Abstract
Several studies have reported short-term results for post-cholecystectomy symptoms and quality of life (QoL). However, reports on long-term results are still limited. This study aimed to identify risk factors affecting short- and long-term patient-reported outcome (PRO) following laparoscopic cholecystectomy.
From 2016 to 2017, a total of 476 patients from 5 institutions were enrolled. PRO was examined using the Numeric Rating Scale (NRS) pain score and the Gastrointestinal (GI) QoL Index questionnaire at postoperative 1 month and 1 year.
Most of patients recovered well at postoperative 1 year compared to postoperative 1 month for the NRS pain score, QoL score, and GI symptoms. A high operative difficulty score (HR 1.740, P = .031) and pathology of acute or complicated cholecystitis (HR 1.524, P = .048) were identified as independent risk factors for high NRS pain scores at postoperative 1 month. Similarly, female sex (HR 1.571, P = .003) at postoperative 1 month and postoperative complications (HR 5.567, P = .001) at postoperative 1 year were independent risk factors for a low QoL. Also, age above 50 (HR 1.842, P = .001), female sex (HR 1.531, P = .006), and preoperative gallbladder drainage (HR 3.086, P = .001) were identified as independent risk factors for GI symptoms at postoperative 1 month.
Most patients showed improved long-term PRO measurement in terms of pain, QoL, and GI symptoms. There were no independent risk factors for long-term postoperative pain and GI symptoms. However, postoperative complications were identified to affect QoL adversely at postoperative 1 year. Careful and long-term follow up is thus necessary for patients who experienced postoperative complications.
Keywords: laparoscopic cholecystectomy, patient-reported outcome, quality of life
1. Introduction
Since its introduction in 1986, laparoscopic cholecystectomy (LC) has become more widely used and is now considered the treatment of choice for various gallbladder (GB) diseases.[1–5] However, after cholecystectomy, patients often experience various symptoms from the immediate postoperative period to even years after, which can independently predict changes in prognosis, quality of life (QoL), and functional status.[1,6–11] As a result, it is important to recognize that patient-reported outcomes (PRO) incorporate postoperative pain, and various gastrointestinal (GI) symptoms in addition to QoL.[12–14] The Gastrointestinal Quality of Life Index (GIQLI) is 1 of the most widely used questionnaires for the objective measurement of QoL in GI surgery, and its use is validated in gallstone disease.[13,15–17] The European Association for Endoscopic Surgery also recommends the GIQLI questionnaire for the evaluation of QoL for GB disease[18]; thus, it should be utilized as a vital measure of outcome for studies on cholecystectomy. There have been various reports that anatomical factors may contribute to PRO and these include sphincter of Oddi dysfunction,[19,20] cystic-duct remnant neuroma,[21] and retained cystic-duct remnant calculi.[22] However, there is limited and inconsistent information available about PRO in these patients.[23,24] For these reasons, the clinical management of these patients is frequently without an evidence-based approach. The purpose of this prospective multicenter observational study is to analyze which factors have the greatest impact on short- and long-term PRO including various postoperative symptoms and QoL using the GIQLI questionnaire following cholecystectomy.
2. Methods
2.1. Patients
Patients over 18 years with suspected GB diseases were evaluated at the outpatient clinic or emergency room. After a thorough examination that included a physical examination, laboratory testing, and abdominal imaging such as ultrasonography, or a computed tomography scan, the patients who were diagnosed with symptomatic gallstone disease, cholecystitis, GB polyp, or early GB cancer with LC were enrolled. Patients who underwent combined surgery with other gastrointestinal organs were excluded, as were patients with planned radical cholecystectomy for GB cancer, or those with lack of informed consent. All the operations were conducted by experienced biliary surgeons. Operative difficulty was assessed using GB adhesion, distension or contraction, access, severe sepsis or complication, and time to identify the cystic artery or duct.[25] Postoperative complications consisted of bile duct injury (BDI), bleeding, surgical site infection, fluid collection or biloma, bile leak, bile duct obstruction, or bowel injury. LC was performed by single or multiport methods at all institutes.
