Graphical abstract
Keywords: Buparvaquone, Parvaquone, HPLC, Drug potency, Drug stability, Theileria parva, Theileria annulata, Theileria equi
Highlights
-
•
Buparvaquone and parvaquone are used to treat livestock infected with Theileria spp.
-
•
Air exposure had a significant impact on the stability of buparvaquone and parvaquone.
-
•
Parvaquone was more stable than buparvaquone.
-
•
Drug degradation was related with loss of potency by an in vitro viability assay.
Abstract
Buparvaquone and parvaquone are hydroxynaphthoquinone compounds commonly used to treat livestock infected with Theileria species such as T. parva and T. annulata. In many (sub)tropical regions, chromatic changes in medicines can result from extreme environmental conditions and improper drug storage or handling, raising the possibility of drug degradation and loss of potency. We evaluated the effects of UV light, elevated temperature, and atmospheric air on the stability and potency of both buparvaquone and parvaquone by using a combination of high performance liquid chromatography (HPLC) and a T. equi based in vitro parasite growth inhibition assay (to measure potency). Aliquots (1 ml; 3 replicates per treatment) of each compound were subjected to a variety of treatments that varied in duration and intensity followed by HPLC and potency assays. Exposure to ambient air for 50 days was correlated with a significant loss of potency for both buparvaquone (4535%, P < 0.05) and parvaquone (247%, P < 0.05), while elevated temperature (37°C) and UV light exposure (24 h) had no significant impact (P > 0.05). The decrease in potency of both buparvaquone and parvaquone correlated with drug degradation (r = -0.74 and -0.88, respectively) as measured by HPLC. In practice, if there is headspace present in the vial, then ambient air will invariably enter the vial and contribute to degradation of these compounds. Such degradation may contribute to increasing drug resistance, economic losses for farmers, and animal welfare concerns for animals that are treated for Theileria infections.
1. Introduction
Buparvaquone (BPQ) and parvaquone (PQ) are hydroxynaphthoquinone compounds that are used to treat theilerial parasitic infections. These compounds are widely used to clear cattle infected with Theileria parva (Muraguri et al., 1999) and Theileria annulata (Hashemifesharki, 1991), with several studies also demonstrating some efficacy for treating horses infected with Theileria equi (Kumar et al., 2003, Kuttler et al., 1987, Salib et al., 2012, Zaugg and Lane, 1989, Zaugg and Lane, 1992). In (sub)tropical geographical regions, the ability to properly store medicinal drugs is often compromised, and consequently such drugs may be exposed to conditions that do not adhere to the manufacturers’ recommendations. As a result, the potency (or “efficacy”) of drugs like BPQ and PQ could be adversely affected.
T. parva is the causative agent of East Coast Fever (ECF) in cattle, a disease of serious clinical and economic importance in eastern and southern Africa. Historically it is estimated that over a million cattle are lost annually to ECF in this region (Mukhebi et al., 1992). The fatality rate is approximately 50–60% in zebu cattle (Bos indicus), but approaches 100% in European cattle breeds (Bos taurus) (Muraguri et al., 1999, Muraguri et al., 2006). In Kenya, ECF is the leading cause of mortality in zebu cattle during the first year of life (Thumbi et al., 2013). T. parva is lymphodestructive, causing dramatic immunosuppression (Dolan et al., 1988). Prompt treatment with either BPQ or PQ can achieve success rates of 90–100% in uncomplicated cases of ECF (Muraguri et al., 1999). This approach is more cost effective and efficacious than the alternative and expensive process of controlling the primary disease vector Rhipicephalus appendiculatus (brown ear tick) (Mukhebi et al., 1992). Theileria annulata causes tropical theileriosis in cattle, which can result in up to 90% mortality in susceptible breeds. Similar to ECF, both BPQ and PQ are effective in treating tropical theileriosis, however, emergence of in vivo T. annulata resistance to BPQ treatment has been reported (Mhadhbi et al., 2010, Sharifiyazdi et al., 2012).
In addition to T. parva and T. annulata in cattle, both BPQ and PQ have been evaluated for efficacy against the related tick-borne hemoprotozoan T. equi, a causative agent of equine piroplasmosis. Acute T. equi infection is characterized by intravascular hemolysis and associated fever, anemia, and thrombocytopenia. Following resolution of acute disease, horses remain persistently infected, inapparent carriers and serve as reservoirs of transmission to naïve horses (Ueti et al., 2012, Wise et al., 2012). Although the goal of treatment in endemic regions is to mitigate clinical disease, the goal in nonendemic regions such as the United States is to clear infection. The current drug of choice for both purposes is imidocarb dipropionate (Ueti et al., 2012, Wise et al., 2012). Although BPQ and PQ are effective against T. equi in vitro, neither drug is consistently effective in eliminating T. equi from infected horses (Kumar et al., 2003, Kuttler et al., 1987, Salib et al., 2012, Zaugg and Lane, 1989, Zaugg and Lane, 1992). Therefore, hydroxynaphthoquinone drugs are not routinely used to treat T. equi infections in vivo.
In (sub)tropical regions, drugs are often handled in less than ideal conditions. They can be exposed to extreme temperatures (both low and high) that may fluctuate extensively in a 24 -h period. Between these extremes and uncontrolled exposure to ambient air and ultraviolet (UV) light, drugs are likely subjected to conditions outside of recommended storage conditions. Although these drugs are manufactured in airtight vials for injection, multi-dose vials invariably allow introduction of atmospheric air, particularly when the bottles experience frequent swings from hot to cold temperatures. It is also possible to inadvertently or intentionally inject air when withdrawing drugs from such vials. The manner in which medicines are handled and use of expired drugs (potentially driven by socioeconomic reasons) can lead to use of drugs that present dramatic chromatic changes [e.g., oxidized oxytetracycline collected from households in northern Tanzania, Fig S1. (Caudell et al., 2017)
Drug stability has been an issue of importance in human medicine, with particular concern for the stability of anti-malarial and other drugs vital for human health in tropical and (sub)tropical regions. Many medications do not reach their labeled shelf life with adequate active drug content due to extreme environmental conditions (Amin and Kokwaro, 2007, Ballereau et al., 1997, Kayumba et al., 2004). Exposure to UV light, elevated temperature, and atmospheric air are all known factors that can affect drug stability, although the degree of effect on a specific drug varies (Amin and Kokwaro, 2007, Ammann, 2011, Chatzitakis et al., 2008, Jaffe et al., 1976, Kayumba et al., 2004, Langner and Maibach, 2009, Risha et al., 2002, Risha et al., 2003, Twagirumukiza et al., 2009). Given the above challenges, the purpose of the present study was to determine the effects of UV light, elevated temperature, and atmospheric air on the stability and potency of both PQ and BPQ using a combination of high performance liquid chromatography (HPLC) and an in vitro T. equi inhibition assay.
