Abstract
Study Objectives
Much of what we assume about the effects of short sleep duration on neural reward processing derives from total sleep deprivation studies. Although total sleep deprivation appears rare, habitual short sleep is common: 30% of working US adults report habitually sleeping ≤ 6 hours/night. It remains largely unknown whether habitual short sleepers exhibit similar reward processing brain activation patterns to those observed following total sleep deprivation in prior studies. Therefore, our aim was to test objectively reward processing brain activation patterns associated with self-reported habitual short sleep duration in a large sample.
Methods
Nine hundred and fifty-two adult participants from the Human Connectome Project database were grouped on reported habitual short (≤6 hours) vs. medium-length (7–9 hours) sleep duration using the Pittsburgh Sleep Quality Index (PSQI). Reward processing brain activation was examined using a gambling task during functional magnetic resonance imaging (fMRI). Subject-level covariates for age, sex, continuous sleep duration, daytime dysfunction, and PSQI total score are provided as supplemental analyses.
Results
Brain activation patterns revealed expected reward processing-related activation for age and sex. However, activation for sleep duration, dysfunction, and PSQI score did not correspond to those evident in previous total sleep deprivation studies.
Conclusions
Self-reported short sleep duration, perceived sleep-related dysfunction, and sleep quality via PSQI do not appear to be meaningfully associated with activation in well-described regions of the human neurobiological reward circuit. As these findings are counter to prior results using experimental sleep deprivation, future work focused on more direct comparisons between self-reported sleep variables and experimental sleep deprivation appears warranted.
Keywords: habitual short sleep duration, reward processing, fMRI, total sleep deprivation
Statement of Significance.
Approximately one-third of the adults report routinely sleeping 6 hours or less each night—widely considered to be a public health epidemic. Understanding the biopsychosocial changes affecting this population has notable public health implications. Unfortunately, much of our understanding about neurobiological consequences of habitual short sleep derives from experimental sleep deprivation studies of otherwise healthy, nonshort sleeping individuals. Counter to prior experimental sleep deprivation studies, current findings indicate that self-reported short sleep duration, perceived sleep-related dysfunction, and global sleep quality may not meaningfully impact reward processing at the level of the brain. Current findings suggest that further examination of biopsychosocial differences between experimental sleep deprivation and habitual short sleep duration is warranted.
Introduction
How much sleep do humans need to thrive? Although the National Sleep Foundation recommends that adults (ages 18–64 years) sleep 7–9 hours each day [1], approximately 30% of employed US adults report sleeping 6 hour or less each day [2]. Self-report data from the Human Connectome Project (HCP) database [3] supports this prevalence estimate; with 291 of 970 US adults (30.0%) reporting 6 hours or less sleep each night over the past month [4]. Despite the apparent common prevalence of habitual short sleep duration, much of what we assume about the effects of habitual short sleep duration are derived from studies using experimental total sleep deprivation [5]. Therefore, it remains largely unknown whether individuals with self-reported habitual short sleep duration exhibit similar outcomes as those previously observed following experimental total sleep deprivation. As an initial step to address this gap in our knowledge, the current study used data from the HCP database [3] to examine reward processing brain activation patterns among self-reported habitual short sleepers.
Neural reward processing studies indicate that 24–27 hours of experimental total sleep deprivation is associated with increased medial prefrontal cortex and nucleus accumbens activation following monetary wins [6, 7] and decreased anterior insula [6, 8] and orbitofrontal cortex [8] activation following monetary losses. Interpretation of these findings has included suggestions that sleep deprivation results in less disappointment (decreased anterior insula [6, 8]) and an impaired ability to learn from negative outcomes (decreased orbitofrontal cortex [8]) in response to monetary losses. An amplified attentional bias to positive outcomes coupled with reduced concern for losses (increased medial prefrontal cortex and nucleus accumbens [6]) that may contribute to the development and maintenance of compulsive gambling, eating, substance abuse, and mood disorders (increased medial prefrontal cortex and nucleus accumbens [7]) have also been suggested implications. Whether these effects generalize beyond presumably rare instances of 24–27 hours of total sleep deprivation to the larger population of habitual short sleepers remains an important unresolved question. Evidence from actigraphy-verified adolescent habitual short sleepers (N = 58; ages 11–13 years) provide preliminary evidence that questions this assumption, with dissimilar brain activation patterns observed in response to monetary rewards (reduced bilateral caudate) and losses (no differences in brain activation) [9]. To our knowledge, whether adult habitual short sleepers exhibit similar reward processing brain activation patterns to those observed following experimental total sleep deprivation in prior studies remains unknown.
A promising approach to objective characterization of reward processing in habitual short sleepers is the use of reward decision-making tasks during functional magnetic resonance imaging (fMRI). These motivated behavioral tasks require participants to select one of several options, each with a distinct likelihood of reward or loss. This approach enables investigation of behavioral response times and brain activation patterns associated with the outcome of rewarding and losing trials [10]. A useful theoretical framework for conceptualizing the neural substrates underlying motivated behaviors is the Triadic Model of motivated behavior [11]. In this model, three neural systems map to distinct, but overlapping neural circuits: (1) approach (striatum, orbitofrontal cortex); (2) avoidance (amygdala, hippocampus, insula); and (3) regulatory (dorsolateral and ventral prefrontal cortices, anterior cingulate cortex). A synthesis of the extensive literature examining human reward processing using fMRI revealed considerable overlap with the Triadic Model [12, 13].