2.2. Study design
This prospective multicenter observational study evaluated risk factors affecting short- and long-term PRO after LC. Between October 2016 to March 2017, a total of 3,002 patients in 18 institutions were screened for eligibility for the Korea Surgical Improvement Program. Among them, 496 consecutive patients were observed prospectively at 5 tertiary referral centers which were Samsung Medical Center (SMC), Ewha Womans University Hospital, Chung-Ang University Hospital, Seoul National University Hospital, and Dongguk University Ilsan Hospital in South Korea. After exclusion of 20 patients who refused the survey, the results from 476 patients were placed into final analysis (Fig. 1). The Institutional Review Board at each hospital approved the study protocol (SMC No. 2013–10-122-001). This study was also registered under clinicaltrials.gov (NCT02983474) as a part of Korea Surgical Improvement Program before patient recruitment commenced.
Figure 1.
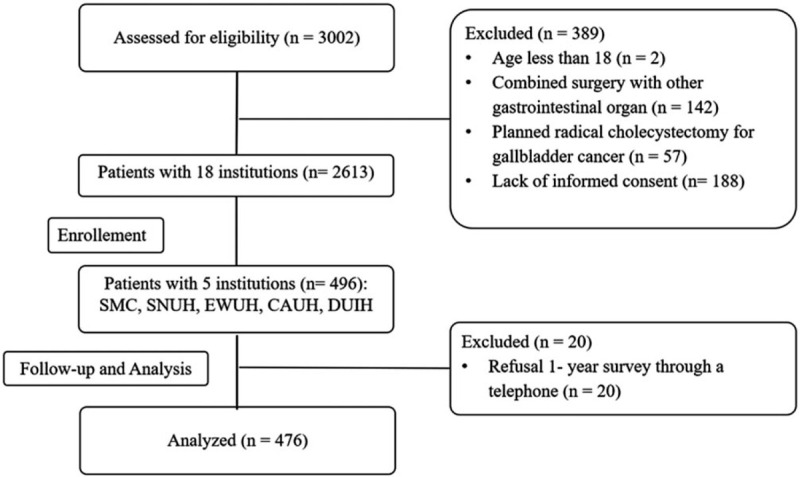
Patients flow according to STROBE statement. CAUH = Chung-Ang University Hospital, DUIH = Dongguk University Ilsan Hospital, EWUH = Ewha Womans University Hospital, SMC = Samsung Medical Center, SNUH = Seoul National University Hospital.
2.3. Patient- reported outcome measurements
PROs were evaluated with postoperative pain using the Numeric Rating Scale (NRS) pain score and GI symptoms and QoL using the GIQLI questionnaire at postoperative 1 and 12 months. The survey of PRO at postoperative 1 month was performed at the outpatients’ clinic whereas the survey at postoperative 1 year was performed through a telephone. The NRS pain score is a segmented numeric version of the visual analog scale in which a respondent selects a whole number (0–10 integers) that best reflects the intensity of their pain.[26,27] GIQLI is an instrument that was designed in the early 1990 s by Eypasch, et al[28] to assess health-related QoL in clinical studies of GI disease and in daily clinical practice. The GIQLI questionnaire for GI symptoms consisted of 19 questions. Each question consists of 5 response categories. Questions are scored using a response scale ranging from 0 (worst appraisal) to 4 (best appraisal) points for each question. The questionnaires were self-administered, and patients were given privacy and time to complete the survey. A trained nurse was available for patients that required help in completing the surveys. Outcomes were assessed prospectively by dedicated study nurses who submitted the data to a web-based database (MDB, Seoul, KOR).
