Abstract
Background:
To compare the effects of 3% hypertonic saline solution and 20% mannitol solution on intracranial hypertension.
Methods:
WAN-FANGDATA, CNKI, and CQVIP databases were searched, and relevant literatures of randomized controlled trials comparing 3% hypertonic saline solution with mannitol in reducing intracranial hypertension from 2010 to October 2019 were collected. Meta-analysis was performed using RevMan software.
Results
: As a result, 10 articles that met the inclusion criteria were finally included. A total of 544 patients were enrolled in the study, 270 in the hypertonic saline group and 274 in the mannitol group. There was no significant difference in the decrease of intracranial pressure and the onset time of drug between the 2 groups after intervention (all P > .05). There was a statistically significant difference between the hypertonic saline group and the mannitol group in terms of duration of effect in reducing intracranial pressure (95% confidence interval: 0.64–1.05, Z = 8.09, P < .00001) and cerebral perfusion pressure after intervention (95% confidence interval: 0.15–0.92, Z = 2.72, P = .007).
Conclusion:
Both 3% hypertonic saline and mannitol can effectively reduce intracranial pressure, but 3% hypertonic saline has a more sustained effect on intracranial pressure and can effectively increase cerebral perfusion pressure.
Keywords: hypertonic, intracranial hypertension, intracranial pressure, mannitol, meta-analysis, saline
1. Introduction
Many neurosurgical diseases especially craniocerebral trauma can cause brain edema, and then leading to increased intracranial pressure (ICP). The increased pressure is considered to be the leading cause of death in patients with acute cerebral edema. As a frequently used drug to reduce ICP, the effectiveness of mannitol has been widely recognized.[1] However, serious side effects are more and more obvious, such as acute renal insufficiency and rebound cerebral edema.[2] Hypertonic saline was first reported for the treatment for alteration of brain bulk by Weed and McKibben.[3] Because of its effect of reducing ICP, hypertonic saline has become more and more widely used in clinical practice. In the treatment of cerebral edema and increased ICP caused by neurosurgical diseases, the use of mannitol and hypertonic saline is a hot spot of present research. Currently used hypertonic saline concentrations are 3.0% to 23.4%, the dose of hypertonic saline is from 1.0 to 4.0 mL/kg.[4] Compare different concentration for treatment with hypertonic saline dehydration, currently around the world, there is no unified conclusion. If the concentration is too low, the osmotic pressure is insufficient, which affects the curative effect. Some meta-analyses tend to suggest that hypertonic saline reduces ICP better than mannitol, and in pediatric head injury, there is class II evidence to support the use of 3% hypertonic saline for the treatment of intracranial hypertension, with insufficient evidence to support or refute the use of mannitol or other hyperosmolar agents for the treatment of severe head injury.[5] But the Traumatic Brain Injury Foundation's 4th edition of the guidelines for the management of severe traumatic brain injury (TBI) states that there is insufficient evidence from comparative studies to support a formal recommendation and no evidence to support the use of any specific hyperosmolar medication in patients with severe TBI.[6] The aim of this study was to compare the effects of 3% hypertonic saline and 20% mannitol on brain injury.
1.1. Literature search strategy
We searched WAN-FANGDATA, CNKI, CQVIP databases until October 13, 2019 using the text words or MeSH: “Saline Solution, Hypertonic,” “mannitol,” “intracranial pressure.” Two investigators independently screened the titles and abstracts of all researches and eliminated those that were not in accord to eligibility criteria. Any disagreement was solved by a 3rd investigator.
1.2. Selection criteria and data extraction
Inclusion criteria were randomized controlled trial, intervention of hypertonic saline and mannitol, elevated ICP, treatment including 3% hypertonic saline and 20% mannitol, full text available, and literature published between 2010 and 2019. The following exclusion criteria were applied: case report, comments, cohort studies, animal studies, and reviews; studies that did not give quantitative data at the 1st 6 hours.