2. Materials and methods
2.1. Chemical reagents
To induce chromatic changes in PQ and BPQ in a timely manner and under controlled experimental conditions, commercially available PQ and BPQ were initially diluted by a factor of 10 in either DMSO or a relevant excipient as described below. The purpose of this dilution is to decrease the stability window of the drugs. For the primary exposure experiment, chemical grade parvaquone (BOC Sciences) was diluted to saturation in dimethyl sulfoxide (DMSO) to 15 mg/mL (Sigma-Aldrich, St. Louis, MO, USA) as the commercial formulation was not available at the time, this value corresponds to the saturation concentration in DMSO. For later replication of the air-exposure experiment, we obtained a 150 mg/mL commercial product, Parvexon (Biomeda, Dublin, Ireland). In both experiments, the BPQ commercial product Bupaquone (Eagle Vet. Tech Co. Ltd, Chungnam, Korea) was used. Commercial BPQ was also diluted 10 times in DMSO (to a final concentration of 5 mg/ml) to match the 1:10 dilution that was used for Parvaquone. Both manufacturers recommend storage at temperatures below 25 °C and protected from light. Reagents for T. equi culture medium (HL2A-NHS) included HL-1 and HEPES (Fisher Scientific, Waltham, MA), HB101 supplement (Irvine Scientific, Santa Ana, CA, USA), L-glutamine and AlbuMax (Gibco, Grand Island, NY, USA) and penicillin/streptomycin and gentamicin (Sigma-Aldrich, St. Louis, MO, USA). Hydroethidine that was used for flow cytometric evaluation was acquired from Invitrogen (Carlsbad, CA, USA) as a 5 mM solution solubilized in DMSO. Acetonitrile and sodium acetate used for HPLC were purchased from Fisher Scientific (Pittsburg, PA, USA).
2.2. Drugs exposure conditions
Each sample consisted of a 1-mL aliquot of drug (5 mg/mL BPQ or 15 mg/mL PQ, diluted in DMSO) placed into 1.5-mL microcentrifuge tubes. A total of 12 samples were used for each drug in each storage condition (Ambient air, UV light, 37 °C, and Combination), with triplicate samples for each designated length of exposure. All samples were wrapped in aluminum foil and stored at room temperature, unless under active exposure to UV light or storage at 37 °C. Tubes containing samples for air exposure had 2 mm holes punched through each cap to simulate puncture by a 14-16-gauge needle such as those typically used for bovine injections. Three samples were removed and stored in fully sealed microcentrifuge tubes at seven (Air7d), 14 (Air14d), 30 (Air30d), and 50 days (Air50d). Exposure to UV light was accomplished by using a BSL-II hood UV lamp (UV—A and UV—B). Three samples were removed after three (UV3h), six (UV6h), 12 (UV12h), and 24 h (UV24h). Environmental temperature was simulated by placing samples in a 37 °C stationary incubator (dark inside) for eight hours per exposure. Three samples were removed after five (37 °C5exp), 10 (37 °C10exp), 20 (37 °C20exp), and 40 exposures (37 °C40exp). Between exposures, samples were stored at 4 °C in the dark. Finally, for the Combination exposure group each condition was set up as described above, and three samples were exposed to each of the following combinations of conditions: 1.) five exposures 37 °C/three hours UV light/seven days ambient air (Combo7d); 2.) 10 exposures 37 °C/six hours UV light/14 days ambient air (Combo14d); 3.) 20 exposures 37 °C/12 h UV light/30 days ambient air (Combo30d); 4.) 40 exposures 37 °C/24 h UV light/50 days ambient air (Combo50d).
2.3. BPQ/PQ assay development and determination of drug susceptibility of exposed samples
To evaluate loss of BPQ and PQ potency, we used laboratory cultures of T. equi-infected erythrocytes. T. equi parasites were originally isolated and adapted to culture from a splenectomized horse that was infected with T. equi from a 2009 field outbreak in Texas (Hines et al., 2015, Ueti et al., 2012). The potency of fresh PQ and BPQ were both evaluated in this system. The initial concentrations were based on the described pharmacokinetics of PQ and BPQ in cattle (Kinabo and Bogan, 1988, Muraguri et al., 2006), in vitro efficacy for T. parva in lymphoblastoid culture (Mchardy et al., 1985), and in vitro efficiency for Leishmania donovani (Croft et al., 1992, Venkatesh et al., 2008). This involved two-fold dilutions initially ranging from 0.00019 to 0.048 μg/mL for PQ and 0.0768 to 0.3 g/mL for BPQ. These dilutions were prepared by using the stock solutions of PQ (15 mg/mL) and BPQ (5 mg/mL) in DMSO, serially diluted in HL2A-NHS media to reach the appropriate concentrations for the assay. The final concentration of DMSO in all drug dilutions was < 0.02%, which has no effect on parasite growth (Wise et al., 2012). The assay was adapted from previously described procedures (Hines et al., 2015, Silva et al., 2018); briefly, each assay was performed over a 48-h period in a 96-well plate, with infected/untreated erythrocytes, uninfected/untreated erythrocytes, and infected/treated erythrocytes (at each tested concentration of drug) used as controls. Each sample and control was evaluated in triplicate, with approximately one percent of parasitized erythrocytes (PPE) infected at the beginning of the assay. Hydroethidine staining with flow cytometry was used to evaluate the final PPE for each sample (Hines et al., 2015). Each of the three independent exposure replicates for every exposure type and length was assessed in tandem by using three technical replicates for each drug concentration. The concentrations initially tested included two-fold dilutions ranging from 0.00075 to 0.048 μg/mL for PQ, and from 0.0006 to 0.0096 μg/mL for BPQ. This range was expanded upwards as necessary to accommodate samples with an increase in IC50. Evaluation of exposed samples began with examination of maximum Combo exposures (BPQ Combo50d and PQ Combo50d) to determine the expected maximum possible effect from all factors. This was followed by the other treatment exposures. A similar process was used for individual conditions, starting with UV24h, 37 °C40exp, and Air50d. If no treatment effects were evident upon maximum exposure, lesser exposures were not evaluated.
