Abstract
Rationale
Comorbid use of heroin and cocaine is highly prevalent among drug users and can greatly increase addiction risk. Nonetheless, little is known regarding how a multi-drug history impacts motivation and cue responsivity to individual drugs.
Objective
We used behavioral-economic procedures to examine motivation to maintain drug consumption and tests of drug-seeking to drug-associated cues to assess sensitivity to heroin and cocaine-associated cues in rats that had a self-administration history of heroin, cocaine, or both drugs.
Results
Unexpectedly, we found that groups with a polydrug history of heroin and cocaine did not have higher levels of motivation or cue-induced reinstatement of drug-seeking for either cocaine or heroin compared to single drug groups. Nonetheless, we did find drug-specific differences in both economic price and cue sensitivity. Specifically, demand elasticity was lower for cocaine compared to heroin in animals with a single drug history, but not with polydrug groups. In addition, cocaine demand was predictive of the degree of cue-induced reinstatement of drug-seeking for cocaine following extinction, whereas heroin demand was predictive of the degree of reactivity to a heroin-associated cue. Furthermore, although cue reactivity following the initial self-administration phase did not differ across cues and drug history, reactivity to both heroin and cocaine cues was greater during subsequent heroin use compared to cocaine use, and this enhanced reactivity to heroin cues persisted during forced abstinence.
Conclusions
These results indicate that there is a greater motivation to maintain cocaine consumption, but higher sensitivity to drug-associated cues with a history of heroin use, suggesting that cocaine and heroin may drive continued drug use through different behavioral processes.
Keywords: Drug addiction, Rodent, Behavioral economics, Reward
Introduction
Comorbid use of heroin and cocaine is frequently reported in clinical research of various populations with substance use disorders (Leri et al. 2003, 2004; Williamson et al. 2007; Wang et al. 2017). In fact, studies have found that up to 80% of people with a heroin use disorder also use cocaine (Leri et al. 2003), and are 15 times more likely to develop a cocaine use disorder than people that only use cocaine (Substance Abuse and Mental Health Services Administration 2014). Notably, involvement of opioids in cocaine overdoses has increased from 29 to 63% from 2000 to 2015 (McCall Jones et al. 2017). Thus, cocaine and heroin co-use is not only widespread, but it is also associated with unmet physical and mental healthcare needs, poorer treatment outcomes, and greater addiction severity, including persistence of drug use and risk of overdose (Evans et al. 2017; Hartel et al. 1995; Kerr et al. 2005; Kinner et al. 2012; Lorvick et al. 2018). It is critical, therefore, to have a better systematic understanding of the neurobiological alterations resulting from polysubstance use, as well as how drug history impacts addiction severity. To date, the majority of animal studies investigating these questions have examined simultaneous use of these drugs, such as with “speedball” administration of heroin and cocaine (Pattison et al. 2014), but have largely ignored sequential use, although this pattern of multi-drug taking is reportedly more ubiquitous (Leri et al. 2004; Hayden et al. 2014).
The purpose of this study was to begin to address this gap in the research by investigating the effects of drug history on two measures of addiction severity. Accordingly, we established a paradigm whereby rats were trained to self-administer single (cocaine or heroin) or multiple (alternating sessions of cocaine and heroin) drugs before undergoing assessment on several tests of addiction-like behavior, including motivation to maintain drug consumption and sensitivity to drug-associated cues. Motivation was examined using a behavioral economics paradigm which permits quantitative comparison of demand for different rewards across normalized measures (Hursh 1991). This method is advantageous over the more commonly used progressive ratio (PR) schedules of reinforcement because price is increased by decreasing the unit dose of a drug rather than increasing effort, thereby avoiding confounds of delay on cost and ceiling effects of limited consumption that are inherent to PR schedules (Bickel et al. 1990; Schelp et al. 2017). Drug-seeking was assessed by both cue-induced reinstatement following extinction and by cue reactivity tests administered at multiple timepoints after forced abstinence from rewards. Given reports that heroin users show inelasticity to heroin and cocaine price, but cocaine users show elastic demand for cocaine (Jofre-Bonet and Petry 2008), we hypothesized that a polydrug history would result in greater demand for cocaine and heroin compared to single drug histories, with all groups showing inelastic demand for heroin consumption. In addition, we predicted that polydrug groups would show greater cue-induced responding for both cocaine and heroin compared to single drug groups.
Methods
Animals
All experiments were approved by the Seattle Children’s Research Institute Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health guidelines. Male Sprague-Dawley rats (n = 38 Envigo) weighing 250–274 g upon arrival were pair-housed in a temperature- and humidity-controlled vivarium on a 12-h light/dark cycle. Rats were acclimated for at least 3 days prior to any experimental manipulations. Food was provided ad libitum until the start of the experiment, after which rats were mildly food restricted and fed 40 ± 1 g of chow per day per cage. Water was available ad libitum. Prior to the start of self-administration, rats were divided into three groups pseudorandomly: rats that self-administered a single drug daily (single drug high (SDH); cocaine, n = 7 or heroin, n = 6), rats that alternated between drug and flavored food pellet self-administration sessions (single drug low (SDL); cocaine + flavored food pellets, n = 6 or heroin + flavored food pellets, n = 6), and rats that alternated administration of two drugs (polydrug (PD): cocaine + heroin, n = 7 (experiment 1) and n = 6 (experiment 2)). Experimenters were not blinded to group assignment.
Drugs
Cocaine HCl and Diamorphine HCl were obtained from the National Institute on Drug Abuse and were dissolved in sterile 0.9% saline.
Surgery
Rats were anesthetized with isoflurane (2–5% inhaled; Patterson Veterinary) during the procedure and received an injection of meloxicam (0.1 mg/kg SC; Patterson Veterinary) prior to surgery for analgesia. Rats were monitored for a minimum of three recovery days following surgery prior to the start of self-administration. Chronic indwelling catheters were placed into the right jugular vein and attached to a back-mounted port as previously described (Crombag et al. 2000). Following surgery, catheters were flushed daily with 0.1 mL of sterile saline containing 10 mg/mL gentamicin sulfate (Patterson Veterinary) to prevent occlusions and minimize risk of infection. Catheter patency was tested prior to the first self-administration session and following completion of each experimental phase by IV injection of up to 0.3 mL of methohexital sodium (Brevital sodium: 10 mg/mL in sterile water; Patterson Veterinary). Rats that became ataxic within 5 se were considered to have patent catheters. If catheter patency was lost during any phase of the paradigm, the rat was removed from subsequent analysis.
Behavior
Self-administration chambers
Behavioral testing occurred in 20 standard operant self-administration chambers equipped with retractable levers, stimulus lights, a house light, a feeder, and a metal grid floor (Med Associates ENV-007CT). The front wall housed two white stimulus lights, one located above each lever, with an additional green stimulus light above the white stimulus light on one side. The back wall contained a white house light. A syringe pump (Med Associates PHM-100VS), located outside on top of the chamber, delivered either cocaine or heroin via tubing attached to the catheter backport. All tubing was attached to a suspended swivel (Instech 375/22) to allow rats to move freely within the chambers.
Self-administration training
A timeline of the full behavioral paradigm is shown in Fig. 1a. Depending on group assignment and session, a lever response resulted in presentation of a cue light and either a 0.4 mg/kg infusion of cocaine, a 0.15 mg/kg infusion of heroin (each delivered in 50 μL over 2.8 s), or administration of four food pellets (banana- or chocolate-flavored), followed by a 20-s timeout period during which the cue light above the lever was illuminated. Sessions occurred 7 days per week during the light cycle and alternated every other day between administration of cocaine (experiment 1) or heroin (experiment 2) + presentation of a white cue light on one lever (lever A) and administration of cocaine, heroin, or flavored food pellets + presentation of a green cue light on the alternate lever (lever B). This design resulted in the following groups: rats that self-administered a single drug daily (single drug high (SDH); cocaine or heroin), rats that alternated between drug and flavored food pellet self-administration sessions (single drug low (SDL); cocaine + flavored food pellets or heroin + flavored food pellets), and rats that alternated administration of two drugs (polydrug (PD): cocaine + heroin). Once a reinforcer was assigned to lever A and lever B, it was not changed for the duration of the experiment. No inactive lever was presented. Sessions lasted 2 h on a fixed ratio (FR1) schedule to establish modest drug intake without escalation so that potential group differences in ad libitum consummatory behavior could be observed while avoiding confounds from differences in appetitive seeking that are inherent to higher-order ratio schedules (Roberts et al. 2013). The doses of heroin and cocaine were selected because they produce reliable levels of self-administration; both doses are on descending limbs of the dose-response curve, which provides greater intake compared to other doses (Kerstetter et al. 2016; Leri et al. 2001; Yager et al. 2019).
Fig. 1.
