Highlights
-
•
The ISI was applied to evaluate histologic alterations specific of Eimeria infection.
-
•
The ISI histological score show high correlation with broilers performance.
-
•
The higher the ISI scores the worse are the performance results of birds challenged.
Keywords: Coccidiosis, Small intestine, Histological lesions, Performance
Abstract
The present study evaluated the effects of coccidiosis on histological parameters and performance of broilers submitted to a mild challenge with Eimeria sp. A total of 132 broilers were randomly divided into two groups with 6 replicates of 11 birds each: negative control (NC) – birds uninfected and challenge (CH) - birds infected by gavage at day-one with 10x the manufacturer recommended dose of Bio-Coccivet R® live vaccine by Biovet, with strains of E. acervulina, E. brunetti, E. maxima, E. necatrix, E. praecox, E. tenella and E. mitis. From 1 to 28 d of age, weekly the zootechnical performance was evaluated and samples of the liver, duodenum, jejunum and ileum were collected and submitted to histological analysis by the I See Inside methodology (ISI). The ISI methodology is a metric evaluation of histological alterations in the intestine and liver. Which translates macroscopic and microscopic alterations into numbers and allows their correlation with the animal zootechnical performance. Pearson’s correlation was used to demonstrates how the damages to the gut, evaluated by ISI method, could affect negatively the performance of broilers. The presence of oocysts in histological analysis of the jejunum and ileum at 7d indicate a negative effect on performance over the next 2 weeks (14d and 21d). When the ISI total score was measured in the ileum at 14d, we observed a strong negative effect on zootechnical performance from that period on 14d, 21d and 28d. The obtained data demonstrates that the higher the ISI scores, the worse are the zootechnical performance results of broilers challenged with Eimeria sp. and the I See Inside scores could be applied to evaluate coccidiosis effect on performance in future.
1. Introduction
Chicken coccidiosis is a disease caused by several protozoan parasites of the genus Eimeria; its economic impacts could reach up to 3 billion dollars per year worldwide (Dalloul and Lillehoj, 2006). Seven Eimeria species have been recognized to infect chickens: Eimeria acervulina, E. maxima, E. tenella, E. necatrix, E. mitis and E. praecox. Each species has its own characteristics regarding lesion, site of infection in the intestine and immunogenicity (Vermeulen et al., 2001). Some species of Eimeria invades intestinal epithelial cells, eliciting a variety of clinical manifestations, including necrotic gut lesion, inefficient feed utilization, impaired growth rate and, in several cases, mortality (Min et al., 2013).
The most common zootechnical aspects observed during infection are poor growth rate and worse feed conversion without mortality (Lee et al., 2013) even in the absence of clinical symptoms (sub-clinical coccidiosis). The pathological changes vary from local disintegration of the mucosa barrier (associated with inflammation on the underlying tissue) to systemic effects such as blood loss, shock syndrome and even death (Vermeulen et al., 2001).
The diagnosis of sub-clinical coccidiosis is very difficult, provided that there are only mild, non-specific macroscopic histological alterations. These are sometimes labeled as “non important lesions” during pathological analysis and therefore neglected. The I See Inside methodology (ISI), described by Kraieski et al (2017) and adapted by Belote et al. (2018), is a metric evaluation of histological alterations in the intestine and liver. ISI translates macroscopic and microscopic alterations into numbers and allows their correlation with the animal zootechnical performance. For this reason, we believe that the ISI methodology could be a good tool to determine, in raw numbers, mild tissue alterations caused by this disease and their correlation with performance results.
The objective of the present study was to determine the macroscopic and microscopic alterations in intestine and liver of broilers, both non-challenged and challenged with Eimeria sp. and to correlate these results with their performance applying the ISI methodology.
2. Materials and methods
2.1. Ethics statement
The study protocol and the use of all animal studies were approved by the Animal Use Ethics Commission (Comissão de Ética no Uso de Animais – CEUA), Agricultural Sciences Sector of the Federal University of Paraná – Brazil (Permission number 032/2015).
2.2. Housing chickens
One-day-old male Cobb 500 broilers were housed from 1 to 28 days of age in negative pressure facilities previously cleaned and disinfected with quaternary ammonia, as well as nipple drinkers. The littler was sterilized by 121 °C for 15 min. Automatic temperature control was set according to comfort conditions for each life stage. The temperature was gradually decreased from 34 °C on day 1 to 24 °C on day 28 and then kept constant. During the whole experiment, birds received water and feed ad libitum and the diet followed the Brazilian nutritional recommendations for poultry (Rostagno et al., 2011), without any anticoccidials drug or antibiotic growth promoter.
