Abstract
Imbalances in the gut microbiota mediate the progression of neurodegenerative diseases such as Parkinson's disease (PD). Fecal microbiota transplantation (FMT) is currently being explored as a potential therapy for PD. The objective of this study was to assess the efficacy and safety of FMT on PD. Fifteen PD patients were included, 10 of them received FMT via colonoscopy (colonic FMT group) and 5 received FMT via nasal-jejunal tube (nasointestinal FMT group). The score of PSQI, HAMD, HAMA, PDQ-39, NMSQ and UPDRS-III significantly decreased after FMT treatment (all P < .05). Colonic FMT group showed significant improvement and longer maintenance of efficacy compared with nasointestinal FMT (P = .002). Two patients achieved self-satisfying outcomes that last for more than 24 months. However, nasointestinal FMT group had no significant therapeutic effect, although UPDRS-III score slightly reduced. There were no patients were satisfied with nasointestinal FMT for more than 3 months. Among 15 PD patients, there were 5 cases had adverse events (AEs), including diarrhea (2 cases), abdominal pain (2 cases) and flatulence (1 case). These AEs were mild and self-limiting. We conclude that FMT can relieve the motor and non-motor symptoms with acceptable safety in PD. Compared with nasointestinal FMT, colonic FMT seems better and preferable.
Keywords: colonoscopy, efficacy, fecal microbiota transplantation, Parkinson's disease, safety
1. Introduction
Parkinson's disease (PD) is a type of chronic progressive disease of the nervous system with a loss of DA-producing neurons. It is the second most prevalent neurodegenerative disease worldwide after Alzheimers disease, and increasing more rapidly.[1] An estimated 6.1 million individuals globally had a PD diagnosis in 2016.[1] Approximately 930,000 people will be living with a PD diagnosis in the United States in 2020.[2] In China, PD constitutes a serious public health burden, affecting approximately 1.7% of the population over 65 years old.[3]
The PD patients often suffer from motor symptoms including rigidity, bradykinesia, tremor, impaired balance, and also non-motor symptoms such as sleep disturbance, anxiety, depression, cognitive deficits.[4] These symptoms worsen over time, becoming severe enough to interfere with daily common activities, thus necessitating the help of caregivers. Although current treatments can alleviate symptoms, there are few treatments available to slow or stop the deterioration of brain cells. As dopaminergic neurons are degraded and dopamine levels in the striatum decrease, the symptoms may worsen.
A direct and bidirectional channel of communication exists between the gut and the brain, dubbed the microbiome-gut-brain axis, which includes a connection between the enteric and central nervous systems.[5] Imbalances in the gut microbiota promote altered host-microbial interactions that mediate the progression of many neurological diseases, including depression, autism, PD, etc.[6] It has been reported that the gut microbiota in PD patients significantly differ from that of healthy individuals.[7–9] In addition, a recent study shows that pathogenic a-synuclein can spread to brain along the vagus nerve.[10] The findings of these studies provide a basis for the treatment of PD by targeting the gut.
Manipulation of gut microbiota to influence health has recently received greater attention in humans through procedures such as fecal microbiota transplantation (FMT).[11] FMT refers to infusion of a fecal suspension from a healthy donor into the gut of a patient to restore a healthy microbiota and treat disease.[12] It can change gut microbiota more robustly in comparison to food or probiotics. FMT is a new strategy to treat a variety of dysbiosis-associated gut diseases, such as Clostridium difficile infection (CDI)[11,13] and inflammatory bowel disease (IBD)[14–16] with satisfactory outcomes. In a case report, FMT was shown to improve PD symptoms.[17] However, FMT is not paid enough attention by clinicians and lacks systemic study right now. To the best of our knowledge, no study has yet ascertained the efficacy and safety of FMT in patients with PD.