In summary, our aim was to test objectively reward processing brain activation patterns associated with self-reported habitual short sleep duration in a large sample. To accomplish this aim, we examined data using the HCP database [3] 1200 Participants Release. This database includes objective behavioral and functional neuroimaging data of high-resolution and image quality [14], providing a unique opportunity to overcome prior limitations of relatively small sample sizes in reward processing fMRI studies following experimental total sleep deprivation (Ns = 27–39 adult participants [6–8]) and habitual short sleep duration in adolescents (N = 58 [9]) compared to the present study (N = 952 adult participants).
Materials and Methods
We analyzed data from 952 participants with full gambling decision-making task fMRI data [14] from the HCP database [3] 1200 Participants Release. Participants ranged in age from 22 to 37 years (mean = 28.8; standard deviation = 3.7) with 444 males and 508 females. The primary HCP participant pool comprised healthy individuals, irrespective of known sleep disturbances, who were selected to capture a wide range of variability with respect to behavior, ethnicity, and socioeconomic status [3]. Exclusion criteria included severe neurodevelopmental disorders (e.g. autism), neurological disorders (e.g. Parkinson’s disease), or documented neuropsychiatric disorders (e.g. schizophrenia or depression) [3]. Participants were grouped based on whether they reported habitual short (≤6 hours) vs. medium-length (7–9 hours) sleep duration over the past month, consistent with National Sleep Foundation (NSF) recommendations [1]. These data were derived from self-report answers to the Pittsburgh Sleep Quality Index (PSQI), a 24-item questionnaire comprising 7 component scores, including sleep duration (component 3) [15]. Sleep duration was obtained from question #4: “During the past month, how many hours of actual sleep did you get at night? (This may be different than the number of hours you spend in bed.)” [15]. This strategy resulted in the following groups: (1) all habitual short sleepers (All HSS; n = 319); (2) all medium-length sleepers (All MLS; n = 633).
Images were all obtained on a single Siemens Skyra 3T scanner in similar lighting conditions with 32 channel head coil using standard multiband BOLD acquisition (TR = 720 ms, TE = 33.1 ms, 72 slices, 2.0 mm isotropic resolution, 52 degree flip angle, multiband factor = 8) [3]. As described [3], scans were conducted for all participants over two days following a regimented schedule at similar times of day and in the same order.
fMRI gambling decision-making task
The HCP database [3] uses a modified incentive processing task originally developed by Delgado and colleagues [16]. Participants play a card guessing game in which they are asked to guess possible numbers ranging from 1 to 9 on a mystery card, represented by a “?”, by pressing one of two buttons on a response box (i.e. is the mystery card more or less than 5) in order to win or lose money during fMRI [14]. As described [17], the mystery card is presented for up to 1,500 ms followed by feedback for 1,000 ms. Feedback consists of either a green up arrow next to “$1” for reward trials, a red down arrow next to “−$0.50” for loss trials, or a gray double headed arrow next to the number 5 for neutral trials. Intertrial intervals last 1,000 ms with a “+” presented on the fMRI screen. The task is presented in blocks of eight trials consisting of mostly reward or mostly loss trials. Mostly reward trials consist of six reward trials pseudo randomly interspaced with one neutral and one loss trial, two neutral trials, or two loss trials. Mostly loss trials consist of six loss trials pseudo randomly interspaced with one neutral and one reward trial, two neutral trials, or two reward trials. In each of the two task runs, there are two mostly reward and two mostly loss blocks interspaced with four fixation blocks lasting 15 seconds each. Unknown to participants, reward, punishment, and neutral outcomes were predetermined, with card values selected only after participants made their responses [16]. Although participants gambled for potential monetary rewards while in the scanner [18], all participants were provided a standard amount of money for completing the task [14]. Initial analyses of HCP brain activation data during this gambling task revealed activation of many expected brain regions across participants, including bilateral striatum, insula, caudate, putamen, medial prefrontal cortex, and orbitofrontal cortex [14].
Brain regions of interest (ROIs)
ROIs were selected a priori based on previous findings from reward-related brain activation following total sleep deprivation during monetary rewards (e.g. ventral striatum [6–8], medial prefrontal cortex [6, 7]) and losses (e.g. insula [6, 8], orbitofrontal cortex [8]) and canonical components of the human reward circuit (e.g. Triadic Model [11], whereby three systems map to distinct but overlapping neural circuits: (1) approach [striatum, orbitofrontal cortex]; (2) avoidance [amygdala, hippocampus, insula]; and (3) regulatory [dorsolateral and ventral prefrontal cortices, anterior cingulate cortex]). Exploratory ROIs were included based on prior resting fMRI findings in Internet gaming disorder [19]: supplementary motor area, motor cortex, intraparietal sulcus, frontal eye fields, posterior cingulate cortex, and temporoparietal junction.
Continuous voxelwise analysis
In addition to the ROI-level reporting, we include in Supplementary Information a report of a voxelwise analysis using a one-sample T-test design with five subject-level covariates: age, sex, reported sleep duration as a continuous variable, reported daytime dysfunction (PSQI component 7) as a continuous variable, and PSQI total score as a continuous variable. This was performed in two separate analyses, one with age, sex, sleep duration, and daytime dysfunction as covariates, and one with age, sex, and PSQI total score. Activation for each of these five variables was reported for familywise error cluster-corrected results with initial cluster-defining threshold of p < 0.001 and images displayed at p < 0.001.