2.4. Risk factor analysis for patients- reported outcomes
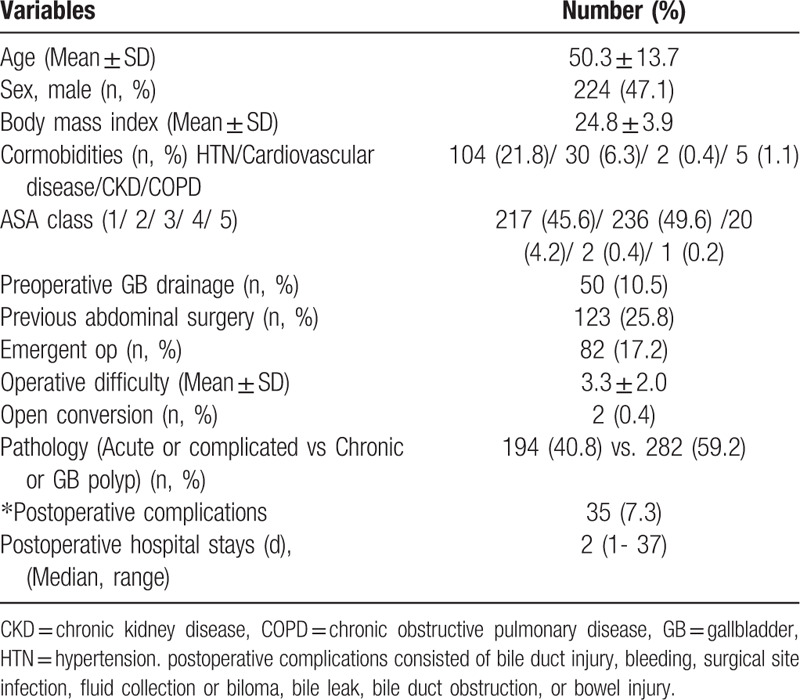
All of the study population was analyzed according to age, sex, body mass index, preoperative percutaneous transhepatic GB drainage (PTGBD), previous abdominal surgery, emergent surgery, operative difficulty, pathology, postoperative complications, and postoperative hospital stay for evaluating short- and long-term pain, QoL, and GI symptoms (Table 1).
Table 1.
Patients characteristics of enrolled patients.
2.5. Statistical analysis
The data were analyzed using SPSS ver. 25.0 (SPSS, Chicago, IL). Continuous and normally distributed variables are presented as the mean ± standard deviation. Continuous parameters in each group were compared using the independent t-test, and categorical parameters using the Chi-square test or Fisher exact test. Multivariate analysis was performed using a proportional hazards regression model including a 95% confidence interval (CI) and P- value. P-values of .05 or less were considered statistically significant.
3. Results
3.1. Patient characteristics and overall status of PRO measurement
The mean age of the study population was 50.3 years, and women- to men ratio was 1.14: 1. The mean operative difficulty score was 3.3 ± 2.0, and median postoperative hospital stays were 2 days (range 1–37 days) (Table 1). Most of the patients reported improved long-term PRO measurement compared to postoperative 1 months in terms of NRS pain score (.51 vs 1.80, P = .004), QoL score (4.15 vs. 2.50, P < .001), and GI symptoms (88.1 vs 82.1, P = .012) (Fig. 2).
Figure 2.
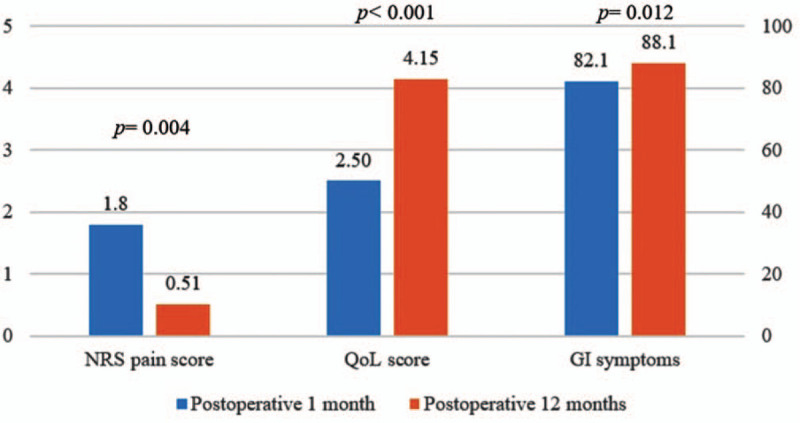
Overall status of patients reported outcomes. GI = gastrointestinal, NRS = Numeric Rating Scale, QoL = quality of life.
3.2. NRS pain score
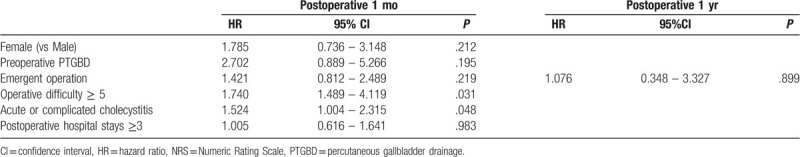
Based on the NRS pain score at postoperative 1 month, women, preoperative PTGBD insertion, emergent operation, high operative difficulty score, pathology of acute or complicated cholecystitis, and a longer hospital stay were identified as short-term risk factors for postoperative pain after univariate analysis (Table 2). Similarly, emergent operation was the only risk factor for long-term follow-up of pain (Table 2). After multivariate analysis, high operative difficulty score (HR 1.740, 95% CI 1.489- 4.119, P = .031) and pathology of acute or complicated cholecystitis (HR 1.524, 95% 1.004–2.315, P = .048) were identified as independent risk factors for NRS pain score at postoperative 1 month (Table 3). No independent risk factor was identified for long-term follow-up of pain (Table 3).
