Abstract
Background:
This study aimed to provide reliable estimates for dietary antioxidant vitamin (vitamins A, C, and E) intake and their effect on fracture risk at various sites.
Methods:
The PubMed, EMBASE, and Cochrane Library databases were searched to identify prospective cohort studies published throughout October 2019. The pooled relative risk (RR) with its 95% confidence interval (CI) was calculated using a random-effects model.
Results:
In total, 13 prospective cohort studies involving 384,464 individuals were selected for this meta-analysis. The summary RR indicated that increased antioxidant vitamin intake was associated with a reduced fracture risk (RR: 0.92; 95% CI: 0.86–0.98; P = .015). When stratified by the vitamin types, increased vitamin E intake was found to be associated with a reduced fracture risk (RR: 0.66; 95% CI: 0.46–0.95; P = .025), whereas increased vitamin A and C intake did not affect this risk. Increased antioxidant vitamin intake was associated with a reduced fracture risk, irrespective of fracture sites (HR: 0.90; 95% CI: 0.86–0.94; P < .001); however, it did not affect hip fracture risk. Furthermore, increased antioxidant vitamin intake was associated with a reduced fracture risk in men (RR: 0.81; 95% CI: 0.68–0.96; P = .017) and combined men and women (RR: 0.83; 95%CI: 0.73–0.93; P = .002); however, it did not affect fracture risk in women.
Conclusion:
Fracture risk at any site is significantly reduced with increased antioxidant vitamin intake, especially vitamin E intake and in men.
Keywords: fractures, meta-analysis, vitamin A, vitamin C, vitamin E
1. Introduction
Osteoporosis is a chronic multifactorial disease characterized by low bone mass and impaired bone microarchitecture.[1,2] The prevalence of osteoporosis is increased in people with advanced age, coronary heart diseases, cancer, respiratory system diseases, depression, and neurodegenerative diseases.[3] Individuals with osteoporosis have an increased risk of bone fracture, and the common fracture sites include the hip, spine, forearm, and proximal humerus, especially among older women.[4] Moreover, individuals with low bone mineral density (BMD) have an increased risk of falls and decline in muscle strength, balance, mobility, and physical functioning, resulting in an excess fracture risk.[5–7] Studies have already demonstrated that genetics, age, lifestyle habits, and sex are significant factors affecting fracture risk.[2,8–12] Moreover, the etiology of osteoporosis could be affected by nutrition intake.[13] Furthermore, studies have reported that vitamins have potent antioxidant effects that can counteract the effects of high levels of reactive oxygen species.[14–16] However, the potential role of dietary antioxidant vitamin (vitamins A, C, and E) intake in the progression of a fracture is unknown.
Numerous studies have already demonstrated that increased fruit and vegetable intake plays a critical role in determining the bone mineral status.[17–20] This could be correlated with reactive oxygen intermediates involved in the bone resorptive process; furthermore, antioxidant intake can reduce oxidative stress.[21–23] Moreover, antioxidants are essential cofactors for the formation of collagen and synthesis of hydroxyproline and hydroxylysine, which constitute 90% of the proteins in the bone matrix.[24] Therefore, increased antioxidant intake may result in bone strengthening and a reduced fracture risk. Numerous studies have already investigated the potential effect of antioxidant vitamins on fracture risk; however, inconsistent results have been obtained.[25–37] The present meta-analysis included prospective cohort studies and aimed to evaluate the role of antioxidant vitamins in the progression of fractures and to determine whether the associations differ according to the vitamin types, fracture sites, and sex.
2. Methods
2.1. Data sources, search strategy, and selection criteria
This meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis protocol of 2009.[38] The ethical approval was not applicable. The study was designed as a prospective cohort study to investigate the role of dietary antioxidant vitamin intake on fracture risk. Studies were considered eligible irrespective of the language and publication status (in press or published). The PubMed, EMBASE, and Cochrane Library electronic databases were systematically screened for studies published throughout October 2019. Vitamin A OR retinol OR vitamin C OR acid ascorbic OR vitamin E OR tocopherol AND fracture were the core search terms. After choosing the included studies, the reference lists of the retrieved studies were reviewed to select any additional eligible studies.
The literature search and study selection were performed by 2 authors who followed a standardized approach, and mutual consensus was obtained after discussion if disagreements occurred between the 2. This study was restricted to a prospective cohort design to eliminate selection and recall bias related to retrospective observational studies. A study was considered eligible if it met the following criteria: the study had a prospective cohort design; participants had no fractures at any sites before the study; participants were exposed to vitamin A, C, or E; and the outcomes were the effect estimates (risk ratio [RR], hazard ratio [HR], or odds ratios [ORs]) and 95% confidence intervals (CIs) for comparisons of high and low antioxidant vitamin intake and fracture risk.
