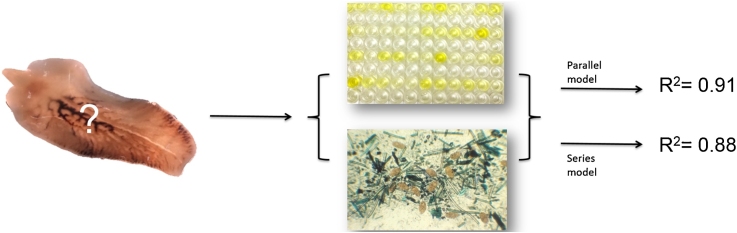
Graphical abstract
Keywords: Coproantigen, ELISA, Efficacy, Fasciola hepatica, Triclabendazole, Prevalence
Highlights
-
•
FFEC and cELISA explained by parallel (R2 = 0.91) and series (R2 = 0.88) models.
-
•
Kappa agreement of FFEC and cELISA higher in cattle than sheep.
-
•
Infection intensity did not affect agreement of diagnostics in sheep.
-
•
Application of Ollerenshaw Index or alternate model requires further investigation.
Abstract
The diagnosis, monitoring and flukicide efficacy testing of fasciolosis on-farm is reliant on non-terminal methods. The coproantigen ELISA (cELISA) has been recommended for diagnosis of fasciolosis and associated flukicide efficacy testing as an alternative to fluke egg counts for monitoring parasitism. Recently experimental multi-age infections have suggested that the reliability of efficacy results can be improved by a second cELISA testing at 6 weeks post-treatment (wpt) in addition to the generally accepted 1 wpt. A field study was conducted to determine the suitability of faecal fluke egg counts (FFEC) and cELISA as diagnostic, drug efficacy testing and epidemiological tools on Australian sheep and cattle farms. Faecal samples from sheep and/or cattle on three endemic farms were taken at monthly intervals for 12 months and examined by both methods. Normal farm management was maintained during the study period and opportunistic efficacy testing, in line with each farm’s normal flukicide management was undertaken. Additionally, the suitability of the Ollerenshaw Index as a predictive model for fasciolosis under Australian conditions was examined. While both diagnostics demonstrated their value in the farm environment, the current data demonstrate a distinct and significant increase in diagnostic sensitivity for epidemiological studies by using the two tests in parallel. The agreement between the two diagnostics was found to be higher in cattle, despite the poor sensitivity of FFEC in this species. Similar levels of agreement between the two tests were demonstrated at both sheep properties, regardless of the marked difference in the intensity of F. hepatica challenge. Linear regression models demonstrated the results of the two diagnostics utilized in parallel were explained substantially (R2 = 0.91) as were series data (R2 = 0.88) when the respective models were fitted. In contrast, the fitted models for FFEC (R2 = 0.54) and cELISA (R2 = 0.58) were poor explanations for test outcomes. The outcomes of these models support previous findings that suggest that the two diagnostic tests are best utilized together, particularly in parallel. The application of the Ollerenshaw Index to Australian conditions requires further investigation.
1. Introduction
The Digenean trematode Fasciola hepatica is important to both human and ruminant health across the globe due to its inducement of the clinical disease fasciolosis. Ninety-one million humans and 700 million sheep and cattle in 50 countries are considered at risk of infection (Keiser and Utzinger, 2005, Martinez-Perez et al., 2012, Mehmood et al., 2017). Adverse impacts on global livestock industries due to clinical disease include decreased weight gains, reduced wool/milk production, poor reproduction, costs of treatment and mortalities (Howell et al., 2015, Young et al., 2010). The global economic impact of these losses are estimated at $US 2–3 billion (Brockwell et al., 2013, Diab et al., 2010, McManus and Dalton, 2006).
Prevalence studies of fasciolosis in ruminant species globally were summarized by Mehmood et al. (2017) with 108 peer-reviewed reports since the year 2000 identified. Of these, only two reported prevalence data for Australia (Elliott et al., 2015, Molloy et al., 2005). Serum antibody testing in 2005 reported F. hepatica prevalence of 60.2% in sheep and 41.4% in cattle (Molloy et al., 2005), whilst Elliot et al. (2015) described a mean cattle herd prevalence of 81.0% using cELISA.
In Australia, available data are predominantly from abattoir detection, with the state of Victoria having the highest prevalence in cattle and New South Wales (NSW) the highest for sheep. Necropsy data for NSW indicates prevalence at approximately 9% of slaughtered sheep, affecting 34% of properties and an average on-farm prevalence of 26% (GHD Pty Ltd, 2009, Lane et al., 2015). Victorian abattoir surveillance in the mid-1900s indicated 36.2% of slaughtered cattle were affected by liver fluke (Seddon, 1950) and more recently, coproantigen ELISA (cELISA) of dairy cattle in regions known to be endemically infected demonstrated a mean herd prevalence of 81.0% (Elliott et al., 2015).
Effective management of liver fluke requires an understanding of the epidemiology and more particularly the seasonal influences. Stochastic modelling may assist with management. A predictive model and index (risk) of infection has been developed in the United Kingdom (UK): the Ollerenshaw Index (Ollerenshaw and Rowlands, 1959). This has been utilized in the UK by the National Animal Disease Information Service (NADIS) until at least 2017 (NADIS, 2017). Other predictive approaches utilise Geographic Information System (GIS) mapping and spatial distribution (Malone et al., 1992, Rapsch et al., 2008), growing degree-day / climate change models combined (Haydock et al., 2016) and the recent mechanistic Hydro-epidemiological model for Liver Fluke (HELF) (Beltrame et al., 2018). At present, there is limited information on predictive models in Australian conditions.
Diagnosis of fasciolosis is hindered by non-specific clinical signs, variable faecal fluke egg counts (FFEC) combined with long pre-patent periods, limitations of ELISA methods and molecular technologies and heavy reliance on necropsy for the diagnosis of acute fasciolosis (Brunsdon, 1967, George et al., 2017, Gordon et al., 2012a). While traditional faecal flotation techniques (Happich and Boray, 1969) continue to be utilized due to basic equipment and low costs, efficiency per sample is questionable (Gordon et al., 2012b). PCR methods demonstrate increased analytical and diagnostic sensitivity, but still rely on patency (Calvani et al., 2017). The cELISA has been reported as a more efficient diagnostic tool with earlier detection of F. hepatica, a higher specificity and sensitivity, and has been shown to be useable for drug efficacy testing and resistance diagnosis on-farm (Brockwell et al., 2014, Flanagan et al., 2011b, Gordon et al., 2012b). Whilst the controlled studies of Martinez-Valladares et al. (2010) promoted the cELISA to assess drench efficacy, subsequent reports from Gordon et al. (2012b) and Novobilsky et al. (2012) highlighted the sensitivity (particularly when compared with FFEC) under field conditions required optimisation. The Bio-K201 cELISA has since been released as a second generation kit based on the work of Martínez-Sernández et al. (2016), which significantly lowered the limit of detection in cattle in the parent cELISA (MM3−COPRO). This second generation kit utilises of streptavidin-polymerised horseradish peroxidase conjugate in place of avidin-horseradish peroxidase as per the original kit. As the previously discussed studies were performed using the original kit it is likely that the modifications to the current kit would have improved sensitivity, although this needs to be confirmed.
George et al. (2017) recently described the quantitative phenology of faecal antigen and determined that surviving immature parasites would not be detected in the 6 weeks post-treatment in concurrent multiple age F. hepatica infections. The objective of the current study was to determine the suitability of FFEC and cELISA as diagnostic, drug efficacy testing and epidemiological tools on Australian sheep and cattle farms.