1.3. Quality assessment
Two independent reviewers were required to finish the retrieval work. We used the risk-of-bias assessment tool in the Cochrane Handbook for Systematic Reviews of Interventions to assess the methodologic quality of the studies we selected. Evaluate the risk of bias of included studies, including: whether the random allocation method is correct; whether there is allocation concealment scheme; whether blinding method is used; whether blinding of outcome assessment is used; whether the outcome data are completely reported; whether the study results are selectively reported; other sources of bias and each indicator is divided into 3 levels of “yes” (low bias), “no” (high bias), and “unclear” (lack of relevant information). Disagreements can be settled well by discussion of 2 reviewers or with a 3rd reviewer. All data were entered twice and discrepancies resolved (Fig. 1).
Figure 1.
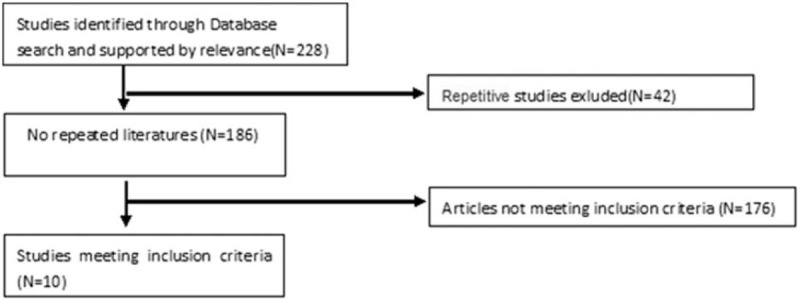
Flow diagram through the systematic review.
1.4. Statistical analysis
In this study, we compared the maximum changes of ICP and cerebral perfusion pressure (CPP), onset time, and maintenance time between 3% hypertonic saline and 20% mannitol. Patients treated with either mannitol (20%) or hypertonic saline (3%) were compared during each time. We use RevMan 5.3 software to analyze all data. A P value <.5 or I2 > 50% was regarded significant heterogeneity. The random-effect model was applied if heterogeneity was detected. The difference in means with 95% confidence intervals (CIs) and P value <.05 were used to indicated statistical significance.
1.5. Ethical approval