2. Methods
2.1. Patients and donors
All patients treated with FMT for PD between September 2017 and March 2019 were included. Written informed consent was taken from study participants prior to recruitment. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in an approval by the ethical review board of The Affiliated Huaian No. 1 People's Hospital of Nanjing Medical University. Patient characteristics and baseline condition were assessed thoroughly before FMT. All patients failed to achieve satisfactory effectiveness from the previous medications. Stool donors were screened according to the selection criteria which were described in detail in our previous report.[18] Healthy donors (ranged from 18 to 24 years old) were selected from patients’ non-relatives and carefully screened by the following exclusion criteria: history of drug use (e.g. antibiotic, laxative or diet pill use within the past 3 months; prior immunomodulator or chemotherapy use) and history of disease (e.g. infectious diseases, obesity, diabetes, IBD, irritable bowel syndrome, chronic diarrhea, constipation or autoimmune diseases, as well as any other diseases or conditions related to the disturbance of gut microbiota). In addition, all donors accepted lab examinations, such as regular blood test, biochemical tests, hepatitis associated indices, HIV, syphilis, and stool testing.
2.2. FMT procedure
Fecal microbiota from donors was purified and isolated in our laboratory. Single feces from a donor is used to isolate microbiota to treat a PD patient. Five Chinese Han donors with an mean age of 22 years, including 3 men and 2 women, participated in this study. There was no clear selectivity between donors and PD patients before FMT that means the selection of the donor was random. The purification process of fecal microbiota includes stirring, mixing, filtering step by step, and resuspending in the laboratory.[18] The purified fecal microbiota suspension was then transplanted into the gut of the patient in a short time. The time between feces released and been transplanted into the gut of a PD patient was less than one hour. This means that we use fresh fecal microbiota to treat PD patients. Two delivery routes were adopted to transplant the microbiota, one was colonic route that the fecal microbiota suspension was transplanted into the ascending colon of the patient via colonoscopy (Patient No. 1–10, called “colonic FMT group”), and the other one was nasointestinal route that the suspension was transplanted into jejunum via nasal-jejunal tube (Patient No. 11–15, called “nasointestinal FMT group”). All patients continued their previous medication before FMT and the following 3 months after FMT.
2.3. The short-term and extended follow-up
Three months after FMT were the set follow-up period. During this period, the treatment medication was the same as before FMT. Any treatment that may affect the symptoms of PD was not allowed unless the patient requested to withdraw from the study. Three patients refused to continue follow-up because of unsatisfactory efficacy after 1 month of FMT treatment. Therefore, all patients (15 patients with PD) completed 1-month follow-up, but only 12 patients completed the 3-month follow-up after FMT. After the third month of FMT, the patients were further followed. If the patients were not self-satisfied with the efficacy of FMT, changing the treatment drugs or receiving deep brain stimulation (DBS) surgery was allowed at any time during the extended follow-up period (3–24 months). Self-satisfaction was marked as “green”, while dissatisfaction was “gray” in Figure 1.
Figure 1.
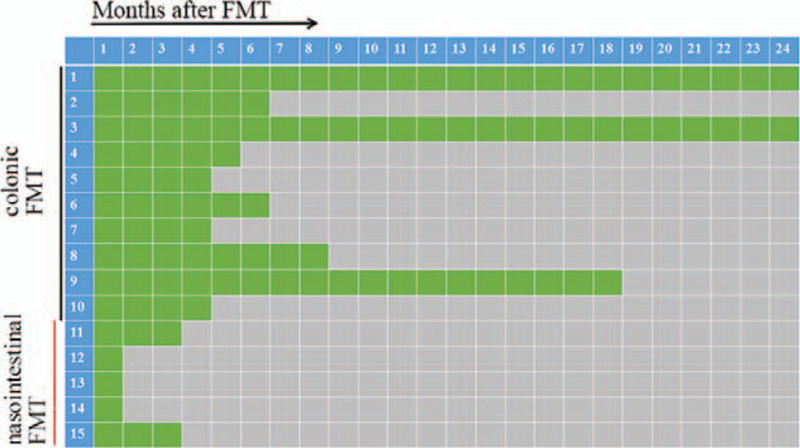
The extended follow-up of self-satisfaction assessment during the 24-mo follow-up. Self-satisfaction was marked as “green”, while dissatisfaction was “gray”. Colonic FMT showed longer maintenance of efficacy compared with nasointestinal FMT. Patient 1–10 were colonic FMT group, and Patient 11–15 were nasointestinal FMT group.