2.4. Flow cytometry
Cell suspensions were evaluated using a FACSCaliber flow cytometer equipped with CellQuest computer software (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). The inclusion gate was based on the forward and side scatter features of uninfected erythrocytes stained with hydroethidine and 50,000–150,000 events per sample were collected. Ethidium bromide fluoresces in the FL-2 channel, and correspondingly argon-laser fluorescence excitation at 488 nm and emission at 585 nm (range 563–607 nm) were used for analysis in log FL-2 data mode. Fluorescent profiles were recorded for later analysis with FCS Express software (De Novo software, Los Angeles, CA, USA). Quadrant gating of generated dot plots (FL-2 vs. side scatter) was based on stained uninfected erythrocyte controls to delineate between infected and uninfected cell populations. The PPE of each well was determined based on the percentage of the cell population characterized as RBCs that exhibited FL-2 fluorescence on flow cytometry.
2.5. BPQ and PQ degradation determined by reversed-phase high performance chromatography
High Performance Liquid Chromatography (HPLC) was performed by using an ÄKTA™ Avant system (GE Healthcare, Pittsburg, PA, USA) coupled with a Kinetex C18, 5 μm particle size, 100A, 100 x 4.6 mm stainless steel column, manufactured by Phenomenex (Phenomenex Gemini, Torrance, CA, USA). Mobile phases were 0.05 M Na-acetate buffer (pH 3.6)-Acetonitrile (35:65 v/v) and 0.05 M Na-acetate buffer (pH 3.6)-Acetonitrile (20:80, v/v) for BPQ and PQ, respectively. Elution was performed at 1 ml/min flow rate with an equilibration phase for 4 min followed by an elution step for 10 min for PQ and 8 min for BPQ, using protocols adapted from Kinabo (1988) and Venkatesh (2007). Samples were injected using a 20-μl loop and detection was achieved by measuring simultaneously the UV absorption at 251 and 281 nm. Integration was used to determine the area under the 251 nm chromatogram curve, which correlated with recovery of the drug. Data collection and analysis were completed using UNICORN™ 6.3 control software (GE Healthcare Life Sciences). Calibration curves were constructed using the injectable drug due to unavailability of the pure compound for both drugs. The BPQ calibration curve was linear in the 20–1000 ng range (Fig. S3) and the PQ calibration curve was linear in the 30–1500 ng range (Fig. S4). PQ samples in all treatment groups were diluted 1/100 prior to injection while BPQ samples were diluted 1/200 in their respective mobile phase. The samples were diluted to ensure that undegraded samples were detected well inside the linear range of the calibration curve, with only samples that underwent complete degradation falling outside the linear range of detection.
2.6. Confirmation of air exposure as the main cause for BPQ and PQ degradation
BPQ and PQ were diluted to 5 mg/mL and 15 mg/mL (respectively) in N-methyl-2-pyrrolidone. Triplicate samples of 1.4 mL (completely filled) and 0.2 mL (partially filled) were prepared in brown 1.5 mL microcentrifuge tubes and left at room temperature for 50 days, protected from light. Samples included those with air exposure in the form of “headspace” in partially filled tubes, while the completely filled tubes had no obvious headspace. These samples were then evaluated for drug recovery using HPLC in comparison with a 1/10 dilution of a fresh, unexposed sample taken from the drug vial just before HPLC analysis.
2.7. Data analysis
Mean PPE for each drug concentration and controls was calculated by averaging the PPE of three independent replicates. Percent of maximum PPE (%-max-PPE) was then calculated for each concentration using the mean PPE value in comparison to the highest PPE obtained in the assay for a given isolate. The 50% inhibitory concentrations (IC50) were estimated by fitting response curves using nonlinear regression and determining interpolated X values for Y = 50, including 95% confidence intervals for a significance level of P < 0.05 (GraphPad Prism version 6.3, GraphPad Software, La Jolla, CA). The IC50 values for each exposure replicate were compiled to calculate the mean, standard deviation, and percent change in IC50 (relative to the control of no exposure) for each exposure type and duration. Mean %-max-PPE values were also calculated to generate one mean IC50 curve for each exposure type and duration to compare against a mean control curve and determine if there were statistical differences between each treatment compared to unexposed drug. Exposure replicates deemed significant outliers via Grubb’s test were eliminated from analysis (http://graphpad.com/quickcalcs/Grubbs1.cfm). Data from HPLC analysis was evaluated by one-way ANOVA (F-test level of significance) using pairwise comparisons between each exposure duration for the content of each drug under each exposure condition. This was also performed to evaluate the relationships between the Air and Combo samples from each drug. Mean values were grouped according to Tukey HSC with simultaneous 95% confidence intervals. Average percent of intact drug was calculated for each time point as a function of the time zero unexposed control. A nonlinear regression plot with one phase decay was then generated and Pearson’s correlation coefficient calculated to determine the relationship between these values and the corresponding mean IC50 values; that is, depicting the correlation between drug quantity recovered and potency.
3. Results
3.1. In vitro susceptibility of T. equi to BPQ and PQ
Initial tests using our in vitro assay indicated that the 50% inhibitory concentration (IC50) for unexposed BPQ and PQ was 0.0019 μg/mL and 0.0042 μg/mL, respectively. When no-treatment control data was pooled across independent experiments, the results remained very consistent with a mean IC50 of 0.002 μg/mL (9.5% coefficient of variation; 10 replicates) for unexposed BPQ and 0.0048 μg/mL (9.0% coefficient of variation; seven replicates) for unexposed PQ (Fig. S1)
3.2. Effect of simulated environmental exposures on the in vitro susceptibility of T. equi to BPQ and PQ
3.2.1. Exposure to UV light and elevated temperature (37°C)
There was no significant difference between the mean IC50 of triplicate BPQ samples with maximum exposures to ultraviolet light (UV24h) and to elevated temperature (37C40exp) relative to controls (P > 0.05; all 95% confidence intervals overlapped). The same result was evident for PQ, and thus no further testing was pursued with respect to UV light or elevated temperature.