2.2. Data collection and quality assessment
Two authors extracted all the data from the included studies according to a standardized protocol, and any disagreements were settled by discussion until a consensus was reached. The data included the first author's name, publication year, country, sample size, mean age, participants’ sex ratio, number of fractures, antioxidant vitamin types, follow-up duration, adjusted factors, and investigated outcomes. For studies reporting several multivariable adjusted effect estimates, the effect estimate that was maximally adjusted for potential confounders was selected. The Newcastle-Ottawa Scale (NOS) was used to evaluate the methodological quality; it is a comprehensive tool and has been partially validated for evaluating the quality of observational studies in a meta-analysis.[39] The scoring system of NOS ranges from 0 to 9 and is based on selection (4 items), comparability (1 item), and outcome (3 items). Quality assessment was performed by 2 authors, and any conflicts were resolved by another author by referring to the original article.
2.3. Statistical analysis
The association between antioxidant vitamin intake and subsequent fracture risk was examined based on the effect estimate with its 95% CI in each individual study; the pooled RR and 95%CI for the high versus low antioxidant vitamin intake were calculated using a random-effects model.[40,41] Heterogeneity across the included studies was assessed using the I2 and P values for Q statistics, with I2 > 50.0% or P < .10 being considered as a significant heterogeneity.[42,43] The robustness of the pooled conclusion was assessed using a sensitivity analysis.[44] Subgroup analyses were performed based on the vitamin types, fracture sites, and sex. Publication bias for fracture risk was assessed using several methods, including funnel plots and Egger [45] and Begg [46] test results. The inspection level was 2-sided, and P < .05 was considered statistically significant for pooled results. STATA software (version 12.0; Stata Corporation, College Station, TX) was used to perform all statistical analyses in this study.
3. Results
3.1. Literature search
The flow chart of the study selection process is shown in Figure 1. In total, 1372 articles were identified during the initial electronic searches, and 1327 records were excluded because they were duplicates or unrelated studies. The remaining 45 studies were retrieved for further full-text evaluations, and of them, 13 studies were selected for the final meta-analysis.[25–37] The manual searches of reference lists yielded seven studies, and all of them were included in the initial electronic searches.
Figure 1.
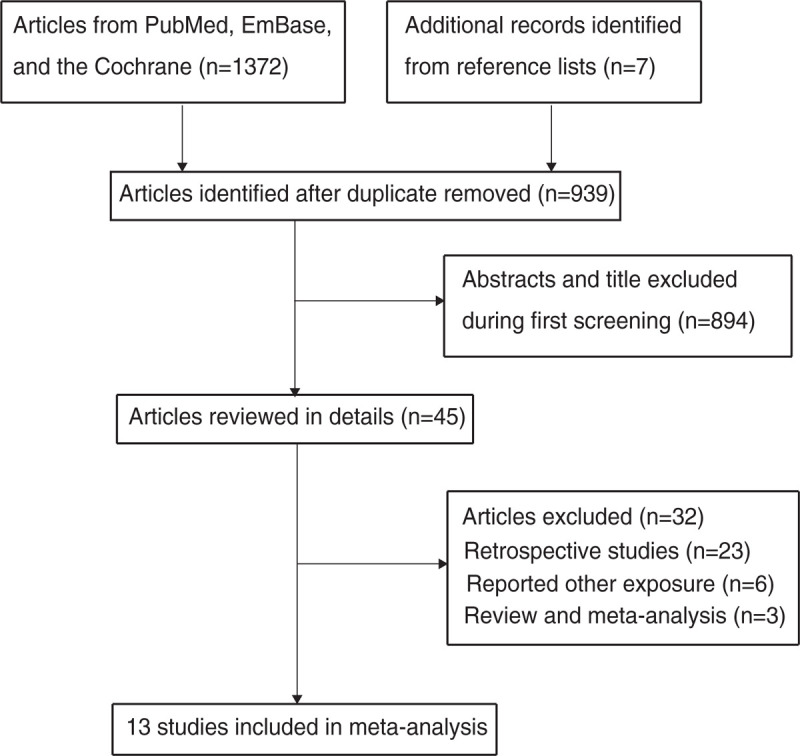
Flow diagram of the literature search and study selection process.