Table 2.
The NRS pain score at postoperative 1 and 12 mo.
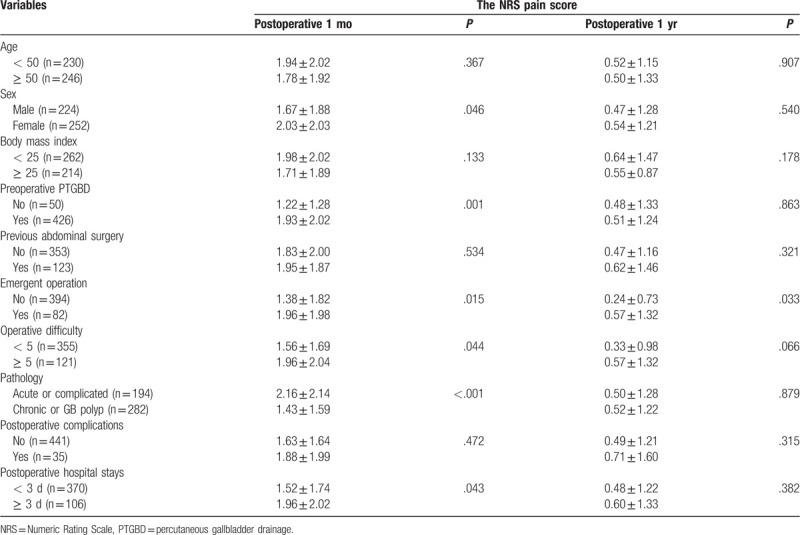
Table 3.
Multivariate risk factor analysis of pain.
3.3. QoL score using the GIQLI questionnaire
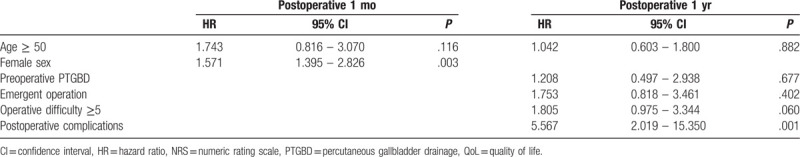
After univariate analysis, older age and women were identified as risk factors for QoL at postoperative 1month, whereas older age, preoperative PTGBD, emergent operation, high operative difficulty score, and postoperative complications were risk factors at postoperative 12 months (Table 4). Multivariate analysis revealed that female sex (HR 1.571, 95% CI 1.395- 2.826, P = .003) at postoperative 1 month and postoperative complications (HR 5.567, 95% CI 2.019- 15.350, P = .001) at postoperative 1 year were independent risk factors for lower QoL (Table 5).
Table 4.
QoL score and gastrointestinal symptoms using GIQLI questionnaire.
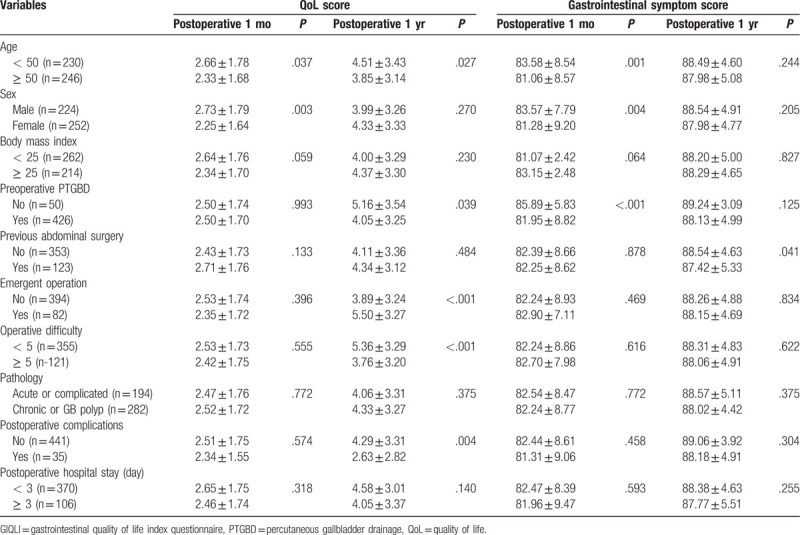
Table 5.