2.4. Efficacy and safety assessment
The efficacy was evaluated by scale scores at 1 month and 3 months after FMT. Motor symptoms were evaluated using the Unified PD rating scale III (UPDRS-III) in the on state. The quality of sleep was assessed using the Pittsburgh sleep quality index (PSQI), and the quality of life using the PD 39-items questionnaire (PDQ-39). The anxiety and depression were assessed using the Hamilton anxiety scale (HAMA) and the Hamilton depression scale (HAMD). Self-satisfaction in the extended follow-up was not evaluated by scale scores. Self-satisfaction relied on the patient's subjective evaluation of the efficacy of FMT. All adverse events (AEs) were recorded after FMT. Intensity of AEs was classified as mild, moderate, severe, or disabling. It was described using Common Terminology Criteria for Adverse Events (CTCAE version 4.0).
2.5. Statistical analysis
Data were analyzed by using SPSS 23.0. Analyses included paired Student's t test. Two tailed P value was calculated with each test. P values <.05 were considered significant.
3. Results
3.1. The clinical characteristics of PD patients
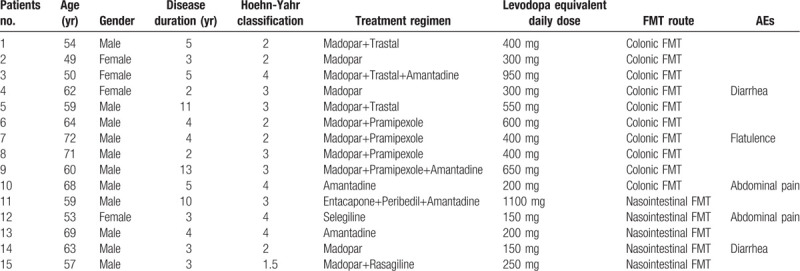
A total of 15 PD patients received FMT treatment, 11 males and 4 females. The median age was 61 years old. Patient condition was assessed thoroughly, including disease duration, Hoehn-Yahr classification, treatment drugs, and levodopa equivalent daily dose. The median disease duration was 4 years, and the median Hoehn-Yahr classification was 3. PD treatment drugs included Madopar, Trastal, Amantadine, Pramipexole, Entacapone, Peribedil, Selegiline, and Rasagiline. The levodopa equivalent daily dose of each patient was calculated and showed in Table 1.
Table 1.
Clinical characteristics of the patients with PD.
3.2. The efficacy of FMT in short-term follow-up
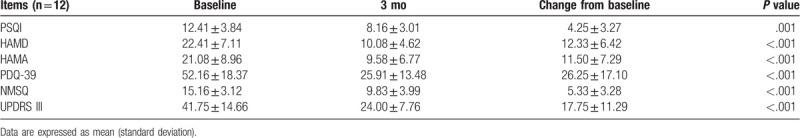
The motor and non-motor symptoms were evaluated by scale scores during the 3-month follow-up (Fig. 2). The score of PSQI, HAMD, HAMA, PDQ-39, NMSQ and UPDRS-III significantly decreased at 1 month after FMT (P < .05) (Table 2). Three patients refused to continue follow-up after 1 month of FMT. Therefore, only 12 patients were followed up and evaluated at 3 month (Table 3). The mean score of UPDRS-III decreased from 41.75 before FMT to 24.00 at 3 month after FMT. The quality of sleep and life improved after FMT treatment, with a decrease in PSQI score (12.41 vs 8.16) and PDQ-39 score (52.16 vs 25.91). In addition, anxiety and depression were also partially relieved, with a significant decrease in HAMA score (21.08 vs 9.58) and HAMD score (22.41 vs 10.08).