3.2. Study characteristics
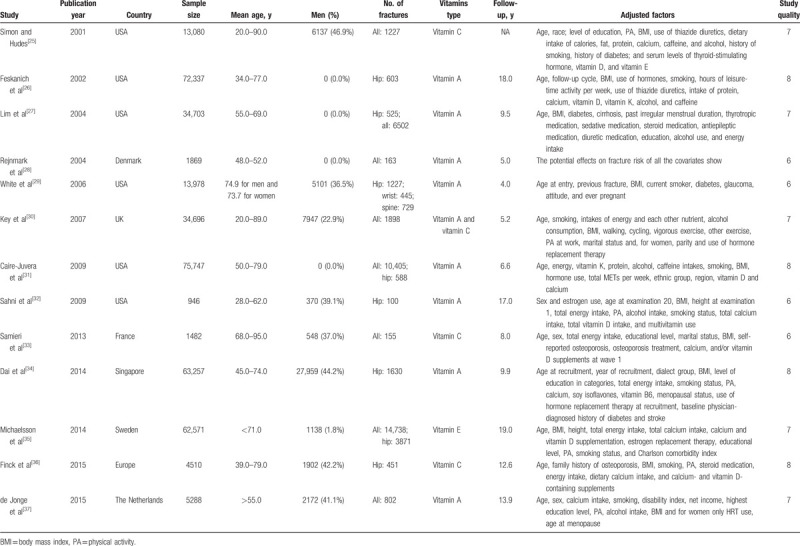
The baseline characteristics of the included studies and participants are summarized in Table 1. In total, 384,464 individuals were included in the 13 studies. The follow-up duration ranged from 4.0 to 19.0 years, and the number of participants in the individual studies ranged from 946 to 75,747. Eight studies investigated the role of vitamin A, 3 studies evaluated the role of vitamin C, 1 study included both vitamin A and vitamin C evaluation, and the remaining study assessed the role of vitamin E in 2 cohorts. Six studies were conducted in the United States and 6 studies in Europe, and the remaining study was conducted in Singapore. Study quality was assessed using NOS: 4 studies were awarded 8 stars, 5 studies were awarded 7 stars, and the remaining 4 studies were awarded 6 stars.
Table 1.
Baseline characteristics of the included studies and participants.
3.3. Meta-analysis
After pooling all the included studies, we noted that high antioxidant vitamin intake was associated with a reduced fracture risk (RR: 0.92; 95% CI: 0.86–0.98; P = .015; Fig. 2). Moreover, a significant heterogeneity was observed across these studies (I2 = 49.4%; P = .008). After this, a sensitivity analysis was performed to assess the robustness of the pooled conclusion; the results indicated that the conclusion was stable and did not change after the sequential exclusion of individual studies (data not shown).
Figure 2.
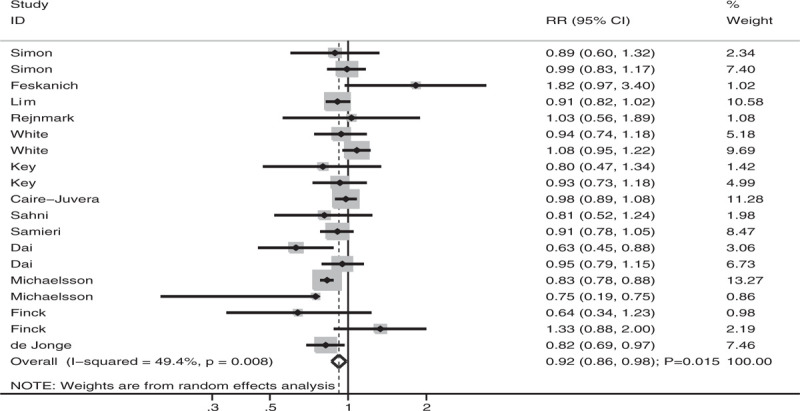
Association of antioxidant vitamin intake with fracture risk.
3.4. Subgroup analysis
Subgroup analyses of the association between antioxidant vitamin intake and fracture risk were performed based on the vitamin types, fracture sites, and sex. When stratified according to the vitamin types (Fig. 3), fracture risk was significantly reduced if individuals had high vitamin E intake (RR: 0.66; 95% CI: 0.46–0.95; P = .025; significant heterogeneity). However, there were no significant associations of vitamin A (HR: 0.93; 95% CI: 0.86–1.01; P = .089; significant heterogeneity) and vitamin C (HR: 0.95; 95%CI: 0.85–1.05; P = .273; mild heterogeneity) intake with fracture risk. When stratified according to fracture sites (Fig. 4), fracture risk at all sites was significantly reduced in individuals with high antioxidant vitamin intake (HR: 0.90; 95% CI: 0.86–0.94; P < .001; mild heterogeneity). However, there was no significant association between antioxidant vitamin intake and hip fracture risk (HR: 0.87; 95% CI: 0.69–1.08; P = .202; significant heterogeneity). When stratified according to sex (Fig. 5), fracture risk was significantly reduced in men (RR: 0.81; 95% CI: 0.68–0.96; P = .017; mild heterogeneity), with another study reporting that the risk was significantly reduced in both men and women (RR: 0.83; 95% CI: 0.73–0.93; P = .002; mild heterogeneity), with high antioxidant vitamin intake. There was no significant association between antioxidant vitamin intake and fracture risk in women (HR: 0.96; 95% CI: 0.84–1.09; P = .505; significant heterogeneity).