Ethical approval was not necessary because we integrate and analyze the existing research data.
2. Results
The flow diagram through the systematic review is shown in the Figure 2. Our research of the database included 228 records, 218 records were excluded and thus the 10 studies[7–16] were enrolled in the systematic and meta-analysis.
Figure 2.
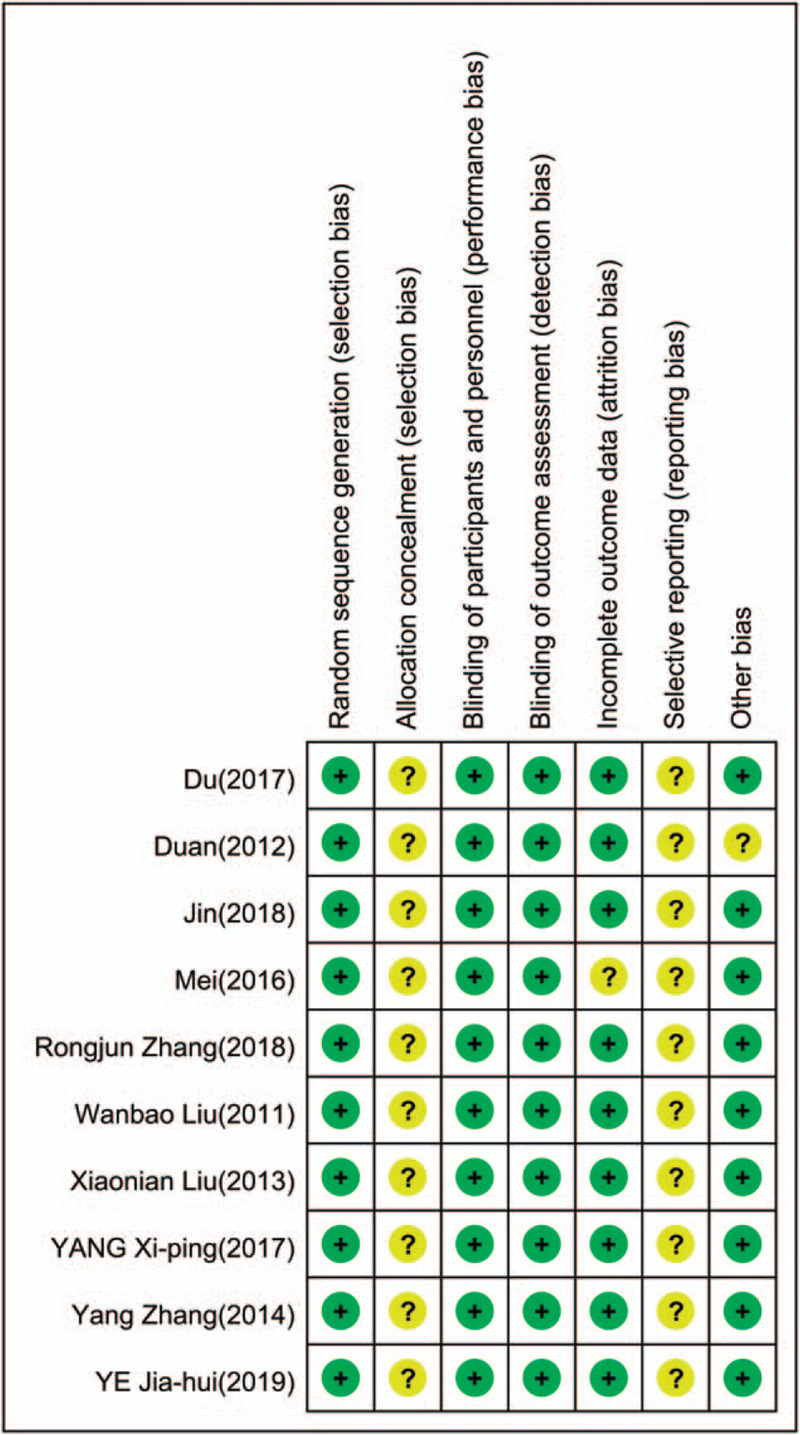
Risk-of-bias summary.
2.1. Intracranial pressure
Nine of the ten studies provided complete data of the maximum changes of ICP after termination of the infusion, and were included in the meta-analysis. There was evidence of heterogeneity (I2 = 0%, P = .57); therefore, a fixed effect model of analysis was used. The pooled difference in means = −0.19 (95% CI: −0.37 to −0.02, P = .03) indicated that mannitol reduces ICP more than hypertonic saline (Fig. 3).
Figure 3.
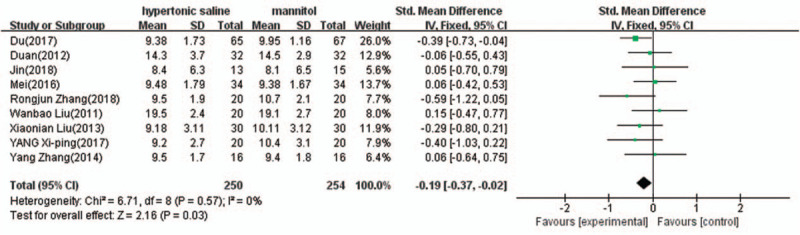
Comparison of intracranial pressure reduction between 3% hypertonic saline and 20% mannitol (mm Hg). CI = confidence interval.
Three of the 10 studies provided complete data of cerebral perfusion pressure changes after termination of the infusion, and were included in the meta-analysis. There was evidence of heterogeneity (I2 = 15%, P = .31); therefore, a fixed effect model of analysis was used. The pooled difference in means = 0.54 (95% CI: 0.15–0.92, P = .007) indicated that 3% hypertonic saline is more effective than 20% mannitol in increasing CPP (Fig. 4).
Figure 4.
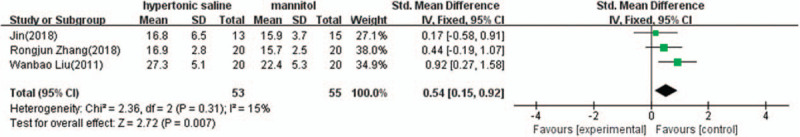
Comparison of cerebral perfusion pressure changes between 3% hypertonic saline and 20% mannitol (mm Hg). CI = confidence interval, SD = standard deviation.