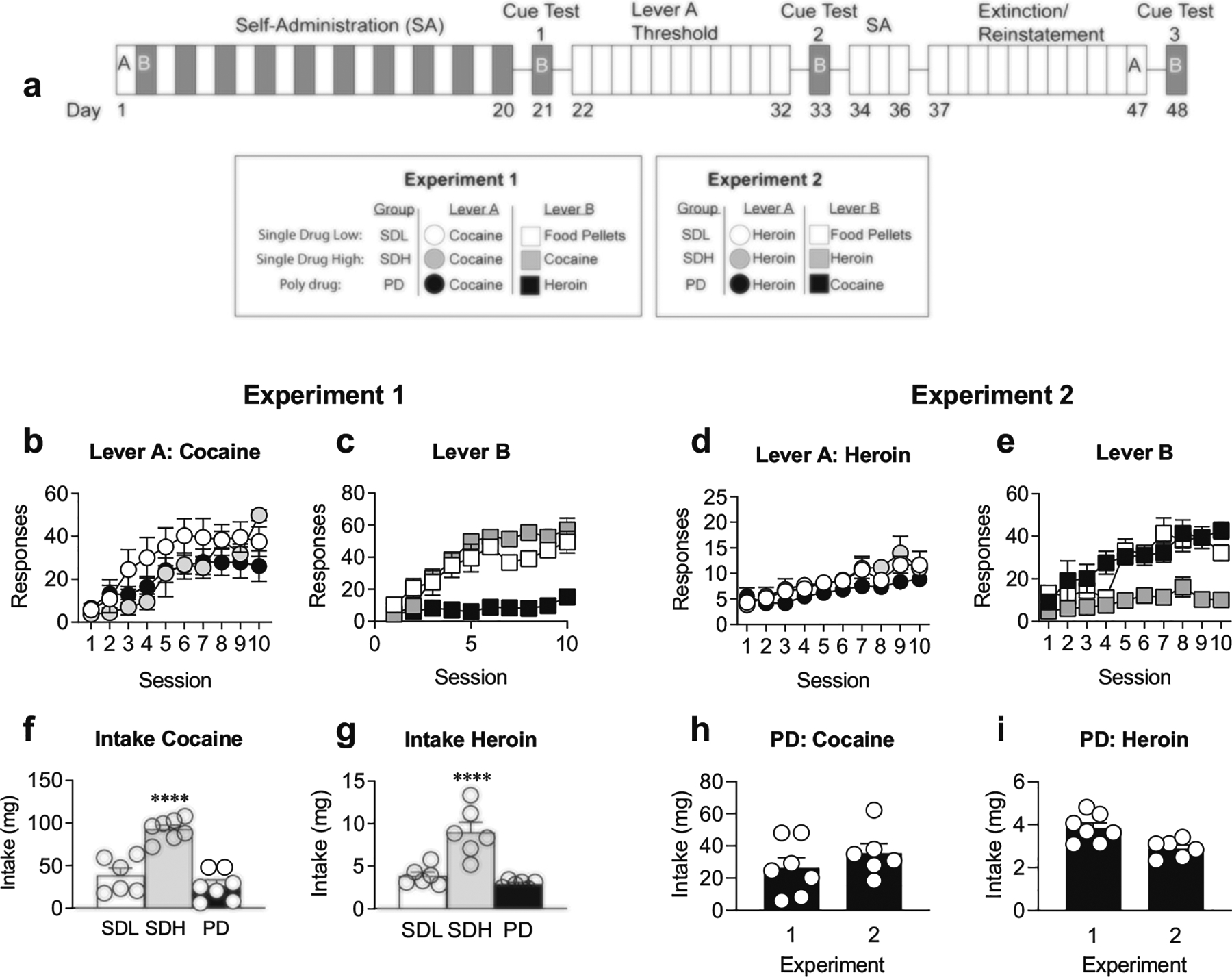
Self-administration paradigm. a Timeline of experimental paradigm. Lever A (white boxes) was associated either with an infusion of cocaine (experiment 1) or heroin (experiment 2) across all groups. Lever B (gray boxes) was paired with either: flavored food pellets (SDL), cocaine (experiment 1: SDH; experiment 2: PD), or heroin (experiment 1: PD, experiment 2: SDH). b Experiment 1 SDL (white circles), SDH (gray circles), and PD (black circles) groups acquire cocaine self-administration across ten total sessions on lever A. c Experiment 1 SDL (white squares), SDH (gray squares), and PD (black squares) groups acquire food pellet, cocaine, and heroin administration, respectively, across ten sessions on lever B. d Experiment 2 SDL (white circles), SDH (gray circles), and PD (black circles) groups acquire heroin self-administration across ten sessions on lever A. e Experiment 2 SDL (white squares), SDH (gray squares), and PD (black squares) groups acquire food, heroin, and cocaine self-administration, respectively, across ten sessions on lever B. f Intake for cocaine is greater for SDH history than SDL or PD history across all self-administration sessions. g SDH intake for heroin is higher than SDL and PD groups. h Total intake of cocaine in PD rats across ten sessions did not differ between experiments 1 and 2. i Total intake of heroin in PD rats across ten sessions did not differ between experiments 1 and 2. ****p < 0.0001, across drug history groups. Data are presented as mean ± SEM. Experiment 1: SDL n = 6, SDH n = 7, PD n = 7; experiment 2: SDL n = 6, SDH n = 6, PD n = 6
Cue reactivity tests
Following the end of the initial self-administration phase, rats underwent three, 2-h sessions of cue responding on the lever where the reinforcer was previously paired with the green stimulus light (i.e., lever B). For these tests, rats were connected to the infusion line, placed in the chamber, and each session began with extension of lever B. Responses on a FR1 schedule resulted in illumination of the green cue light above the lever for 20 s, but no reward administration. Tests occurred at three timepoints: 24 h following the final self-administration session, 24 h following the final day of the threshold procedure, and 2 days after cue-induced reinstatement on lever A (i.e., at 1, 13, and 29 days following the end of initial self-administration training). Unlike the cue-induced reinstatement test on lever A, no extinction sessions were performed for lever B prior to each cue reactivity session.
Behavioral economics
Twenty-four hours after the first cue reactivity test, rats underwent a between-session threshold procedure. In this paradigm, the unit price (responses/mg of drug) of cocaine or heroin was increased each session by reducing the drug concentration (mg/kg) that was infused for each lever press by a quarter-log scale (0.421, 0.237, 0.133, 0.075, 0.041, 0.024, 0.013, 0.075, 0.0041, 0.0024, 0.0013 mg/kg per infusion) for cocaine, or a third-log scale (0.533, 0.247, 0.115, 0.053, 0.025, 0.012, 0.005, 0.002, 0.001, mg/kg per infusion) for heroin. Rats had access to drug for the entirety of each 2-h self-administration session, with no timeout periods between infusion. Because drug concentration was changed between sessions, the flow rate, volume, and infusion time were kept constant across sessions, unlike previous studies which decreased infusion time to change dose (Oleson and Roberts 2009). Infusions were administered on an FR1 schedule and were paired with illumination of the white cue light during each infusion (50 μL in 2.8 s).
Demand curve analysis
Demand curve analysis was performed by graphically extracting parameters (Qo, Pmax, Omax) for each animal as previously described (Oleson and Roberts 2009). Q0 was determined from the average responding at the highest unit dose of drug (average intake during first three threshold sessions) when drug price is minimal. Pmax was graphically determined as the inverse of the unit dose eliciting maximal levels of responding prior to reduction in responding, and decreased intake from Q0 (apex of the response curve; derivative of intake curve at − 1). Omax was defined as the number of responses at Pmax. Alpha (α) is a measure of consumption that is sensitive to price, with higher values corresponding to faster declines in the demand curve, indicating higher price sensitivity and elasticity (Schelp et al. 2017). This value was calculated using the exponential demand equation (Hursh and Silberberg 2008).
Extinction
Twenty-four hours after the second cue reactivity test on lever B, rats underwent three additional days of self-administration of cocaine or heroin on lever A (depending on their initial drug assignment to that lever) followed by extinction training on that lever. Extinction sessions lasted for 2 h and consisted of extension of lever A (i.e., the lever that had been paired with the white cue light). Responses on the lever had no programmed consequence (i.e., no drug infusion or stimulus light illumination). Extinction training lasted for 10 days.
Cue-induced reinstatement
Following the final extinction session, rats underwent a 2-h cue-induced reinstatement test for cocaine or heroin on lever A (depending on their initial drug assignment to that lever). The session began with extension of lever A into the chamber. Responses on the lever resulted in illumination of the white cue light above the lever but no drug infusion.
