Abstract
The aim of this study is to investigate the levels of 25(OH)D, inflammation markers and glucose and fat metabolism indexes in pregnant women with Gestational diabetes mellitus (GDM).
One hundred and ten cases GDM and 100 cases healthy pregnant women in the First People's Hospital of Lianyungang City from October 2016 to December 2018 were recruited for this observational cross-sectional study. Each participant's anthropometric and demographic data was recorded. Blood samples were collected and analyzed to determine the levels of 25(OH)D, high sensitivity C-reactive protein (hs-CRP), tumor necrosis factor-alpha (TNF-α), fasting blood glucose, fasting blood insulin, hemoglobin A1c (HbA1c), homeostasis model assessment of insulin resistance (HOMA-IR), cholesterol and triglycerides.
Inflammatory markers and glucose and fat metabolism indexes were all significantly higher in the GDM group than that in the control group, while Serum 25(OH)D level in the GDM group was significantly lower. Serum 25(OH)D levels were negatively correlated with hs-CRP, while not with TNF-α. Furthermore, Serum 25(OH)D, hs-CRP and TNF-α levels were all associated with increased risk of developing GDM.
Nowadays, the reports on the association between 25(OH)D level and GDM were controversial. Our results are consistent with the view that there was association between 25(OH)D level and GDM, and expand the literature by showing the roles of 25(OH)D, inflammation markers as well as glucose and fat metabolism indexes in the risk of developing GDM in the pregnant women with the low overall levels of 25(OH)D before delivery. This broadens our knowledge on the pathophysiology of GDM, which may be helpful in prevention and treatment of GDM.
Keywords: 25-dihydroxyvitamin D, gestational diabetes mellitus, high sensitivity C-reactive protein, tumor necrosis factor-alpha
1. Introduction
Gestational diabetes mellitus (GDM) is defined as hyperglycemia diagnosed in the second or third trimester of pregnancy that is not clearly overt diabetes.[1] The prevalence of GDM varies from 1% to 14% of all pregnancies, depending on the population studied and the diagnostic tests employed.[2] The prevalence of GDM in high risk population is even higher, 25% in midtrimester pregnancies[3] and 33% in third trimester pregnancies.[4] According to the medical records of 17,186 pregnant women who received care at the GDM centers established in 13 hospitals in China, 17.5% women were diagnosed with GDM.[5] GDM has both short-term and long-term complications for mothers and their children.[6] Thus, it is valuable to investigate the pathophysiology and identify potential risk factors of GDM.
Beta β-cell dysfunction and insulin resistance play important roles in the pathophysiology of GDM.[6] It is well known that some risk factors such as advanced maternal age, ethnicity and overweight/obesity are associated with development of GDM.[7] Vitamin D plays an important role in bone mineralization and calcium/phosphorus homeostasis.[8] Recently, more and more evidence has shown that Vitamin D also involves in many aspects for human health especially in chronic diseases.[9–11] Vitamin D deficiency may relate to glucose intolerance, altered insulin secretion and type 2 diabetes.[12] However, the reports on the association between vitamin D level and GDM were controversial.
Inflammation, especially chronic inflammation, is a state of persistent secretion of cytokines and chemokines, which interferes with normal metabolism including disrupting insulin signaling.[12] Chronic low-grade inflammation is linked to many risk factors of GDM.[6] Vitamin D is converted to 25(OH)D in the liver, which is further converted to 1,25(OH)2D3 and 24,25(OH)2D3, the active form of Vitamin D with biological activity, in the kidney. Interestingly, 1,25(OH)2D3 reduces inflammation through multiple mechanism and serum 25(OH)D has been found to have cross sectional relationships with some inflammation markers such as C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α).[13] We wonder what is the relationship between 25(OH)D and inflammation in GDM. However, such reports are limited.
In this study, we performed an observational cross-sectional study including 210 pregnant women (110 cases GDM and 100 cases normoglycemic women) and investigated the levels of 25(OH)D, inflammation markers and glucose and fat metabolism indexes in these participants. Our results confirmed the association between 25(OH)D level and GDM and further expanding the literature by showing the roles of 25(OH)D, inflammation markers as well as glucose & fat metabolism indexes in the risk of developing GDM.