Eight of the 10 studies provided complete data of the onset time after termination of the infusion, and were included in the meta-analysis. There was evidence of heterogeneity (I2 = 37%, P = .13); therefore, a fixed effect model of analysis was used. The pooled difference in means = 0.05 (95% CI: −0.14 to 0.23, P = .64) indicated that There was no significant difference in onset time between 3% hypertonic saline and 20% mannitol (Fig. 5).
Figure 5.
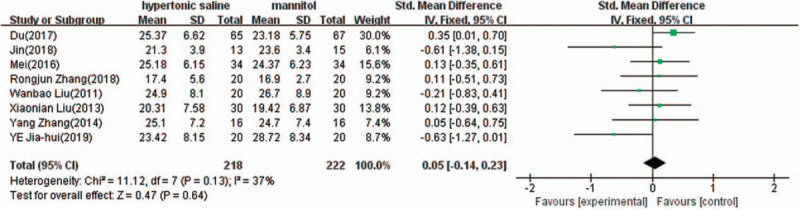
Comparison of onset time between 3% hypertonic saline and 20% mannitol (minutes). CI = confidence interval, SD = standard deviation.
Nine of the 10 studies provided complete data of continuous ICP reduction time after termination of the infusion, and were included in the meta-analysis. There was evidence of heterogeneity (I2 = 45%, P = .07); therefore, a fixed model of analysis was used. The pooled difference in means = 0.84 (95% CI: 0.64–1.05, P < .00001) indicated that 3% hypertonic saline lasts longer time for ICP reduction than 20% mannitol (Fig. 6).
Figure 6.
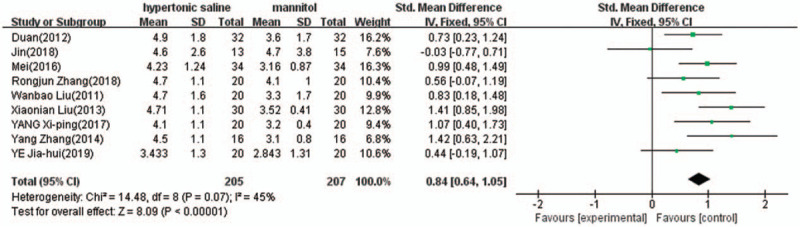
Comparison of duration of continuous intracranial pressure reduction between 3% hypertonic saline and 20% mannitol (hours). CI = confidence interval, SD = standard deviation.
2.2. Bias analysis
RevMan 5.3 was used to test the possibility of publication bias of included studies. Figures 7–10 show the results of publication bias detection of ICP reduction, changes in CPP, drug onset time, and drug duration in turn. As shown in the figures, the points on the funnel plot presented in this included studies are symmetrically scattered around the estimated true value of each independent study effect point, considering that the bias is not great. The results of sensitivity analysis of ICP reduction, changes in CPP, drug onset time, and drug duration showed good stability of the results (Figs. 7–10).
Figure 7.
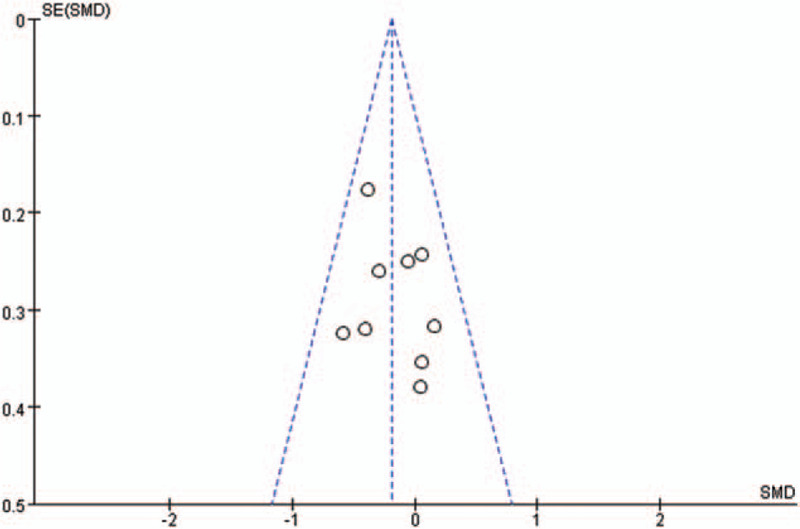
Funnel plot for intracranial pressure comparison of 3% hypertonic saline and 20% mannitol. SE = standard error, SMD = standard mean difference.