2. Materials and methods
2.1. Experimental design
A field study was conducted to monitor the natural infection of F. hepatica in sheep and cattle for one year at three sites across two geographic locations with known endemic infection in NSW, Australia. Utilising a similar approach as would be conducted for assessment of flukicide burdens and treatment requirements, tracer sheep or cattle (n = 10 per site) grazing in these localities had faeces collected and analysed at approximately monthly intervals. Blood samples were collected on four occasions (months 3 or 4, 7, 9 and 11) from the same sheep. Faecal samples were analysed by FFEC and cELISA, whilst a serum antibody ELISA was utilized for blood samples. Meteorological data from each location was accessed via the public domain.
Normal farm management practices were followed at each site and opportunistic faecal egg count reduction tests (FECRT) and coproantigen reduction tests (CRT) were conducted in line with treatment for F. hepatica.
2.2. Animals, management and property history
Site 1 was located at Taralga (34.40 °S, 149.82 °E) in the Southern Tablelands of NSW. Naturally infected enrolled tracer sheep (n = 10) were 5 year-old Border Leicester x Merino ewes, which were maintained in a flock of approximately 150 breeders. Additionally, 10 heifer (˜14 months old) cattle, maintained in a herd of 30 Hereford replacement heifers, at an adjoining property in Taralga (Site 2) were enrolled.
At Site 3, located at Guyra (30.22 °S, 151.67 °E) in the Northern Tablelands of NSW, a mixed breed flock of approximately 50 breeder ewes was utilized and the 10 mature ewes selected were either Border Leicester (n = 5) or Dorper cross (n = 5).
The sheep and cattle were confirmed as being infected with F. hepatica prior to enrolment by positive FFEC and/or positive cELISA.
The sheep and cattle were maintained as per normal husbandry by their owners at pasture and supplemented as allowing for seasonal requirements (Site 1; July 2015–August 2016, Site 2; September 2015–October 2016, Site 3; October 2015–October 2016). Water was available ad libitum via dams, springs and creeks.
2.3. Anthelmintics and dosing
The anthelmintics and doses utilized at each property are described in Table 1.
Table 1.
Anthelmintic information and efficacy (%) for FFEC and cELISA by study site.
| Site | Month | Treatment | Dose | Trade name | Registrant | Diagnostic | Efficacy (%) |
|
|---|---|---|---|---|---|---|---|---|
| <5 wpt | 8+ wpt | |||||||
| 1 | September 2015 | TCBZ | 10 mg/kg | Flukare C | Virbac (Australia) Pty Ltd | FFEC | 100 | 78.9 |
| cELISA | 0 | 0 | ||||||
| 2 | August 2016 | TCBZ | 30 mg/kg | Genesis Ultra | Boehringer Ingelheim Animal Health Australasia Pty. Ltd. | FFEC | 100 | 99.7 |
| Abamectin | 0.5 mg/kg | cELISA | 89.7 | 91.2 | ||||
| 3 | November 2015 | TCBZ | 10 mg/kg | Tremacide 120 | Jurox Pty Limited | FFEC | 15.5 | 0 |
| cELISA | 46.8 | 27.2 | ||||||
| 3 | May 2016 | Closantel Albendazole |
7.5 mg/kg 5 mg/kg |
Q-drench | Jurox Pty Limited | FFEC | 49.2 | 0 |
| Abamectin Levamisole hydrochloride |
0.2 mg/kg 8 mg/kg |
cELISA | 42.5 | 48.2 | ||||
| 3 | August 2016 | Closantel Albendazole |
7.5 mg/kg 5 mg/kg |
Q-drench | Jurox Pty Limited | FFEC | 0 | – |
| Abamectin Levamisole hydrochloride |
0.2 mg/kg 8 mg/kg |
cELISA | 0 | – | ||||
wpt = weeks post treatment.
The sheep at Site 1 received a single triclabendazole (TCBZ) oral drench (Flukare C, Virbac) before the second monthly visit in September 2015. Cattle at Site 2 had a triclabendazole/abamectin (TCBZ/ABA) pour-on applied (Genesis Ultra, Boehringer Ingelheim) in August 2016 (month 9). Site 3 sheep were treated with oral drenches on three occasions; initially in November 2015 (month 2) with TCBZ (Tremacide 120, Jurox) and subsequently with a combination of levamisole, closantel, albendazole and abamectin (Q-drench, Jurox) in May and August 2016, months 8 and 11, respectively. All treatments were administered according to the heaviest animal in the group, as recommended.
2.4. Faecal fluke egg counts
Faecal samples collected monthly at each site were analysed by a modified (Happich and Boray, 1969) sedimentation test as described by George et al. (2017) and recorded as eggs per gram faeces (epg).
2.5. Coproantigen ELISA
Faecal samples were collected directly from the rectum of each sheep and cow prior to enrolment and at approximate monthly intervals for the ensuing year. Faecal samples (0.5 g for sheep; 2 g for cattle) were stored at -20 °C until analysis. Individual samples were analysed in duplicate using a commercially available cELISA (BIOK 201; BIO-X Diagnostics, Belgium) as per the manufacturer’s direction with overnight antigen extraction and 1.3% cut-off as described by Brockwell et al. (2013). Optical densities (ODs) were corrected by subtracting the negative values of individual samples. Analysis of data was completed on the corrected OD value. Results of replicated samples were averaged prior to statistical analysis.
2.6. Serum ELISA
Blood samples were obtained from sheep enrolled at Sites 1 and 3 at months 3 (Site 1 only), 4 (Site 2 only), 7, 9 and 11 to confirm parasite exposure status via serum ELISA (IDEXX Fasciolosis Verification Test).
2.7. Meteorological data
Data summaries of the monthly climate variables (rainfall, minimum and maximum temperature), including monthly averages of the preceding 30 years (1984–2014), were obtained from weather stations located at Taralga Post Office (station 070,080) and Guyra Hospital (station 056,229) and reproduced by permission of Bureau of Meteorology, © 2018 Commonwealth of Australia (http://www.bom.gov.au/).
2.8. Calculation of efficacy
Efficacy was calculated using arithmetic mean FFEC or cELISA reductions in treated animals when compared with the pre-treatment counts of the same individuals. Percentage efficacy was determined from the FFEC using the formula: Efficacy [%] = 100 × (C–T)/C, where C and T are the FFEC or cELISA means for the untreated and treated animals, respectively (Abbott, 1925). The efficacy was calculated for each treatment at the initial post-treatment visit (<5 wpt) and a secondary test of efficacy at 8+ wpt was conducted at the following visit.
2.9. Seasonal infection risk
Seasonal infection risk was examined by the methods of Ollerenshaw and Rowlands (1959), with adjustments as described by Fox et al. (2011). Monthly fasciolosis values were calculated as below:
Where:
Mt = Fasciolosis risk value
n = Number of rain days per month
R = Rainfall (mm/month)
P = Potential evapotransformation per month
Potential evapotransformation was calculated using the Hargreaves equations for evapotransformation as described by (Droogers and Allen, 2002), where Ra is extraterrestrial radiation [MJ m −2 day -1].