2. Materials and methods
2.1. Study participants
This is an observational cross-sectional study. It was performed among the pregnant women who underwent routine prenatal examination and delivered in the First People's Hospital of Lianyungang City, which is a tertiary hospital, from October 2016 to December 2018. The inclusion criteria were Han nationality, singleton pregnancy, aged 18 to 40 years, showing normal results of routine blood tests, routine urine tests, coagulation tests, liver & kidney function check (blood test) and electrocardiogram test. The exclusion criteria were history of diabetes, hypertension during pregnancy, thyroid disease, premature rupture of membranes, autoimmune disease and taking hormone therapy. For the diagnosis of GDM, 75 g Oral Glucose Tolerance Test (OGTT) is performed in the morning after overnight fast of > 8 hours in the pregnant women after 24 weeks of gestation. GDM was confirmed if at least one of the three glucose level results exceeded the following: fasting < 5.1 mmol/L, 1 h < 10.0 mmol/L and 2 h < 8.5 mmol/L.
The participants were included in this study after their admittance to maternity ward in the First People's Hospital of Lianyungang City. One hundred ten pregnant women with GDM who met all the inclusion criteria and did not meet any item of the exclusion criteria were included in the study group (GDM group). These women did not receive insulin therapy during pregnancy. One hundred healthy pregnant women without GDM were randomly selected as the control. A written informed consent was obtained from each participant after explaining the procedure of the study. This study was approved by the local ethics committee (No. YJ-20160926001).
2.2. Anthropometric measurements
Each participant's anthropometric and demographic data was recorded. We obtained the information on the age, gestational age, pregnancy and birth history, pre-pregnancy weights, history of pregestational diabetes mellitus, family history of diabetes and education through face to face interview. Weight was measured with light clothing and without shoes, while height was measured using pointer body weight scale with height meter (RGZ-120-RT, Wuxi Xiheng). Body mass index (BMI) was calculated as Weight (kg) / [Height (m) × Height (m)]. The amount of weight gain during pregnancy was calculated as current weight (kg) − pre-pregnancy weight (kg).
2.3. Blood collection
We collected blood samples from all participants at indicated time points after overnight fasting. The blood samples were centrifuged. Then serum was separated and stored at -80°C until analysis. Hemoglobin A1c (HbA1c) was measured in whole blood collected in an Ethylene Diamine Tetraacetic Acid (EDTA)-2K tube.
2.4. Blood sampling analyses
Serum 25(OH)D concentration was determined using electrochemiluminescence binding assay in Cobas E601 mass analyzer (Roche Diagnostics). The method had a sensitivity below 3.0 ng/ml and an analytic range to 70.0 ng/ml. Based on Institute of Medicine (IoM) criteria, Vitamin D status was categorized as sever deficiency (< 10 ng/ml), deficiency (10–20 ng/ml) and insufficiency (20–30 ng/ml).[14] Serum Hs-CRP, cholesterol and triglycerides concentrations were measured by immunoturbidimetry on the IMMAGE 800 System (Beckman Coulter, Brea, CA). The method had a sensitivity below 0.1 mg/l and an analytic range to 8.0 mg/l for Hs-CRP, an analytic range from 0 to 12.9 mmol/l for cholesterol and an analytic range from 0 to 0∼11.3mmol/l for triglycerides. Serum TNF-α concentration was detected with Human TNF-α enzyme-linked immunosorbent assay (ELISA) Kit (Absin (Shanghai) Biotechnology Co., Ltd, China). The method had a sensitivity below 0.68 pg/ml. Blood glucose level was monitored using Glucose oxidase method on VITROS 350 Chemistry System (Johnson & Johnson, USA), which had an analytic range from 1.00 mmol/l to 34.69 mmol/l. Serum insulin was determined using electrochemiluminescence binding assay in Cobas E601 mass analyzer (Roche Diagnostics). The method had sensitivity below 0.2 uIU/ml and an analytic range to 1000.0uIU/ml. HbA1c level was detected using electrochemiluminescence assay on D-10 Hemoglobin Testing System (Bio-Rad, USA). The method had sensitivity below 3.8% and an analytic range to 18.5%. Homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated as fasting plasma glucose level (mmol/L) × fasting serum insulin (mIU/L)/22.5.