Figure 10.
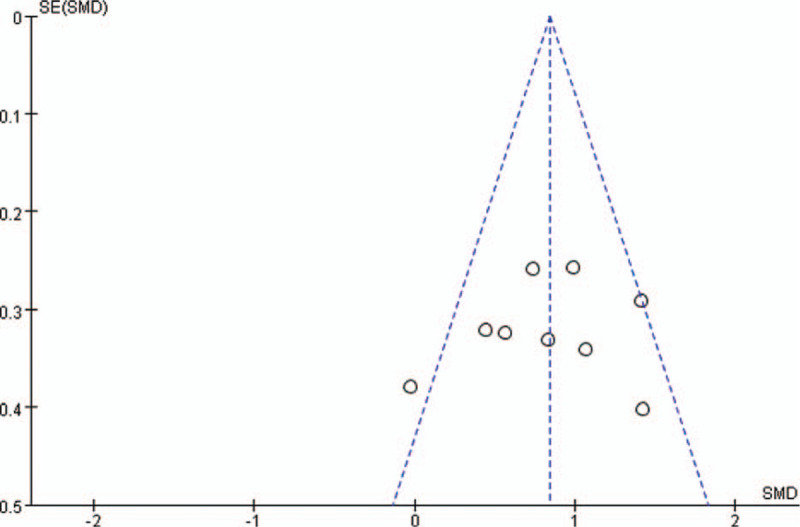
Funnel plot for duration of continuous intracranial pressure reduction comparison of 3% hypertonic saline and 20% mannitol. SE = standard error, SMD = standard mean difference.
Figure 8.
Funnel Plot for cerebral perfusion pressure Comparison of 3% Hypertonic Saline and 20% Mannitol. SE = standard error, SMD = standard mean difference.
Figure 9.
Funnel plot for onset time comparison of 3% hypertonic saline and 20% mannitol. SE = standard error, SMD = standard mean difference.
3. Discussion
The 10 literatures included in this study were all randomized controlled studies. The research direction and focus of each literature were not completely consistent. The number of comparable studies was small after screening according to the inclusion of the detection indicators in this study. We compared the effect of 3% hypertonic saline with that of 20% mannitol in reducing ICP in 9 of 10 studies. Since there are few articles providing data on CPP, we took 3 of them to compare the difference between 3% hypertonic saline and 20% mannitol in reducing CPP. There are many studies comparing the onset time and duration of the 2 drugs, and the total number of patients in the experimental group and control group reached more than 400. The main findings are as follows: For patients with intracranial hypertension, 20% mannitol was slightly more effective than 3% hypertonic saline in reducing ICP. In the aspect of increasing CPP, both 20% mannitol and 3% hypertonic saline had the effect of increasing CPP, and 3% hypertonic saline perform better. There was no significant difference in onset time between 20% mannitol and 3% hypertonic saline. There was a significant difference in duration between the 2, with 3% hypertonic saline having a more sustained effect than 20% mannitol in patients with intracranial hypertension.