3.2.2. Exposure to air
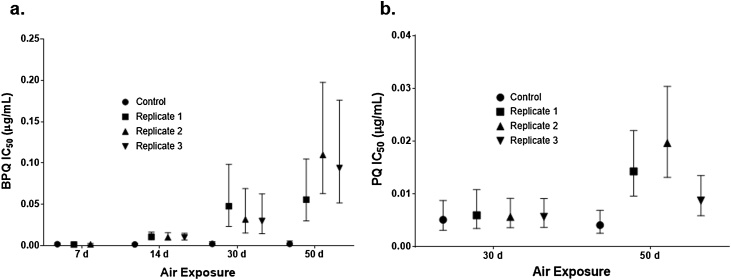
Exposure of BPQ to air altered the IC50 dramatically, with increases in the mean IC50 for Air14d, Air30d, and Air50d of 538%, 1531%, and 4535%, respectively (P < 0.05), relative to no-air exposure control (Fig. 1a). The Air7d IC50 was not significantly different from the unexposed control (P > 0.05). The mean IC50 of PQ increased 247% (P < 0.05) when exposed to air for 50 days but by day 30 the increase was only 12.6% (Fig. 1b; PQ Air14d and Air7d were not evaluated).
Fig. 1.
Mean IC50 after exposure to ambient air for 7, 14, 30 or 50 days for (a) BPQ, and for 30 and 50 days for (b) PQ. Error bars represent the estimated 95% confidence interval that was based on the goodness-of-fit growth inhibition curve for each replicate.
3.2.3. Combination of exposures
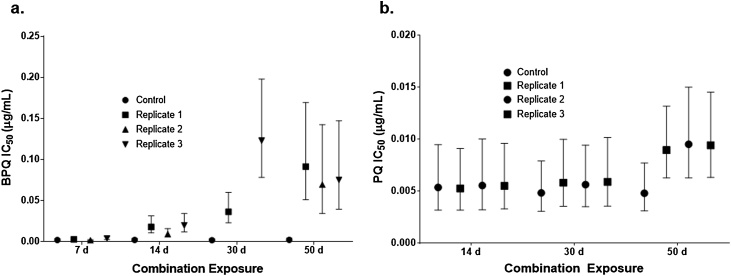
Combination exposures were subjected to each of the following combinations of conditions, designated by length of air exposure: Combo7d (five exposures 37 °C/three hours UV light/seven days ambient air); Combo14d (10 exposures 37 °C/six hours UV light/14 days ambient air); Combo30d (20 exposures 37 °C/12 h UV light/30 days ambient air); and Combo50d (40 exposures 37 °C/24 h UV light/50 days ambient air). When exposed to multiple environmental conditions, the mean IC50 of BPQ increased an average 43% (BPQ Combo7d), 806% (BPQ Combo14d), 4233% (BPQ Combo30d), and 3526% (BPQ Combo50d) (Fig. 2a). The mean IC50 of PQ Combo50d increased an average of 93%, however the wide confidence interval observed in this treatment rendered the result statistically insignificant (Fig. 2b). No significant change was found for PQ Combo30d or PQ Combo14d, while PQ Combo7d was not tested.
Fig. 2.
Mean IC50 for combination exposure experiments with (a) BPQ and (b) PQ. 7 d: 5 exposures 37 °C/3 h UV light/7 d ambient air; 14 d: 10 exposures 37 °C/6 h UV light/14 d ambient air; 30 d: 20 exposures 37 °C/12 h UV light/30 d ambient air; 50 d: 40 exposures 37 °C/24 h UV light/50 d ambient air. Error bars represent the estimated 95% confidence interval based on the goodness-of-fit growth inhibition curve for each replicate.
3.3. Effect of simulated environmental exposures on the stability of BPQ and PQ determined by HPLC
3.3.1. Exposure to UV light or elevated temperature (37°C)
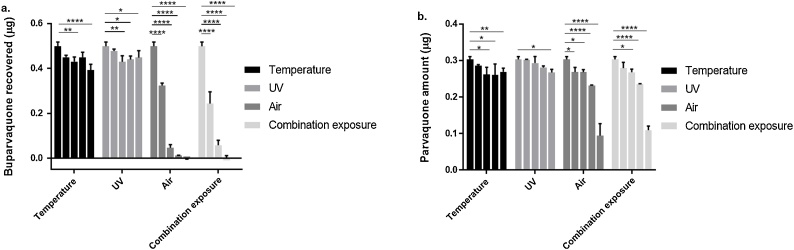
BPQ exposed to 37 °C for five or 20 exposures of eight hours each resulted in no change in recovered drug while the 37 °C10exp and 37 °C40exp samples resulted in 13.6% and 21.1% reductions in detectable drug compared to the control (P < 0.01 and P < 0.0001, respectively) (Fig. 3a – Temperature). While statistically significant, these changes were equivalent to 1.2-fold and 1.3-fold reductions, respectively. Ultraviolet light exposure caused a significant decrease in the mean μg of recovered BPQ relative to the control in all tested exposures except for UV3h, but there were no statistical differences between treatments >3 h in duration and the magnitude of change was 1.1-fold relative to the unexposed control (Fig. 3a – UV). PQ was significantly degraded by exposure to 37 °C for ten, 20, or 40 exposure periods of eight hours each, but not after five exposures. Exposures of 10 and 20 periods resulted in a reduction of approximately 13.5% (P < 0.01) where after 40 exposures the medium reduction was of 11.1% (P < 0.05), statistically the exposures of 10, 20 and 40 where not different among each other. This reduction, although statistically significant, represents only a <1.2-fold change decrease in drug quantity (Fig. 3b). Exposure to UV light for 24 h resulted in a significant (11.9%) decrease in recovered PQ relative to the unexposed sample (P < 0.05), while exposures for 3, 6, and 12 h were not statistically different (Fig. 3b – UV) with a 1.1-fold-change in comparison to the control.