Statistical analysis
Analyses were performed using GraphPad Prism 8. FR1 sessions on lever A between groups were analyzed using two-way repeated measures (RM) analyses of variance (ANOVA)s. FR1 sessions were analyzed for each lever using one-way RM ANOVAs, with comparisons against the first self-administration session. Total intake across groups was analyzed using one-way ANOVA. Paired, two-tailed t tests were performed for comparing total infusions on levers A and B. Group comparisons of demand curves were made with two-way (drug history × price) ANOVAs for response and intake curves, and group comparisons of behavioral economic parameters were made using ordinary one-way ANOVAs. Responding during extinction and reinstatement was analyzed with RM two-way (drug history × time) ANOVAs. Cue reactivity was compared for each reinforcer across experiments using a two-way (experiment × drug cued) ANOVA, and cue tests using RM two-way (session × experiment) ANOVA. Linear regressions were performed to determine the relationships between α and Q0 values and extinction day one, reinstatement, cue test two, and cue test three. RM ANOVAs with over two factors were adjusted for unequal variability of differences using Geisser-Greenhouse sphericity corrections. All post-hoc analyses were Bonferroni corrected for multiple comparisons, unless otherwise stated.
Results
No group differences in the acquisition of cocaine or heroin self-administration
A summary of the experimental design and timeline is shown in Fig. 1a. Briefly, rats were divided into three groups prior to beginning each experiment: Rats that self-administered a single drug daily (single drug high (SDH)), rats that alternated between self-administration of drug and flavored food pellets (single drug low (SDL)), and rats that alternated between self-administration of cocaine and heroin (polydrug (PD)). A two-way RM ANOVA of lever A sessions for each drug history group revealed no differences in responding for cocaine or heroin across groups, but did reveal that all groups learned to self-administer the lever A reward across ten sessions (Fig. 1b: main effect of session: experiment 1 (cocaine lever A): F(1.88, 31.97) = 24.69, p < 0.0001; Fig. 1d: experiment 2 (heroin lever A): F(2.66, 39.90) = 12.00, p < 0.0001). For lever A cocaine administration, there was a significant interaction of session × drug history, suggesting there were differences in learning rates across groups for the lever A reward (session × drug history cocaine (lever A): F(18,153) = 2.40, p = 0.002). Post-hoc analyses show greater cocaine intake on the final lever A session in SDH vs. PD groups (Bonferroni-corrected, p < 0.05). RM one-way ANOVAs for self-administration across sessions for lever B rewards revealed that, although the number of responses varied by reinforcer, all groups increased their responses over sessions in experiment 1 (Fig. 1c; lever B; SDL-food: F(3.56, 17.79) = 8.96, p = 0.0005; SDH-cocaine: F(2.31, 13.88) = 27.03, p < 0.0001; PD-Heroin: F(1.98, 11.86) = 3.88, p = 0.051) and in experiment 2 (Fig. 1e; lever B; SDL-food: F(2.68, 13.39) = 14.15, p < 0.0005; SDH-heroin: F(1.37, 6.84) = 4.22, p = 0.07; PD-cocaine: F(2.35, 11.74) = 10.49, p < 0.005). As expected, since SDH groups have 20 self-administration sessions of cocaine or heroin compared to the 10 sessions of the other groups, ordinary one-way ANOVAs for drug history revealed that the SDH groups had significantly higher levels of total drug intake of cocaine (Fig. 1f; F(2,17) = 30.26, p < 0.0001) and heroin (Fig. 1g; F(2, 15) = 19.73, p < 0.0001). However, Bonferroni-corrected post-hoc analyses showed no differences in intake levels between the SDL and PD groups for either cocaine or heroin. In addition, responses on lever A and B did not significantly differ in SDH groups for cocaine or heroin, indicating that responding was not biased toward a particular lever (paired t test for infusions on levers A and B; cocaine (Fig. 1h) or heroin (Fig. 1i)). To determine if the order of heroin and cocaine self-administration impacted total intake in the PD group, intake of this group was compared across experiments. Two-way ANOVA analysis revealed no significant main effect of experiment and no significant interaction of drug × experiment, suggesting that intake of both drugs was not influenced by which drug was initially self-administered. Nonetheless, as noted with other analyses, differences in total intake were found between cocaine and heroin (Fig. 1h, i; main effect of drug: F(1,11) = 38.79, p < 0.0001).
Behavioral economics
Across-session threshold procedures were used to assess motivation as a function of drug history. Multiple demand parameters were extrapolated from the threshold procedures, and include Qo, which is the initial intake of drug at minimal to no cost of consumption; Pmax, which is the maximal price individuals are willing to pay to maintain initial intake levels (i.e., to defend Qo); Omax, which is the level of responding at Pmax; and alpha (α), which is a normalized measure that is inversely related to Pmax normalized to Qo, and is representative of price elasticity. Relative to cocaine, heroin demand curves were noticeably rightward shifted to higher price points for all animals, with some rats persistently increasing responses even at the lowest price points (Fig. 2a, b).
Fig. 2.
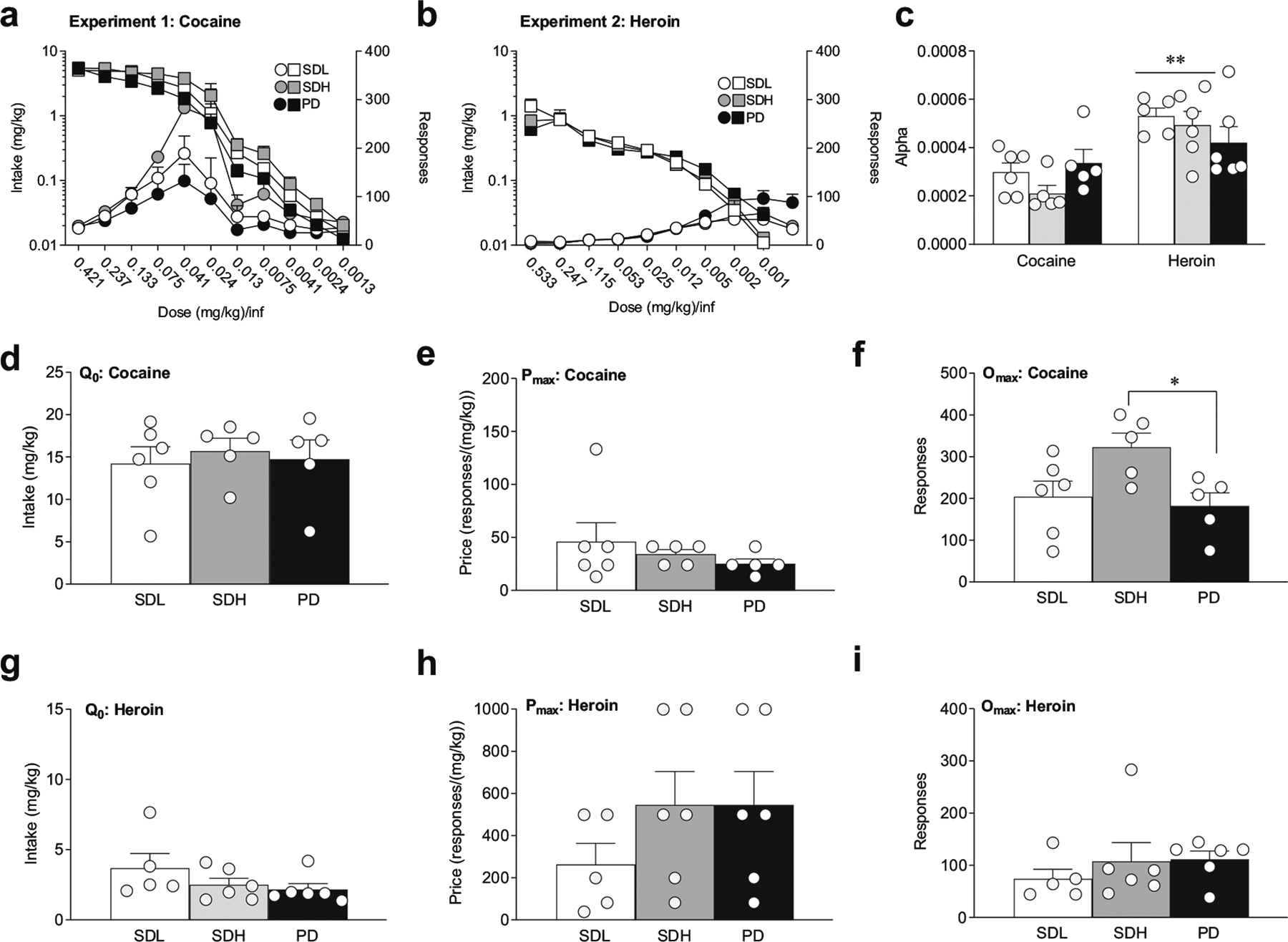
Cocaine and heroin demand as a function of drug history. a, b Intake (squares) and response (circles) curves for cocaine (a) and heroin (b). The number of lever presses (right y-axis) and total mg infused (left y-axis) at each price point are shown for SDL (white), SDH (gray), and PD (black) groups. Price is increased with decreasing doses of cocaine (experiment 1) or heroin (experiment 2) per infusion. c Price elasticity (α) for SDL, SDH, and PD groups for cocaine and heroin threshold. Cocaine α was significantly lower for SDL and SDH compared to heroin α, but not for PD groups d No-cost cocaine intake (Qo) is independent of drug history. e Maximal price to maintain initial intake (Pmax) for cocaine is independent of drug history. f Responding at Pmax for cocaine is larger with greater consumption history than polydrug history (SDH vs. PD). g No-cost heroin intake (Qo) is independent of drug history. h Heroin Pmax is independent of drug history. i Responding at Pmax does not depend on drug history. **p < 0.01, across drugs. Data are presented as mean ± SEM. Cocaine (experiment 1): SDL n = 6, SDH n = 5, PD n = 5; heroin (experiment 2): SDL n = 5, SDH n = 6, PD n = 6
Demand for cocaine is not dependent on cocaine drug history
All groups in experiment 1 exhibited a sensitivity to changes in price for cocaine, as indicated by a decrease in responding at lower doses in the threshold procedure (Fig. 2a; two-way RM ANOVA, main effect of price on responding: F(2.33, 32.65,) = 25.63, p < 0.0001; main effect of price on intake: F(2.13,29.76) = 75.92, p < 0.0001). This sensitivity differed by drug history (Fig. 2a, main effect of drug history on responding: F(2,14) = 5.56, p < 0.05; interaction of price × drug history: F(20,140) = 2.01, p = 0.01), but not by intake; post-hoc Bonferroni-corrected analyses indicate that differences in response at each price occurred between SDH and PD groups (p < 0.05). There were no significant group differences in Qo (Fig. 3d), which averaged between 14 and 16 mg/kg, or Pmax (Fig. 2e). However, Omax was significantly higher in the SDH group compared to the PD group, consistent with response vs. price analyses (Fig. 2f; one-way ANVOA, F(2,13) = 4.47, p < 0.05; Bonferroni-corrected post-hoc comparisons: SDH versus PD, p < 0.05; SDH versus SDL, p = 0.09). Taken together, these results suggest that a higher level of cocaine intake during training elicits greater overall responding for cocaine, but drug history does not alter baseline consumption or motivation to self-administer cocaine.