Extraterrestrial radiation was calculated for each month and location by multiplying the extraterrestrial solar radiation (http://www.engr.scu.edu/˜emaurer/tools/calc_solar_cgi.pl) in equivalent water depth (mm/d) by 2.45 (the equivalent MJ m −2 day -1 for every 1 mm/d evaporation). Latitude of the town in which the properties were most closely located and the first of the month were applied to this calculator. Seasonal infection risk was determined as follows (two infection periods which represent the risk of metacerceriae in those seasons and include months over which development in the snail host occur. These are equivalent to UK methods but adjusted for the southern hemisphere):
-
•
Summer; expanded to include November of one year through to the following April,
-
•
Winter; expanded to include February, March and April with October and November of the same year.
Fasciolosis risk was interpreted as:
Mt ≤ 300 Little to no disease
> Mt ≤ 400 Occasional losses
Mt > 400 Disease prevalent
2.10. Statistical evaluation
Linear models were applied to determine if the effects of a range of variables could explain variation within each diagnostic test (FFEC and cELISA). The FFEC data was transformed via a logit function and examined utilising a GLMM, assuming normal distribution. The data for cELISA (net expression) was transformed via a natural log function and analysed as a GLMM assuming a normal distribution. Additionally, models of binary data (in parallel and series) were modelled via GLMM with binomial (total of 1) distribution and a logit link function. The variables of month/year or season/year were included as the primary fixed effect for models tested. Further variables included were of climatic origin (monthly means of rainfall, minimum and maximum temperatures), locality (region and property), treatment with flukicide prior to visit and species. The individual animal was included as a random effect for all models.
Models were constructed by the reverse stepwise removal of parameters with low explanatory power (Wald test > 0.05). The amount of variance explained by the models was estimated using the marginal coefficient of determination (R2m; fixed effects) and the conditional coefficient of determination (R2c; fixed and random effects) following the method of Nakagawa and Schielzeth (2013) and adapted for GenStat by Marcus et al. (2014). GenStat v 16.1.0.10916 (VSN International Ltd, UK) was used for these analyses.
The estimated true prevalence, positive and negative predictive values, and likelihood ratios were determined at each site, by animal species, utilising the EpiTools epidemiological calculator; ‘Estimated true prevalence and predictive values from survey testing’ (http://epitools.ausvet.com.au/content.php?page=TruePrevalence), and selecting the Rogan-Gladen confidence limits (Rogan and Gladen, 1978). These calculations were performed on binary data for each of the two coprological diagnostic methods, as well as for the two methods considered in parallel (either test positive for animal to be regarded as infected) or series (both tests positive for animal to be regarded as infected), for the entire study period, for seasons and for months. Diagnostic sensitivity (Dsn) and specificity (Dsp) were as defined by George et al. (2017) for FFEC (Dsn = 0.842, Dsp = 0.842) and cELISA (Dsn = 0.842, Dsp = 0.931). Calculations of parallel and series Dsn and Dsp were conducted using the ‘Sensitivity and specificity for two tests in parallel or series’ calculator on Epitools (http://epitools.ausvet.com.au/content.php?page=2Tests).
Additionally, a KAPPA analysis (http://vassarstats.net/kappa.html) was performed to determine the agreement of the two diagnostics in defining the infection status of an animal. The interpretation of KAPPA values was as defined by Viera and Garrett (2005), where values of 0.21–0.40 represent ‘Fair’, 0.41–0.60 ‘Moderate’, 0.61–0.80 ‘Substantial’ and 0.81–0.99 ‘Almost perfect’ agreement.
The infection risk for each season (summer and winter) was compared with calculations based on 30-year annual averages (Mann-Whitney U test; GenStat v 16) to determine if infection risk was significantly higher or lower in the study period.
Variance and mean monthly meteorological data were compared to historical monthly averages using Student’s t-test (Genstat).
2.11. Animal ethics
The Elanco Australasia Pty. Limited Animal Ethics Committee (AEC) approved the conduct of this study (approval NAH-14-420) and the Sydney University AEC ratified the approval.
3. Results
3.1. FFEC, cELISA and serum ELISA diagnostic results
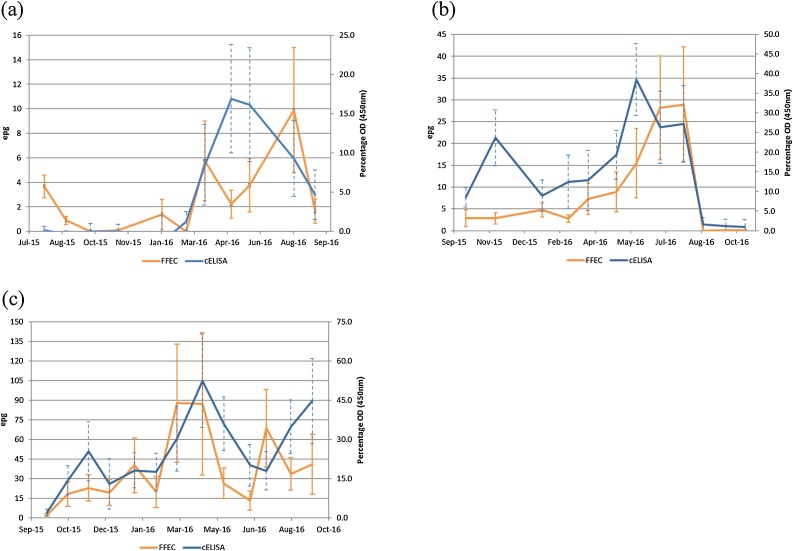
Mean FFEC and cELISA (% OD corrected for the cut-off of 1.3%) results are presented in Fig. 1a-c and the number of positive results for each of the diagnostic methods per site are summarized in Table 2. An initial increase in infection in the spring months was evident at Site 2 (cattle, Taralga; October-November 2015) and Site 3 (sheep, Guyra; September-November 2015), which was more evident by cELISA. An infection surge was evident at all sites over the autumn period (March-May 2016, months 4–6). Increased infection rates became evident approximately one month later in cattle FFEC.
Fig. 1.
Observed mean FFEC (solid orange line) and mean cELISA percentage OD (450 nm) corrected for cut-off of 1.3% (solid blue line) by site. Dashed blue error bars represent SE for cELISA, and solid orange error bars represent SD for FFEC. a) Site 1; sheep grazing at Taralga, b) Site 2; cattle grazing at Taralga and c) Site 3; sheep grazing at Guyra. Note that scales utilized for FFEC and cELISA of each figure are reflective of results obtained for each respective site.
Table 2.
Total samples and percentage (bracketed) shown to be positive for Fasciola hepatica by diagnostic method, including KAPPA of FFEC and cELISA.
| Site/species | Number of Observations | KAPPA value | Number (and percentage) of positive samples by diagnostic method |
||||
|---|---|---|---|---|---|---|---|
| FFEC | cELISA | Parallel | Series | Serum ELISA* | |||
| 1 - sheep | 100 | 0.52 | 42 (42.0) | 39 (39.0) | 52 (52.0) | 29 (29.0) | 18 (66.7) |
| 2 - cattle | 120 | 0.64 | 72 (60.0) | 82 (68.3) | 87 (72.5) | 67 (55.8) | n/a |
| 3 - sheep | 117 | 0.50 | 104 (88.8) | 95 (81.2) | 107 (91.5) | 92 (78.6) | 31 (100) |
Number of observations for serum ELISA were 27 and 31 at Sites 1 and 3, respectively. Not all sheep were sampled on every occasion.