Multivariate risk factor analysis of QoL.
3.4. Gastrointestinal symptoms using the GIQLI questionnaire
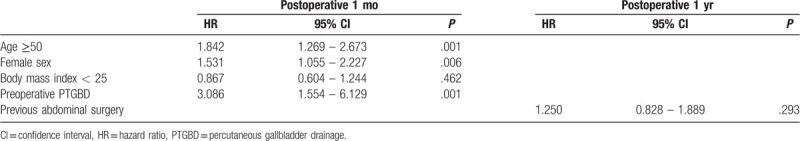
Similarly, older age, female sex, and preoperative PTGBD were risk factors for GI symptoms at postoperative 1 month, and a history of previous abdominal surgery is a risk factor for long-term follow-up (Table 4). Also, age over 50 (HR 1.842, 95% CI 1.269- 2.673, P = .001), female sex (HR 1.531, 95% CI 1.055–2.227, P = .006), and preoperative GB drainage (HR 3.086, HR 1.554- 6.129, P = .001) were identified as independent risk factors for GI symptoms at postoperative 1 month (Table 6). No independent risk factor was identified for gastrointestinal symptoms at postoperative 1 year (Table 6).
Table 6.
Multivariate risk factor analysis of gastrointestinal symptoms.
4. Discussion
When LC is recommended, many patients wonder about the relief of their symptoms including pain and the occurrence of new symptoms after removing the GB.[29,30] As a result, PRO measurement has been useful as a significant determinant of patient satisfaction following cholecystectomy.[12,13] The GIQLI is 1 of the most widely used and validated questionnaires for the objective measurement of QoL including GB disease.[13,15–17] In this prospective multicenter study, most patients showed improved long-term PRO measurement of NRS pain score, QoL score, and GI symptoms using the GIQLI questionnaire compared to short-term PRO measurement (Fig. 2). This positive result following cholecystectomy is consistent with previous published literature.[11,12,24,31] In the case of severe complication such as BDI, several studies reported that extremely long-term follow-up of 8 to 12 years was needed to improve QoL after cholecystectomy.[14,32,33] Also, other studies suggested that the occurrence of a BDI has a great impact on the patient's physical and mental QoL even after excellent functional outcome following repair.[34,35] In this study, postoperative complication including BDI was identified as the only independent risk factor to affect QoL adversely at postoperative 1 year after LC (Tables 4 and 5). As a result, careful and long-term follow up for more than 1 year is necessary for patients who experienced postoperative complications. Cholecystectomy is associated with several physiological changes in the upper GI tract, which may account for persistence of symptoms or worsening QoL after GB removal besides abdominal pain.[1,7,9,10,30] In this study, female sex was an independent risk factor for short-term QoL and GI symptoms after multivariate analysis (Tables 5 and 6). The prevalence of gallstones is known to be higher in female sex,[17,36] and functional causes of abdominal symptoms, such as irritable bowel syndrome,[37] are also more common among women and could possibly resemble gallstone related symptoms. It is therefore not unlikely that for a certain proportion of female patients with GB diseases, cholecystectomy might have had little or no positive effect on QoL or GI symptoms.[17] Postoperative pain is a well-known major determinant for QoL in patients with cholecystectomy, and it is not uncommon with a prevalence of 30% to 50%.[3,6–11,31] After multivariate analysis, high operative difficulty score and pathology of acute or complicated cholecystitis were identified as independent risk factors for the NRS pain score at postoperative 1 month (Tables 2 and 3). We previously reported that a score indicative of higher difficulty in performing LC, in the absence of other definite visceral organ damage, was an independent risk factor in developing short-term postoperative pain.[29] This may be because difficulty in dissection of the triangle formed by the common bile duct, cystic duct, and liver (Calot's triangle) may cause intraoperative nerve damage innervating the visceral structures.[8,29] Preoperative PTGBD has been widely used and has had the benefits of a low complication rate, being a simple operation with early symptom relief and improvements in cases of acute cholecystitis.[38,39] Despite these positive effects on the management of patients, the effects of PTGBD on operative duration and open conversion rates reflecting surgical difficulties have not been identified clearly in patients with acute cholecystitis.[39] Also, as far as we know, the relationship between preoperative PTGBD and PRO has not yet been reported. In this study, preoperative PTGBD was identified as an independent risk factors for GI symptoms at postoperative 1 month (Table 6). As a result, it is worthy to consider short-term symptomatic management in patients who have had preoperative PTGBD. This study has some potential limitations. First, the survey at postoperative 1 year was performed through the telephone which could cause recall bias. Second, in spite of being conducted in a prospective multicenter manner, the study period was relatively short and population number was not large. Thus selection bias cannot be ruled out. A future prospective nationwide study is necessary to evaluate QoL with extremely long-term follow up. In conclusion, most patients reported improved long-term PRO measurement in terms of NRS pain score, QoL score, and GI symptoms using the GIQLI questionnaire. There were no independent risk factors for long-term postoperative pain and gastrointestinal symptoms. However, postoperative complication was identified to affect QoL adversely at postoperative 1 year. Careful and long-term follow up is needed for patients who experienced postoperative complications.