Fig. 3.
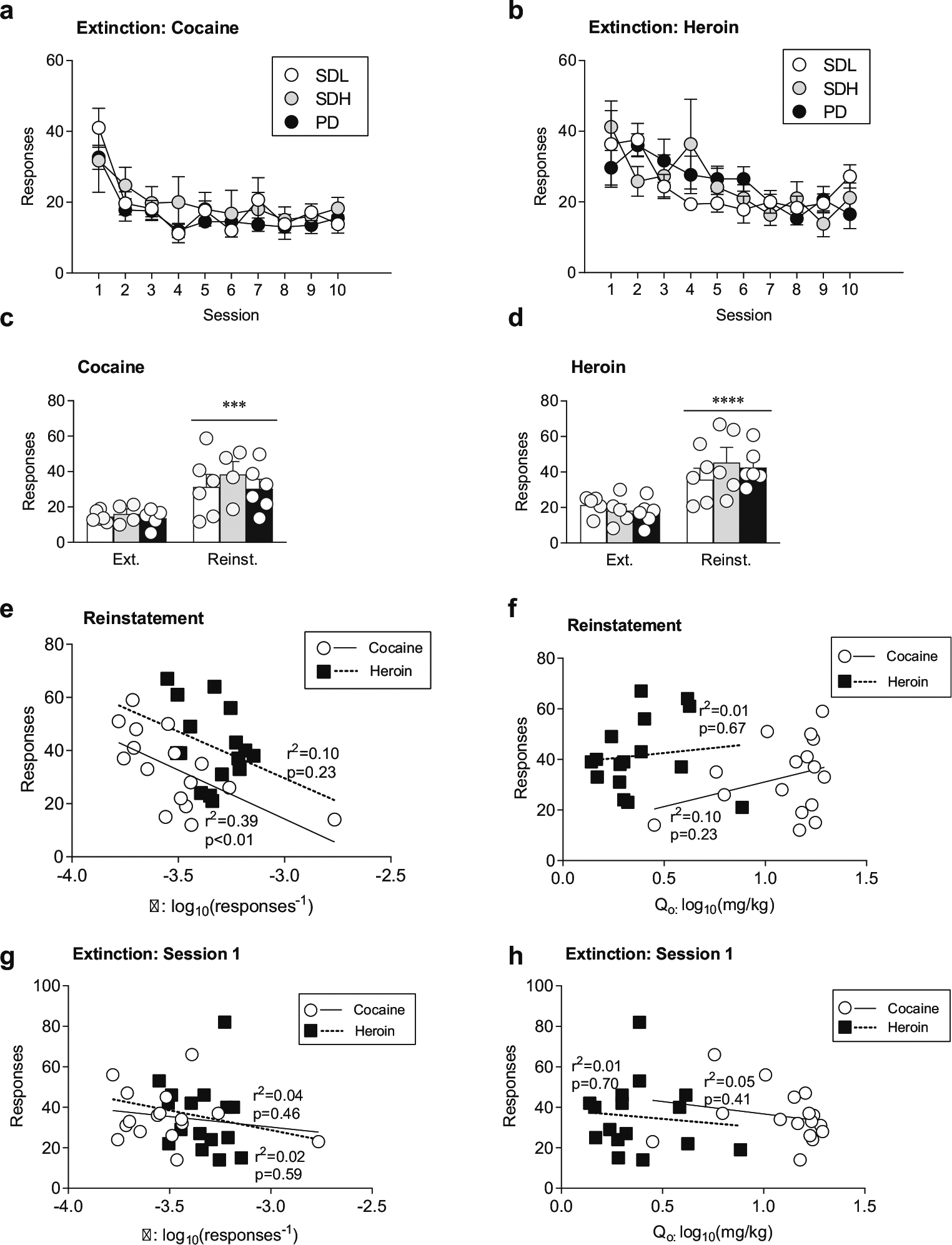
Cue-induced reinstatement as a function of drug history. a, b All groups decrease lever A responding for cocaine (a) or heroin (b) after removal of drug and cue pairing for ten sessions. c. All groups reinstate similarly to a cocaine cue following extinction. d Groups with a history of daily drug administration (SDH and PD), but not with intermittent drug access (SDL) increase responding when presented with heroin cues. The levels of cue responding are similar across groups for cocaine and heroin cues. e Price elasticity (log10(α)) is predictive of the degree of reinstatement of drug-seeking to cocaine cues (white circles, solid regression line), but not heroin cues (black squares, dashed regression line). f No-cost drug intake (log10(Q0)) is not predictive of reinstatement of drug-seeking to cocaine or heroin cues. g α is not predictive of the level of responding during the initial extinction session for cocaine or heroin. h Q0 is not predictive of reinstatement for cocaine or heroin cues. Drug history groups: SDL (White circles, bars), SDH (gray circles, bars), PD (black circles, bars). ***p < 0.001, ****p < 0.0001, compared to extinction. Data are presented as mean ± SEM. Extinction vs. reinstatement testing was performed using the average of the last three days of extinction. Cocaine: SDL n = 6, SDH n = 4, PD n = 6; heroin: SDL n = 5, SDH n = 5, PD n = 6. Linear regressions for cocaine and heroin were from reinstatement and extinction day 1 results collapsed for all groups
Demand for heroin is not dependent on heroin drug history
As with cocaine, all groups in experiment 2 exhibited a sensitivity to changes in price for heroin (Fig. 2b, main effect of price on responding: F(2.05,26.64) = 29.67, p < 0.0001; main effect of price on intake: F(1.65, 21.41) = 22.99, p < 0.0001); however, there were no significant differences in sensitivity between groups, though there was a trend for differences in responding (Fig. 2b). An interaction was found for price × drug history on responding (F(16,104) = 2.51, p < 0.01), but not on intake. Bonferroni-corrected post-hoc analyses, however, did not reveal significant differences in responding between groups at any price. In addition, there were no significant differences in Q0 (Fig. 2g), Pmax (Fig. 2h), or Omax (Fig. 2i) as a function of drug history. These results indicate that neither the degree of heroin intake nor combining heroin use with cocaine use altered the motivation to self-administer heroin.
Price sensitivity is lower for cocaine than heroin
Alpha (α) provides a normalized measure of elasticity to changes in price, which permits comparison of price sensitivities across different reinforcers (Hursh and Winger 1995). Thus, we can use α to assess and compare the relative demand elasticity of heroin and cocaine across drug histories. We found drug-specific differences in α values, with significantly higher heroin values compared to cocaine values (Fig. 2c, two-way ANOVA: main effect of drug α: F(1,27) = 23.31, p < 0.0001). Interestingly, this difference was only in groups with single drug histories, as α values for heroin and cocaine were not significantly different in the PD groups (Fig. 2c, Bonferroni-corrected post-hoc tests: SDL cocaine vs. heroin: p < 0.01; SDH cocaine vs. heroin: p < 0.001). Together, these data suggest that cocaine has a greater motivational value than heroin, but that this distinction is eliminated with a history of self-administering both drugs.