2.5. Statistical analysis
Normally distributed quantitative data were presented as mean ± standard deviation (SD) and compared using independent sample T-test. If the variances were unequal, T’-test was applied. Non-normally distributed quantitative data were presented as median and interquartile range (quartile 1-quartile 3) and compared with nonparametric Mann-Whitney U test. Qualitative data were expressed as numbers & proportions and compared with χ2 or Fisher exact probability methods. As to pairwise comparison, P value was corrected by Bonferroni method. The Spearman rank correlation test was performed to test the correlation of 25(OH)D, Hs-CRP or TNF-α level with other Variables. Risk factors of GDM were analyzed using binary logistic regression model. SPSS (Statistical Package for the Social Sciences) version 25.0 was used for statistical analyses and P < .05 was considered statistically significant.
3. Results
3.1. Anthropometric and demographic data
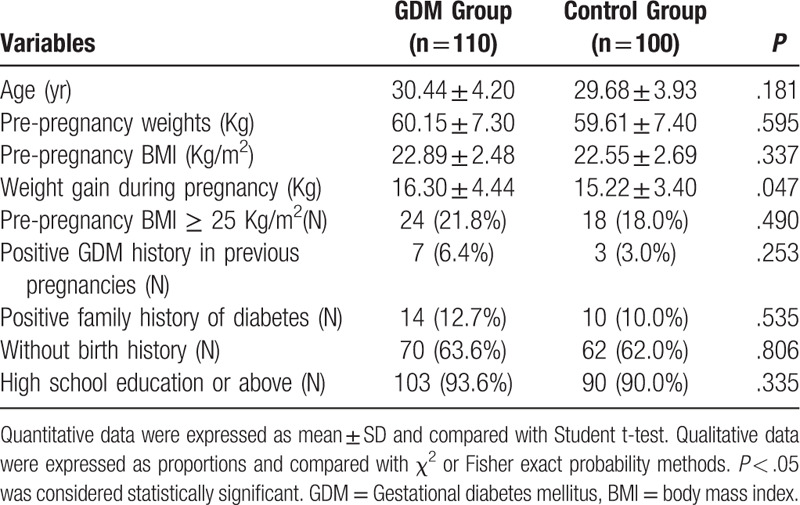
This study included 210 pregnant women (GDM group, 110 women; control group, 100 women). Anthropometric and demographic data of all the participants were shown in Table 1. There were no significant differences in age, pre-pregnancy weights, pre-pregnancy BMI, pregnancy & birth history, history of pregestational diabetes mellitus, family history of diabetes and education between GDM group and the control group. The amount of weight gain during pregnancy was significantly higher in the GDM group than that in the control group (16.30 ± 4.44 kg vs 15.22 ± 3.40 kg, P < .05).
Table 1.
Anthropometric and demographic data of GDM group and the control group.
3.2. Characteristics of 25(OH)D level, inflammatory markers and glucose and fat metabolism indexes
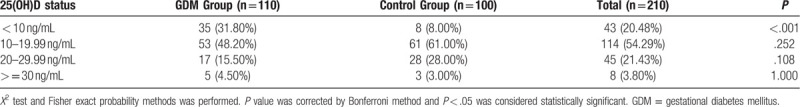
Data for 25(OH)D level, inflammatory markers and glucose & fat metabolism indexes of the GDM group and the control group were shown in Table 2. Inflammatory markers and glucose and fat metabolism indexes, including hs-CRP, TNF-α, fasting blood glucose, fasting blood insulin, HbA1c, HOMA-IR, cholesterol and triglycerides were all significantly higher in the GDM group than that in the control group. Serum median (quartile 1-quartile 3) 25(OH)D level in the GDM group was 13.90 (9.06–18.64) ng/ml, which was significantly lower than that in the control group (17.50 (12.64–22.78) ng/ml, P < .001). According to Institute of Medicine (IoM) criteria, serum 25(OH)D levels were categorized as sever deficiency (< 10 ng/ml), deficiency (10–20 ng/ml) and insufficiency (20–30 ng/ml).[14] In this study, serum 25(OH)D levels in most majorities of the participants in both the GDM group (48.20%) and the control group (61.00%) were 10 to 20 ng/ml. Furthermore, there was a 31.80% prevalence of sever deficiency of serum 25(OH)D level (<10 ng/ml) in the GDM group, while only 8.00% prevalence in the control group (Table 3).