Figure 3.
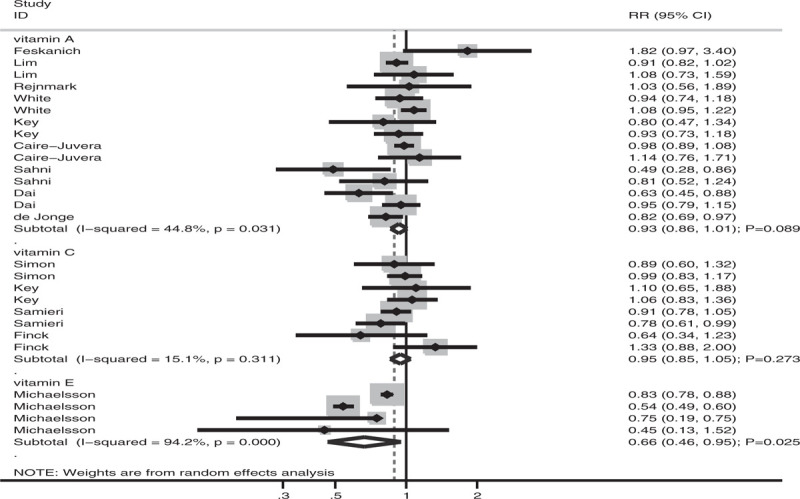
Association of antioxidant vitamin intake with fracture risk stratified by the type of vitamins.
Figure 4.
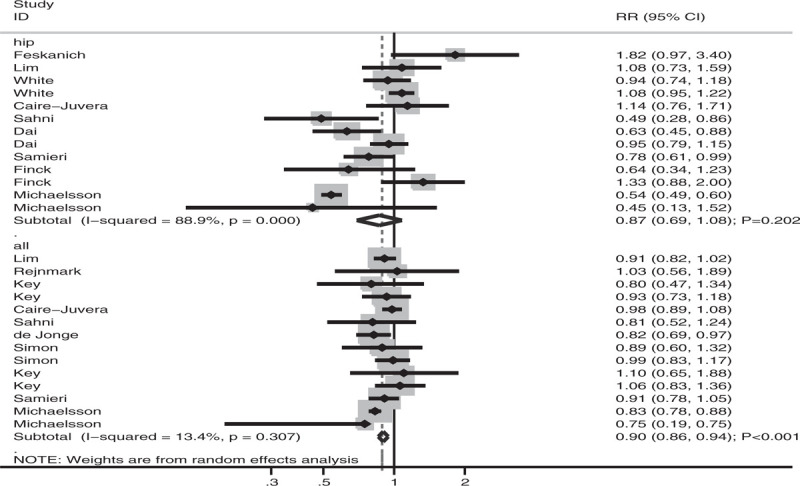
Association of antioxidant vitamin intake with fracture risk stratified by fracture sites.
Figure 5.
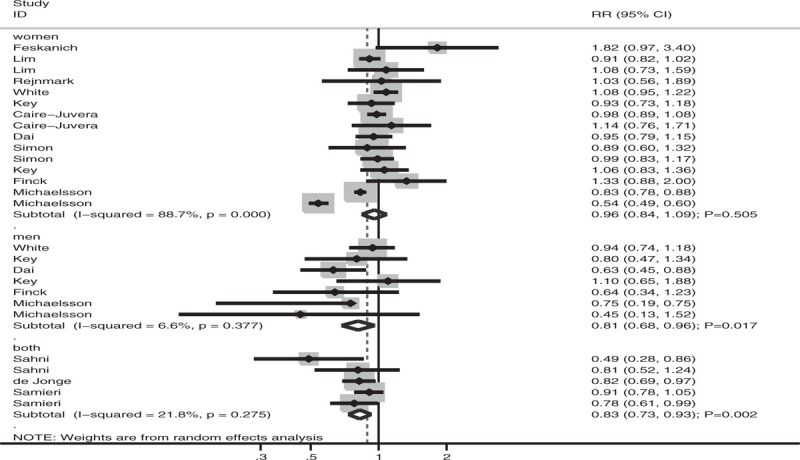
Association of antioxidant vitamin intake with fracture risk stratified by sex.