Figure 2.
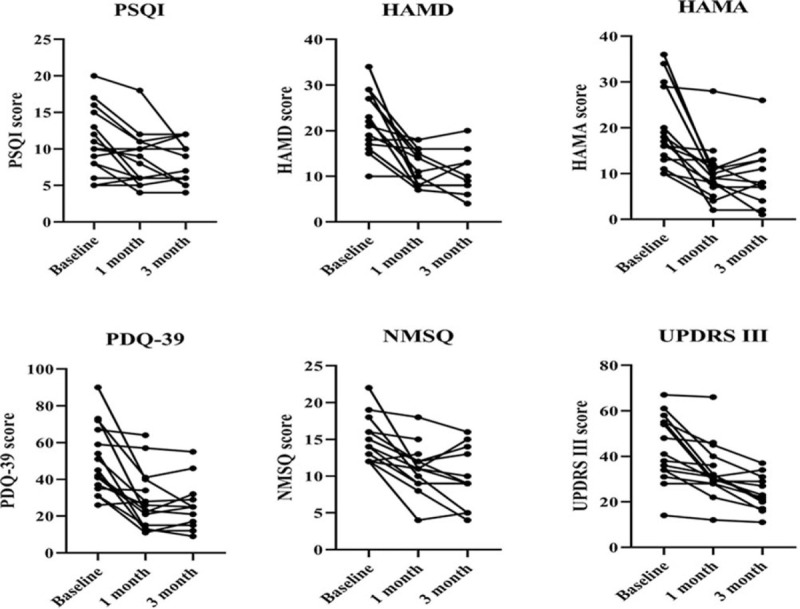
The motor and non-motor symptoms were evaluated by scale scores during the 3-mo follow-up. The score of PSQI, HAMD, HAMA, PDQ-39, NMSQ and UPDRS-III significantly decreased at 1 and 3 mo after FMT.
Table 2.
The changed score of PSQI, HAMD, HAMA, PDQ-39, NMSQ and UPDRS-III at 1 mo after FMT.
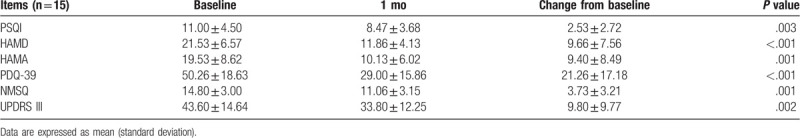
Table 3.
The changed score of PSQI, HAMD, HAMA, PDQ-39, NMSQ and UPDRS-III at 3 mo after FMT.
3.3. The delivery of microbiota and the efficacy of FMT
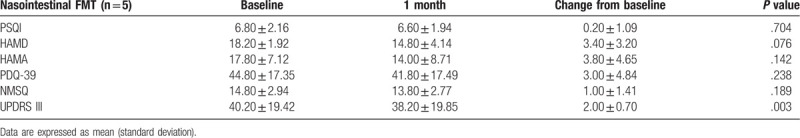
Of these 15 patients, 10 received FMT via the colonoscopy route (colonic FMT), and 5 received FMT via the nasal-jejunal tube (nasointestinal FMT). Colonic FMT significantly reduced the score of PSQI, HAMD, HAMA, PDQ-39, NMSQ and UPDRS-III at 1 month after FMT (P < .05) (Table 4). However, nasointestinal FMT did not have significant improvement, although UPDRS-III score slightly reduced (40.20 vs 38.20) (Table 5).
Table 4.
The changed score of PSQI, HAMD, HAMA, PDQ-39, NMSQ and UPDRS-III at 1 mo after colonic FMT.