3.1.1. Site 1
The level of infection at Site 1 was low, with mean FFEC results not exceeding 10 epg for the entire study duration. Only three individual sheep exceeded this mean, the first on 3 occasions (maximum 46 epg), the second on 2 occasions (maximum 20 epg) and the third on a single occasion (16 epg). Although individual animals tested positive in the cELISA at all monthly visits, the mean % OD of the group was negative on three occasions, i.e. in August and October 2015 and January 2016. The mean % OD ranged from -0.07% (January 2016) to 18.2% (May 2016).
Serum antibody detection at this site was inconsistent with 3 out of 8, 4 out of 7, 4 out of 6 and 5 out of 6 sheep testing positive in November 2015, March, May and August 2016, respectively. In November 2015, at approximately 8 weeks post-treatment, three sheep positive for serum antibodies were negative in both FFEC and cELISA. At the March 2016 sampling, one sheep was positive for FFEC but not for cELISA or serum antibody, suggesting a false positive epg. In the same month, another sheep recorded a positive serum antibody and cELISA but negative FFEC; the following month FFEC became positive in this individual. In May 2016, one sheep recorded negative serum antibody and an epg of 1, again indicating a false positive FFEC. The final serum in August found one sheep to have positive serum antibody and negative cELISA and FFEC.
3.1.2. Site 2
The mean FFEC ranged from 2.8 to 28.9 epg in cattle at Site 2. Over the study period all sampling sessions demonstrated mean coproantigen to be positive with OD% ranging from 2.25% (October 2016) to 39.8% (May 2016).
3.1.3. Site 3
The mean FFEC in sheep at Site 3 ranged from 2.3 epg at selection and peaked at 87.8 epg in March 2016. Coproantigen detection was positive at all visits with lowest levels observed at selection in September 2015 (3.38%) and peak levels in April 2016 (53.8%).
Serum antibody detection was positive for all sheep samples tested at Site 3, confirming the exposure status of enrolled animals.
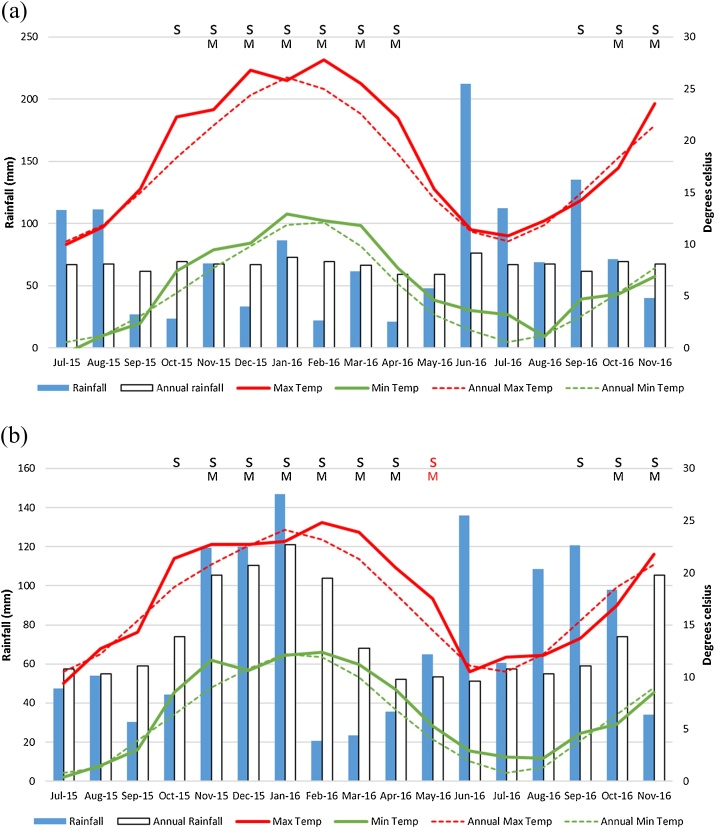
3.2. Meteorological data
The mean monthly rainfall and the minimum and maximum temperatures of Taralga (sites 1 and 2) and Guyra (site 3) are summarized in Fig. 2a and b, respectively.
Fig. 2.
Meteorological trends throughout the study period compared to annual data (average of preceding 30 years), including snail activity (S) and metacercariae presence (M). a) Taralga and b) Guyra (increased snail/metacercariae season in 2016 indicated by red letters). Note that scales utilized for rainfall differ between figures.
Reproduced by permission of Bureau of Meteorology, © 2018 Commonwealth of Australia.
Mean monthly rainfall at Taralga was not significantly different to 30-year monthly mean (μ = 66.98) in 2015 (μ = 70.12; p = 0.840) or 2016 (μ = 78.42; p = 0.478). However, the variance of rainfall was different to the historical mean data (σ2 = 26) in both 2015 (σ2 = 2743; p < 0.001) and 2016 (σ2 = 2896; p < 0.001). No differences for maximum temperature monthly means or variance were found between 30-year averages (μ = 18.29, σ2 = 32.12) and study years (2015: μ = 18.78; p = 0.841, σ2 = 32.64; p = 0.870; 2016: μ = 19.44; p = 0.649, σ2 = 42.33; p = 0.660), or between study years (μ; p = 0.796, σ2; p = 0.780). No differences for minimum monthly temperature means or variance were found between 30-year averages (μ = 6.050, σ2 = 17.38) and study years (2015: μ = 6.250; p = 0.914, σ2 = 22.42; p = 0.680; 2016: μ = 7.142; p = 0.524, σ2 = 16.67; p = 0.950), or between study years (μ; p = 0.626, σ2; p = 0.630).
Guyra mean monthly rainfall was not different to the 30-year monthly average (μ = 75.89) in 2015 (μ = 80.03; p = 0.807) or 2016 (μ = 76.46; p = 0.970). However, the variance of rainfall was significantly different to annual data (σ2 = 700) in 2015 (σ2 = 2651; p = 0.040), but not 2016 (σ2 = 1979; p = 0.100). No differences for maximum temperature monthly means or variance were found between 30-year averages (μ = 17.69, σ2 = 23.62) and study years (2015: μ = 17.82; p = 0.952, σ2 = 26.65; p = 0.850; 2016: μ = 18.52; p = 0.700, σ2 = 29.96; p = 0.700), or between study years (μ; p = 0.750, σ2; p = 0.850). No differences for minimum monthly temperature means or variance were found between 30-year averages (μ = 6.592, σ2 =17.49) and study years (2015: μ = 7.083; p = 0.784, σ2 = 20.36; p = 0.810; 2016: μ = 7.350; p = 0.656, σ2 =17.49; p = 0.910), or between study years (μ; p = 0.880, σ2; p = 0.720).
Average daily temperatures in Taralga were below 10 °C between May-September 2015 and 2016, consistent with the annual averages. At Guyra, average daily temperatures were below 10 °C from May to September 2015, consistent with the annual averages, but in 2016 temperatures remained above 10 °C until May. This indicates an extended period of snail activity and metacercariae release in 2016 at Guyra.
3.3. Efficacy
The efficacy of anthelmintics is summarized in Table 1.
The TCBZ flukicide administered orally to sheep at Site 1 was fully effective as measured by FFEC (100%), but ineffective when measured by cELISA (0%). The secondary test demonstrated a reduction in efficacy as measured by FFEC (78.9%), but no change to the cELISA result. The TCBZ/ABA combination applied as pour-on to the cattle at Site 2 was fully effective as measured by FFEC (100%) and demonstrated marginally reduced efficacy when measured by cELISA (89.7%). After this treatment, very little change in efficacy was observed at the second visit; 99.7% as measured by FFEC and 91.2% as measured by cELISA. The three treatments administered to the sheep at Site 3 were shown to be ineffective by both diagnostic tests, although the TCBZ treatment in November 2015 showed the least agreement between test methods (15.5% as measured by FFEC vs. 46.8% as measured by cELISA).