3.5. Publication bias
Publication bias could not be ruled out by reviewing the funnel plot (Fig. 6). The Egger (P = .447) and Begg (P = .576) test results indicated no significant publication bias for the association between antioxidant vitamin intake and fracture risk.
Figure 6.
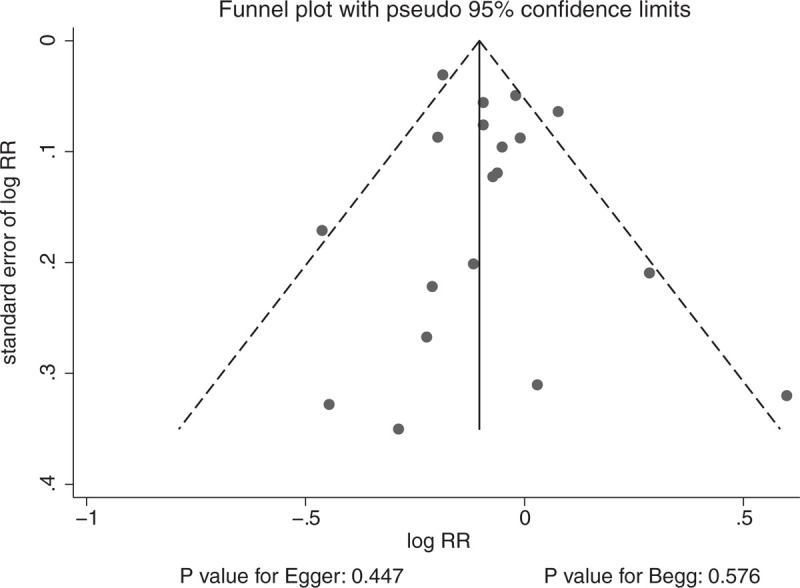
Publication bias.
4. Discussion
The potential effect of antioxidant vitamin intake on fracture risk was investigated in this meta-analysis. A total of 384,464 individuals from 13 studies were included, and the study findings indicated that high antioxidant vitamin intake yielded a protective effect on fracture risk. This conclusion was found to be stable through a sensitivity analysis. Subgroup analyses showed a protective effect of antioxidant vitamins, especially vitamin E, at all fracture sites and in men. Although no significant associations were detected in other subsets, a protective remaining trend existed, which needs further large-scale prospective studies for verification.
A meta-analysis conducted by Wu et al[47] included 8 vitamin A (or retinol or beta-carotene) intake studies and found that high vitamin A and retinol intake was associated with an increased hip fracture risk, whereas beta-carotene intake did not yield a significant association with hip fracture risk. Moreover, Zhang et al[48] conducted a meta-analysis of 13 studies and found that high retinol and total vitamin A intake was associated with low fracture risk at all sites, whereas hip fracture risk was significantly increased. Another group of researchers pointed out that excessive vitamin A intake could alter the metabolism of calcium-regulating hormones and minimize the activity of vitamin D.[49] However, another study reported that BMD levels increase with high vitamin A intake, resulting in a discrepancy.[50] The present study did not find any harmful effects of vitamin A intake on fracture risk. The potential reasons for this include the following: the fracture sites were not differentiated, and the effect estimates for all fractures and hip fractures might have been neutralized; and the sources of vitamin A differed from studies in previous meta-analyses suggesting that vitamin A dietary intake is beneficial for preventing fractures.
Sun et al[51] conducted a meta-analysis including six studies and found that high vitamin C intake was associated with a reduced hip fracture risk, with the risk reducing by 5% per 50 mg/day of vitamin C intake. Malmir et al[52] conducted a meta-analysis and found that high vitamin C intake was associated with a reduced osteoporosis risk and increased BMD levels in the femoral neck and lumbar spine, whereas hip fracture risk was not altered. A nonsignificant association between vitamin C intake and hip fracture risk might be explained by the retrospective studies that were included in their study; furthermore, uncontrolled biases might affect the reliability of a pooled conclusion. Moreover, the protective effect of vitamin C on hip fracture risk is attributable to the stimulation of type I and III collagen synthesis by ascorbic acid, and vitamin C deficiency can stimulate osteoclastogenesis.[53,54] Although a significant association between vitamin C intake and fracture risk was not detected in our study, this result could be due to fracture risk being examined based on the effect estimates related to vitamin C intake.