2.3. Experimental design
A total of 132 one-day-old broilers were randomly divided in two treatments with 6 replicates of 11 birds each. The treatments were negative control (NC) –uninfected, untreated and challenge birds (CH) – birds infected with 10x the manufactured recommended dose of commercial Eimeria vaccine (in order to induce experimental sub-clinical coccidiosis disease).
The challenge was done at the first day of trial by oral gavage with 10X the manufacturer recommended dose of Bio-Coccivet R® live vaccine by Biovet, with strains of E. acervulina, E. brunetti, E. maxima, E. necatrix, E. praecox, E. tenella and E. mitis. Each bird from the CH group received 0.5 mL solution with 330.000 oocysts of Eimeria spp. Each bird of the NC group received 0.5 mL of physiologic water instead.
2.4. Performance parameters
At one day of age, birds were weighted and distributed in each treatment in order to obtain equal initial body weight average per cage (replicate) in each treatment. Birds and feed were weighed weekly (at zero, 7, 14, 21, and 28 days) to evaluate feed intake (FI), body weight gain (BWG), and feed conversion ratio (FCR).
2.5. Macroscopic analysis and sample collection
At 2, 7, 14, 21 and 28 days of age, 6 birds were euthanized per treatment. Macroscopic alterations were evaluated using ISI methodology following Kraieski et al (2017), by three observers. Then, samples of liver, duodenum, jejunum and ileum were collected and fixed in Davidson’s solution (100 mL glacial acetic acid, 300 mL 95% ethyl alcohol, 200 mL 10% neutral buffered formalin and 300 mL distilled water) for at least 24 h. Then, samples were dehydrated, infiltrated and embedded in paraffin following common histological routine. Blocks were cut in 5 μm sections and stained with hematoxylin and eosin with Alcian Blue for goblet cells staining (Rapp and Wurster, 1978).
2.6. I see inside methodology (ISI)
The ISI (I See Inside) methodology applied for microscopy was according to Kraieski et al (2017), while the histological routine was according to Belote et al. (2018), adapted from Kraieski et al. (2017). The “I See Inside” (ISI) methodology, patent pending (INPI BR 1020150036019), is based on a numeric score of alteration with the evaluated parameter listed in Table 1. In this methodology, an impact factor (IF) is defined for each alteration in macroscopic and microscopic analysis. The IF is established according to the reduction of an organ’s functional capacity, based on previous knowledge from the literature and background research (e.g. necrosis has the highest IF because the functional capacity of the affected cells is completely lost). It ranges from 1 to 3, with 3 being the highest impact to organ function. In addition, the extent of each lesion (intensity) or the observed frequency of the lesion compared to non-affected organs is evaluated in each organ/tissue with score (S) ranging from 0 to 3, where score 0: absence of lesion or frequency; score 1: alteration in up to 25% of the area or observed frequency; score 2: alteration ranges from 25.1 to 50% of the area or observed frequency and score 3: alteration extends to more than 50% of the area or observed frequency.
Table 1.
ISI histological alterations evaluated in intestine and liver.
| Organ | Alteration | Impact Factor (IF) | Maximuma Score |
|---|---|---|---|
| Intestine | Lamina propria thickness | 2 | 45 |
| Epithelial thickness | 1 | ||
| Enterocytes proliferation | 1 | ||
| Inflammatory cell infiltration in the epithelium | 1 | ||
| Inflammatory cell infiltration in the lamina propria | 3 | ||
| Goblet cells proliferation | 2 | ||
| Congestion | 2 | ||
| Presence of oocysts | 3 | ||
| Liver | Congestion | 1 | 42 |
| Cell vacuolization | 2 | ||
| Bile-duct proliferation | 2 | ||
| Immune cells infiltration | 1 | ||
| Necrosis | 3 | ||
| Pericholangitis | 3 | ||
| Lymphocytic aggregate | 2 | ||
Maximum score represents the sum of all alterations according to the formula ISI =Σ(IF*S) where IF = impact factor (previously fixed) and S = Score (observed) considering the maximum observed S. For example, the lamina propria thickness has IF = 2, this number is multiplied by the observed score (ranging from 1 to 3). If in a villus, a score S = 3 (maximum score) was observed for lamina propria thickness, the ISI for this parameter in this villi will be ISI = (2*3) = 6. The average of 20 villi in ileum or 10 fields in the liver for each bird will indicate the final ISI value for each bird.