Motivation for cocaine but not heroin is predictive of cue-induced reinstatement of drug-seeking
We next examined whether drug history affects reinstatement of drug-seeking by a drug-associated cue. Following the threshold procedure and three additional days of cocaine (experiment 1) or heroin (experiment 2) self-administration, rats underwent ten sessions of extinction training on lever A. As expected, all groups significantly decreased responding across extinction sessions (Fig. 3a, two-way ANOVA; cocaine: main effect of session, F(2.73, 35.52) = 13.18, p < 0.0001; Fig. 3b, two-way ANOVA; heroin: main effect of session, F(2.87,37.28) = 7.58, p = 0.0005), but extinction responding did not differ across drug histories. There was a session × drug history interaction for heroin extinction (heroin: F(18,117) = 1.73, p < 0.05) though post-hoc analyses did not report differences in responding across any groups for any extinction session. In addition, all groups in experiment 1 made significantly more lever responses during the cocaine cue-induced reinstatement test compared to the last 3 days of extinction (Fig. 3c, main effect of reinstatement, F(1,13) = 24.27, p < 0.001). However, there were no significant differences in lever responses across groups. For experiment 2, there was a difference in responding between extinction and reinstatement (Fig. 3d, heroin: main effect of reinstatement, F(1,13) = 53.67, p < 0.0001). As with cocaine reinstatement, there were no significant differences in lever responses between groups for the cue associated with heroin. Together, these data suggest that neither the total amount of drug intake nor combining drug use with alternate reinforcers impacted the degree of drug-seeking for a cue associated with cocaine or heroin use.
To determine if no-cost drug intake or price elasticity were predictive of cue-induced drug-seeking following extinction, regressions for Qo and α versus responding during the reinstatement tests were performed, as in previous studies (Bentzley et al. 2014; Cox et al. 2017). Groups were collapsed for regression analyses. Price elasticity (i.e., α) was predictive of reinstatement to cues associated with cocaine (Fig. 3e: F(1,14) = 9.12, p < 0.01, R2 = 0.39) but not heroin, with lower α values associated with higher levels of cue-induced responding. In contrast, no-cost intake (i.e., Qo) was not predictive of cue-induced drug-seeking for either drug reward (Fig. 3f). Neither α nor intake were predictive of responding on the initial extinction session (Fig. 3g, h). These data suggest that the degree of motivation for cocaine, but not heroin, is predictive of the degree of cue-induced reinstatement of drug-seeking.
Heroin and cocaine use differentially affect cue reactivity
During the self-administration phase, groups that underwent cocaine (experiment 1) or heroin (experiment 2) self-administration on lever A also underwent self-administration for either cocaine, heroin, or food on lever B. Thus, this paradigm allows us to examine how drug history and continued use of one drug affects drug-seeking of another reinforcer after forced abstinence. Cue reactivity tests were administered on lever B at three time points throughout the paradigm, with no extinction sessions on this lever prior to these cue tests: 24 h after the last self-administration session, 13 days after the last self-administration session (which was 24 h following the final threshold session), and 29 days after the last self-administration session (which was 12 days after cessation of all drug self-administration) (Fig. 4a). There were no differences in cue-induced responding on test 1 across groups or across experiments (Table 1), indicating that baseline cue reactivity was the same for all cues, and was not influenced by drug history. In contrast, groups that were subsequently maintained on heroin (i.e., the SDL, SDH, and PD groups from experiment 2) showed significantly greater responding for all reward-associated cues on test 2 compared to groups that were subsequently maintained on cocaine (i.e., the SDL, SDH, and PD groups from experiment 1) (Table 1; main effect of experiment F(1,29) = 32.19, p < 0.0001). In addition, although responding was not different across cues associated with heroin, cocaine, or food in groups being maintained on cocaine, there was a significant difference in responding across these cues in groups being maintained on heroin (i.e., differences between SDL, SDH, and PD groups in green cue responding from experiment 2) (Table 1; main effect of cue F(2,29) = 3.53, p < 0.05). Similarly, following a period of complete drug abstinence, animals that had a history of heroin use showed significantly greater responding for cues associated with all rewards on test 3 compared to animals that had a history of cocaine (i.e., greater responding across all groups from experiment 2 vs. groups from experiment 1) (Fig. 4d; main effect of experiment F(1,28) = 10.56, p < 0.01). These results suggest that cue reactivity is more persistent during continued heroin use and continues into abstinence.
Fig. 4.
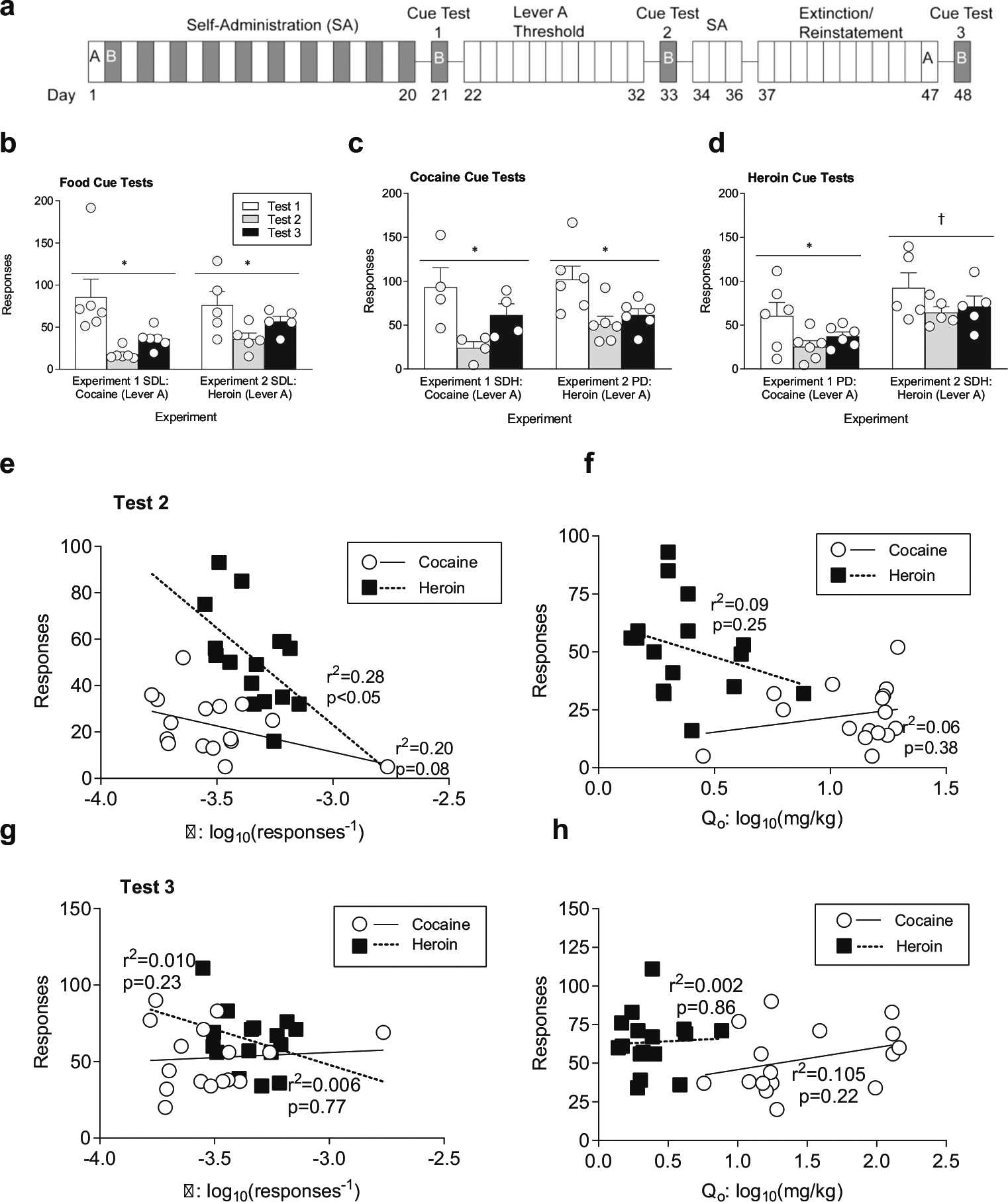
Cue reactivity as a function of drug history. a Lever B cue tests were administered: 1) 24 h after the final self-administration session on lever B (test 1, white bars), 2) 13 days after self-administration (test 2, gray bars), and 3) 29 days after self-administration (test 3, black bars). b With cocaine administration (experiment 1), responding to food cues (SDL group) decreases between the first and second test, and begins to rebound between tests two and three. A history of heroin administration (Experiment 2) decreases food cue reactivity (SDL group) between the first and second tests. c Cocaine cue reactivity decreases during the period of cocaine thresholding (experiment 1, SDH group test 2), and rebounds during complete drug abstinence (test 3). Heroin administration (experiment 2, PD group) results in maintained reduction in cue reactivity to cocaine (test 2), even after complete drug abstinence (test3). d Responding to heroin cues decreases with cocaine administration (experiment 1, PD group, test 2) between cue tests, while maintenance on heroin (experiment 2, SDH group) produces persistent heroin cue reactivity. e Price elasticity (log10(α)) is predictive of cue reactivity following heroin (black squares, dashed regression line), but not cocaine (white circles, solid regression line) thresholding (cue test 2). f No-cost intake (log10(Q0)) of cocaine or heroin is not predictive of cue reactivity following thresholding (cue test 2). g α is not predictive of reactivity to cues following abstinence from lever A rewards (experiment 1: cocaine, experiment 2: heroin; cue test 3). h Q0 is not predictive of reactivity to cues following abstinence from lever A rewards (experiment 1: cocaine, experiment 2: heroin; cue test 3). Linear regressions for experiment 1, cocaine lever A (white circles, solid regression line) and experiment 2, heroin lever B (black squares, dotted regression line) cue reactivity responding with SDL, SDH, and PD groups collapsed for each experiment against behavioral economic measures α and Q0. *p < 0.05, significance between cue tests, †p < 0.05, significance between experiments. Data are presented as mean ± SEM. Cocaine: SDL n = 6, SDH n = 4, PD n = 6; heroin: SDL n = 5, SDH n = 5, PD n = 6. Linear regressions for experiment 1: cocaine and experiment 2: heroin cue reactivity tests 2 and 3 responding were collapsed for all groups
Table 1.