Fig. 3.
Drug quantity (μg) as measured by HPLC for both (a) BPQ and (b) PQ following environmental exposure. Increasing shades of gray from left to right indicate time 0 control, followed by increasing exposures to 37C (5, 10, 20, and 40 exposures), UV light (2, 6, 12, and 24 h), ambient air (7, 14, 30, and 50 days) and combination exposures. (see legend to establish relation between shades of gray and exposure treatments).
3.3.2. Exposure to ambient air
The average recovered quantity of BPQ decreased significantly in a time dependent manner, with virtually no drug being recovered after 30 days of exposure (P < 0.0001; Fig. 3a-Air). PQ stability was also affected by air exposure, with Air30d and Air50d demonstrating significant reductions in recovered drug (23.5% and 68.8% reduction, respectively) (Fig. 3b-Air). All of the combination exposures included ambient air and the results from these experiments reflected te air experiments with a high degree of precision (r = 0.987 for BPQ and r = 0.998 for PQ; Fig. 3). This was also evident by comparing Air and Combo samples for each time point (Fig. 3a and b.).
3.4. Confirmation that air exposure is the primary cause for BPQ and PQ degradation
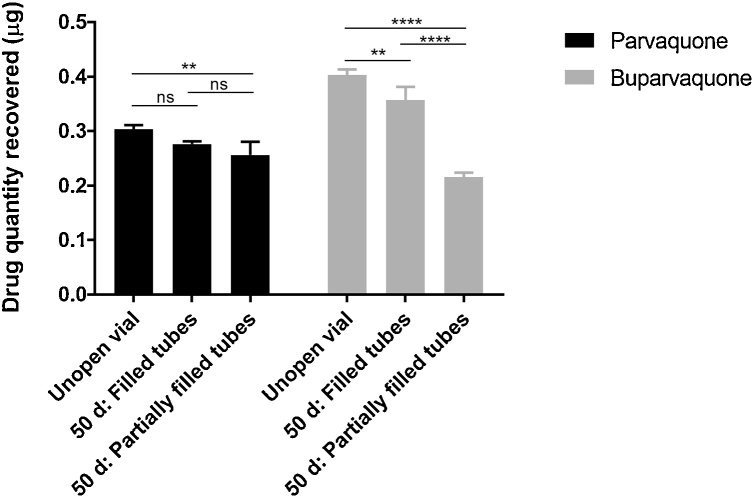
The first set of experiments used PQ and BPQ samples diluted in DMSO as solvent due to the lack of information regarding medicinal drug formulations and because DMSO would not interfere with the bioassays. Once we confirmed N-methyl-2-pyrrolidone as the pharmaceutically-relevant solvent we elected to replicate our initial results by evaluating degradation of BPQ and PQ, pharmacy-grade formulations that were exposed to 50 days of air and compared to unexposed controls. In comparison to the original experiments we opted to leave one tube partially filled (0.2 ml) and other set of triplicates completely filled (1.4 mL). The mean reduction in partially-filled tubes (having ambient air headspace) was 45.12% (P < 0.0001) for BPQ and 15.4% reduction (P < 0.01) for PQ (Fig. 4). These changes were equivalent to 1.9-fold and 1.2-fold reductions, respectively. For completely-filled tubes (no obvious headspace) the reductions were 11% (P < 0.01) and 7.2% (P = 0.12), respectively (Fig. 4) resulting in a 1.1-fold reduction for both drugs. Diluted aliquots (1:10) exhibited color changes after 50 days, but these chromatic changes were not associated with a loss of drug potency (Fig. S4).
Fig. 4.
Drug quantity (μg) as measured by HPLC for both BPQ and PQ in an unopened source vial and 50 days after transfer into tubes with different amounts of air.
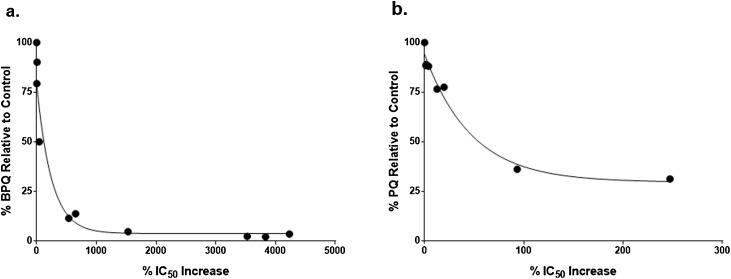
3.5. Correlation of drug degradation with loss of potency
The percent increase in IC50 against T. equi (decrease in drug potency) was negatively correlated with percent drug quantity for both BPQ (r = -0.74, P < 0.01) and PQ (r = -0.88, P < 0.01). A minimal loss of potency was evident with 50% degradation of BPQ quantity, but further degradation caused a significant increase in IC50 (Fig. 5a). For PQ, a statistically significant change in IC50 was not observed until the mean recovered quantity decreased by almost 69% (to ∼31% of the control) (Fig. 5b). Degradation of more than 70% was not observed in this experiment.
Fig. 5.
Correlation between drug degradation and reduced potency. Drug quantity percent of unexposed control sample) versus loss of potency percent increase in IC50) following environmental exposures for (a) BPQ and (b) PQ.
4. Discussion
The motivation for the present study was the observation that in East Africa, veterinary antibiotics are commonly stored at individual households and these injectable drugs often exhibit dramatic changes in color. The hydroxynaphthoquinone compounds investigated in this study, buparvaquone and parvaquone, are the current medications of choice for the treatment of theileriosis in cattle, most notably East Coast Fever and tropical theileriosis. Given that ideal storage conditions are difficult to achieve in many parts of the world, it was important to determine how exposure to variable environmental factors such as temperature, UV light, and ambient air impact the potency of these drugs (Amin and Kokwaro, 2007). These factors were tested initially with undiluted drug products, however our initial attempts to induce chromatic changes in the tested drugs did not produce any observable color changes for either PQ or BPQ. Therefore, we decreased the stability window of both drugs by performing a 10x dilution using either DMSO or N-methyl-2-pyrrolidone - a pharmaceutically-relevant solvent. This type of dilution for “stress tests,” which are also called “forced degradation studies, is a standard practice to assess drugs stability against different conditions in a timely manner (Guidance for IndustryQ1A(R2) Stability Testing of New Drug Substances and Products). In effect, by decreasing the stability window via dilution we are able to assess a “worst case scenario” in terms of drug stability.