Brain activation analyses
Minimally processed gambling task-based fMRI data [20] were used for brain activation analyses. Prior to our analysis, images were previously coregistered and normalized to MNI space and corrected for head motion as described in the HCP 1200 Subjects Release [20]. Images were processed by performing spatial smoothing with 6 mm kernel, and general linear model was performed in SPM12 software (Wellcome Trust, London) to identify brain activation associated with win and loss trial contrasts. Each subject had two task repetitions with reversal of the phase encoding direction (left to right or right to left) within the same session. Activation maps were calculated separately for each task acquisition in each participant. First level (within-subject) analysis was performed by comparing event-related responses to each win trial as one contrast and each loss trial as a second contrast with 12 motion parameters included as covariates. Event-related win and loss trial contrasts were registered using the event timing and duration released for each trial with the Human Connectome Project 1200 subjects release, and convolved with a hemodynamic response function in SPM12 to obtain event-related activation estimate images. For continuous activation estimates, each of the two trials for each subject was included in a second level random effects analysis (one-sample t-test design) with age, sex, reported sleep duration, reported daytime dysfunction, and reported PSQI total score as covariates. For ROI analysis, activation maps for left-right and right-left phase direction acquisitions were averaged for each subject, and t-statistic values were averaged from 39 predefined regions of interest for each subject. Regions consisted of seven bilateral deep gray nuclei (thalamus, caudate, putamen, globus pallidus, hippocampus, amygdala, and accumbens) obtained from subject-specific FreeSurfer parcellation provided with the Human Connectome Project dataset (MNINonLinear/aparc+aseg image). The remaining 25 regions consisted of major hubs of intrinsic connectivity networks from the Yeo seven-network parcellation [21] as previously described [19]. Two-tailed t-tests were used with false discovery rate correction for multiple comparisons to evaluate intergroup differences in brain activation between self-reported short and medium-length sleepers.
Results
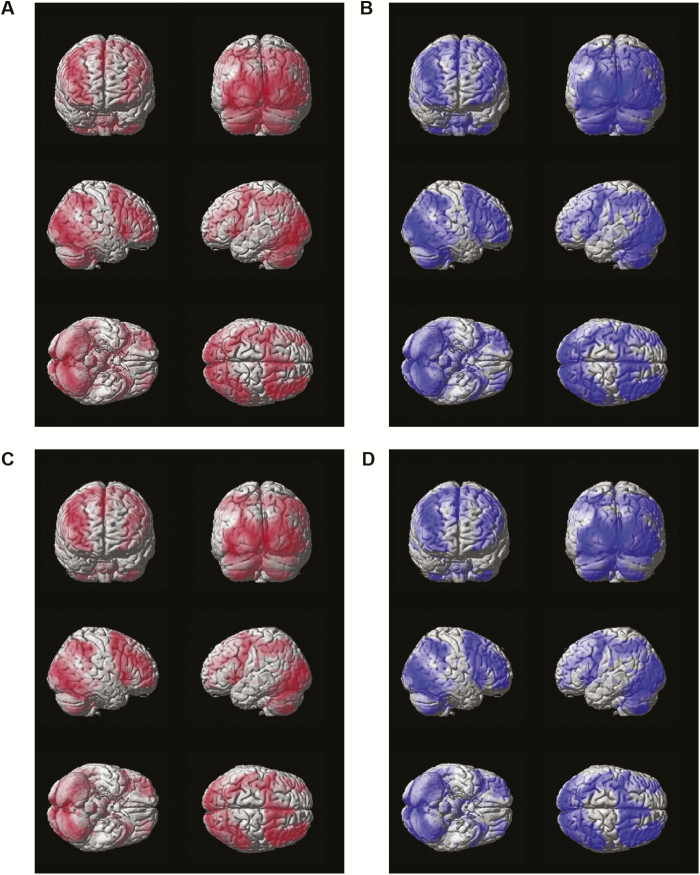
Strikingly similar patterns of brain activation during gambling fMRI win and loss trials were observed between self-reported short sleepers and medium-length sleepers (Figure 1). No significant clusters were identified in subcortical regions, with t-statistic differences being primarily attributable to larger sample size in medium-length sleepers (Supplementary Figure 1). These analyses also functioned as a check to ensure results are consistent with known activation patterns on this reward task (e.g. Figure 6 of Barch and colleagues [14] indicating activation in striatum, insula, caudate, putamen, and medial frontal cortex across HCP participants). Indeed, these regions are activated in short and medium-length sleepers during win and loss trials in the present study (Figure 1 and Supplementary Figure 1).
Figure 1.
Brain activation in self-reported short sleepers and medium-length sleepers during gambling fMRI win and loss trials. (A) Short sleeper win trials. (B) Medium-length sleeper win trials. (C) Short sleeper loss trials. (D) Medium-length sleeper loss trials. Color-coding indicates group identity (i.e. red = short sleepers; blue = medium-length sleepers).
To further examine the overall main effects of reward conditions at the whole brain level, voxelwise results are reported in Supplementary Information for both win trial and loss trial contrasts with age, sex, reported sleep duration as a continuous variable, daytime dysfunction (PSQI component 7) as a continuous variable, and PSQI total score as a continuous variable. Whereas small significant clusters were observed for continuous sleep duration and daytime dysfunction on voxelwise analysis, most notably in the visual cortex (Supplementary Figures 2–3 and 6–7, respectively), only one cluster in primary visual cortex for longer sleep duration on win trials survived familywise error rate (FWE) correction (Supplementary Tables 1–2 and 5–6). No significant clusters were observed on voxelwise analysis for PSQI total score (Supplementary Tables 9–10). However, activation was much more robust for associations with age and sex (Supplemental Figures 4–5 and 8–9 and Supplementary Tables 3–4 and 7–8) in regions with expected associations with reward processing (e.g. ventral striatum, medial, dorsolateral, and orbitofrontal prefrontal cortices, anterior cingulate cortex—refer to Figure 6 of Barch and colleagues [14], neural regions comprising the Triadic model [11], and Tables 2 and 3 of the present manuscript).