Table 2.
25(OH)D level, inflammatory markers and glucose & fat metabolism indexes of GDM group and the control group.
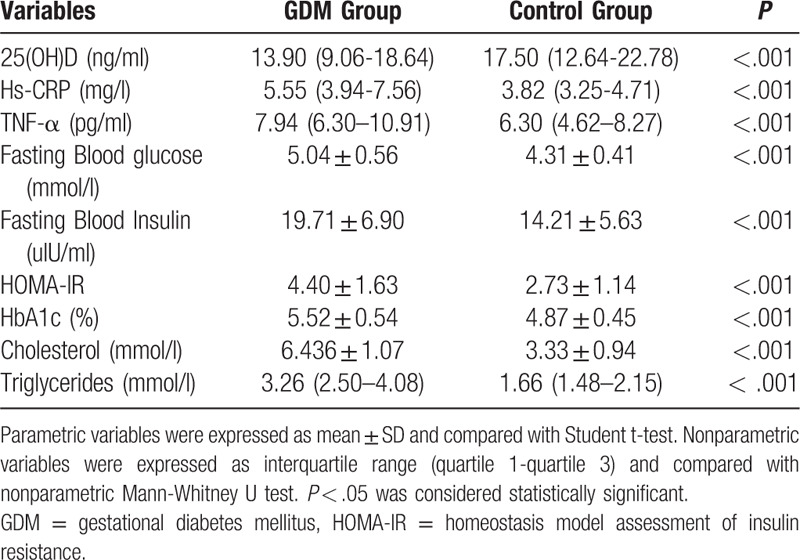
Table 3.
Distribution of 25(OH)D level.
3.3. Correlation of 25(OH)D and inflammatory markers with other variables
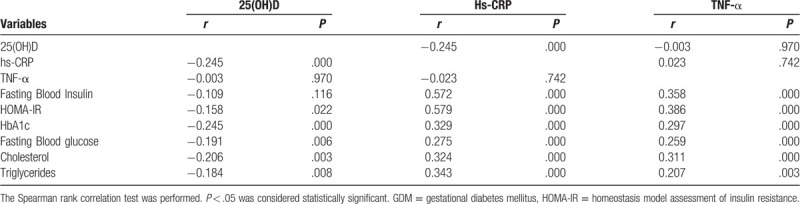
We investigated the correlation of 25(OH)D and inflammatory markers with other variables. As shown in Table 4, serum 25(OH)D levels were negatively correlated with hs-CRP, while not with TNF-α. The correlation of TNF-α with neither 25(OH)D nor hs-CRP was significant. As to glucose & fat metabolism indexes, both hs-CRP and TNF-α were positively correlated with all the glucose & fat metabolism indexes detected. Serum 25(OH)D levels were negatively correlated with fasting blood glucose, HbA1c, HOMA-IR, Cholesterol and triglycerides, while not with fasting blood insulin.
Table 4.
Correlation of 25(OH)D, Hs-CRP or TNF-α level with other Variables.
3.4. Effects of 25(OH)D and inflammatory markers on the risk of developing GDM
We investigated the effects of 25(OH)D and inflammatory markers on the risk of developing GDM. First, univariate binary logistic regression analysis of 25(OH)D, hs-CRP or TNF-α alone was conducted. The results showed that lower 25(OH)D levels were associated with increased risk of developing GDM (OR = 0.934; 95% CI 0.896–0.974; P = .001), higher hs-CRP levels were associated with increased risk of developing GDM (OR = 1.627; 95% CI 1.357–1.951; P < .001) and higher TNF-α levels were associated with increased risk of developing GDM (OR = 1.259; 95% CI 1.134–1.398; P < .001).