Based on our functional assay (T. equi susceptibility) and evaluation of drug degradation (HPLC), BPQ and PQ were relatively stable for the temperature and UV treatments used in this study (<11% and <8% loss, respectively). This degree of reduction, based on HPLC analysis, was not correlated with a detectable change in the IC50 against T. equii cell culture assay. This does not mean that there was no biological impact from this degree of degradation, but this probably reflects limitations in the cell culture assay for which IC50 calculations were based on 2-fold changes in drug concentration. Exposure to ambient air, however, caused much greater degradation to the drugs, with BPQ essentially eliminated and PQ reduced to 31.2% after 50 days of exposure. The larger quantifiable decreases in drug quality were closely correlated with measurable loss of potency based on the T. equi cell culture assay. Fortunately, there appeared to be no synergistic degradation effects when ambient air exposure was coupled with temperature and UV exposure. Overall, BPQ was more sensitive to ambient air exposure compared with PQ.
Theileriosis is a major disease burden of livestock in (sub)tropical regions (Mukhebi et al., 1992, Wise et al., 2013), therefore evaluating factors that affect the stability of these important anti-theilerial drugs is very important. These findings also contribute to the establishment of best practices for storage and handling of veterinary drugs in these areas, thus supporting the human populations that depend on successful treatment outcomes in infected animals. Compromised drug quality negatively affects the ability of veterinarians and farmers to control infectious livestock diseases. Importantly, the use of drugs with decreased potency due to degradation would likely result in sub-therapeutic plasma levels in treated animals, a well-known risk factor for development of antimicrobial drug resistance (Dzinjalamala et al., 2005, Nzila et al., 2000, Tjitra et al., 2002, Watkins and Mosobo, 1993). This is a progressive mechanism whereby insufficient drug concentrations affect highly susceptible organisms while allowing survival of more resistant parasites. This phenomenon is a particular problem for drugs with extended half-lives (Nzila et al., 2000, Watkins and Mosobo, 1993), such as BPQ.. Although resistance to naphthoquinones has not been widely reported, resistance to BPQ has been observed and investigated in T. annulata (Mhadhbi et al., 2015, Mhadhbi et al., 2010, Sharifiyazdi et al., 2012). In human medicine, there is well documented resistance to the naphthoquinone drug atovaquone in apicomplexans such as Plasmodium falciparum (Cottrell et al., 2014), Toxoplasma gondii (McFadden et al., 2000), and Pneumocystis carinii (Kaneshiro, 2001). Despite the lack of reported drug resistance in T. parva, treatment of ECF relies heavily on BPQ and PQ with limited alternative therapeutic options. As such, development of resistance to these drugs would pose a significant problem and the use of PQ may be preferable in situations where ideal storage conditions are not feasible.
Fortunately, despite the potential that these anti-parasitic drugs are commonly stored inappropriately and undergo chromatic changes (Fig. S5), such color changes are not a reliable indicator that the drugs have lost efficacy except under more extreme conditions that were needed to decrease the IC50. The potency of BPQ and PQ could be preserved for longer periods if ambient air can be evacuated from multiple-use vials assuming that the seals remain intact. While temperature per se had much less effect on these compounds, repeated temperature cycles (heating-cooling) is likely to draw ambient air into vials via pressure changes, and thus storing these compounds at a constant temperature will also help. It would also be prudent to market these drugs in single-dose aliquots to eliminate the problem of degradation when the compounds cannot otherwise be protected from ambient air. Without these precautions, decreased potency of BPQ and PQ could have significant detrimental impacts including decreased efficacy for treatment of Theileria-infected cattle and potential development of drug resistance over time, resulting in higher treatment costs, treatment failures, reduced animal welfare, and loss of income and health status that will be particularly problematic for African small-scale farmers and pastoralists (Thumbi et al., 2015).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Siddra A. Hines: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Jacob Brandvold: Data curation, Formal analysis, Investigation, Writing – original draft. Robert H. Mealey: Conceptualization, Methodology, Supervision, Writing – review & editing. Douglas R. Call: Conceptualization, Supervision, Methodology, Writing – review & editing. Telmo Graça: Conceptualization, Formal analysis, Investigation, Supervision, Writing - review & editing.
Acknowledgements
This work was supported in part by Zoetis-Morris Animal Foundation Fellowship Grant No. D12EQ-901 (SAH); USDA-National Institute of Food and Agriculture-Agriculture and Food Research Initiative Grant No. 2013-01149 (RHM); and the National Sciences Foundation Grant number NSF DEB1216040. The authors appreciate the excellent technical support of Lisa Orfe and Christina Macheres.