Author contributions
Conceptualization: In Woong Han, Hyeon Kook Lee, Jin-Young Jang, Huisong Lee, Jin Seok Heo.
Data curation: In Woong Han, Hyeon Kook Lee, Dae Joon Park, Yoo Shin Choi, Seung Eun Lee, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Huisong Lee, Jin Seok Heo.
Formal analysis: In Woong Han, Hyeon Kook Lee, Dae Joon Park, Yoo Shin Choi, Seung Eun Lee, Hongbeom Kim, Wooil Kwon.
Funding acquisition: In Woong Han, Hyeon Kook Lee, Jin Seok Heo.
Investigation: In Woong Han, Hyeon Kook Lee, Jin Seok Heo.
Methodology: In Woong Han, Hyeon Kook Lee, Dae Joon Park, Yoo Shin Choi, Seung Eun Lee, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Huisong Lee, Jin Seok Heo.
Project administration: In Woong Han, Hyeon Kook Lee, Jin Seok Heo.
Resources: In Woong Han, Hyeon Kook Lee, Dae Joon Park, Jin-Young Jang, Jin Seok Heo.
Software: In Woong Han, Hyeon Kook Lee, Jin Seok Heo.
Supervision: In Woong Han, Hyeon Kook Lee, Dae Joon Park, Yoo Shin Choi, Seung Eun Lee, Hongbeom Kim, Wooil Kwon, Jin Seok Heo.
Validation: In Woong Han, Hyeon Kook Lee, Dae Joon Park, Jin-Young Jang, Huisong Lee, Jin Seok Heo.
Visualization: In Woong Han, Hyeon Kook Lee, Jin Seok Heo.
Writing – original draft: In Woong Han, Hyeon Kook Lee, Jin Seok Heo.
Writing – review and editing: In Woong Han, Hyeon Kook Lee, Dae Joon Park, Yoo Shin Choi, Seung Eun Lee, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Huisong Lee, Jin Seok Heo.
Footnotes
Abbreviations: BDI = bile duct injury, GB = gallbladder, GIQLI = Gastrointestinal Quality of Life Index, LC = laparoscopic cholecystectomy, NRS = numeric rating scale, PRO = patient-reported outcome, PTGBD = preoperative percutaneous transhepatic gallbladder drainage, QoL = quality of life.
How to cite this article: Han IW, Lee HK, Park DJ, Choi YS, Lee SE, Kim H, Kwon W, Jang JY, Lee H, Heo JS. Long-term patient-reported outcomes following laparoscopic cholecystectomy: a prospective multicenter observational study. Medicine. 2020;99:35(e21683).
IH and HL equally contributed to this article.