Intracranial hypertension caused by TBI can produce multiple hazards, such as cerebral ischemia, brain shift, Cushing reaction, and neurogenic pulmonary edema.[17] Intracranial hypertension has been defined in the past as ICP >20 mm Hg, a value that is a recognized criterion for initiating clinical intervention. However, 1 study have suggested that ICP >22 mm Hg should be initiated, but this study is a single-center, retrospective study, and the reliability of the conclusions drawn remains to be determined.[6] ICP has been a major predictor of neurologic deterioration in patients with intracranial hypertension and elevated ICP is associated with poor neurologic outcome.[18] It has also been proved that if CPP is extremely low (<50 mm Hg), ICP is no longer a predictor of poor outcome, and maintaining ICP in the range of 18 to 23 mm Hg ensures that CPP remains stable for a longer period of time.[19]
Mannitol has been used to reduce elevated ICP for decades. Current evidence suggests that mannitol is more effective in reducing ICP in patients with TBI compared with barbiturates and is recommended by guidelines.[20] Mannitol also has its side effects, acute renal failure, pulmonary edema, aggravation, and rebound of existing cerebral edema (by accumulation in brain tissue via a traumatically damaged blood–brain barrier), and arterial hypotension resulting in a decrease in CPP by its diuretic action have all been reported.[21] An ideal therapeutic agent to control ICP should reduce ICP while maintaining cerebral perfusion. Adverse effects of hypertonic saline are rarely reported, a retrospective trial in pediatric intensive care patients found that continuous infusion of hypertonic saline targeted to achieve a serum sodium concentration of 170 mmol/L (and concurrent hyperchloremia) was associated with acute renal failure, acute respiratory distress syndrome, thrombocytopenia, neutropenia, and anemia.[22] Three percent hypertonic saline significantly increased serum sodium and osmolality. Excessive increases in sodium levels and osmolarity result in volume overload with heart failure and pulmonary edema, or can induce hyperchloremic metabolic acidosis and coagulopathy.[23,24] Therefore, the use of hypertonic solutions in patients with impaired cardiac function should be performed under close cardiac monitoring. And in experiments, intravenous application of hypertonic saline increased cerebral perfusion and resulted in a rightward shift of the oxygen dissociation curve, thereby improving oxygen delivery. At the same time, cerebral edema decreased, brain compliance increased, and ICP decreased.[25] Current evidence on the use of hypertonic saline in severe TBI is limited to small studies showing its benefit in reducing ICP and mortality.[26]
According to our included studies, all patients should also achieve ICP >20 mm Hg for the criteria of starting treatment. Most patients with intracranial hypertension in hospitals in China use 3% hypertonic saline or 20% mannitol. Through this comparative analysis, we found that there was no significant difference in ICP from baseline to the lowest value between the 2, which was consistent with the results of several previous studies.[27–32] There is evidence that episodes of CPP <60 mm Hg or ICP >20 mm Hg are associated with a worse outcome, and that CPP above 70 mm Hg by treatment is a widely accepted treatment goal.[20] In the results of this data analysis, 3% hypertonic saline perform better than 20% mannitol, and Inclusion of a larger number of studies will increase the credibility of the results.
The duration of mannitol and hypertonic saline was different in the previous studies,[33,34] and 3% hypertonic saline also showed a longer duration of ICP reduction than 20% mannitol in children with head injury in this study. In addition, the optimal infusion rate is one of the controversial points. One study by Battison et al,[35] ICP returned to pretreatment levels after a median time of 90 minutes, mannitol was administered in a 5-minute bolus dose. When mannitol was administered at a slower rate (20–30 minutes), no ICP rebound was observed within 2 hours of infusion. This suggests that the duration of mannitol action may involve the infusion rate: the faster the infusion, the effect is terminated by rapid renal elimination or penetration of mannitol into the brain tissue. In addition, the strength, frequency, timing, and route of administration of hypertonic saline or mannitol need to be justified in multicenter studies. This systematic review is limited by the number and quality of studies available for review. It is important to note that subject inclusion criteria vary between studies, clinical outcomes are inconsistently reported across studies, and more data on long-term clinical outcomes are needed. Reducing ICP alone is a practical goal, but may be a point of controversy if not accompanied by improved patient functional outcomes. Evaluation of the prognosis of children after treatment with hypertonic saline and mannitol requires a randomized study with adequate power and a reasonable follow-up period to confirm that hypertonic saline is superior to mannitol in patient-centered outcomes. In particular, the theoretical benefit of hypertonic saline in hemodynamically unstable patients should be investigated and the optimal concentration and dosage regimen of hypertonic saline should be determined. In addition, the role of regimens that combine 2 drugs in cases where one drug is ineffective also requires more research to demonstrate.
4. Conclusion
In summary, the effect of 3% hypertonic saline and 20% mannitol in reducing ICP was satisfactory and the difference was not significant, but the effect of 3% hypertonic saline in reducing ICP was more sustained and easy to obtain clinically, reducing the economic burden of patients. In clinical work, when selecting mannitol or hypertonic saline for the treatment of intracranial hypertension, it is also necessary to take the infusion rate, frequency, route and mode of administration into account, to obtain the best therapeutic effect.