Table 2.
Gambling task fMRI brain activations following monetary win outcome trials associated with experimental sleep deprivation and habitual short sleep duration
| Experimental sleep deprivation | Habitual short sleep duration | |||||
|---|---|---|---|---|---|---|
| Reference | Mullin et al., 2013 [7] | Venkatraman et al., 2011 [6] | Venkatraman et al., 2007 [8] | Mullin et al., 2013 [7] | Holm et al., 2009 [9] | Present Study |
| Sleep Deprivation | 25.5–27 hours | 24 hours | 24 hours | N/A | N/A | N/A |
| Sample Size | N = 27 | N = 29 | N = 39 | N = 27 | N = 58 | N = 952 |
| Gambling Task | Delgado et al., 2000 [16]; Forbes et al., 2009 [21] | Payne, 2005 [22] | Venkatraman et al., 2007 [8] | Delgado et al., 2000 [16]; Forbes et al., 2009 [21] | Delgado et al., 2000 [16]; Delgado et al., 2004 [23]; Forbes et al., 2009 [21] | Delgado et al., 2000 [16]; Forbes et al., 2009 [21] |
| Monetary Amount | −$0.50 to $1 | −$75 to $80 | $2 to $70 | −$0.50 to $1 | −$0.50 to $1 | −$.50 to $1 |
| Reaction Time | Not Reported | Longer | Longer | Not Reported | Not Reported | No Difference |
| R MPF | ↑* | ↑ | (↑) | |||
| L MPF | ↑* | ↑ | (↑) | |||
| ACC | ↑ | (↑) | ||||
| R Ant Insula | ||||||
| L Ant Insula | ||||||
| R Caudate | ↓ | (↑) | ||||
| L Caudate | ↓ | |||||
| R Accumbens | ↑ | |||||
| L Accumbens | ↑ | ↑ | ||||
↑* = reduced deactivation (i.e. relatively increased activation). (↑) = nonstatistically significant increased activation. ACC, anterior cingulate cortex; Ant, anterior; DLPFC, dorsolateral prefrontal cortex; MPF, medial prefrontal cortex.
Table 3.
Gambling task fMRI brain activations during monetary loss outcome trials associated with experimental sleep deprivation and habitual short sleep duration
| Experimental sleep deprivation (24–27 hours) | Habitual short sleep | |||||
|---|---|---|---|---|---|---|
| Reference | Mullin et al., 2013 [7] | Venkatraman et al., 2011 [6] | Venkatraman et al., 2007 [8] | Mullin et al., 2013 [7] | Holm et al., 2009 [9] | Present study (group comparisons) |
| Sample Size | N = 27 | N = 29 | N = 39 | N = 27 | N = 58 | N = 952 |
| Gambling Task | Delgado et al., 2000 [16]; Forbes et al., 2009 [34] | Payne, 2005 [35] | Venkatraman et al., 2007 [8] | Delgado et al., 2000 [16]; Forbes et al., 2009 [34] | Delgado et al., 2000 [16]; Delgado et al., 2004 [36]; Forbes et al., 2009 [34] | Delgado et al., 2000 [16]; Forbes et al., 2009 [34] |
| Monetary Amount | −$0.50 to $1 | −$75 to $80 | $2 to $70 | −$0.50 to $1 | −$0.50 to $1 | −$.50 to $1 |
| Reaction Time | No Data | Longer | Longer | Not Reported | Not Reported | No Difference |
| R Frontal Eye Field | (↑) | |||||
| L Visual | (↑) | |||||
| R Ant Insula | ||||||
| L Ant Insula | ↓* | ↓ | ||||
| Medial Orbitofrontal Cortex | ↓ a | |||||
| R Amygdala | (↓) | |||||
↓* = reduced activation (i.e. relatively decreased activation). ↓ = decreased activation. aRegion was left lateral orbitofrontal cortex (medial OFC listed to be consistent with regions of interest in the present study).