3.4. Statistical analysis
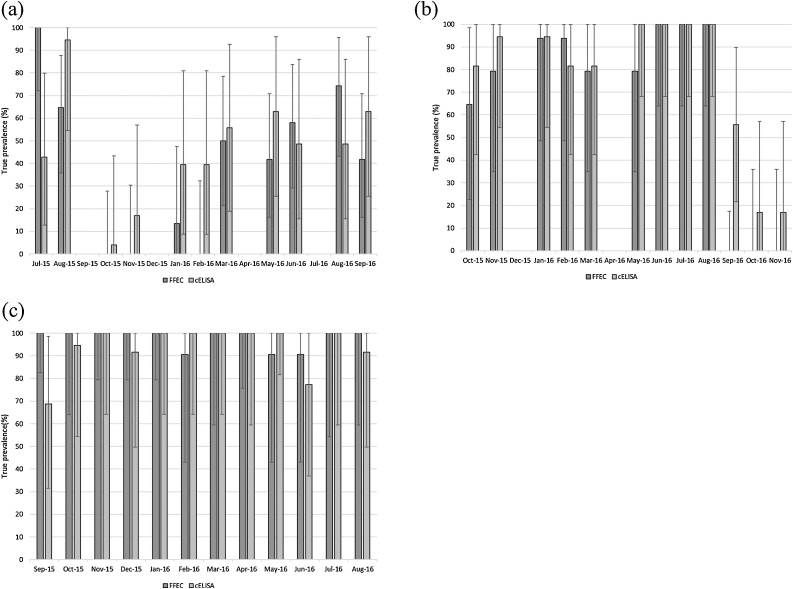
3.4.1. Prevalence
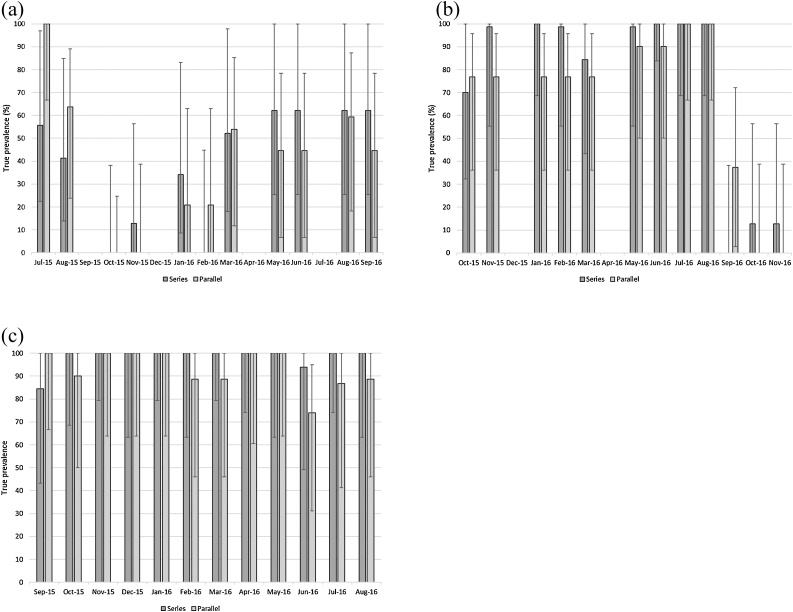
The estimated true prevalence for samples collected each month are summarized by site in Fig. 3a-c and Fig. 4a-c. When considered in parallel, the two diagnostic tests had a Dsn of 0.975 and a Dsp of 0.7839, whilst when considered in series, the Dsn was 0.709 and the Dsp was 0.9891 for FFEC and cELISA combined.
Fig. 3.
Estimated true prevalence and 95% Rogan-Gladen CL (error bar) of FFEC (dark grey) and cELISA (light grey) by month and site. a) Site 1; sheep grazing at Taralga, b) Site 2; cattle grazing at Taralga and c) Site 3; sheep grazing at Guyra.
Fig. 4.
Estimated true prevalence and 95% Rogan-Gladen CL of FFEC and cELISA in Series and in Parallel by site over the study duration. a) Site 1; sheep grazing at Taralga, b) Site 2; cattle grazing at Taralga and c) Site 3; sheep grazing at Guyra.
3.4.2. Linear mixed models
The GLMM for FFEC (R2c = 54%, R2m = 20%) demonstrated the probability of detecting F. hepatica infection in sheep and cattle with this test was significantly associated with season (F5, 181.2 = 8.30, P < 0.001), region (F1, 28.8 = 6.21, P = 0.019), treatment with flukicide prior to visit (F1, 171.7 = 4.71, P = 0.031) and mean monthly rainfall (F1, 173.2 = 9.76, P = 0.002). Individual variability contributed to the model variance (R2c-R2m = 33%). Mean minimum and maximum temperatures, property and species did not contribute significantly to the model fit. Autumn and Winter 2016 were found to be 2.2–2.7 and 2.8–5.9 times (respectively) more likely to have positive FFEC than all other seasons. The likelihood of animals being positive by FFEC was 2.4 (CI 1.1–5.1) and 3.6 times (CI 1.3–10.3) lower if animals were treated prior to sampling visit than when untreated at Taralga and Guyra, respectively. The likelihood of positive FFEC increased by 5.3% with every 5 mm of rainfall.
The probability of detecting F. hepatica infection in sheep and cattle using the cELISA was associated with month (F15, 263.5 = 5.28, P < 0.001) and property (F2, 28.7 = 4.69, P = 0.017). The model explained 58% of diagnostic variance (R2c = 58%, R2m = 23%), with individual variability accounting for 35%. Mean minimum and maximum temperatures, rainfall, region, treatment with flukicide prior to visit, and species did not contribute significantly to the model fit. Samples collected in May 2016 were 4.02 times (CI 2.03–7.97) more likely to be positive on FFEC, which was different (P < 0.05) compared with all other months except for April, July and August 2016. Samples collected in October 2016 were 0.21 times (CI 0.09-0.51) less likely to be positive, which was significantly different compared to all other months except for August, September 2015 and June 2016. Cattle at Site 2 were 4.04 times (CI 1.18–13.90) and sheep at Site 3 were 5.90 (CI 1.69–20.58) more likely to have positive cELISA than sheep at Site 1.
The GLMM for Series data (R2c = 88%, R2m = 24%) demonstrated the probability of detecting F. hepatica infection in sheep and cattle (when both FFEC and cELISA were positive) was significantly associated with season (F5, 308 = 6.41, P < 0.001), mean minimum temperatures (F1, 314 = 11.68, P < 0.001), mean maximum temperatures (F1, 318 = 15.54, P < 0.001) and treatment with flukicide prior to visit (F1, 306 = 9.47, P=0.002). Rainfall, property, region and species did not contribute significantly to the model fit. Each degree increase in maximum daily temperature decreased the likelihood of infection by 39.0% (CI 21.9–52.3). Conversely, each degree increase in minimum temperature increased the likelihood of infection by 75.2% (CI 26.9–142.1). Treated animals were 4.2 (CI 1.7–10.4) times less likely to test positive than untreated animals. Positive tests for Series data in the Spring of 2016 were 0.066–0.083 times less likely than in any other of the seasons monitored.