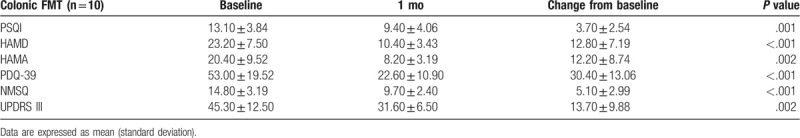
Table 5.
The changed score of PSQI, HAMD, HAMA, PDQ-39, NMSQ and UPDRS-III at 1 mo after nasointestinal FMT.
3.4. The extended follow-up of self-satisfaction assessment
The median “gray” appearance time was 4 months after FMT. In the colonic FMT group, the median time was 6 months, while the time was 1 month in nasointestinal FMT group. Colonic FMT showed longer maintenance of efficacy compared with nasointestinal FMT (P = .002). In colonic FMT group, 2 patients showed satisfactory response for 24 months. However, no patients were satisfied with nasointestinal FMT for more than 3 months (Fig. 2).
3.5. The safety of FMT in PD patients
Among all patients, 5 patients developed FMT-related AEs within one week after FMT (Table 1). These AEs were mild and self-limiting, including diarrhea (2 patients), abdominal pain (2 patients) and flatulence (1 patient). During the long-term follow-up, no serious AE occurred. In the extended follow-up, no FMT-related AEs were reported.
4. Discussion
In this study, FMT significantly decreased the score of PSQI, HAMD, HAMA, PDQ-39, NMSQ, UPDRS III at 1 and 3 months after FMT treatment for PD. Compared to FMT via the nasointestinal tube, the efficacy of FMT via colonoscopy was relatively more satisfactory. Two patients achieved self-satisfying outcomes that last for more than 24 months. Conversely, the effect of FMT via the nasointestinal tube was unsatisfactory, especially for non-motor symptoms. The overall safety of FMT was acceptable. Only 5 cases had AEs, including diarrhea, abdominal pain and flatulence. These AEs were mild and self-limiting. No serious AEs were found during the period.
Anxiety, depression, and sleep disorders is common non-motor symptoms of PD and may precede motor manifestations. Neurodegenerative changes are present in parts of the brainstem reticular core and limbic system before motor circuits are affected to a degree that causes motor symptoms.[19] In the present study, FMT significantly improved the patients’ anxiety (HAMA score), depression (HAMD score), and sleep quality (PSQI score). This therapeutic effect only reflected in the colonic FMT group. In contrast, FMT via the nasointestinal tube did not show a significant decrease in HAMA, HAMD and PSQI score. Predictably, the non-motor symptoms score (NMSQ) decreased significantly after FMT, and only the colonic FMT group showed the improvement effect. Microbiota-gut-brain axis mediates association between gut microbiota disorder and PD. The damaged intestinal barrier causes systemic inflammation that leads to neuroinflammation with changed cognition, behavior, and stress in PD.[20] The underlying mechanism of FMT for PD is unknown. Gut microbiota and its metabolites may regulate neuroinflammation.[21,22] A speculation worth noting is that FMT changes the gut microbiota and its metabolites by optimizing the structure of gut microbiota, and then improves the non-motor symptoms of PD.[23,24]
FMT has a high cure rate for CDI, and has become the recommended treatment for refractory CDI.[25] Regardless of the microbiota transplantation route, there is a satisfactory cure rate.[26] But in IBD, the microbiota transplantation route may affect the efficacy of FMT.[14,15] As reported by Ding et al, colonoscopic FMT may be more effective in UC.[14] In our study, colonoscopic FMT showed better therapeutic effect than nasointestinal FMT in PD. The colonization of the microbiota in the gut may be a factor affecting the efficacy. The fecal microbiota of the donors mainly represents the colonic microbiota. Therefore, the isolated microbiota should be theoretically colonized most, not the small intestine. The nasointestinal FMT transplant the isolated microbiota into the recipient's jejunum. The alkaline environment of the small intestine partially interferes with the colonization of the microbiota, leading to reduced functional microbiota in the recipient's colon. This may be the reason why nasointestinal FMT has not achieved satisfactory results. In fact, it is unclear which kind of microbiota can improve the symptoms of PD. In the future study, confirming the key microbiota for treating PD and artificially cultivating in vitro can greatly promote the treatment of probiotics with PD.