The association between vitamin E intake and fracture risk could not be determined as only one study comprising 2 cohorts was included in the present meta-analysis.[35] This study specifically found that increased vitamin E intake was associated with a reduced fracture risk at all sites in both men and women and a reduced hip fracture risk in women. The potential reason for this could be that vitamin E exerts beneficial effects on both the bones and muscle mass, which are associated with a reduced fracture risk.[55,56] Moreover, the background α-tocopherol levels in individuals could affect the net effect estimate for the association between vitamin E intake and fracture risk.
Several limitations of this meta-analysis should be highlighted: this meta-analysis mostly included studies reporting on the association between vitamin A and fracture risk, and the pooled results for the potential role of vitamins C and E were restricted; a significant heterogeneity was observed across the included studies in view of various patient characteristics and cutoff values for antioxidant vitamin intake; the adjusted models were different across the included studies, which might play an important role in the progression of fractures; publication bias was an inevitable problem due to the analysis being based on published articles; and finally, the present study analyses were performed at a study level, which restricted us from conducting further detailed analyses.
In summary, the findings of this meta-analysis suggest that fracture risk at all sites is significantly reduced with increased antioxidant vitamin intake, especially vitamin E intake and in men. Given the number of included studies and significant heterogeneity across included studies, further large-scale prospective studies should be conducted to verify the present study findings, and a dose–response relationship curve should be constructed for dietary antioxidant vitamin intake.
Author contributions
Conceptualization: Penghe Zhou, Ruyi Shao, Xianhui Wang, Jiaqing Miao.
Data curation: Penghe Zhou, Ruyi Shao, Xianhui Wang, Jiaqing Miao, Hua Wang.
Formal analysis: Penghe Zhou, Ruyi Shao, Xianhui Wang, Jiaqing Miao, Hua Wang.
Funding acquisition: Penghe Zhou, Ruyi Shao, Xianhui Wang.
Investigation: Penghe Zhou, Ruyi Shao, Xianhui Wang.
Methodology: Penghe Zhou, Ruyi Shao, Xianhui Wang.
Project administration: Penghe Zhou, Ruyi Shao, Xianhui Wang.
Software: Jiaqing Miao.
Resources: Xianhui Wang, Jiaqing Miao.
Visualization: Xianhui Wang.
Supervision: Penghe Zhou, Ruyi Shao.
Writing – original draft: Penghe Zhou, Ruyi Shao, Xianhui Wang.
Writing – review & editing: Penghe Zhou, Ruyi Shao, Xianhui Wang.
Correction
Dr. Ruyi Shao's name was originally published as Ruiyi Shao. It has since been corrected. The funding source, Funding Source: Jiangsu University Clinical Medicine Science and Technology Development Fund Project; Award ID: 2019jd005, has been added.
Footnotes
Abbreviations: BMD = bone mineral density, CIs = confidence intervals, HR = hazard ratio, NOS = Newcastle-Ottawa Scale, ORs = odds ratios, RR = risk ratio.
How to cite this article: Zhou P, Shao R, Wang H, Miao J, Wang X. Dietary vitamin A, C, and E intake and subsequent fracture risk at various sites: A meta-analysis of prospective cohort studies. Medicine. 2020;99:35(e20841).
PZ, RS, and HW contributed equally to this work.
The authors report no conflicts of interest.
Funding Source: Jiangsu University Clinical Medicine Science and Technology Development Fund Project; Award ID: 2019jd005.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].McCormick RK. Osteoporosis: integrating biomarkers and other diagnostic correlates into the management of bone fragility. Altern Med Rev 2007;12:113–45.. [PubMed] [Google Scholar]
- [2].Bartolozzi E. The natural approach to osteoporosis. Clin Cases Miner Bone Metab 2015;12:111–5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Springer, Malcomson FC, Mathers JC. Nutrition and ageing. Biochemistry Cell Biology of Ageing: Part I. Biomedical Science. 2018;373–424. [Google Scholar]
- [4].Cashman KD. Diet, nutrition, and bone health. J Nutr 2007;137: 11 suppl: 2507s–12s.. [DOI] [PubMed] [Google Scholar]
- [5].Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med 1995;332:767–73.. [DOI] [PubMed] [Google Scholar]
- [6].Nelson ME, Fiatarone MA, Morganti CM, et al. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures. A randomized controlled trial. JAMA 1994;272:1909–14.. [DOI] [PubMed] [Google Scholar]