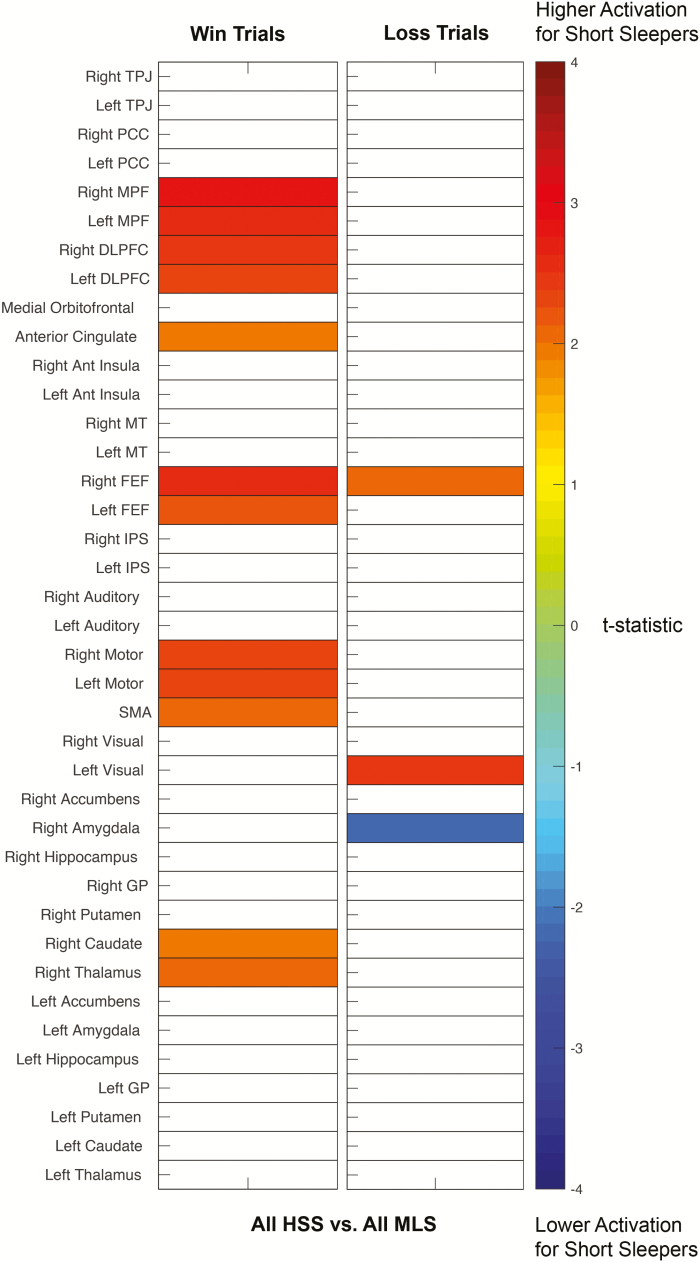
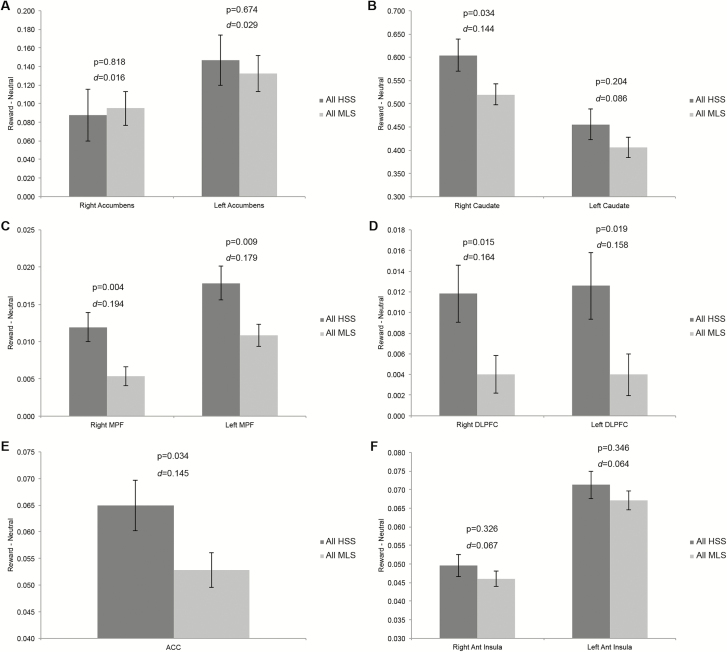
Using an acceptable false discovery rate (FDR) of q < 0.05, relatively increased, but not statistically significant brain activation following win trials for all habitual short sleepers vs. all medium-length sleepers was observed across multiple brain regions; most prominently in bilateral medial prefrontal cortex (Figure 2). Relatively increased, but not statistically significant brain activation following loss trials was observed in left visual cortex and right frontal eye fields for all habitual short sleepers vs. all medium-length sleepers, with decreased activation observed in right amygdala (Figure 2). Relative brain activation differences, significance levels, and effect sizes for a priori regions of interest are shown in Figure 3.
Figure 2.
Differences in brain activation between self-reported short sleepers and medium-length sleepers during gambling fMRI win and loss trials. Colored squares satisfied p < 0.05, uncorrected. No regions were significant for false discovery rate q < 0.05. Color scale represents t-statistic for a two-tailed t-test of brain activation between groups.
Figure 3.
Differences in a priori brain regions between self-reported short and medium-length sleepers during gambling fMRI win trials. Two-tailed t-test. Error bars represent standard error from the mean. ACC, anterior cingulate cortex; Ant, anterior; DLPFC, dorsolateral prefrontal cortex; MPF, medial prefrontal cortex. d = Cohen’s d effect size estimates.
Next, we examined whether behavioral differences existed between groups in terms of median response times during gambling task fMRI. Two-tailed t-test analyses with false discovery rate correction for multiple comparisons revealed no significant differences between groups in median response times for total larger rewards, total smaller rewards, win trial larger rewards, win trial smaller rewards, loss trial larger rewards, or loss trial smaller rewards (Table 1).
Table 1.
Differences in median response times during gambling task fMRI
| All HSS | All MLS | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n | M | SD | N | M | SD | t | P |
| Total Larger | 323 | 404.45 | 109.9 | 641 | 410.3 | 113.9 | −0.762 | 0.45 |
| Total Smaller | 325 | 408.05 | 113.8 | 647 | 415.0 | 113.7 | −0.905 | 0.37 |
| Win Larger | 322 | 413.57 | 118.2 | 633 | 420.8 | 118.2 | −0.890 | 0.37 |
| Win Smaller | 325 | 414.32 | 119.0 | 642 | 424.4 | 120.8 | −1.234 | 0.22 |
| Loss Larger | 323 | 395.31 | 114.5 | 641 | 399.9 | 120.1 | −0.569 | 0.57 |
| Loss Smaller | 325 | 401.78 | 119.0 | 647 | 406.2 | 118.9 | −0.551 | 0.58 |
n = subsample size. M = mean (in milliseconds). SD, standard deviation.