The safety of FMT in the treatment of PD is a matter of concern. FMT is not without risks.[27] In IBD, Ding and Wang reported long-term evaluation on the safety of FMT in the treatment of IBD.[14,15] Both real-world studies showed that the occurrence of AEs was related to the method of fecal microbiota preparation. The fecal microbiota purification by GenFMTer system greatly reduced the rate of AEs without reducing the curative effect. Strict donor screening, proper recipient selection, washed microbiota transplantation (WMT), and standardized transplantation procedures may reduce the risk of AEs.[28] In our study, 15 patients with PD were treated with FMT, and 4 patients developed AEs with diarrhea, abdominal pain, and abdominal distension. These AEs were mild and self-limiting.
There are some limitations in this study. First, this study is a self-controlled study before and after FMT treatment. There is a lack of placebo as a randomized control. Second, this is a preliminary study with few cases. There were 15 cases with PD were included, and only 5 cases received nasointestinal FMT. Third, the defined follow-up period is short. After 3 months of FMT treatment, the patients were allowed to change their medication if they feel that FMT is not effective. Fourth, we focused on the clinical efficacy and safety of FMT in PD, but did not analyze the composition of gut microbiota and blood inflammatory factors. Although there are some limitations, the preliminary study confirms the potential clinical value of FMT in PD. In the future, we will design a placebo randomized controlled trial to further study the efficacy and safety of FMT.
In conclusion, the results of this preliminary study indicate that FMT can relieve the motor and non-motor symptoms with acceptable safety in PD. Compared with nasointestinal FMT, colonic FMT may be more effective and may become a new option for the treatment of PD.
Author contributions
Investigation: Liu-Jun Xue, Xiao-Zhong Yang, Jin-Long Zheng, Hong-Gang Wang.
Methodology: Qiang Tong, Peng Shen, Shi-Jie Ma.
Project administration: Hong-Gang Wang, Xiao-Zhong Yang, Shang-Nong Wu, Peng Shen, Shi-Jie Ma.
Writing – original draft: Liu-Jun Xue, Xiao-Zhong Yang.
Writing – review & editing: Jin-Long Zheng, Hong-Gang Wang.
Footnotes
Abbreviations: AEs = adverse events, CDI = Clostridium difficile infection, FMT = fecal microbiota transplantation, HAMA = Hamilton anxiety scale, HAMD = Hamilton depression scale, IBD = inflammatory bowel disease, PD = Parkinson's disease, PDQ-39 = PD 39-items questionnaire, PSQI = Pittsburgh sleep quality index, UPDRS-III = Unified PD rating scale III.
How to cite this article: Xue LJ, Yang XZ, Tong Q, Shen P, Ma SJ, Wu SN, Zheng JL, Wang HG. Fecal microbiota transplantation therapy for Parkinson's disease: a preliminary study. Medicine. 2020;99:35(e22035).
L-JX and X-ZY contributed equally to this work.
This study was reviewed and approved by The Affiliated Huaian No. 1 People's Hospital of Nanjing Medical University Institutional Review Board (KY-P-2017-003-01). The patients provided written informed consents prior to participation in this study.