- [7].Nguyen T, Sambrook P, Kelly P, et al. Prediction of osteoporotic fractures by postural instability and bone density. BMJ 1993;307:1111–5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Recker RR, Deng HW. Role of genetics in osteoporosis. Endocrine 2002;17:55–66.. [DOI] [PubMed] [Google Scholar]
- [9].Duncan EL, Danoy P, Kemp JP, et al. Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. PLoS Genet 2011;7:e1001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rizzoli R, Bonjour JP, Ferrari SL. Osteoporosis, genetics and hormones. J Mol Endocrinol 2001;26:79–94.. [DOI] [PubMed] [Google Scholar]
- [11].Henry MJ, Pasco JA, Nicholson GC, et al. Prevalence of osteoporosis in Australian women: Geelong Osteoporosis Study. J Clin Densitom 2000;3:261–8.. [DOI] [PubMed] [Google Scholar]
- [12].Alswat KA. Gender disparities in osteoporosis. J Clin Med Res 2017;9:382–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Heaney RP. Calcium, dairy products and osteoporosis. J Am Coll Nutr 2000;19: 2 suppl: 83s–99s.. [DOI] [PubMed] [Google Scholar]
- [14].Gheorghe G, Stoian AP, Gaman M-A, et al. The benefits and risks of antioxidant treatment in liver diseases. Revista de Chimie 2019;70:651–5.. [Google Scholar]
- [15].Gaman AM, Buga AM, Gaman MA, et al. The role of oxidative stress and the effects of antioxidants on the incidence of infectious complications of chronic lymphocytic leukemia. Oxid Med Cell Longev 2014;2014:158135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Elsevier, Găman AM, Egbuna C, Găman M-A. Egbuna C, Kumar S, Ifemeje JC, Ezzat SM, Kaliyaperumal S. Natural bioactive lead compounds effective against haematological malignancies. Phytochemicals as Lead Compounds for New Drug Discovery. 2020;95–115. [Google Scholar]
- [17].Macdonald HM, New SA, Golden MH, et al. Nutritional associations with bone loss during the menopausal transition: evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am J Clin Nutr 2004;79:155–65.. [DOI] [PubMed] [Google Scholar]
- [18].Prynne CJ, Mishra GD, O’Connell MA, et al. Fruit and vegetable intakes and bone mineral status: a cross sectional study in 5 age and sex cohorts. Am J Clin Nutr 2006;83:1420–8.. [DOI] [PubMed] [Google Scholar]
- [19].Tucker KL, Hannan MT, Chen H, et al. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr 1999;69:727–36.. [DOI] [PubMed] [Google Scholar]
- [20].New SA, Robins SP, Campbell MK, et al. Dietary influences on bone mass and bone metabolism: further evidence of a positive link between fruit and vegetable consumption and bone health? Am J Clin Nutr 2000;71:142–51.. [DOI] [PubMed] [Google Scholar]
- [21].Garrett IR, Boyce BF, Oreffo RO, et al. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest 1990;85:632–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Basu S, Michaelsson K, Olofsson H, et al. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun 2001;288:275–9.. [DOI] [PubMed] [Google Scholar]
- [23].Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition 1996;12:274–7.. [DOI] [PubMed] [Google Scholar]
- [24].Peterkofsky B. Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. TheAm J Clin Nutr 1991;54: 6 suppl: 1135s–40s.. [DOI] [PubMed] [Google Scholar]
- [25].Simon JA, Hudes ES. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. Am J Epidemiol 2001;154:427–33.. [DOI] [PubMed] [Google Scholar]
- [26].Feskanich D, Singh V, Willett WC, et al. Vitamin A intake and hip fractures among postmenopausal women. JAMA 2002;287:47–54.. [DOI] [PubMed] [Google Scholar]
- [27].Lim LS, Harnack LJ, Lazovich D, et al. Vitamin A intake and the risk of hip fracture in postmenopausal women: the Iowa Women's Health Study. Osteoporos Int 2004;15:552–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rejnmark L, Vestergaard P, Charles P, et al. No effect of vitamin A intake on bone mineral density and fracture risk in perimenopausal women. Osteoporos Int 2004;15:872–80.. [DOI] [PubMed] [Google Scholar]
- [29].White SC, Atchison KA, Gornbein JA, et al. Risk factors for fractures in older men and women: The Leisure World Cohort Study. Gend Med 2006;3:110–23.. [DOI] [PubMed] [Google Scholar]
- [30].Key TJ, Appleby PN, Spencer EA, et al. Calcium, diet and fracture risk: a prospective study of 1898 incident fractures among 34 696 British women and men. Public Health Nutr 2007;10:1314–20.. [DOI] [PubMed] [Google Scholar]