Cue reactivity tests of lever B rewards after forced abstinence. Calculated least squares mean for both experiment 1 (cocaine lever A) and experiment 2 (heroin lever A), and two-way ANOVA of drug cued on lever B × experiment 1 vs. 2 for each cue test. Data are presented as mean ± standard error of the mean (SEM).
| Least squares mean of experiment 1: cocaine (lever A) | Least squares mean of experiment 2: heroin (lever A) | Mean difference (SEM of difference) | Two-way ANOVA: experiment | Two-way ANOVA: reward cued (lever B) | Two-way ANOVA: interaction (experiment × reward cued) | |
|---|---|---|---|---|---|---|
| Cue test 1 (day 1) | 80.28 | 90.70 | − 10.42 (14.77) | F (1,26) = 0.4979 | F (2, 26) = 0.7171 | F (2, 26) = 0.6866 |
| Cue test 2 (day 13) | 21.65 | 51.02 | − 29.37 (5.177) | F(1, 29) = 32.19**** | F (2, 29) = 3.525* | F (2, 29) = 1.531 |
| Cue test 3 (day 29) | 44.35 | 63.79 | − 19.44 (5.982) | F (1,28) = 10.56** | F (2, 28) = 1.774 | F (2, 28) = 2.549 |
p < 0.05,
p < 0.01,
p <0.0001 with respect to differences in responding between experiments or the reward corresponding to the lever B cue (i.e., responding for lever B rewards for SDL, SDH, and PD groups)
All animals underwent all three cue tests; therefore, this paradigm can also be used to determine how continued drug use and abstinence affect reactivity to the same cue over time. For both food- and cocaine-associated cues, there was a significant decrease in responding following a period of cocaine or heroin maintenance compared to initial responding (i.e., differences between SDL groups in experiments 1 and 2 for food cues and differences between the SDH group from experiment 1 and the PD group from experiment 2 for cocaine cues) (Fig. 4b, c; food cue: main effect of session: F(1.18,10.58) = 13.39, p < 0.01; cocaine cue: main effect of session: F(1.53,12.23) = 14.76, p = 0.001). In contrast, responses to a cue associated with heroin decreased following cocaine maintenance (i.e., the PD group from experiment 1) (Fig. 4d, main effect of session, F(1.35,12.10) = 5.52, p < 0.05; post-hoc Bonferroni corrected for row effect: test 2 p < 0.05), and this effect persisted following abstinence. However, responding to a heroin-associated cue did not change across tests in animals with a history of heroin use (i.e., the SDH group from experiment 2) (Fig. 4d, main effect of experiment: F(1,9) = 10.03, p < 0.05). Together, these results suggest that cued seeking is blunted for all rewards during ongoing cocaine use but only for non-heroin rewards during ongoing heroin use. In addition, this cued seeking begins to rebound during forced abstinence from cocaine but not heroin.
Linear regressions were calculated to determine the predictive validity of α and Q0 to drug-seeking following threshold testing for cocaine (experiment 1) or heroin (experiment 2). We found that α for heroin was significantly correlated with cue reactivity (Fig. 4e; α: F(1,14) = 5.38, p < 0.05, R2 = 0.28) and trended toward significance for cocaine elasticity and cue reactivity (Fig. 4e; α: F(1,14) = 3.48, p = 0.08, R2 = 0.20) when cue responsivity was tested 24 h after the threshold procedure. However, α did not correlate with cue responsivity following periods of total drug abstinence (i.e., during the third cue test) (Fig. 4g). In contrast, Q0 was not correlated with reactivity to reward-associated cues for cocaine or heroin when tested either at 24 h post-thresholding (Fig. 4f, cue test 2) or following complete drug abstinence (Fig. 4h, cue test 3). These data suggest that the degree of motivation for heroin, but not cocaine, is predictive of the degree of reactivity to reward-associated cues.
Discussion
In order to examine the effects of cocaine and heroin polydrug use on measures of addiction severity (economic demand parameters, cue-induced reinstatement following extinction, cue reactivity), we compared responses of rats that had a self-administration history of heroin, cocaine, or sequential use of both drugs. We found that groups with a polydrug history did not have higher levels of motivation or cue-induced reinstatement of drug-seeking for either cocaine or heroin when compared to single drug groups. This was surprising as previous work has found that extended periods of heroin self-administration enhance the motivating properties of cocaine, as demonstrated by increased breakpoints under a progressive ratio schedule of reinforcement (Ward et al. 2005). Nonetheless, given that studies have shown positive correlations between non-fatal overdoses and heroin/cocaine polydrug users (Kerr et al. 2007), as well as increased susceptibility to pulmonary disease and mechanical ventilation with cocaine use in heroin users (de Wit et al. 2008; Levine et al. 2005), understanding how the concurrent use of these drugs impacts other aspects of use, including the aversive consequences of withdrawal and overdose, would improve our understanding of potential risks and is essential for developing effective treatment options for patients with multi-drug dependencies.
Polydrug self-administering groups were compared to groups that self-administered either equivalent or larger amounts of cocaine or heroin, which allowed us to assess how total amount of drug intake, as well as multi-drug use, affects economic demand. Interestingly, we found that the total amount of drug consumed had no effect on demand elasticity for either cocaine or heroin. However, demand elasticity was predictive of the degree of cue-induced reinstatement of drug-seeking for cocaine, but not heroin. Previous studies have found that intermediate training doses of cocaine (0.5 and 1 mg/kg per infusion) and heroin (50 and 100 μg/kg per infusion) produce higher breakpoints on a progressive ratio schedule (Arnold and Roberts 1997) and greater persistence in responding to drug-associated levers during extinction (Leri and Stewart 2001) compared to lower or higher training doses. These inverted U-shaped dose-response curves are consistent with our findings for cocaine, with demand curves exhibiting a similar pattern and Pmax coinciding with a unit dose around 41 μg/kg/infusion. However, the demand curves for heroin were markedly different, as they continued to rise up to prices that are indicative of doses as low as 1 μg/kg/infusion, whereas previous work with multiple training doses found peak progressive ratio responding at a 50 μg/kg/infusion training dose (Arnold and Roberts 1997).
The present work utilized a 2-h continuous access schedule of sequential polydrug administration of cocaine and heroin; future studies utilizing self-administration paradigms that consistently produce escalation of drug intake, such as 6-h or intermittent access, may help to reveal whether demand elasticity of heroin can be modulated by drug consumption patterns, as is the case with cocaine. Intermittent access schedules, which produce cocaine intake levels that are comparable to short access schedules but under a different temporal pattern, lead to higher Pmax values and greater responding (Omax) during within-threshold procedures than either long- or short-access trained rats (Zimmer et al. 2012), suggesting pattern can supersede impact of total intake. Furthermore, environmental factors can also impact drug self-administration preference and affective valence in both human polydrug users and rodent polydrug self-administration models, with cocaine consumption preferred in non-resident environments and, conversely, preference for heroin in home environments (Caprioli et al. 2009; De Luca et al. 2018; De Pirro et al. 2018). In addition, polydrug users report differential cravings for cocaine and heroin throughout the day (Phillips et al. 2013). Thus, modulating the temporal and environmental conditions in our sequential use model may impact demand elasticity as well as sensitivity to drug-associated cues not currently captured in our paradigm.