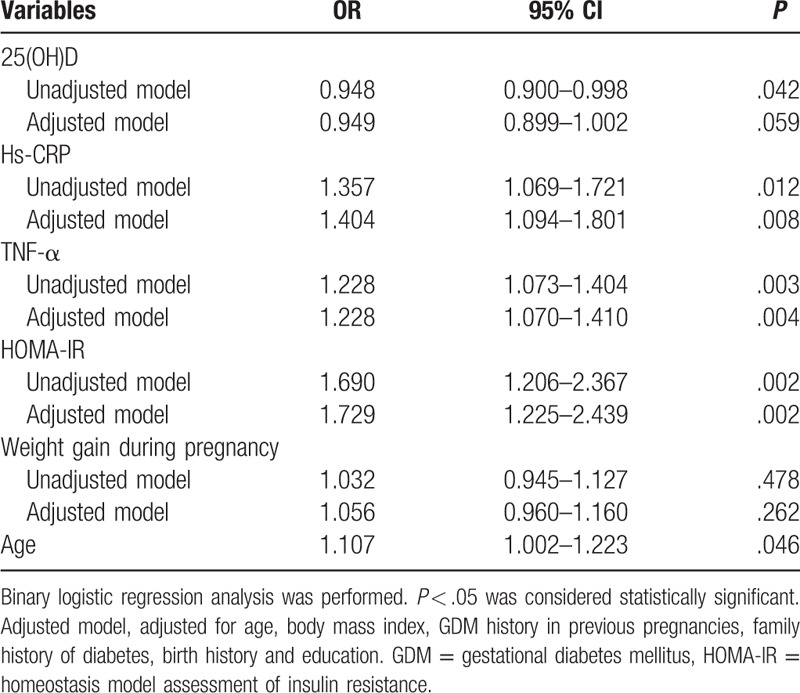
Then we established the multivariable binary logistic regression models with multiple variables including 25(OH)D, hs-CRP, TNF-α, HOMA-IR, weight gain during pregnancy and age. As shown in Table 5, lower 25(OH)D levels were associated with increased risk of developing GDM in the unadjusted model (OR = 0.948; 95% CI 0.900–0.998; P = .042). While the association was not statistically significant after adjustment for age, body mass index, GDM history in previous pregnancies, family history of diabetes, birth history and education (OR = 0.949; 95% CI 0.899–1.002; P = .059). Furthermore, higher hs-CRP, TNF-α and HOMA-IR levels were associated with increased risk of developing GDM in both the unadjusted model and the adjusted model. As to weight gain during pregnancy, the association was not statistically significant.
Table 5.
Multivariable logistic regression models using 25(OH)D and other variables.
4. Discussion
In this study, we compared the levels of 25(OH)D and inflammatory markers (hs-CRP and TNF-α) between the GDM group and the control group. Our results indicated that both hs-CRP and TNF-α were significantly higher in the GDM group than that in the control group, while 25(OH)D level was significantly lower in the GDM group. Serum 25(OH)D and hs-CRP levels were negatively correlated with each other. However, the correlation of TNF-α with neither 25(OH)D nor hs-CRP was significant. Furthermore, lower 25(OH)D levels were associated with increased risk of developing GDM, and higher hs-CRP or TNF-α levels were associated with increased risk of developing GDM.
Nowadays, the association between vitamin D level and GDM has aroused much attention. However, the conclusions are inconsistent. Some studies reported that lower 25(OH)D level was associated with elevated risk of GDM.[15–19] But some studies reported that 25(OH)D deficiency was not associated with GDM.[20–23] In a case-sectional study of 723 pregnant women, of which 97% were vitamin D sufficient [25(OH)D ≥50 nmol/L], there was no difference in 25(OH)D concentration between GDM and non-GDM group.[21] Baker et al also reported that in a cohort of pregnant women with mostly sufficient levels of serum25 (OH)D, vitamin D deficiency was not associated with GDM.[24] In this study, we found that 25(OH)D level was significantly lower in the GDM group than that in the control group and lower 25(OH)D levels were associated with increased risk of developing GDM. It should be noticed that in our study, serum median 25(OH)D levels in the GDM group and the control group were 13.90 ng/ml and 17.50 ng/ml respectively, which were categorized as deficiency (10–20 ng/ml) according to Institute of Medicine (IoM) criteria. This result reflected the low overall levels of 25(OH)D in the pregnant women enrolled in this study. It seems that as to the effects of 25(OH)D on the risk of GDM, more attention will be needed in populations with lower levels of 25(OH)D.