Discussion
In this study, we examined the association between self-reported habitual short sleep duration and neural reward processing. Results indicated strikingly similar patterns of brain activation between self-reported short sleepers and medium-length sleepers (Figure 1) in expected reward-related regions (Supplementary Figure 1). Subtle, but nonsignificant increased brain activation was observed across multiple brain regions (most prominently in bilateral medial prefrontal cortex) following gambling win trials for all habitual short sleepers vs. all medium-length sleepers (Figures 2 and 3C). Given the large sample size of the present study (N = 952), these relative increases in brain activation were quite small, with the largest effect size in medial prefrontal cortex being 0.19 (Figure 3C). Relative, but nonstatistically significant increases following loss trials were observed in left visual cortex and right frontal eye fields for all habitual short sleepers vs. all medium-length sleepers, with decreased activation observed in right amygdala (Figure 2). Median response times did not differ between groups (Table 1).
Given that much of what we know about the effects of short sleep duration on neural reward processing is derived from experimental total sleep deprivation fMRI studies, the aim of this study was to examine objectively reward processing brain activation patterns associated with self-reported habitual short sleep duration in a large sample. Comparisons between the present findings and prior studies examining neural reward processing outcomes following monetary wins and losses based on experimental total sleep deprivation and habitual short sleep duration are summarized in Tables 2 and 3.
Interpretations of the effects of experimental total sleep deprivation on human reward processing suggest that short sleepers may show less disappointment to monetary losses (reduced left anterior insula activation [6, 8]; Table 3), an impaired ability to learn from negative outcomes (reduced orbitofrontal cortex activation [8]; Table 3), and an increased bias in attention to positive outcomes while minimizing concern for losses [6]. This bias has been hypothesized to contribute to the development and maintenance of compulsive gambling, eating, substance abuse, and mood disorders [7] (increased activation in medial prefrontal cortex and ventral striatum [6, 7]; Table 2). The reproducible nature of these neurobiological changes and their potential negative functional impact raises a fundamental question: Are these findings reproducible across degrees of short sleep duration, or might they be exclusive to ≥ 24 hours of total sleep deprivation?
Mullin and colleagues noted uncertainty regarding whether more prevalent and naturalistic chronic sleep restriction would mirror their findings of increased nucleus accumbens activation following rewarding outcomes after a single acute dose of experimental total sleep deprivation (p. 8; [7]). Using the identical gambling task, the present findings of expectedly increased, but not significantly different bilateral accumbens activation following monetary reward (Figure 3A) provides preliminary evidence that the answer to this question is no. This is consistent with Mullin and colleagues’ own finding of no relationship between accumbens activation and actigraphy-determined shorter sleep durations over the five nights prior to experimental total sleep deprivation [7].
Following monetary losses, the present findings did not find evidence for reduced activation of left anterior insula and left lateral orbitofrontal cortex found by Venkatraman and colleagues after 24 hours of total sleep deprivation [6, 8]. This may indicate that exhibiting less disappointment in response to monetary losses (insula) and an impaired ability to learn from negative outcomes (orbitofrontal cortex) may be a feature of individuals experiencing ≥ 24 hours of total sleep deprivation, but not by habitual short sleepers. However, as summarized in Table 3, these findings may also be attributable to differences in gambling task complexity and monetary reward amounts used by Venkatraman and colleagues and the present study. Support for this latter interpretation comes from Mullin and colleagues, who used the same gambling task as the present study and also observed no differences in insula or orbitofrontal cortical activation following monetary losses after 25.5–27 hours of total sleep deprivation [7]. Therefore, future efforts to examine the effects of habitual short sleep duration on neural reward processing using the gambling tasks of Venkatraman et al., or similar tasks, appear warranted.
Subtly increased activation of bilateral medial prefrontal cortex in habitual short sleepers vs. medium-length sleepers following win trials in the present study (Figures 2 and 3C) is the only consistent finding with prior experimental sleep deprivation studies (Table 2). Venkatraman and colleagues interpreted their finding of increased ventromedial prefrontal cortical activation as representing an attentional valuation bias that sleep deprived individuals place on selecting higher-ranked, highest gain outcomes [6]. This interpretation is unlikely to account for the subtle increases in medial prefrontal cortical activation observed in habitual short sleepers in the present study, as the present gambling task lacks the option for higher gain outcomes (i.e. wins are rewarded solely with monetary gains of $1). Using the same gambling task as the present study, Mullin and colleagues observed decreased medial prefrontal cortex activation following monetary win trials in well-rested participants, with this deactivation being significantly reduced following 25.5–27 hours of total sleep deprivation (Figure 2A of their study [7]). This finding was interpreted as suggesting that an absence of decreased medial prefrontal cortex activation may be a general effect of sleep deprivation, possibly indicating a failure to properly deactivate the default mode network during active portions of cognitive tasks [7]. Contrary to this interpretation, the present findings based on self-report in a large sample suggest that deactivation of medial prefrontal cortex may neither be a common feature of well-rested individuals (i.e. bilateral increases in medial prefrontal cortex in N = 633 medium-length sleepers in the present study, Figure 3C, vs. N = 27 medium-length sleepers [7]) nor a common feature of habitual short sleep duration (i.e. bilateral increases in medial prefrontal cortex in N = 319 habitual short sleepers, Figure 3C, vs. N = 27 medium-length sleepers undergoing 25.5–27 hours of total sleep deprivation [7]). Further work directly comparing habitual short sleep duration to experimental sleep deprivation appears warranted for a more rigorous examination of these questions.