Author contributions
Linhua Tan contributed to the conception of the study.
Lei Hu, Jing Ye contributed significantly to quality assessment.
Jiamin Shi performed the data analyses and wrote the manuscript.
Linhua Tan helped perform the analysis with constructive discussions.
Footnotes
Abbreviations: CPP = cerebral perfusion pressure, ICP = intracranial pressure, TBI = traumatic brain injury.
How to cite this article: Shi J, Tan L, Ye J, Hu L. Hypertonic saline and mannitol in patients with traumatic brain injury: a systematic and meta-analysis. Medicine. 2020;99:35(e21655).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Diringer MN. The evolution of the clinical use of osmotic therapy in the treatment of cerebral edema. Acta Neurochir Suppl 2016;121:3–6.. [DOI] [PubMed] [Google Scholar]
- [2].Boone MD, Oren-Grinberg A, Robinson TM, et al. Mannitol or hypertonic saline in the setting of traumatic brain injury: what have we learned? Surg Neurol Int 2015;6:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Weed LH, Mckibben PS. Experimental alteration of brain bulk. 1919. [Google Scholar]
- [4].Patil H, Gupta R. A comparative study of bolus dose of hypertonic saline, mannitol, and mannitol plus glycerol combination in patients with severe traumatic brain injury. World Neurosurg 2019;125:e221–8.. [DOI] [PubMed] [Google Scholar]
- [5].Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med 2012;13:S1–82.. [DOI] [PubMed] [Google Scholar]
- [6].Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 2017;80:6–15.. [DOI] [PubMed] [Google Scholar]
- [7]. Liu Wanbao, Joe, Liu Jian. Clinical Study on Hypertonic Saline Rescue Acute Intracranial Hypertension Caused by Craniocerebral Injury. Kunming. [Google Scholar]
- [8].Duan Longxi, Zhan Xiquan, Wu, et al. A comparative study on dehydration effect of hypertonic saline and mannitol after peak edema in craniocerebral trauma. Contemporary Medicine 2012;38–9.. [Google Scholar]
- [9].Zhang Yang. Comparison of hypertonic saline and mannitol in the treatment of intracranial hypertension. Henan Med Res 2014;23:74–5.. [Google Scholar]
- [10].Ye ahui, Ding, Yanjing Effect of different concentrations of hypertonic saline on intracranial hypertension in patients with head injury. J Mudanjiang Med Coll 2019;40:23–6.. [Google Scholar]
- [11].Du DY, Sun LT, Zhang WS, et al. Hypertonic saline can reduce intracranial pressure in patients with severe traumatic brain injury. Nerve Injury Funct Reconstr 2017;12:215–7.. [Google Scholar]
- [12].Yang XP, Zhang XY, Tu Y, et al. Effect of different concentrations of hypertonic saline on intracranial hypertension after craniocerebral trauma. Tianjin Med J 2017;45:810–4.. [Google Scholar]
- [13].Clinical study of 3% hypertonic saline in treating cerebral edema of severe craniocerebral injury. Zhejiang J Trauma Surg 2018;23:37–8.. [Google Scholar]
- [14].Liu Xiaonan, Huang, Jiafu Clinical observation on treatment of 30 cases of traumatic brain edema combined with intracranial hypertension with hypertonic saline. Chin Folk Med 2013;22:68. [Google Scholar]
- [15].Mei LQ, Wang L, Yu JB. Comparative analysis of clinical efficacy of hypertonic saline and mannitol in the treatment of intracranial hypertension. China Med Eng 2016;126–7.. [Google Scholar]
- [16].Zhang Rongjun, Wang Xiaofeng, Luo Wenying, et al. A comparative study on the effect of hypertonic saline and mannitol in reducing intracranial pressure under intracranial pressure monitoring. Chin J Neurosurg 2018;34:630–2.. [Google Scholar]
- [17].Maas AIR, Menon DK, Adelson PD, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 2017;16:987–1048.. [DOI] [PubMed] [Google Scholar]