The GLMM for parallel data (R2c = 91%, R2m = 48%) demonstrated the probability of detecting F. hepatica infection in sheep and cattle (when either FFEC or cELISA was positive) was associated with season (F5, 309 = 10.89, P < 0.001), region (F1, 31 = 9.59, P=0.004), treatment with flukicide prior to visit (F1, 302 = 8.27, P = 0.004), rainfall (F1, 305 = 15.42, P < 0.001) and mean minimum temperatures (F1, 303 = 7.88, P=0.005). Mean maximum temperatures, property and species did not contribute to the model fit. The likelihood of F. hepatica diagnosis increased by 12.3% (CI 6.0–19.0%) for each 5 mm increase in mean monthly rainfall, with animals in Taralga 13.1 (CI 2.4–71.9) times less likely and treated animals 4.3 (CI 1.6–11.5) times less likely to be confirmed infected when compared with animals in Guyra and untreated counterparts, respectively. Animals tested in the Spring of 2016 were 0.014–0.100 times less likely to have positive parallel tests than all other seasons monitored. Animals tested in Winter 2016 were 0.14 (CI 0.02–0.98) and 0.19 (CI 0.05–0.77) times less likely to have F. hepatica infection detected than those monitored in Summer 2015/2016 and Autumn 2016.
3.4.3. KAPPA
The KAPPA value for diagnostic test agreement in sheep was moderate at Sites 1 (0.52) and 3 (0.50). The KAPPA value for diagnostic agreement in cattle was substantial at Site 2 (0.64), as was the agreement when data from all sites were considered together (0.62).
3.4.4. Seasonal infection risk
Calculations of Ollerenshaw Indices are shown in Table 3. No significant differences were found in this index between historical data and study period data (2015–2016) at Taralga (Sites 1 and 2). A decrease in infection risk was evident at Guyra in winter 2016 when compared to historical averages (p = 0.008).
Table 3.
Calculations of Ollerenshaw Index.
| Region | Season | Ollerenshaw Index | Risk category | p-value (Mann-Whitney) |
|---|---|---|---|---|
| Taralga | Summer – Annual | 407.46 | Disease prevalent | |
| Summer – 2014/2015* | 399.17 | Occasional losses | 1.000 | |
| Summer – 2015/2016 | 296.22 | Little to no disease | 0.394 | |
| Winter – Annual | 344.49 | Occasional losses | ||
| Winter – 2015 | 263.89 | Little to no disease | 0.690 | |
| Winter – 2016 | 204.78 | Little to no disease | 0.056 | |
| Guyra | Summer – Annual | 538.89 | Disease prevalent | |
| Summer – 2014/2015* | 478.83 | Disease prevalent | 0.545 | |
| Summer – 2015/2016 | 423.92 | Disease prevalent | 0.113 | |
| Winter – Annual | 416.31 | Disease prevalent | ||
| Winter – 2015* | 368.86 | Occasional losses | 0.413 | |
| Winter – 2016 | 230.63 | Little to no disease | 0.008 |
Pre-study period.
4. Discussion
These investigations addressed whether the two diagnostic tests FFEC and cELISA could reliably discriminate the seasonal differences in the level of fasciolosis. Whilst both diagnostic tests have their value in the farm environment, the field data demonstrated that the diagnostic sensitivity for epidemiological studies can be increased by using the two tests in parallel. The agreement between the two diagnostics was found to be higher in cattle, despite the poor sensitivity of FFEC in this species. Similar levels of agreement between the two tests were demonstrated at both sheep properties, regardless of the clear difference in the intensity of F. hepatica challenge.
Full agreement between FFEC and cELISA was observed in 10% of enrolled animals, with the poor agreement at Site 1 (apparent low infection/challenge) consistent with previous investigations where burdens of <7 live fluke exhibited poor diagnostic agreement in sheep (George et al., 2017, Gordon et al., 2012b). Given the multiple treatments and poor efficacy at Site 3, the lack of agreement despite evidence of heavy infection in the majority of sheep is likely due to inconsistent antigen release post-treatment (i.e. false-negative rates of 33–40%;(George et al., 2017). This reinforces the necessity of a secondary cELISA test at 6 weeks or more to clarify the initial result.
The discrepancies in efficacy results across all three study sites highlight the impact of F. hepatica maturation. The efficacy was demonstrated as effective for sites 1 and 2 by FFEC but either ineffective (site 1) or poorly effective (site 2) when measured by cELISA. The change in FFEC and associated drop in efficacy at both these sites at the second visit (8+ weeks post treatment [wpt]) confirms that the cELISA was detecting immature F. hepatica. At site 3 initial poor efficacy across both tests (<5 wpt) was followed by a reduction in efficacy for FFEC at 8+ wpt, as did the secondary cELISA for the TCBZ drench. However, the combination drench administered in May 2016 remained at a similar efficacy to <5wpt. This would reflect the greater exposure to metacercariae over spring/summer resulting in aquired parasites post treatment. This suggests that in contrast to the observations of Gordon et al. (2012b) the cELISA is capable of detecting aquired immature parasites prior to patency in the field.
The epidemiological trends displayed during the entire study period were consistent with previous regional data (Boray, 1969, Boray, 2017), which records infection rising in both autumn (March through May) and spring months (September through November). Given that detection of F. hepatica is expected from 6 to 8 wpi by cELISA and 8–12 wpi by FFEC (Brockwell et al., 2013, Flanagan et al., 2011a, Mezo et al., 2004), it is expected that rises in cELISA would precede FFEC by 1–2 months. In contrast to the findings utilising the original cELISA of Gordon et al. (2012b), this was evident in sheep at Site 1 and cattle at Site 2, but this was not clear in the sheep from Site 3 where infection levels were higher. This may suggest that the substitution of the streptavidin conjugate in the second generation kit has improved the in-field sensitivity of the diagnostic.
The application of the Ollerenshaw Index was found to require modification for Australian conditions, possibly as acute disease is minimal and stocking density tends to be low; which was previously noted by Ollerenshaw (1966) to require Mt values much higher than 300 in similar UK stocking density regions. When applied, the minimal disease risk predicted at the Taralga site was consistent with the study data at Site 1 and Site 2, with no significant difference to annual data in the Ollerenshaw Index observed. In contrast, significant differences for Ollerenshaw Index were found in Guyra for winter 2016, at which point the anticipated challenge was predicted as significantly lower than annual averages. However, actual infection levels at Site 2 were maintained throughout this period, this may be related to higher than average autumn 2016 temperatures resulting in extension of snail activity (and metacercarial availability) by one month. This could account, in part, for the continued high infection demonstrated in the current study.
Overall, true prevalence at each property was consistent with the infection trends demonstrated in raw data. Interestingly, true prevalence of infection was frequently higher by cELISA for both Taralga sites, whilst true prevalence of cELISA exceeded FEC on only two occasions at the Guyra site. At Site 1 where infection levels appeared to be low, the difference was substantial in the months following treatment but not as extreme and ultimately reversed once patent infection returned. The poorer detection reliability at this site reflects the poor sensitivity of cELISA for diagnosing sheep with low infection levels (George et al., 2017). In contrast at Site 3 where infection was persistent, the estimates of true prevalence were found to be more consistent between the diagnostics. Only the estimate immediately post-treatment at Site 2 in cattle suggested a significant difference (as confidence limits were separated) between the two diagnostics for true prevalence estimates. These data would suggest that while suitable for cattle under variable F. hepatica challenges, the cELISA is only suitable under heavy challenge conditions in sheep and may be a poor diagnostic test if infectivity is low.