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].GBD 2016 Parkinson's Disease Collaborators. Global, regional, and national burden of Parkinson's disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018;17:939–53.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Marras C, Beck JC, Bower JH, et al. Prevalence of Parkinson's disease across North America. NPJ Parkinsons Dis 2018;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen S, Chan P, Sun S, et al. The recommendations of Chinese Parkinson's disease and movement disorder society consensus on therapeutic management of Parkinson's disease. Transl Neurodegener 2016;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA 2020;323:548–60.. [DOI] [PubMed] [Google Scholar]
- [5].Carabotti M, Scirocco A, Maselli MA, et al. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 2015;28:203–9.. [PMC free article] [PubMed] [Google Scholar]
- [6].Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol 2020;19:179–94.. [DOI] [PubMed] [Google Scholar]
- [7].Heintz-Buschart A, Pandey U, Wicke T, et al. The nasal and gut microbiome in Parkinson's disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord 2018;33:88–98.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aho VTE, Pereira PAB, Voutilainen S, et al. Gut microbiota in Parkinson's disease: temporal stability and relations to disease progression. EBioMedicine 2019;44:691–707.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cirstea MS, Yu AC, Golz E, et al. Microbiota composition and metabolism are associated with gut function in Parkinson's disease. Mov Disord 2020;35:1208–17.. 10.1002/mds.28052. [DOI] [PubMed] [Google Scholar]
- [10].Kim S, Kwon SH, Kam TI, et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson's disease. Neuron 2019;103:627–41.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].D’Haens GR, Jobin C. Fecal microbial transplantation for diseases beyond recurrent Clostridium difficile infection. Gastroenterology 2019;157:624–36.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang F, Cui B, He X, et al. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell 2018;9:462–73.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mullish BH, Quraishi MN, Segal JP, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut 2018;67:1920–41.. [DOI] [PubMed] [Google Scholar]
- [14].Ding X, Li Q, Li P, et al. Long-term safety and efficacy of fecal microbiota transplant in active ulcerative colitis. Drug Saf 2019;42:869–80.. [DOI] [PubMed] [Google Scholar]
- [15].Wang H, Cui B, Li Q, et al. The safety of fecal microbiota transplantation for Crohn's disease: findings from a long-term study. Adv Ther 2018;35:1935–44.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sokol H, Landman C, Seksik P, et al. Fecal microbiota transplantation to maintain remission in Crohn's disease: a pilot randomized controlled study. Microbiome 2020;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Huang H, Xu H, Luo Q, et al. Fecal microbiota transplantation to treat Parkinson's disease with constipation: a case report. Medicine (Baltimore) 2019;98:e16163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang HG, Liu SP, Ma TH, et al. Fecal microbiota transplantation treatment for refractory ulcerative colitis with allergy to 5-aminosalicylic acid: a case report. Medicine (Baltimore) 2018;97:e0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Seritan AL, Rienas C, Duong T, et al. Ages at onset of anxiety and depressive disorders in Parkinson's disease. J Neuropsychiatry Clin Neurosci 2019;31:346–52.. [DOI] [PubMed] [Google Scholar]
- [20].Sun MF, Shen YQ. Dysbiosis of gut microbiota and microbial metabolites in Parkinson's disease. Ageing Res Rev 2018;45:53–61.. [DOI] [PubMed] [Google Scholar]
- [21].Jameson KG, Hsiao EY. Linking the gut microbiota to a brain neurotransmitter. Trends Neurosci 2018;41:413–4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lach G, Schellekens H, Dinan TG, et al. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics 2018;15:36–59.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sun MF, Zhu YL, Zhou ZL, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun 2018;70:48–60.. [DOI] [PubMed] [Google Scholar]
- [24].Maini Rekdal V, Bess EN, Bisanz JE, et al. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science 2019;364:eaau6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hvas CL, Dahl Jørgensen SM, Jørgensen SP, et al. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent Clostridium difficile infection. Gastroenterology 2019;156:1324–32.. [DOI] [PubMed] [Google Scholar]
- [26].Kao D, Roach B, Silva M, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 2017;318:1985–93.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Giles EM, D’Adamo GL, Forster SC. The future of faecal transplants. Nat Rev Microbiol 2019;17:719. [DOI] [PubMed] [Google Scholar]
- [28].Zhang T, Lu G, Zhao Z, et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell 2020;11:251–66.. [DOI] [PMC free article] [PubMed] [Google Scholar]