Supplemental voxelwise analyses revealed robust patterns of brain activation for participant age and sex (Supplementary Figures 4–5 and 8–9 and Supplementary Tables 3–4 and 7–8) in expected reward processing-related regions (e.g. ventral striatum, medial frontal cortices, anterior cingulate cortex). These regions are consistent with the Triadic model [11], gambling task performance following experimental sleep deprivation [6–8] (see Tables 2–3 of the present study), and prior analyses using the same gambling task in the HCP database (see Figure 6 of Barch and colleagues [14]). In contrast to the robust activation patterns observed with participant age and sex, no significant clusters were observed for PSQI total score (Supplementary Tables 9–10) or continuous perceived sleep-related daytime dysfunction (Supplementary Figures 3 and 7 and Supplementary Tables 2 and 6). For continuous reported sleep duration, only one cluster in primary visual cortex survived FWE correction for longer sleep duration on win trials (Supplementary Tables 1 and 5). Given the very large sample size of these analyses, these data support the overall conclusion that self-reported habitual short sleep duration, perceived sleep-related daytime dysfunction, and sleep quality via PSQI total score do not appear to be meaningfully associated with activation of the human reward circuit.
Using the HCP database, our research team recently reported evidence that habitual short sleep duration is associated with increased delay discounting regardless of perceived sleep-related daytime dysfunction [22]. Although these findings were consistent with one study using 21 hours of total sleep deprivation [23], two separate studies found that 24 hours of total sleep deprivation was insufficient to demonstrate a meaningful association with delay discounting behavior [24, 25]. As described [22], candidate explanations for these discrepancies include methodological differences in monetary amounts of delay discounting tasks ($0.30 to $40,000), sample sizes (N = 12 to N = 1,070 participants), and time delays between task rewards (60 seconds to 120 months). As described in this report, similar discrepancies may account for neurobiological differences in reward processing brain activation between self-reported habitual short sleep duration in the present study and prior studies using experimental sleep deprivation (Tables 2 and 3). An interesting question arises between previously reported discounting results and current brain activation findings using the HCP database: Why might habitual short sleep duration be associated with increased delay discounting (a behavioral measure of reward-related cognitive impulsivity) but have no observable association with reward processing-related brain activation? One possible explanation involves task differences between HCP delay discounting ($200 and $40,000 conditions awarded over 1–120 months) and gambling fMRI tasks ($1 reward and $−.50 losses for up to 1.5 seconds). Although it is possible that a delay discounting task with $200 and $40,000 monetary values and 1–120-month time intervals would result in notable reward-related brain activation changes, fMRI data were not collected during delay discounting in the HCP database. A second explanation includes the possible masking of individual differences in sleep need and response to sleep deprivation among heterogeneous “subtypes” of habitual short sleepers examined in delay discounting and gambling task fMRI populations in the HCP database. Arguably, an individual differences framework would allow for more precise explication of mechanisms to inform our growing understanding of the development and perpetuation of habitual short sleep duration [26].
It is important to note that data in the HCP database were not collected with specific sleep-related hypotheses in mind. Indeed, the HCP’s stated objectives include capturing “a large amount of information about each subject across many behavioral domains, especially for measures that have the potential to covary in interesting ways (across subjects) with brain connectivity and function” (p. 3 [3]). Accordingly, data mining approaches similar to the present study and our prior efforts using the HCP database [4, 22] are necessarily limited by data collection on a large number of variables that are not optimized to isolate the effects of sleep on specific behavioral or neurobiological outcomes. For example, data on habitual caffeine use and caffeine intake before gambling fMRI scanning are not available in the HCP database. Measures of sleep duration and quality were not provided or controlled for the night before fMRI task performance. Although the large sample size of the HCP database and absence of brain activation differences based on reported sleep duration, daytime dysfunction, and PSQI total score appear to mitigate such concerns in the present study, these limitations likely add to interindividual variability, which can decrease effect sizes of brain activation differences.
Methodologically, experimental sleep deprivation protocols have a number of strengths, including the ability to explore questions of causation (vs. the cross-sectional/correlational nature of data available in the HCP database used in the present study) and the objective, quantifiable, and reproducible nature of sleep deficits across research participants (vs. retrospective sleep durations based on self-report in the present study). Indeed, self-reports of sleep have been shown to be less reliable indicators of objective sleep measures compared to more objective methods such as actigraphy [27, 28] and polysomnography [29]. However, as detailed by Grandner and colleagues [5], laboratory sleep studies tend to have several important limitations. These include relatively small sample sizes (e.g. 27–39 participants [6–8] vs. 952 participants in the present study; Tables 2 and 3), examination of healthy individuals with otherwise medium-length sleep durations (rather than individuals with habitual short sleep durations), and the study of sleep in an artificial setting (i.e. laboratory) with less ecological validity than one’s home environment (e.g. use of caffeine and alcohol, the presence of environmental noise from pets and/or children).