- [31].Caire-Juvera G, Ritenbaugh C, Wactawski-Wende J, et al. Vitamin A and retinol intakes and the risk of fractures among participants of the Women's Health Initiative Observational Study. Am J Clin Nutr 2009;89:323–30.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sahni S, Hannan MT, Blumberg J, et al. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: a 17-year follow-up from the Framingham Osteoporosis Study. J Bone Miner Res 2009;24:1086–94.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Samieri C, Ginder Coupez V, Lorrain S, et al. Nutrient patterns and risk of fracture in older subjects: results from the Three-City Study. Osteoporos Int 2013;24:1295–305.. [DOI] [PubMed] [Google Scholar]
- [34].Dai Z, Wang R, Ang LW, et al. Protective effects of dietary carotenoids on risk of hip fracture in men: the Singapore Chinese Health Study. J Bone Miner Res 2014;29:408–17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Michaelsson K, Wolk A, Byberg L, et al. Intake and serum concentrations of alpha-tocopherol in relation to fractures in elderly women and men: 2 cohort studies. Am J Clin Nutr 2014;99:107–14.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Finck H, Hart AR, Lentjes MA, et al. Cross-sectional and prospective associations between dietary and plasma vitamin C, heel bone ultrasound, and fracture risk in men and women in the European Prospective Investigation into Cancer in Norfolk cohort. Am J Clin Nutr 2015;102:1416–24.. [DOI] [PubMed] [Google Scholar]
- [37].de Jonge EA, Kiefte-de Jong JC, Campos-Obando N, et al. Dietary vitamin A intake and bone health in the elderly: the Rotterdam Study. Eur J Clin Nutr 2015;69:1360–8.. [DOI] [PubMed] [Google Scholar]
- [38].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Wells G SB, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa (ON): Ottawa Hospital Research Institute. 2009: available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [Google Scholar]
- [40].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88.. [DOI] [PubMed] [Google Scholar]
- [41].Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making 2005;25:646–54.. [DOI] [PubMed] [Google Scholar]
- [42].Deeks JJ, Higgins J, Altman DG. Higgins J, Green S. Analysing data and undertaking meta-analyses. Oxford, Cochrane Handbook for Systematic Reviews of Interventions.. UK: 2008. [Google Scholar]
- [43].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327:557–60.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull 1999;47:15–7.. [Google Scholar]
- [45].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed) 1997;315:629–34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101.. [PubMed] [Google Scholar]
- [47].Wu AM, Huang CQ, Lin ZK, et al. The relationship between vitamin A and risk of fracture: meta-analysis of prospective studies. J Bone Miner Res 2014;29:2032–9.. [DOI] [PubMed] [Google Scholar]
- [48].Zhang X, Zhang R, Moore JB, et al. The effect of vitamin A on fracture risk: a meta-analysis of cohort studies. Int J Environ Res Public Health 2017;14(9.): [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rohde CM, Manatt M, Clagett-Dame M, et al. Vitamin A antagonizes the action of vitamin D in rats. J Nutr 1999;129:2246–50.. [DOI] [PubMed] [Google Scholar]
- [50].Barker ME, McCloskey E, Saha S, et al. Serum retinoids and beta-carotene as predictors of hip and other fractures in elderly women. J Bone Miner Res 2005;20:913–20.. [DOI] [PubMed] [Google Scholar]
- [51].Sun Y, Liu C, Bo Y, et al. Dietary vitamin C intake and the risk of hip fracture: a dose-response meta-analysis. Osteoporos Int 2018;29:79–87.. [DOI] [PubMed] [Google Scholar]
- [52].Malmir H, Shab-Bidar S, Djafarian K. Vitamin C intake in relation to bone mineral density and risk of hip fracture and osteoporosis: a systematic review and meta-analysis of observational studies. Br J Nutr 2018;119:847–58.. [DOI] [PubMed] [Google Scholar]
- [53].Carinci F, Pezzetti F, Spina AM, et al. Effect of Vitamin C on pre-osteoblast gene expression. Arch Oral Biol 2005;50:481–96.. [DOI] [PubMed] [Google Scholar]
- [54].Hie M, Tsukamoto I. Vitamin C-deficiency stimulates osteoclastogenesis with an increase in RANK expression. J Nutr Biochem 2011;22:164–71.. [DOI] [PubMed] [Google Scholar]
- [55].Shuid AN, Mohamad S, Muhammad N, et al. Effects of alpha-tocopherol on the early phase of osteoporotic fracture healing. J Orthop Res 2011;29:1732–8.. [DOI] [PubMed] [Google Scholar]
- [56].Mehat MZ, Shuid AN, Mohamed N, et al. Beneficial effects of vitamin E isomer supplementation on static and dynamic bone histomorphometry parameters in normal male rats. J Bone Miner Metab 2010;28:503–9.. [DOI] [PubMed] [Google Scholar]