The outcomes of the two diagnostics utilized in parallel were explained substantially (R2 = 0.91) when season, region, rainfall, pre-visit treatment and mean minimum temperature were included in the model. Use of the two diagnostics in series were also explained substantially (R2 = 0.88) when season, mean minimum temperatures, mean maximum temperatures and pre-visit treatment were included in the model. This contrasts to the fitted models for FFEC (R2 = 0.54) and cELISA (R2 = 0.58). The outcomes of these models support the previous findings of George et al. (2017) that the two diagnostics are best utilized together, particularly in parallel.
The on-farm application of the two diagnostic tests demonstrated that the cELISA is potentially more sensitive in detecting infection changes than the FFEC, but is unreliable in cases of low parasite challenge. Infection trends based on known epidemiology were contemporaneous utilising this diagnostic, whereas due to patency delays this was not so for FFEC. Therefore, results from the cELISA require minimal interpretation in comparison to FFEC when investigating the epidemiology of F. hepatica. The two Taralga properties highlighted the localized nature of parasite challenge despite the close proximity, albeit the use of different species and timing of treatment application confound the interpretations. These data would suggest if fasciolosis is an issue for a producer, investigation of epidemiology and parasite challenge at the individual property level may be advisable, although costs may be prohibitive. Furthermore, as reported previously, studies with the cELISA should highlight cases of potential resistance earlier than reliance on FFEC alone (Brockwell et al., 2014, Flanagan et al., 2011b).
Whilst the current body of work highlights the potential for the cELISA utilisation on farm, the outcomes must be interpreted with caution due to the low number of sites (n = 3) and sample population at each property (n = 10). This is particularly evident when one considers the true prevalence data and the extreme confidence limits. The data here presented may misrepresent the true situation in terms of prevalence of fasciolosis in Australia and also the performance of both diagnostics. More extensive studies with a larger number of sites and sample population are required for further validation of the current findings.
5. Conclusion
The prevalence of endemic F. hepatica may be successfully monitored utilising cELISA or FFEC. Utilisation of the techniques together (in parallel) allows the best explanation of individual animal variance.
Both methods are also suitable for monitoring drug efficacy on farm, however, the interpretation of the results requires expert knowledge of the epidemiology and biology of the parasites as well as understanding of the diagnostic limitations of each test. Further investigation into ideal sampling points on-farm and standard testing regimes for the determination of drug performance against F. hepatica would be recommended.
The application of the Ollerenshaw Index or development of a unique Australian predictive index for F. hepatica infection risk require further investigation, however, the lack of standardized monitoring and data collation for fasciolosis may inhibit this requirement. The implementation of management practices combined with knowledge of specific property risk areas and normal epidemiology for the region may provide a more economical path for producers in this region.
Conflict of interest statement
Several authors (SG, AG and PR) are paid employees of Elanco Australasia Pty Limited, who funded this study.
Acknowledgements
The authors acknowledge the support of Dominique Marendy, Matthew Van der Saag and Vanessa Sluyter at Yarrandoo R&D Centre. Navneet Dhand and Alan Marcus are thanked for their advice on the statistical analysis. Meat and Livestock Australia (MLA) is thanked for the award of a post-graduate scholarship technical assistance grant (B.STU.0298). Barry Hosking, Alan Marcus and Kim Baker are thanked for their constructive comments on the draft manuscript.
References
- Abbott W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–267. [Google Scholar]
- Beltrame L., Dunne T., Vineer H.R., Walker J.G., Morgan E.R., Vickerman P., McCann C.M., Williams D.J.L., Wagener T. A mechanistic hydro-epidemiological model of liver fluke risk. J. Roy. Soc. Interface. 2018;15 doi: 10.1098/rsif.2018.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boray J.C. Experimental fascioliasis in Australia. Adv. Parasitol. 1969;7:95–210. doi: 10.1016/s0065-308x(08)60435-2. [DOI] [PubMed] [Google Scholar]
- Boray J.C. 4 ed. NSW Department of Primary Industries; 2017. Liver Fluke Disease in Sheep and Cattle, Primefact 446. [Google Scholar]
- Brockwell Y.M., Spithill T.W., Anderson G.R., Grillo V., Sangster N.C. Comparative kinetics of serological and coproantigen ELISA and faecal egg count in cattle experimentally infected with Fasciola hepatica and following treatment with triclabendazole. Vet. Parasitol. 2013;196:417–426. doi: 10.1016/j.vetpar.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Brockwell Y.M., Elliott T.P., Anderson G.R., Stanton R., Spithill T.W., Sangster N.C. Confirmation of Fasciola hepatica resistant to triclabendazole in naturally infected Australian beef and dairy cattle. Int. J. Parasitol. Drugs Drug Resist. 2014;4:48–54. doi: 10.1016/j.ijpddr.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunsdon R.V. Liver fluke Fasciola hepatica in sheep and cattle in New Zealand and its control. N.Z. Vet. J. 1967;15:12–23. doi: 10.1080/00480169.1976.33678. [DOI] [PubMed] [Google Scholar]
- Calvani N.E.D., Windsor P.A., Bush R.D., Slapeta J. Scrambled eggs: a highly sensitive molecular diagnostic workflow for Fasciola species specific detection from faecal samples. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005931. e0005931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diab T.M., Mansour H.H., Mahmoud S.S. Fasciola gigantica: parasitological and scanning electron microscopy study of the in vitro effects of ivermectin and/or artemether. Exp. Parasitol. 2010;124:279–284. doi: 10.1016/j.exppara.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Droogers P., Allen R.G. Estimating reference evapotranspiration under inaccurate data conditions. Irrig. Drain. Syst. Eng. 2002;16:33–45. [Google Scholar]
- Elliott T.P., Kelley J.M., Rawlin G., Spithill T.W. High prevalence of fasciolosis and evaluation of drug efficacy against Fasciola hepatica in dairy cattle in the Maffra and Bairnsdale districts of Gippsland, Victoria, Australia. Vet. Parasitol. 2015;209:117–124. doi: 10.1016/j.vetpar.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Flanagan A., Edgar H.W., Gordon A., Hanna R.E., Brennan G.P., Fairweather I. Comparison of two assays, a faecal egg count reduction test (FECRT) and a coproantigen reduction test (CRT), for the diagnosis of resistance to triclabendazole in Fasciola hepatica in sheep. Vet. Parasitol. 2011;176:170–176. doi: 10.1016/j.vetpar.2010.10.057. [DOI] [PubMed] [Google Scholar]