- [18].Juul N, Morris GF, Marshall SB, et al. Intracranial hypertension and cerebral perfusion pressure: influence on neurological deterioration and outcome in severe head injury. The Executive Committee of the International Selfotel Trial. J Neurosurg 2000;92:1–6.. [DOI] [PubMed] [Google Scholar]
- [19].Robba C, Citerio G. How I manage intracranial hypertension. Crit Care 2019;23:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Carney NA, Ghajar J. Guidelines for the management of severe traumatic brain injury. J Neurotraum 2007;1:1–06.. [DOI] [PubMed] [Google Scholar]
- [21].Schwimmbeck F, Voellger B, Chappell D, et al. Hypertonic saline versus mannitol for traumatic brain injury: a systematic review and meta-analysis with trial sequential analysis. J Neurosurg Anesth 2019. [DOI] [PubMed] [Google Scholar]
- [22].Gonda DD, Meltzer HS, Crawford JR, et al. Complications associated with prolonged hypertonic saline therapy in children with elevated intracranial pressure. Pediatr Crit Care Med 2013;14:610–20.. [DOI] [PubMed] [Google Scholar]
- [23].Moon PF, Kramer GC. Hypertonic saline-dextran resuscitation from hemorrhagic shock induces transient mixed acidosis. Crit Care Med 1995;23:323–31.. [DOI] [PubMed] [Google Scholar]
- [24].Treib J, Haass A, Pindur G. Coagulation disorders caused by hydroxyethyl starch. Thromb Haemost 1997;78:974–83.. [PubMed] [Google Scholar]
- [25].Kempski O, Obert C, Mainka T, et al. “Small volume resuscitation” as treatment of cerebral blood flow disturbances and increased ICP in trauma and ischemia. Acta Neurochir Suppl 1996;66:114–7.. [DOI] [PubMed] [Google Scholar]
- [26].Singhi SC, Tiwari L. Management of intracranial hypertension. Indian J Pediatr 2009;76:519–29.. [DOI] [PubMed] [Google Scholar]
- [27].Berger-Pelletier E, Emond M, Lauzier F, et al. Hypertonic saline in severe traumatic brain injury: a systematic review and meta-analysis of randomized controlled trials (vol 18, pg 112, 2016). Can J Emerg Med 2016;18:243. [DOI] [PubMed] [Google Scholar]
- [28].Kumar SA, Devi BI, Reddy M, et al. Comparison of equiosmolar dose of hyperosmolar agents in reducing intracranial pressure-a randomized control study in pediatric traumatic brain injury. Child Nerv Syst 2019;35:999–1005.. [DOI] [PubMed] [Google Scholar]
- [29].Hooman K, Navi BB, Kazuma N, et al. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta-analysis of randomized clinical trials. Crit Care Med 2011;39:554–9.. [DOI] [PubMed] [Google Scholar]
- [30].Rickard AC, Smith JE, Newell P, et al. Salt or sugar for your injured brain? A meta-analysis of randomised controlled trials of mannitol versus hypertonic sodium solutions to manage raised intracranial pressure in traumatic brain injury. Emerg Med J 2014;31:679–83.. [DOI] [PubMed] [Google Scholar]
- [31].Burgess S, Abu-Laban RB, Slavik RS, et al. A systematic review of randomized controlled trials comparing hypertonic sodium solutions and mannitol for traumatic brain injury: implications for emergency department management. J Infrared Millimeter Terahertz Waves 2016;32:1350–66.. [DOI] [PubMed] [Google Scholar]
- [32].Nikolaos S, Elias P, Stylianos K, et al. Comparison of mannitol and hypertonic saline in the treatment of severe brain injuries. J Neurosurg 2011;114:545–8.. [DOI] [PubMed] [Google Scholar]
- [33].Christos L, Ron N, Jeffrey B, et al. High-osmolarity saline in neurocritical care: systematic review and meta-analysis. Crit Care Med 2013;41:1353–60.. [DOI] [PubMed] [Google Scholar]
- [34].Ware ML, Nemani VM, Michele M, et al. Effects of 23.4% sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: a preliminary study. Neurosurgery 2005;57:727–36.. [PubMed] [Google Scholar]
- [35].Battison C, Andrews PJ, Graham C, et al. Randomized, controlled trial on the effect of a 20% mannitol solution and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury. Crit Care Med 2005;33:196–202.. [DOI] [PubMed] [Google Scholar]