All statistical analysis of this study was performed by statistician at Samsung Medical Information and Media Services at Samsung Medical Center. This study was performed with a grant from Phambio Korea Co., Ltd.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Jaunoo SS, Mohandas S, Almond LM. Postcholecystectomy syndrome (PCS). Int J Surg 2010;8:15–7.. [DOI] [PubMed] [Google Scholar]
- [2].McPherson K, Wennberg JE, Hovind OB, et al. Small-area variations in the use of common surgical procedures: an international comparison of New England, England, and Norway. N Engl J Med 1982;307:1310–4.. [DOI] [PubMed] [Google Scholar]
- [3].Lirici MM, Califano AD, Angelini P, et al. Laparo-endoscopic single site cholecystectomy versus standard laparoscopic cholecystectomy: results of a pilot randomized trial. Am J Surg 2011;202:45–52.. [DOI] [PubMed] [Google Scholar]
- [4].Reynolds W., Jr The first laparoscopic cholecystectomy. JSLS 2001;5:89–94.. [PMC free article] [PubMed] [Google Scholar]
- [5].Tiong L, Oh J. Safety and efficacy of a laparoscopic cholecystectomy in the morbid and super obese patients. HPB (Oxford) 2015;17:600–4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Okoro N, Patel A, Goldstein M, et al. Ursodeoxycholic acid treatment for patients with postcholecystectomy pain and bile microlithiasis. Gastrointest Endosc 2008;68:69–74.. [DOI] [PubMed] [Google Scholar]
- [7].Filip M, Saftoiu A, Popescu C, et al. Postcholecystectomy syndrome - an algorithmic approach. J Gastrointestin Liver Dis 2009;18:67–71.. [PubMed] [Google Scholar]
- [8].Blichfeldt-Eckhardt MR, Ording H, Andersen C, et al. Early visceral pain predicts chronic pain after laparoscopic cholecystectomy. Pain 2014;155:2400–7.. [DOI] [PubMed] [Google Scholar]
- [9].Lamberts MP, Lugtenberg M, Rovers MM, et al. Persistent and de novo symptoms after cholecystectomy: a systematic review of cholecystectomy effectiveness. Surg Endosc 2013;27:709–18.. [DOI] [PubMed] [Google Scholar]
- [10].Berger MY, Olde Hartman TC, Bohnen AM. Abdominal symptoms: do they disappear after cholecystectomy? Surg Endosc 2003;17:1723–8.. [DOI] [PubMed] [Google Scholar]
- [11].Finan KR, Leeth RR, Whitley BM, et al. Improvement in gastrointestinal symptoms and quality of life after cholecystectomy. Am J Surg 2006;192:196–202.. [DOI] [PubMed] [Google Scholar]
- [12].Lamberts MP, Den Oudsten BL, Gerritsen JJ, et al. Prospective multicentre cohort study of patient-reported outcomes after cholecystectomy for uncomplicated symptomatic cholecystolithiasis. Br J Surg 2015;102:1402–9.. [DOI] [PubMed] [Google Scholar]
- [13].Mak MHW, Chew WL, Junnarkar SP, et al. Patient reported outcomes in elective laparoscopic cholecystectomy. Ann Hepatobiliary Pancreat Surg 2019;23:20–33.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rystedt JM, Montgomery AK. Quality-of-life after bile duct injury: intraoperative detection is crucial. A national case-control study. HPB (Oxford) 2016;18:1010–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yu H, Chan EE, Lingam P, et al. Index admission laparoscopic cholecystectomy for acute cholecystitis restores Gastrointestinal Quality of Life Index (GIQLI) score. Ann Hepatobiliary Pancreat Surg 2018;22:58–65.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sandblom G, Videhult P, Karlson BM, et al. Validation of Gastrointestinal Quality of Life Index in Swedish for assessing the impact of gallstones on health-related quality of life. Value Health 2009;12:181–4.. [DOI] [PubMed] [Google Scholar]
- [17].Wanjura V, Lundstrom P, Osterberg J, et al. Gastrointestinal quality-of-life after cholecystectomy: indication predicts gastrointestinal symptoms and abdominal pain. World J Surg 2014;38:3075–81.. [DOI] [PubMed] [Google Scholar]
- [18].Korolija D, Sauerland S, Wood-Dauphinee S, et al. Evaluation of quality of life after laparoscopic surgery: evidence-based guidelines of the European Association for Endoscopic Surgery. Surg Endosc 2004;18:879–97.. [DOI] [PubMed] [Google Scholar]
- [19].Quallich LG, Stern MA, Rich M, et al. Bile duct crystals do not contribute to sphincter of Oddi dysfunction. Gastrointest Endosc 2002;55:163–6.. [DOI] [PubMed] [Google Scholar]
