oncol Biol Phys 2014;90:126–33.. [DOI] [PubMed] [Google Scholar]
- [11].Yoneyama T, Tateishi U, Endo I, et al. Staging accuracy of pancreatic cancer: comparison between non-contrast-enhanced and contrast-enhanced PET/CT. Eur J Radiol 2014;83:1734–9.. [DOI] [PubMed] [Google Scholar]
- [12].Parlak C, Topkan E, Onal C, et al. Prognostic value of gross tumor volume delineated by FDG-PET-CT based radiotherapy treatment planning in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Radiat Oncol 2012;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lee JW, Kang CM, Choi HJ, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med 2014;55:898–904.. [DOI] [PubMed] [Google Scholar]
- [14].Xi Y, Guo R, Hu J, et al. 18F-fluoro-2-deoxy-D-glucose retention index as a prognostic parameter in patients with pancreatic cancer. Nucl Med Commun 2014;35:1112–8.. [DOI] [PubMed] [Google Scholar]
- [15].Yamamoto T, Sugiura T, Mizuno T, et al. Preoperative FDG-PET predicts early recurrence and a poor prognosis after resection of pancreatic adenocarcinoma. Ann Surg Oncol 2015;22:677–84.. [DOI] [PubMed] [Google Scholar]
- [16].Choi HJ, Lee JW, Kang B, et al. Prognostic significance of volume-based FDG PET/CT parameters in patients with locally advanced pancreatic cancer treated with chemoradiation therapy. Yonsei Med J 2014;55:1498–506.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee WW, Chung JH, Jang SJ, et al. Consideration of serum glucose levels during malignant mediastinal lymph node detection in non-small-cell lung cancer by FDG-PET. J Surg Oncol 2006;94:607–13.. [DOI] [PubMed] [Google Scholar]
- [18].Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005;92:2076–83.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA 2005;294:2872–8.. [DOI] [PubMed] [Google Scholar]
- [20].Li D. Diabetes and pancreatic cancer. Mol Carcinog 2012;51:64–74.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Andersen DK. Diabetes and cancer: placing the association in perspective. Curr Opin Endocrinol Diabetes Obes 2013;20:81–6.. [DOI] [PubMed] [Google Scholar]
- [22].Holli K, Laaperi AL, Harrison L, et al. Characterization of breast cancer types by texture analysis of magnetic resonance images. Acad Radiol 2010;17:135–41.. [DOI] [PubMed] [Google Scholar]
- [23].Cook GJ, Yip C, Siddique M, et al. Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med 2013;54:19–26.. [DOI] [PubMed] [Google Scholar]
- [24].Rahim MK, Kim SE, So H, et al. Recent trends in PET image interpretations using volumetric and texture-based quantification methods in nuclear oncology. Nucl Med Mol Imaging 2014;48:1–5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moon SY, Joo KR, So YR, et al. Predictive value of maximum standardized uptake value (SUVmax) on 18F-FDG PET/CT in patients with locally advanced or metastatic pancreatic cancer. Clin Nucl Med 2013;38:778–83.. [DOI] [PubMed] [Google Scholar]
- [26].Yue Y, Osipov A, Fraass B, et al. Identifying prognostic intratumor heterogeneity using pre-and post-radiotherapy 18F-FDG PET images for pancreatic cancer patients. J Gastrointest Oncol 2017;8:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hackert T, Ulrich A, Büchler MW. Borderline resectable pancreatic cancer. Cancer Lett 2016;375:231–7.. [DOI] [PubMed] [Google Scholar]
- [28].Lee SJ, Lee WW, Yoon HJ, et al. Regional PET/CT after water gastric inflation for evaluating loco-regional disease of gastric cancer. Eur J Radiol 2013;82:935–42.. [DOI] [PubMed] [Google Scholar]
- [29].HM Stationery Office, James WPT, Waterlow JC. Research on Obesity: A Report of the DHSS/MRC Group. 1976. [Google Scholar]
- [30].HamidianJahromi A, Fallahzadeh MK, Takalkar A, et al. Impact of plasma glucose level at the time of fluorodeoxyglucose administration on the accuracy of FDG-PET/CT in the diagnosis of pancreatic lesions. Int J Endocrinol Metab 2014;12:e16429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Langen K-J, Braun U, Kops ER, et al. The influence of plasma glucose levels on fluorine-18-fluorodeoxyglucose uptake in bronchial carcinomas. J Nucl Med 1993;34:355–9.. [PubMed] [Google Scholar]
- [32].Lindholm P, Minn H, Leskinen-Kallio S, et al. Influence of the blood glucose concentration on FDG uptake in cancer—a PET study. J Nucl Med 1993;34:1–6.. [PubMed] [Google Scholar]
- [33].Diederichs CG, Staib L, Glatting G, et al. FDG PET: elevated plasma glucose reduces both uptake and detection rate of pancreatic malignancies. J Nucl Med 1998;39:1030–3.. [PubMed] [Google Scholar]
- [34].Shibata C, Kobari M, Tsuchiya T, et al. Pancreatectomy combined with superior mesenteric-portal vein resection for adenocarcinoma in pancreas. World J Surg 2001;25:1002–5.. [DOI] [PubMed] [Google Scholar]
- [35].Zhou Y, Zhang Z, Liu Y, et al. Pancreatectomy combined with superior mesenteric vein–portal vein resection for pancreatic cancer: a meta-analysis. World J Surg 2012;36:884–91.. [DOI] [PubMed] [Google Scholar]
- [36].Ravikumar R, Sabin C, Hilal MA, et al. Portal vein resection in borderline resectable pancreatic cancer: a United Kingdom multicenter study. J Am Coll Surg 2014;218:401–11.. [DOI] [PubMed] [Google Scholar]
- [37].Evans DB, George B, Tsai S. Non-metastatic pancreatic cancer: resectable, borderline resectable, and locally advanced-definitions of increasing importance for the optimal delivery of multimodality therapy. Ann Surg Oncol 2015;22:3409–13.. [DOI] [PubMed] [Google Scholar]
- [38].Okamoto K, Koyama I, Miyazawa M, et al. Preoperative 18[F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts early recurrence after pancreatic cancer resection. Int J Clin Oncol 2011;16:39–44.. [DOI] [PubMed] [Google Scholar]
- [39].Hwang JP, Lim I, Chang KJ, et al. Prognostic value of SUVmax measured by fluorine-18 fluorodeoxyglucose positron emission tomography with computed tomography in patients with pancreatic cancer. Nucl Med Mol Imaging 2012;46:207–14.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ha S, Choi H, Cheon GJ, et al. Autoclustering of non-small cell lung carcinoma subtypes of 18F-FDG PET using texture analysis: a preliminary result. Nucl Med Mol Imaging 2014;48:278–86.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