This study did not examine whether polydrug use produces distinct or over-lapping molecular and circuit alterations, or if the effects are synergistic from those induced by exposure to a single drug. However, in support of over-lapping alterations, behavioral studies have found cross-locomotor sensitization between cocaine and heroin, as well as enhanced cocaine self-administration in rats undergoing protracted heroin withdrawal (Leri et al. 2003). Additionally, using a sequential heroin and cocaine self-administration paradigm, similar levels of glutamatergic neuronal activation were found following exposure to heroin- or cocaine-associated cues (Rubio et al. 2018) and methadone maintenance reduces cocaine-induced increases in mu-opioid receptor expression in the nucleus accumbens (Leri et al. 2006). In support of synergistic effects, sequential application of heroin and cocaine directly to cortical neurons induces greater neurotoxicity compared to exposure to either drug alone (Cunha-Oliveira et al. 2010).
Demand elasticity provides a normative measure of motivation across rewards; therefore, we can use this measure to directly compare motivation to cocaine and heroin in comorbid users. It was surprising that group differences were not found between the single- and polydrug groups as human studies have found differences in demand curves for drug purchases as a function of drug history, with cocaine purchases being inelastic in heroin users but elastic in cocaine users (Petry and Bickel 1998). Nonetheless, there was a stark contrast in demand curve patterns between cocaine and heroin. In particular, demand curves were shifted upward for cocaine and more sigmoidal in shape, indicating that cocaine produced a larger range of responses across the threshold procedure. In addition, responding for heroin was maintained by negligible doses per infusion, suggesting that heroin elicits perseverative responding. Of note, α values, which adjust for different no-cost intake levels, were lower for cocaine than heroin, indicating that cocaine demand is more price inelastic and has a greater motivational value than heroin. These findings are consistent with human studies of price elasticity, which found that polysubstance users make more cocaine purchases compared to heroin purchases across prices and that demand for heroin is largely inelastic across drug history, but are inconsistent with reports of elastic demand in single-substance cocaine users (Petry and Bickel 1998; Jofre-Bonet and Petry 2008). These results are also in-line with studies that found that rats will almost exclusively choose cocaine when given access to different combinations of cocaine or heroin (Ward et al. 2005) as well as show greater responding on a cocaine-associated lever when both cocaine- and heroin-associated levers are presented during extinction following a polydrug history (Leri and Stewart 2001).
Interestingly, we found that a polydrug history appears to remove the differences in price elasticity observed with single drug histories, which suggests that multi-substance use changes price valuation across drugs. Consistent with this, cross-price elasticity in human polydrug users has been reported to be drug specific, with increases in cocaine price reducing both cocaine and heroin consumption, while increases in heroin price augment cocaine consumption (Jofre-Bonet and Petry 2008). In addition, a study of heroin price changes in heroin users found that demand was equally inelastic for heroin and cocaine (Petry and Bickel 1998). Similarly, studies in rhesus monkeys comparing dose-dependent preferences for remifentanil and cocaine found minimal bias shifts at comparable doses, similar demand elasticity, and similar Pmax values (Koffarnus and Woods 2008; Wade-Galuska et al. 2007). Together, these data suggest that heroin and cocaine are highly substitutable, which in turn may explain the similarities in demand elasticity between cocaine and heroin that we observed in the PD group.
In order to examine sensitivity to the same cues over time, we used a forced abstinence model. Unlike traditional extinction and reinstatement paradigms, this paradigm does not extinguish action-response and environmental contingencies prior to drug-seeking tests where lever responding elicits delivery of cues previously associated with rewards. This model is thought to mimic scenarios in clinical populations where drug users undergo periods of voluntary or involuntary removal from drug access (See et al. 2007; Reichel and Bevins 2009). Following the end of the initial self-administration phase, we tested cue responsivity at baseline (i.e., 24 h later), as well as cue responsivity following continued use of cocaine or heroin (which occurred on a different lever and was associated with a different cue), and following a period of complete drug abstinence. Of note, baseline cue reactivity was not different across cues, nor was it affected by self-administration history. However, unlike with cues associated with food or cocaine, responsivity to cues associated with heroin was maintained across all three cue tests, but only in animals that continued to self-administer heroin between the first and second cue tests (i.e., during the threshold procedure). These results suggest a persistent sensitivity to heroin cues that was driven by ongoing heroin use. The reductions in responsivity to cues associated with cocaine over multiple tests are consistent with a previous study that found reduced responding on subsequent relapse tests for methamphetamine (Reichel and Bevins 2009). We also found that, unlike the relationship between demand elasticity and cue-induced reinstatement following extinction, demand elasticity was predictive of the degree of responsivity to heroin-associated cues, but not cocaine-associated cues. The heightened and persistent reactivity to heroin-associated cues is particularly concerning as reports indicate that the increases in putative relapse triggers (i.e., mood changes and exposure to drug-associated cues) that normally only precede cocaine use in single drug users are increased prior to the initiation of heroin craving in comorbid users (Epstein et al. 2009).
In summary, the present work demonstrates that compared to single drug use, polydrug use reduces differences in price elasticity to cocaine or heroin without altering motivation or cue-induced reinstatement. In addition, this work reveals that cocaine elicits higher motivation for maintaining drug use than heroin, but cue sensitivity is heightened to heroin-associated cues compared to cocaine-associated cues. Taken together, these data suggest that cocaine and heroin may drive continued drug use through different behavioral processes. Thus, further elucidation of the mechanisms that underlie cocaine and heroin use in polysubstance users is essential for addressing the increasing prevalence of polysubstance use, as well as to develop effective treatments to mitigate its effects. Finally, it is worth noting that although sex-specific differences were not examined in the present work, recent studies have reported a higher frequency of polysubstance use in females compared to males (Lorvick et al. 2018; Cropsey et al. 2015). Therefore, future work examining sex-specific effects on the measures studied here may reveal further distinctions in poly- versus single drug history.
Acknowledgements
The authors would like to thank Ferguson lab members Dr. Aaron F. Garcia, Dr. Kanichi Garcia-Nakata, Timothy O’Neal, Grayson Baden, and Jordyn Richardson for their suggestions and feedback when designing, performing, and analyzing the results of these experiments, as well as Dr. Dave Roberts for his suggestions and insight while designing these experiments. The authors would also like to thank the staff of Seattle Children’s Research Institute Office of Animal Care for their support of behavioral experiments, and Dr. Scott Ng-Evans, for his assistance with MED-PC programming and operant chamber support.
Funding information Financial support for this work was provided by the National Institute on Drug Abuse R01DA036582 to SMF. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1762114 to EAC. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Conflict of interest The authors declare that they have no competing interest.