- [20].Cheon YK, Cho YD, Moon JH, et al. Effects of vardenafil, a phosphodiesterase type-5 inhibitor, on sphincter of Oddi motility in patients with suspected biliary sphincter of Oddi dysfunction. Gastrointest Endosc 2009;69:1111–6.. [DOI] [PubMed] [Google Scholar]
- [21].Topazian M, Salem RR, Robert ME. Painful cystic duct remnant diagnosed by endoscopic ultrasound. Am J Gastroenterol 2005;100:491–5.. [DOI] [PubMed] [Google Scholar]
- [22].Walsh RM, Ponsky JL, Dumot J. Retained gallbladder/cystic duct remnant calculi as a cause of postcholecystectomy pain. Surg Endosc 2002;16:981–4.. [DOI] [PubMed] [Google Scholar]
- [23].Talseth A, Edna TH, Hveem K, et al. Quality of life and psychological and gastrointestinal symptoms after cholecystectomy: a population-based cohort study. BMJ Open Gastroenterol 2017;4:e000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rydbeck D, Anesten B, Barje T, et al. Health-Related Quality-of-Life in a cohort undergoing cholecystectomy. Ann Med Surg (Lond) 2015;4:22–5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sugrue M, Sahebally SM, Ansaloni L, et al. Grading operative findings at laparoscopic cholecystectomy- a new scoring system. World J Emerg Surg 2015;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jensen MP, McFarland CA. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain 1993;55:195–203.. [DOI] [PubMed] [Google Scholar]
- [27].Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: visual analog scale for pain (VAS Pain), numeric rating scale for pain (NRS Pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res 2011;63: Suppl 11: S240–252.. [DOI] [PubMed] [Google Scholar]
- [28].Eypasch E, Williams JI, Wood-Dauphinee S, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg 1995;82:216–22.. [DOI] [PubMed] [Google Scholar]
- [29].Han IW, Kwon OC, Oh MG, et al. Effects of Rowachol on prevention of postcholecystectomy pain after laparoscopic cholecystectomy: prospective multicenter randomized controlled trial. HPB (Oxford) 2016;18:664–70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kim GH, Lee HD, Kim M, et al. Fate of dyspeptic or colonic symptoms after laparoscopic cholecystectomy. J Neurogastroenterol Motil 2014;20:253–60.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Thistle JL, Longstreth GF, Romero Y, et al. Factors that predict relief from upper abdominal pain after cholecystectomy. Clin Gastroenterol Hepatol 2011;9:891–6.. [DOI] [PubMed] [Google Scholar]
- [32].Sarmiento JM, Farnell MB, Nagorney DM, et al. Quality-of-life assessment of surgical reconstruction after laparoscopic cholecystectomy-induced bile duct injuries: what happens at 5 years and beyond? Arch Surg 2004;139:483–8.. discussion 488-489. [DOI] [PubMed] [Google Scholar]
- [33].Hogan AM, Hoti E, Winter DC, et al. Quality of life after iatrogenic bile duct injury: a case control study. Ann Surg 2009;249:292–5.. [DOI] [PubMed] [Google Scholar]
- [34].Boerma D, Rauws EA, Keulemans YC, et al. Impaired quality of life 5 years after bile duct injury during laparoscopic cholecystectomy: a prospective analysis. Ann Surg 2001;234:750–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Flores-Rangel GA, Chapa-Azuela O, Rosales AJ, et al. Quality of life in patients with background of iatrogenic bile duct injury. World J Surg 2018;42:2987–91.. [DOI] [PubMed] [Google Scholar]
- [36].Sun H, Tang H, Jiang S, et al. Gender and metabolic differences of gallstone diseases. World J Gastroenterol 2009;15:1886–91.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cain KC, Jarrett ME, Burr RL, et al. Gender differences in gastrointestinal, psychological, and somatic symptoms in irritable bowel syndrome. Dig Dis Sci 2009;54:1542–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lee R, Ha H, Han YS, et al. Percutaneous transhepatic gallbladder drainage followed by elective laparoscopic cholecystectomy for patients with moderate to severe acute cholecystitis. Medicine (Baltimore) 2017;96:e8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Okamoto K, Suzuki K, Takada T, et al. Tokyo Guidelines 2018: flowchart for the management of acute cholecystitis. J Hepatobiliary Pancreat Sci 2018;25:55–72.. [DOI] [PubMed] [Google Scholar]