References
- Amin A.A., Kokwaro G.O. Antimalarial drug quality in Africa. J. Clin. Pharm. Ther. 2007;32:429–440. doi: 10.1111/j.1365-2710.2007.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann C. Stability studies needed to define the handling and transport conditions of sensitive pharmaceutical or biotechnological products. AAPS PharmSciTech. 2011;12:1264–1275. doi: 10.1208/s12249-011-9684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballereau F., Prazuck T., Schrive I., Lafleuriel M.T., Rozec D., Fisch A., Lafaix C. Stability of essential drugs in the field: results of a study conducted over a two-year period in Burkina Faso. Am. J. Trop. Med. Hyg. 1997;57:31–36. doi: 10.4269/ajtmh.1997.57.31. [DOI] [PubMed] [Google Scholar]
- Caudell M.A., Quinlan M.B., Subbiah M., Call D.R., Roulette C.J., Roulette J.W., Roth A., Matthews L., Quinlan R.J. Antimicrobial use and veterinary care among agro-pastoralists in Northern Tanzania. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzitakis A., Berberidou C., Paspaltsis I., Kyriakou G., Sklaviadis T., Poulios I. Photocatalytic degradation and drug activity reduction of Chloramphenicol. Water Res. 2008;42:386–394. doi: 10.1016/j.watres.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Cottrell G., Musset L., Hubert V., Le Bras J., Clain J., Atovaquone-Proguanil Treatment Failure Study G. Emergence of resistance to atovaquone-proguanil in malaria parasites: insights from computational modeling and clinical case reports. Antimicrob. Agents Chemother. 2014;58:4504–4514. doi: 10.1128/AAC.02550-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft S.L., Hogg J., Gutteridge W.E., Hudson A.T., Randall A.W. The activity of hydroxynaphthoquinones against Leishmania donovani. J. Antimicrob. Chemother. 1992;30:827–832. doi: 10.1093/jac/30.6.827. [DOI] [PubMed] [Google Scholar]
- Dolan T.T., Linyonyi A., Mchardy N., Bond A.L., Clampitt R.B. Chemotherapy of East Coast Fever - Parvaquone Treatment ofTheileria-Parva-Parva at Intervals after Infection. Res. Vet. Sci. 1988;44:15–20. [PubMed] [Google Scholar]
- Dzinjalamala F.K., Macheso A., Kublin J.G., Taylor T.E., Barnes K.I., Molyneux M.E., Plowe C.V., Smith P.J. Association between the pharmacokinetics and in vivo therapeutic efficacy of sulfadoxine-pyrimethamine in Malawian children. Antimicrob Agents Ch. 2005;49:3601–3606. doi: 10.1128/AAC.49.9.3601-3606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemifesharki R. Chemotherapeutic Value of Parvaquone and Buparvaquone against Theileria-Annulata Infection of Cattle. Res. Vet. Sci. 1991;50:204–207. doi: 10.1016/0034-5288(91)90107-y. [DOI] [PubMed] [Google Scholar]
- Hines S.A., Ramsay J.D., Kappmeyer L.S., Lau A.O.T., Ojo K.K., Van Voorhis W.C., Knowles D.P., Mealey R.H. Theileria equi isolates vary in susceptibility to imidocarb dipropionate but demonstrate uniform in vitro susceptibility to a bumped kinase inhibitor. Parasite Vector. 2015;8 doi: 10.1186/s13071-014-0611-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe J.M., Certo N.M., Pirakitikulr P., Colaizzi J.L. Stability of several brands of ampicillin and penicillin V potassium oral liquids following reconstitution. Am. J. Health-system Pharm. 1976:1005–1010. [PubMed] [Google Scholar]
- Kaneshiro E.S. Are cytochrome b gene mutations the only cause of atovaquone resistance in Pneumocystis? Drug Resist. Updat. 2001;4:322–329. doi: 10.1054/drup.2001.0221. [DOI] [PubMed] [Google Scholar]
- Kayumba P.C., Risha P.G., Shewiyo D., Msami A., Masuki G., Ameye D., Vergote G., Ntawukuliryayo J.D., Remon J.P., Vervaet C. The quality of essential antimicrobial and antimalarial drugs marketed in Rwanda and Tanzania: influence of tropical storage conditions on in vitro dissolution. J. Clin. Pharm. Ther. 2004;29:331–338. doi: 10.1111/j.1365-2710.2004.00568.x. [DOI] [PubMed] [Google Scholar]
- Kinabo L.D., Bogan J.A. Parvaquone and buparvaquone: HPLC analysis and comparative pharmacokinetics in cattle. Acta Trop. 1988;45:87–94. [PubMed] [Google Scholar]
- Kumar S., Gupta A.K., Pal Y., Dwivedi S.K. In-vivo therapeutic efficacy trial with artemisinin derivative, buparvaquone and imidocarb dipropionate against Babesia equi infection in donkeys. J. Vet. Med. Sci. 2003;65:1171–1177. doi: 10.1292/jvms.65.1171. [DOI] [PubMed] [Google Scholar]
- Kuttler K.L., Zaugg J.L., Gipson C.A. Imidocarb and parvaquone in the treatment of piroplasmosis (Babesia equi) in equids. Am. J. Vet. Res. 1987;48:1613–1616. [PubMed] [Google Scholar]
- Langner M.D., Maibach H.I. Many common drugs in dermatology are light, temperature, or moisture-sensitive. Skin Therapy Lett. 2009;14:3–5. [PubMed] [Google Scholar]
- McFadden D.C., Tomavo S., Berry E.A., Boothroyd J.C. Characterization of cytochrome b from Toxoplasma gondii and Q(o) domain mutations as a mechanism of atovaquone-resistance. Mol Biochem Parasit. 2000;108:1–12. doi: 10.1016/s0166-6851(00)00184-5. [DOI] [PubMed] [Google Scholar]
- Mchardy N., Wekesa L.S., Hudson A.T., Randall A.W. Antitheilerial Activity of Bw720c (Buparvaquone) - a Comparison with Parvaquone. Res. Vet. Sci. 1985;39:29–33. [PubMed] [Google Scholar]
- Mhadhbi M., Chaouch M., Ajroud K., Darghouth M.A., BenAbderrazak S. Sequence Polymorphism of Cytochrome b Gene in Theileria annulata Tunisian Isolates and Its Association with Buparvaquone Treatment Failure. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhadhbi M., Naouach A., Boumiza A., Chaabani M.F., BenAbderazzak S., Darghouth M.A. In vivo evidence for the resistance of Theileria annulata to buparvaquone. Vet. Parasitol. 2010;169:241–247. doi: 10.1016/j.vetpar.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Mukhebi A.W., Perry B.D., Kruska R. Estimated economics of theileriosis control in Africa. Prev. Vet. Med. 1992;12:73–85. [Google Scholar]