References
- Arnold JM, Roberts DC (1997) A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav 57(3):441–447. 10.1016/S0091-3057(96)00445-5 [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G (2014) Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci U S A 111(32):11822–11827. 10.1073/pnas.1406324111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, Hughes JR (1990) Behavioral economics of drug self-administration. I. Functional equivalence of response requirement and drug dose. Life Sci 47(17):1501–1510. 10.1016/0024-3205(90)90178-T [DOI] [PubMed] [Google Scholar]
- Caprioli D, Celentano M, Dubla A, Lucantonio F, Nencini P, Badiani A (2009) Ambience and drug choice: cocaine- and heroin-taking as a function of environmental context in humans and rats. Biol Psychiatry 65(10):893–899. 10.1016/j.biopsych.2008.12.009 [DOI] [PubMed] [Google Scholar]
- Cox BM, Bentzley BS, Regen-Tuero H, See RE, Reichel CM, Aston-Jones G (2017) Oxytocin acts in nucleus accumbens to attenuate methamphetamine seeking and demand. Biol Psychiatry 81(11): 949–958. 10.1016/j.biopsych.2016.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE (2000) The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res 116(1):1–22. 10.1016/S0166-4328(00)00243 [DOI] [PubMed] [Google Scholar]
- Cropsey KL, Stevens EN, Valera P, Brendan Clark C, Bulls HW, Nair P, Lane PS (2015) Risk factors for concurrent use of benzodiazepines and opioids among individuals under community corrections supervision. Drug Alcohol Depend 154:152–157. 10.1016/j.drugalcdep.2015.06.038 [DOI] [PubMed] [Google Scholar]
- Cunha-Oliveira T, Rego AC, Garrido J, Borges F, Macedo T, Oliveira CR (2010) Neurotoxicity of heroin-cocaine combinations in rat cortical neurons. Toxicology 276(1):11–17. 10.1016/j.tox.2010.06.009 [DOI] [PubMed] [Google Scholar]
- De Luca MT, Montanari C, Meringolo M, Contu L, Celentano M, Badiani A (2018) Heroin versus cocaine: opposite choice as a function of context but not of drug history in the rat. Psychopharmacology 236(2):787–798. 10.1007/s00213-018-5115-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pirro S, Galati G, Pizzamiglio L, Badiani A (2018) The affective and neural correlates of heroin versus cocaine use in addiction are influenced by environmental setting but in opposite directions. J Neurosci 38(22):5182–5195. 10.1523/JNEUROSCI.0019-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit M, Gennings C, Zilberberg M, Burnham EL, Moss M, Balster RL (2008) Drug withdrawal, cocaine and sedative use disorders increase the need for mechanical ventilation in medical patients. Addiction 103(9):1500–1508. 10.1111/j.1360-0443.2008.002267.x [DOI] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL (2009) Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry 66(1):88–94. 10.1001/archgenpsychiatry.2008.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EA, Grella CE, Washington DL, Upchurch DM (2017) Gender and race/ethnic differences in the persistence of alcohol, drug, and polysubstance use disorders. Drug Alcohol Depend 174:128–136. 10.1016/j.drugalcdep.2017.01.021 [DOI] [PubMed] [Google Scholar]
- Hartel DM, Schoenbaum EE, Seliiyn P, Kline J, Davenny K, Klein RS, Friedland GH (1995) Heroin use during methadone maintenance treatment: the importance of methadone dose and cocaine use. Am J Public Health 85(1):83–88. 10.2105/AJPH.85.1.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden A, Hayashi K, Dong H, Milloy MJ, Kerr T, Montaner JSG, Wood E (2014) The impact of drug use patterns on mortality among polysubstance users in a Canadian setting: a prospective cohort study. BMC Public Health 14:1153–1153. 10.1186/1471-2458-14-1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR (1991) Behavioral economics of drug self-administration and drug abuse policy. J Exp Anal Behav 56(2):377–393. 10.1901/jeab.1991.56-377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A (2008) Economic demand and essential value. Psychol Rev 115(1):186–198. 10.1037/0033-295X.115.1.186 [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G (1995) Normalized demand for drugs and other reinforcers. J Exp Anal Behav 64(3):373–384. 10.1901/jeab.1995.64-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofre-Bonet M, Petry NM (2008) Trading apples for oranges? Results of an experiment on the effects of heroin and cocaine price changes on addicts’ polydrug use. J Econ Behav Organ 66:281–311. 10.1016/j.jebo.2006.05.002 [DOI] [Google Scholar]
- Kerr T, Marsh D, Li K, Montaner J, Wood E (2005) Factors associated with methadone maintenance therapy use among a cohort of polysubstance using injection drug users in Vancouver. Drug Alcohol Depend 80:329–335. 10.1016/j.drugalcdep.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Kerr T, Fairbairn N, Tyndall M, Marsh D, Li K, Montaner J, Wood E (2007) Predictors of non-fatal overdose among a cohort of polysubstance-using injection drug users. Drug Alcohol Depend 87:39–45. 10.1016/j.drugalcdep.2006.07.009 [DOI] [PubMed] [Google Scholar]
- Kinner SA, Milloy MJ, Wood E, Qi J, Zhang R, Kerr T (2012) Incidence and risk factors for non-fatal overdose among a cohort of recently incarcerated illicit drug users. Addict Behav 37(6):691–696. 10.1016/j.addbeh.2012.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Woods JH (2008) Quantification of drug choice with the generalized matching law in rhesus monkeys. J Exp Anal Behav 89(2):209–224. 10.1901/JEAB.2008.89-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Stewart J (2001) Drug-induced reinstatement to heroin and cocaine seeking: a rodent model of relapse in polydrug use. Exp Clin Psychopharmacol 9(3):297–306. 10.1037/1064-1297.9.3.297 [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rajabi H, Stewart J (2003) Effects of cocaine in rats exposed to heroin. Neuropsychopharmacology 28(12):2102–2116. 10.1038/sj.npp.1300284 [DOI] [PubMed] [Google Scholar]
- Leri F, Stewart J, Tremblay A, Bruneau J (2004) Heroin and cocaine co-use in a group of injection drug users in Montréal. J Psychiatry Neurosci 29(1):40–47 https://www.ncbi.nlm.nih.gov/pubmed/14719049 [PMC free article] [PubMed] [Google Scholar]
- Leri F, Zhou Y, Goddard B, Cummins E, Kreek MJ (2006) Effects of high-dose methadone maintenance on cocaine place conditioning, cocaine self-administration, and mu-opioid receptor mRNA expression in the rat brain. Neuropsychopharmacology 31(7):1462–1474. 10.1038/sj.npp.1300927 [DOI] [PubMed] [Google Scholar]
- Levine M, Iliescu ME, Margellos-Anast H, Estarziau M, Ansell DA (2005) The effects of cocaine and heroin use on intubation rates and hospital utilization in patients with acute asthma exacerbations. Chest 128(4):1951–1957. 10.1378/chest.128.4.1951 [DOI] [PubMed] [Google Scholar]
- Lorvick J, Browne EN, Lambdin BH, Comfort M (2018) Polydrug use patterns, risk behavior and unmet healthcare need in a community-based sample of women who use cocaine, heroin or methamphetamine. Addict Behav 85:94–99. 10.1016/j.addbeh.2018.05.013 [DOI] [PubMed] [Google Scholar]
- McCall Jones C, Baldwin GT, Compton WM (2017) Recent increases in cocaine-related overdose deaths and the role of opioids. Am J Public Health 107(3):430–432. 10.2105/AJPH.2016.303627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Roberts DC (2009) Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology 34(3):796–804. 10.1038/npp.2008.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison LP, McIntosh S, Sexton T, Childers SR, Hemby SE (2014) Changes in dopamine transporter binding in nucleus accumbens following chronic self-administration cocaine: heroin combinations. Synapse 68(10):437–444. 10.1002/syn.21755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Bickel WK (1998) Polydrug abuse in heroin addicts: a behavioral economic analysis. Addiction 93(3):321–335. 10.1046/j.1360-0443.1998.9333212.x [DOI] [PubMed] [Google Scholar]
- Phillips KA, Epstein DH, Preston KL (2013) Daily temporal patterns of heroin and cocaine use and craving: relationship with business hours regardless of actual employment status. Addict Behav 38(10):2485–2491. 10.1016/j.addbeh.2013.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Bevins RA (2009) Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr Drug Abuse Rev 2(2):184–194. 10.2174/1874473710902020184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DCS, Gabriele A, Zimmer BA (2013) Conflation of cocaine seeking and cocaine taking responses in IV self-administration experiments in rats: methodological and interpretational considerations. Neurosci Biobehav Rev 37(9 Pt A):2026–2036. 10.1016/j.neubiorev.2013.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio FJ, Quintana-Feliciano R, Warren BL, Li X, Witonsky KFR, Valle FS, Selvam PV, Caprioli D, Venniro M, Bossert JM, Shaham Y, Hope BT (2018) Prelimbic cortex is a common brain area activated during cue-induced reinstatement of cocaine and heroin seeking in a polydrug self-administration rat model. Eur J Neurosci 49(2):165–178. 10.1111/ejn.14203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelp SA, Pultorak KJ, Rakowski DR, Gomez DM, Krzystyniak G, Das R, Oleson EB (2017) A transient dopamine signal encodes subjective value and causally influences demand in an economic context. PNAS 114(52):E11303–E11312. 10.1073/pnas.1706969114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW (2007) The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology 194(3):321–331. 10.1007/s00213-007-0850-8 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2014) Results from the 2013 national survey on drug use and health: summary of national findings, NSDUH series H-48, HHS publication no. (SMA) 14–4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; https://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf [Google Scholar]
- Wade-Galuska T, Winger G, Woods JH (2007) A behavioral economic analysis of cocaine and remifentanil self-administration in rhesus monkeys. Psychopharmacology 194(4):563–572. 10.1007/s00213-007-0858-0 [DOI] [PubMed] [Google Scholar]
- Wang L, Min JE, Krebs E, Evans E, Huang D, Liu L, Hser YI, Nosyk B (2017) Polydrug use and its association with drug treatment outcomes among primary heroin, methamphetamine, and cocaine users. Int J Drug Policy 49:32–40. 10.1016/j.drugpo.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Morgan D, Roberts DC (2005) Comparison of the reinforcing effects of cocaine and cocaine/heroin combinations under progressive ratio and choice schedules in rats. Neuropsychopharmacology 30(2):286–295. 10.1038/sj.npp.1300560 [DOI] [PubMed] [Google Scholar]
- Williamson A, Darke S, Ross J, Teesson M (2007) The effect of baseline cocaine use on treatment outcomes for heroin dependence over 24 months: findings from the Australian treatment outcome study. J Subst Abus Treat 33(3):287–293. 10.1016/j.jsat.2006.12.009 [DOI] [PubMed] [Google Scholar]
- Yager LM, Garcia AF, Donckels EA, Ferguson SM (2019) Chemogenetic inhibition of direct pathway striatal neurons normalizes pathological, cue-induced reinstatement of drug-seeking in rats. Addict Biol 24(2):251–264. 10.1111/adb.12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Oleson EB, Roberts DC (2012) The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology 37(8):1901–1910. 10.1038/npp.2012.37 [DOI] [PMC free article] [PubMed] [Google Scholar]