It seems reasonable to expect differences in various physiological processes, including brain activation, if one were to, for example, stay awake all night studying for an exam (e.g. 24–27 hours of total sleep deprivation [6–8]) or if one was to regularly sleep 6 hours per night or less, on average, during the past month (present study). Although we are unaware of the prevalence of total sleep deprivation ≥ 24 hours outside of the laboratory, epidemiological research indicates that approximately 30% of employed US adults report sleeping 6 hours or less each day [2], a prevalence consistent with the HCP database utilized in the current study (30.0%). Given notable differences in reward processing-related brain activation in the present study compared to prior experimental sleep deprivation studies, future efforts to more directly examine the neurobiological effects of total sleep deprivation vs. associations with habitual short sleep duration appears warranted. As habitual short sleep duration has been associated with multiple adverse health outcomes (e.g. impaired cognition [30], affect [31], obesity [32], and all-cause mortality [33]), these efforts have important public health implications. Utilizing objective experimental methods, leveraging large-scale shared resources, such as the HCP database, as well as adopting an individual differences framework will inform our understanding of the development and consequences of habitual short sleep duration.
Funding
This study was supported by the University of Utah Neuroscience Initiative and support from the National Institute of Mental Health (K08 MH080826).
Conflict of interest statement. None declared.
Supplementary Material
Acknowledgments
Data were provided (in part) by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- 1. Hirshkowitz M, et al.. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Heal. 2015;1(4):233–243. [DOI] [PubMed] [Google Scholar]
- 2. Luckhaupt SE, et al.. The prevalence of short sleep duration by industry and occupation in the National Health Interview Survey. Sleep. 2010;33(2):149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Essen DC, et al.. The WU-Minn Human Connectome Project: an overview. Neuroimage. 2013;80:62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Curtis BJ, et al.. Sleep duration and resting fMRI functional connectivity: examination of short sleepers with and without perceived daytime dysfunction. Brain Behav. 2016;6(12):e00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grandner MA, et al.. Pack AI. Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep Med Rev. 2010;14(4):239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Venkatraman V, et al.. Sleep deprivation biases the neural mechanisms underlying economic preferences. J Neurosci. 2011;31(10):3712–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mullin BC, et al.. Sleep deprivation amplifies striatal activation to monetary reward. Psychol Med. 2013;43(10):2215–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Venkatraman V, et al.. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30(5):603–609. [DOI] [PubMed] [Google Scholar]
- 9. Holm SM, et al.. Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. J Adolesc Health. 2009;45(4):326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richards JM, et al.. A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: the impact of task design and implications for understanding neurodevelopment. Neurosci Biobehav Rev. 2013;37(5):976–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ernst M, et al.. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33(3):367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Camara E, et al.. Reward networks in the brain as captured by connectivity measures. Front Neurosci. 2009;3(3):350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haber SN, et al.. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barch DM, et al.. Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage. 2013;80:169–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buysse DJ, et al.. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 16. Delgado MR, et al.. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84(6):3072–3077. [DOI] [PubMed] [Google Scholar]
- 17. The Human Connectome Project Task fMRI Files and Protocol Details. http://www.humanconnectome.org/documentation/Q1/task-fMRI-protocol-details.html. Published 2013. Accessed May 9, 2018.
- 18. Lancaster TM, et al.. Preliminary evidence for genetic overlap between body mass index and striatal reward response. Transl Psychiatry. 2018;8(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han DH, et al.. Brain connectivity and psychiatric comorbidity in adolescents with Internet gaming disorder. Addict Biol. 2017;22(3):802–812. [DOI] [PubMed] [Google Scholar]
- 20. Glasser MF, et al.. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas Yeo BT, et al.. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Curtis BJ, et al.. Objective cognitive functioning in self-reported habitual short sleepers not reporting daytime dysfunction: examination of impulsivity via delay discounting. Sleep. 2018;41(9):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reynolds B, et al.. Measuring state changes in human delay discounting: an experiential discounting task. Behav Processes. 2004;67(3):343–356. [DOI] [PubMed] [Google Scholar]
- 24. Acheson A, et al.. Effects of sleep deprivation on impulsive behaviors in men and women. Physiol Behav. 2007;91(5):579–587. [DOI] [PubMed] [Google Scholar]
- 25. Libedinsky C, et al.. Sleep deprivation alters effort discounting but not delay discounting of monetary rewards. Sleep. 2013;36(6):899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams PG, et al.. Toward an individual differences approach to habitual short sleep duration: a reply to Massar and Chee. Sleep. 2019;42(4). doi: 10.1093/sleep/zsz035 [DOI] [PubMed] [Google Scholar]
- 27. Cespedes EM, et al.. Comparison of Self-Reported sleep duration with actigraphy: results From the Hispanic Community Health Study/Study of Latinos Sueño Ancillary Study. Am J Epidemiol. 2016;183(6):561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lauderdale DS, et al.. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weaver EM, et al.. Polysomnography vs self-reported measures in patients with sleep apnea. Arch Otolaryngol Head Neck Surg. 2004;130(4):453–458. [DOI] [PubMed] [Google Scholar]
- 30. Lim J, et al.. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136(3):375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watson NF, et al.. Sleep duration and depressive symptoms: a gene-environment interaction. Sleep. 2014;37(2):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grandner MA, et al.. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 2014;15(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grandner MA, et al.. Mortality associated with short sleep duration: the evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14(3):191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Forbes EE, et al.. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Payne JW. It is whether you win or lose: the importance of the overall probabilities of winning or losing in risky choice. J Risk Uncertain. 2005;30(1):5–19. [Google Scholar]
- 36. Delgado MR, et al.. Fiez JA. Motivation-dependent responses in the human caudate nucleus. Cereb Cortex. 2004;14(9):1022–1030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.