GDM is a metabolic disease and characterized as high blood glucose level. Accordingly, we observed increased fasting blood glucose and HbA1c in the GDM group. One of the key factors in the pathophysiology of GDM is insulin resistance. In this study, both fasting blood insulin level and HOMA-IR were significantly higher in the GDM group. It seems that increased secretion of insulin in the GDM group cannot compensate for the impaired function of insulin. It has been known that altered lipid metabolism during pregnancy is one of the causes of insulin resistance.[25] Alyas et al reported significantly higher levels of total plasma cholesterol and triglycerides in the GDM group during both the second and the third trimester.[26] In this study, we also observed increased levels of cholesterol and triglycerides in the pregnant women with GDM before delivery.
The role of inflammation in the development of GDM also aroused much attention.[6] The obesity-associated chronic inflammation plays an important role in the pathogenesis of insulin resistance, in which process, TNF-α is one of the key factors.[27,28] CRP is synthesized in response to inflammation cytokines and can be used as indicator of systematic inflammation.[29] In women with GDM, elevated TNF-α or hs-CRP levels have been reported by several studies.[26,30–33] In this study, we observed that both hs-CRP and TNF-α levels were significantly higher in the GDM group than that in the control group and they were positively correlated with all the glucose & fat metabolism indexes detected. Higher hs-CRP and TNF-α levels were associated with increased risk of developing GDM, further supporting the association of inflammation with GDM.
In general, 25(OH)D reduces inflammation through switching the immune system from more inflammatory response to the less inflammatory status.[13,34] CRP is one of the mostly used inflammatory markers to determine the inflammatory status in clinical practice.[35] In this study, lower 25(OH)D levels were associated with increased risk of developing GDM and serum 25(OH)D levels were negatively correlated with hs-CRP. It seems that the role of 25(OH)D in GDM may associate with its correlation with inflammatory status, which deserves further investigation. At a cellular level, 25(OH)D inhibits production of TNF-α in monocyte.[36] Bellia et al reported that serum 25(OH)D was inversely correlated with TNF-α in 147 morbidly obese subjects.[37] However, the study on the correlation of 25(OH)D with TNF-α in GDM was rare. In this study, we observed that serum 25(OH)D levels were not correlated with TNF-α. Fatemeh et al also reported that the relationship between 25(OH)D levels and TNF-α were not significant.[38] Thus, 25(OH)D and TNF-α may affect the risk of developing GDM through different mechanism.
There are some limitations concerning this study. First, we only collected blood samples of the participants before delivery, which could not reflect the changes throughout the pregnancy. Second, we examined the correlation of 25(OH)D levels with TNF-α and hs-CRP. More inflammation markers should be detected in the future work.
In conclusion, in this study we investigated the levels and correlation of 25(OH)D, inflammation markers as well as glucose & fat metabolism indexes in GDM in the population with the low overall levels of 25(OH)D before delivery. This broadens our knowledge on the pathophysiology of GDM, which may be helpful in prevention and treatment of GDM.
Author contributions
Conceptualization: Yaqiong Liu, Guohua Wang.
Methodology: Yaqiong Liu.
Project Administration: Wei Lu.
Formal analysis: Yaqiong Liu.
Investigation: Yaqiong Liu, Dan Shi, Yi Zhang.
Funding Acquisition: Yaqiong Liu.
Resources: Fuyan Yang, Dan Shi, Yi Zhang.
Data Curation: Fuyan Yang, Dan Shi, Yi Zhang.
Supervision: Guohua Wang, Wei Lu.
Validation: Guohua Wang.
Visualization: Guohua Wang.
Writing-Original Draft: Yaqiong Liu.
Writing-Review & Editing: Guohua Wang, Fuyan Yang, Wei Lu.
Footnotes
Abbreviations: GDM = gestational diabetes mellitus, hs-CRP = high sensitivity C-reactive protein, IL-6 = interleukin-6, TNF-α = tumor necrosis factor-alpha, HbA1c = Hemoglobin A1c, HOMA-IR = homeostasis model assessment of insulin resistance, OGTT = Oral Glucose Tolerance Test, BMI = Body mass index, EDTA = Ethylene Diamine Tetraacetic Acid, Institute of Medicine (IoM), ELISA = enzyme-linked immunosorbent assay, SD = standard deviation, SPSS = Statistical Package for the Social Sciences.