- Muraguri G.R., Kiara H.K., McHardy N. Treatment of East Coast fever: a comparison of parvaquone and buparvaquone. Vet. Parasitol. 1999;87:25–37. doi: 10.1016/s0304-4017(99)00154-5. [DOI] [PubMed] [Google Scholar]
- Muraguri G.R., Ngumi P.N., Wesonga D., Ndungu S.G., Wanjohi J.M., Bang K., Fox A., Dunne J., McHardy N. Clinical efficacy and plasma concentrations of two formulations of buparvaquone in cattle infected with East Coast fever (Theileria parva infection) Res. Vet. Sci. 2006;81:119–126. doi: 10.1016/j.rvsc.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Nzila A.M., Nduati E., Mberu E.K., Hopkins Sibley C., Monks S.A., Winstanley P.A., Watkins W.M. Molecular evidence of greater selective pressure for drug resistance exerted by the long-acting antifolate Pyrimethamine/Sulfadoxine compared with the shorter-acting chlorproguanil/dapsone on Kenyan Plasmodium falciparum. J. Infect. Dis. 2000;181:2023–2028. doi: 10.1086/315520. [DOI] [PubMed] [Google Scholar]
- Risha P.G., Shewiyo D., Msami A., Masuki G., Vergote G., Vervaet C., Remon J.P. In vitro evaluation of the quality of essential drugs on the Tanzanian market. Trop. Med. Int. Health. 2002;7:701–707. doi: 10.1046/j.1365-3156.2002.00937.x. [DOI] [PubMed] [Google Scholar]
- Risha P.G., Vervaet C., Vergote G., Van Bortel L., Remon J.P. Drug formulations intended for the global market should be tested for stability under tropical climatic conditions. Eur. J. Clin. Pharmacol. 2003;59:135–141. doi: 10.1007/s00228-003-0587-1. [DOI] [PubMed] [Google Scholar]
- Salib F.A., Youssef R.R., Rizk L.G., Said S.F. Epidemiology, diagnosis, and therapy of Theileria equi infection in Giza. Egypt. Veterinary World. 2012;6:76–82. [Google Scholar]
- Sharifiyazdi H., Namazi F., Oryan A., Shahriari R., Razavi M. Point mutations in the Theileria annulata cytochrome b gene is associated with buparvaquone treatment failure. Vet. Parasitol. 2012;187:431–435. doi: 10.1016/j.vetpar.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Silva M.G., Villarino N.F., Knowles D.P., Suarez C.E. Assessment of Draxxin((R)) (tulathromycin) as an inhibitor of in vitro growth of Babesia bovis, Babesia bigemina and Theileria equi. Int. J. Parasitol. Drugs Drug Resist. 2018;8:265–270. doi: 10.1016/j.ijpddr.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumbi S.M., Bronsvoort M.B., Kiara H., Toye P.G., Poole J., Ndila M., Conradie I., Jennings A., Handel I.G., Coetzer J.A., Steyl J., Hanotte O., Woolhouse M.E. Mortality in East African shorthorn zebu cattle under one year: predictors of infectious-disease mortality. BMC Vet. Res. 2013;9:175. doi: 10.1186/1746-6148-9-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumbi S.M., Njenga M.K., Marsh T.L., Noh S., Otiang E., Munyua P., Ochieng L., Ogola E., Yoder J., Audi A., Montgomery J.M., Bigogo G., Breiman R.F., Palmer G.H., McElwain T.F. Linking human health and livestock health: a "one-health" platform for integrated analysis of human health, livestock health, and economic welfare in livestock dependent communities. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjitra E., Suprianto S., Anstey N.M. Higher gametocyte prevalence following failure of treatment of Plasmodium falciparum malaria with sulfadoxine-pyrimethamine and the combination of chloroquine plus sulfadoxine-pyrimethamine: implications for progression of anti-folate resistance. Trans. R. Soc. Trop. Med. Hyg. 2002;96:434–437. doi: 10.1016/s0035-9203(02)90385-8. [DOI] [PubMed] [Google Scholar]
- Twagirumukiza M., Cosijns A., Pringels E., Remon J.P., Vervaet C., Van Bortel L. Influence of tropical climate conditions on the quality of antihypertensive drugs from rwandan pharmacies. Am. J. Trop. Med. Hyg. 2009;81:776–781. doi: 10.4269/ajtmh.2009.09-0109. [DOI] [PubMed] [Google Scholar]
- Ueti M.W., Mealey R.H., Kappmeyer L.S., White S.N., Kumpula-McWhirter N., Pelzel A.M., Grause J.F., Bunn T.O., Schwartz A., Traub-Dargatz J.L., Hendrickson A., Espy B., Guthrie A.J., Fowler W.K., Knowles D.P. Re-emergence of the apicomplexan theileria equi in the United States: elimination of persistent infection and transmission risk. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh G., Majid M.I., Ramanathan S., Mansor S.M., Nair N.K., Croft S.L., Navaratnam V. Optimization and validation of RP-HPLC-UV method with solid-phase extraction for determination of buparvaquone in human and rabbit plasma: application to pharmacokinetic study. Biomed. Chromatogr. 2008;22:535–541. doi: 10.1002/bmc.965. [DOI] [PubMed] [Google Scholar]
- Watkins W.M., Mosobo M. Treatment of Plasmodium-FalciparumMalaria with Pyrimethamine-Sulfadoxine - Selective Pressure for Resistance Is a Function of Long Elimination Half-Life. Trans. R. Soc. Trop. Med. Hyg. 1993;87:75–78. doi: 10.1016/0035-9203(93)90431-o. [DOI] [PubMed] [Google Scholar]
- Wise L.N., Kappmeyer L.S., Mealey R.H., Knowles D.P. Review of equine piroplasmosis. J. Vet. Intern. Med. 2013;27:1334–1346. doi: 10.1111/jvim.12168. [DOI] [PubMed] [Google Scholar]
- Wise L.N., Ueti M.W., Kappmeyer L.S., Hines M.T., White S.N., Davis W., Knowles D.P. In vitro activity of ponazuril against Theileria equi. Vet. Parasitol. 2012;185:282–285. doi: 10.1016/j.vetpar.2011.10.036. [DOI] [PubMed] [Google Scholar]
- Zaugg J.L., Lane V.M. Evaluations of Buparvaquone as a Treatment for Equine Babesiosis (Babesia-Equi) Am. J. Vet. Res. 1989;50:782–785. [PubMed] [Google Scholar]
- Zaugg J.L., Lane V.M. Efficacy of Buparvaquone as a Therapeutic and Clearing Agent ofBabesia-Equi of European Origin in Horses. Am. J. Vet. Res. 1992;53:1396–1399. [PubMed] [Google Scholar]