- Flanagan A.M., Edgar H.W., Forster F., Gordon A., Hanna R.E., McCoy M., Brennan G.P., Fairweather I. Standardisation of a coproantigen reduction test (CRT) protocol for the diagnosis of resistance to triclabendazole in Fasciola hepatica. Vet. Parasitol. 2011;176:34–42. doi: 10.1016/j.vetpar.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Fox N.J., White P.C., McClean C.J., Marion G., Evans A., Hutchings M.R. Predicting impacts of climate change on Fasciola hepatica risk. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016126. e16126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S.D., Vanhoff K., Baker K., Lake L., Rolfe P.F., Seewald W., Emery D.L. Application of a coproantigen ELISA as an indicator of efficacy against multiple life stages Fasciola hepatica infections in sheep. Vet. Parasitol. 2017;246:60–69. doi: 10.1016/j.vetpar.2017.08.028. [DOI] [PubMed] [Google Scholar]
- GHD Pty Ltd . Meat and Livestock Australia Limited; North Sydney: 2009. Cost Benefits of e-surveillance System for Animal Health Monitoring. [Google Scholar]
- Gordon D., Zadoks R., Skuce P., Sargison N. Confirmation of triclabendazole resistance in liver fluke in the UK. Vet. Rec. 2012;171:159–160. doi: 10.1136/vr.e5381. [DOI] [PubMed] [Google Scholar]
- Gordon D.K., Zadoks R.N., Stevenson H., Sargison N.D., Skuce P.J. On farm evaluation of the coproantigen ELISA and coproantigen reduction test in Scottish sheep naturally infected with Fasciola hepatica. Vet. Parasitol. 2012;187:436–444. doi: 10.1016/j.vetpar.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Happich F.A., Boray J.C. Quantitative diagnosis of chronic fasciolosis. 1. Comparative studies on quantitative faecal examinations for chronic Fasciola hepatica infection in sheep. Aust. Vet. J. 1969;45:326–328. doi: 10.1111/j.1751-0813.1969.tb05009.x. [DOI] [PubMed] [Google Scholar]
- Haydock L.A.J., Pomroy W.E., Stevenson M.A., Lawrence K.E. A growing degree-day model for determination of Fasciola hepatica infection risk in New Zealand with future predictions using climate change models. Vet. Parasitol. 2016;228:52–59. doi: 10.1016/j.vetpar.2016.05.033. [DOI] [PubMed] [Google Scholar]
- Howell A., Baylis M., Smith R., Pinchbeck G., Williams D. Epidemiology and impact of Fasciola hepatica exposure in high-yielding dairy herds. Prev. Vet. Med. 2015;121:41–48. doi: 10.1016/j.prevetmed.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J., Utzinger J. Emerging foodborne trematodiasis. Emerg. Infect. Dis. 2005;11:1507–1514. doi: 10.3201/eid1110.050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J., Jubb T., Shephard R., Webb-Ware J., Fordyce G. Meat and Livestock Australia; 2015. B.AHE.0010 Final Report: Priority List of Endemic Disease for the Red Meat Industries. [Google Scholar]
- Malone J.B., Fehler D.P., Loyacano A.F., Zukowski S.H. Use of LANDSAT MSS imagery and soil type in a geographic information system to assess site-specific risk of fascioliasis on Red River Basin farms in Louisiana. Ann. N.Y. Acad. Sci. 1992;653:389–397. doi: 10.1111/j.1749-6632.1992.tb19667.x. [DOI] [PubMed] [Google Scholar]
- Marcus A.D., Higgins D.P., Gray R. Epidemiology of hookworm (Uncinaria sanguinis) infection in free-ranging Australian sea lion (Neophoca cinerea) pups. Parasitol. Res. 2014;113:3341–3353. doi: 10.1007/s00436-014-3997-3. [DOI] [PubMed] [Google Scholar]
- Martinez-Perez J.M., Robles-Perez D., Rojo-Vazquez F.A., Martinez-Valladares M. Comparison of three different techniques to diagnose Fasciola hepatica infection in experimentally and naturally infected sheep. Vet. Parasitol. 2012;190:80–86. doi: 10.1016/j.vetpar.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Martínez-Sernández V., Orbegozo-Medina R.A., González-Warleta M., Mezo M., Ubeira F.M. Rapid enhanced MM3-COPRO ELISA for detection of Fasciola coproantigens. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004872. e0004872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Valladares M., del Rosario Famularo M., Fernandez-Pato N., Castanon-Ordonez L., Cordero-Perez C., Rojo-Vazquez F.A. Efficacy of nitroxynil against Fasciola hepatica resistant to triclabendazole in a naturally infected sheep flock. Parasitol. Res. 2010;107:1205–1211. doi: 10.1007/s00436-010-1989-5. [DOI] [PubMed] [Google Scholar]
- McManus D.P., Dalton J.P. Vaccines against the zoonotic trematodes Schistosoma japonicum, Fasciola hepatica and Fasciola gigantica. Parasitology. 2006;(133 Suppl):S43–61. doi: 10.1017/S0031182006001806. [DOI] [PubMed] [Google Scholar]
- Mehmood K., Zhang H., Sabir A.J., Abbas R.Z., Ijaz M., Durrani A.Z., Saleem M.H., Ur Rehman M., Iqbal M.K., Wang Y., Ahmad H.I., Abbas T., Hussain R., Ghori M.T., Ali S., Khan A.U., Li J. A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microb. Pathog. 2017;109:253–262. doi: 10.1016/j.micpath.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Mezo M., Gonzalez-Warleta M., Carro C., Ubeira F.M. An ultrasensitive capture ELISA for detection of Fasciola hepatica coproantigens in sheep and cattle using a new monoclonal antibody (MM3) J. Parasitol. 2004;90:845–852. doi: 10.1645/GE-192R. [DOI] [PubMed] [Google Scholar]
- Molloy J.B., Anderson G.R., Fletcher T.I., Landmann J., Knight B.C. Evaluation of a commercially available enzyme-linked immunosorbent assay for detecting antibodies to Fasciola hepatica and Fasciola gigantica in cattle, sheep and buffaloes in Australia. Vet. Parasitol. 2005;130:207–212. doi: 10.1016/j.vetpar.2005.02.010. [DOI] [PubMed] [Google Scholar]
- NADIS . 2017. NADIS Parasite Forecast - September 2017.http://webinars.nadis.org.uk/media/42721/42717-42709_parasite_forecast_sqp_.pdf pp. Available at: (Accessed 42726.42704.42018) [Google Scholar]
- Nakagawa S., Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013;4:133–142. [Google Scholar]
- Novobilsky A., Averpil H.B., Hoglund J. The field evaluation of albendazole and triclabendazole efficacy against Fasciola hepatica by coproantigen ELISA in naturally infected sheep. Vet. Parasitol. 2012;190:272–276. doi: 10.1016/j.vetpar.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Ollerenshaw C.B. The approach to forecasting the incidence of fascioliasis over England and Wales 1958–1962. Agr. Meteorol. 1966;3:35–53. [Google Scholar]
- Ollerenshaw C.B., Rowlands W.T. A method of forecasting the incidence of fascioliasis in Anglesey. Vet. Rec. 1959;71:591–598. [Google Scholar]
- Rapsch C., Dahinden T., Heinzmann D., Torgerson P.R., Braun U., Deplazes P., Hurni L., Bar H., Knubben-Schweizer G. An interactive map to assess the potential spread of Lymnaea truncatula and the free-living stages of Fasciola hepatica in Switzerland. Vet. Parasitol. 2008;154:242–249. doi: 10.1016/j.vetpar.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Rogan W.J., Gladen B. Estimating prevalence from the results of a screening test. Am. J. Epidemiol. 1978;107:71–76. doi: 10.1093/oxfordjournals.aje.a112510. [DOI] [PubMed] [Google Scholar]
- Seddon H.R. Division of Veterinary Hygiene, Department of Health; Canberra, ACT, Australia: 1950. Diseases of Domestic Animals in Australia, Part 1: Helminth Infestations. [Google Scholar]
- Viera A.J., Garrett J.M. Understanding interobserver agreement: the kappa statistic. Fam. Med. 2005;37:360–363. [PubMed] [Google Scholar]
- Young N.D., Hall R.S., Jex A.R., Cantacessi C., Gasser R.B. Elucidating the transcriptome of Fasciola hepatica - a key to fundamental and biotechnological discoveries for a neglected parasite. Biotechnol. Adv. 2010;28:222–231. doi: 10.1016/j.biotechadv.2009.12.003. [DOI] [PubMed] [Google Scholar]