How to cite this article: Yaqiong L, Guohua W, Fuyan Y, Wei L, Dan S, Yi Z. Study on the levels of 25(OH)D, inflammation markers and glucose and fat metabolism indexes in pregnant women of Han nationality in Jiangsu province with gestational diabetes mellitus. Medicine. 2020;99:35(e21654).
The authors report no conflicts of interest.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Feng H, Zhu W-W, Yang H-X, et al. Relationship between oral glucose tolerance test characteristics and adverse pregnancy outcomes among women with gestational diabetes mellitus. Chin Med J 2017;130:1012–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].American Diabetes Association Gestational Diabetes Mellitus. Am Diabetes Assoc Diab Care 2002;25:s94–6.. [Google Scholar]
- [3].Perovic M, Gojnic M, Arsic B, et al. Relationship between mid-trimester ultrasound fetal liver length measurements and gestational diabetes mellitus. J Diabetes 2015;7:497–505.. [DOI] [PubMed] [Google Scholar]
- [4].Perović M, Garalejić E, Gojnić M, et al. Sensitivity and specificity of ultrasonography as a screening tool for gestational diabetes mellitus. J Matern Fetal Neonatal Med 2012;25:1348–53.. [DOI] [PubMed] [Google Scholar]
- [5].Zhu WW, Yang H-x, Wei Y-m, et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in China. Diabetes Care 2013;36:586–90.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Khambule L, George JA. The role of inflammation in the development of GDM and the use of markers of inflammation in GDM screening. In: Guest P. (eds) Reviews on Biomarker Studies of Metabolic and Metabolism-Related Disorders. Adv Exp Med Biol. 2019;28:1134. [DOI] [PubMed] [Google Scholar]
- [7].Zhang C, Rawal S, Chong YS. Risk factors for gestational diabetes: is prevention possible? Diabetologia 2016;59:1385–90.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Moon RJ, Harvey NC, Cooper C. Endocrinology In Pregnancy: Influence of maternal vitamin D status on obstetric outcomes and the fetal skeleton. Eur J Endocrinol 2015;173:R69–83.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mousa A, Abell S, Scragg R, et al. Vitamin D in reproductive health and pregnancy. Semin Reprod Med 2016;34:e1–3.. [DOI] [PubMed] [Google Scholar]
- [10].Sacerdote A, Dave P, Lokshin V, et al. Type 2 diabetes mellitus, insulin resistance, and Vitamin D. Curr Diabetes Rep 2019;19:101. [DOI] [PubMed] [Google Scholar]
- [11].Bivona G, Lo Sasso B, Iacolino G, et al. Standardized measurement of circulating vitamin D [25(OH)D] and its putative role as a serum biomarker in Alzheimer's disease and Parkinson's disease. Clin Chim Acta 2019;497:82–7.. [DOI] [PubMed] [Google Scholar]
- [12].Chagas CEA, Borges MC, Martini LA, et al. Focus on Vitamin D, inflammation and type 2 diabetes. Nutrients 2012;4:52–67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cannell JJ, Grant WB, Holick MF. Vitamin D and inflammation. Dermato-Endocrinol 2014;6:e983401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Olmos-Ortiz A, Avila E, Durand-Carbajal M, et al. Regulation of calcitriol biosynthesis and activity: focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients 2015;7:443–80.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rajput R, Vohra S, Nanda S, et al. Severe 25(OH)VITAMIN-D deficiency: a risk factor for development of gestational diabetes mellitus. Diabetes Metab Syndr Clin Res Rev 2019;13:985–7.. [DOI] [PubMed] [Google Scholar]
- [16].Xia J, Song Y, Rawal S, et al. Vitamin D status during pregnancy and the risk of gestational diabetes mellitus: a longitudinal study in a multiracial cohort. Diabetes Obes Metabol 2019;8:1895–905.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ede G, Keskin U, Cemal Yenen M, et al. Lower vitamin D levels during the second trimester are associated with developing gestational diabetes mellitus: an observational cross-sectional study. Gynecol Endocrinol 2019;35:525–8.. [DOI] [PubMed] [Google Scholar]
- [18].Al-Ajlan A, Al-Musharaf S, Fouda MA, et al. Lower vitamin D levels in Saudi pregnant women are associated with higher risk of developing GDM. Bmc Pregnancy Childbirth 2018;18:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xu C, Ma H-h, Wang Y. Maternal early pregnancy plasma concentration of 25-Hydroxyvitamin D and risk of gestational diabetes mellitus. Calcif Tissue Int 2018;102:280–6.. [DOI] [PubMed] [Google Scholar]
- [20].Ruth ES, Wiegels WC, Line SL, et al. Vitamin D, gestational diabetes, and measures of glucose metabolism in a population-based multiethnic cohort. J Diabetes Res 2018;2018:8939235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hauta-Alus HH, Viljakainen HT, Holmlund-Suila EM, et al. Maternal vitamin D status, gestational diabetes and infant birth size. Bmc Pregnancy Childbirth 2017;17:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Piotr D, Pawel S, Grazyna O-S, et al. Serum 25(OH) Vitamin D levels in polish women during pregnancies complicated by hypertensive disorders and gestational diabetes. Int J Mol Sci 2016;17:1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Al-Shaikh GK, Ibrahim GH, Fayed AA, et al. Impact of vitamin D deficiency on maternal and birth outcomes in the Saudi population: a cross-sectional study. Bmc Pregnancy Childbirth 2016;16:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Arthur M, Sina H, Carlos A, et al. First-trimester maternal vitamin D status and risk for gestational diabetes (GDM) a nested case-control study. Diabetes/Metab Res Rev 2011;28:164–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Taschereau-Charron A, Da Silva MS, Bilodeau J-F, et al. Alterations of fatty acid profiles in gestational diabetes and influence of the diet. Maturitas 2017;99:98–104.. [DOI] [PubMed] [Google Scholar]
- [26].Alyas S, Roohi N, Ashraf S, et al. Early pregnancy biochemical markers of placentation for screening of gestational diabetes mellitus (GDM). Diabetes Metab Syndr 2019;13:2353–6.. [DOI] [PubMed] [Google Scholar]
- [27].Borst SE. The role of TNF-α in insulin resistance. Endocrine 2004;23:177–82.. [DOI] [PubMed] [Google Scholar]
- [28].Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm 2013;2013:139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mugabo Y, Li L, Renier G. The connection between C-reactive protein (CRP) and diabetic vasculopathy. focus on preclinical findings. Curr Diabetes Rev 2010;6:27–34.. [DOI] [PubMed] [Google Scholar]
- [30].Salmi AA, Zaki NMN, Zakaria R, et al. Arterial stiffness, inflammatory and pro-atherogenic markers in gestational diabetes mellitus. J Vasc Dis 2012;41:96–104.. [DOI] [PubMed] [Google Scholar]
- [31].Gao XL, Yang HX, Zhao Y. Variations of tumor necrosis factor-α, leptin and adiponectin in mid-trimester of gestational diabetes mellitus. Chin Med J 2008;121:701–5.. [PubMed] [Google Scholar]
- [32].Guillemette L, Lacroix M, Battista MC, et al. TNFalpha dynamics during the oral glucose tolerance test vary according to the level of insulin resistance in pregnant women. J Clin Endocrinol Metabol 2014;99:1862–9.. [DOI] [PubMed] [Google Scholar]
- [33].Alamolhoda SH, Yazdkhasti M, Namdari M, et al. Association between C-reactive protein and gestational diabetes: a prospective study. J Obstetr Gynaecol 2020;40:349–53.. [DOI] [PubMed] [Google Scholar]
- [34].Danescu LG, Levy S, Levy J. Vitamin D and diabetes mellitus. Endocrine 35:11–7.. [DOI] [PubMed] [Google Scholar]
- [35].Eklund CM. Proinflammatory cytokines in CRP baseline regulation. Adv Clin Chem 2009;48:111–36.. [DOI] [PubMed] [Google Scholar]
- [36].Aranow C. Vitamin D and the immune system. J Investig Med 2011;59:881–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bellia A, Garcovich C, D’Adamo M, et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern Emerg Med 2013;8:33–40.. [DOI] [PubMed] [Google Scholar]
- [38].Haidari F, Jalali MT, Shahbazian N, et al. Comparison of serum levels of Vitamin D and inflammatory markers between women with gestational diabetes mellitus and healthy pregnant control. J Family Reprod Health 2016;10:1–8.. [PMC free article] [PubMed] [Google Scholar]