To obtain the final value of the ISI index referred as ISI total score, the IF of each alteration is multiplied by its respective score number and the results of all alterations are summed according with the formula ISI =Σ(IF*S), where IF = impact factor and S = Score. For example, the increase of lamina propria thickness has an IF = 2. This number is multiplied by the observed score (ranging from 1 to 3). If a S = 3 (maximum score) is given to the increase of lamina propria thickness in a villus, the ISI final value for this parameter in this villus will be ISI = (2 * 3) = 6. An average of 20 intestinal villi per slide was evaluated per bird in 10X objective (using 20X and 40X objective to confirm alterations) of an optical microscope (Nikon Eclipse E200, Sao Paulo-SP- Brazil). Liver samples were evaluated in 10 fields per bird in 10X objective. The ISI scales range from 0 to 45 for the intestine and from 0 to 42 for the liver.
2.7. Statistical analysis
Data were presented as mean plus minus standard error. At first, all data were tested for normality using Shapiro-Wilk normality test. Parametric rates were compared using one-way analysis of variance (ANOVA), followed by Tukey test (P < 0.05). For performance, each cage was used as a replicate (n = 4) while each bird was used as a sample for the remaining analysis (n = 6). All analysis were performed at Statistix 9 software for Windows. For correlation analysis, Pearson’s correlation coefficient (r) was used and the software provided the P values.
3. Results
3.1. Performance parameters
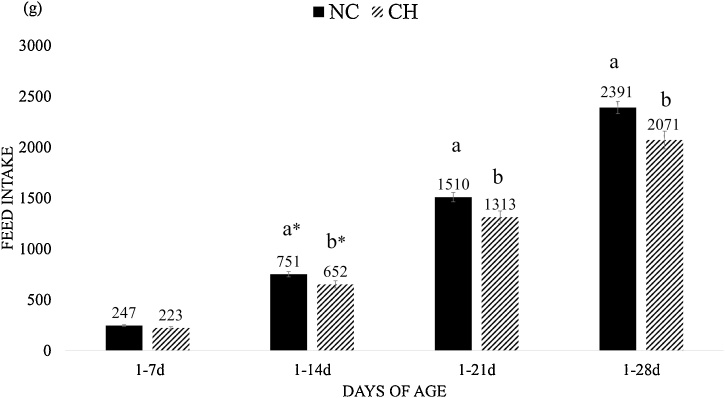
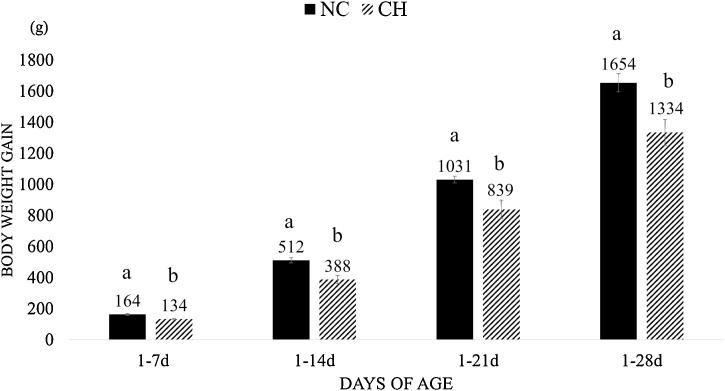
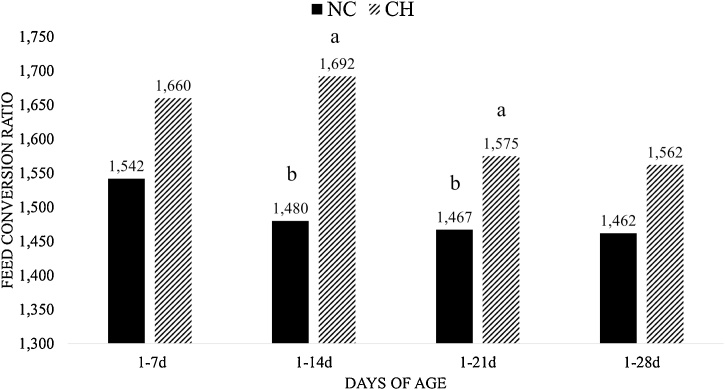
The CH group showed FI reduction (P < 0.05) at times 1–14d, 1–21d and 1–28d when compared with the NC group (Fig. 1), and there was no significant difference among all groups at time 1-7d. The BWG was lower in the CH group for intervals 1–7d, 1–14d, 1–21d and 1–28d when compared to the NC group (P < 0.05) (Fig. 2). The FCR was worst (P < 0.10) in the CH group when compared with the NC group at intervals 1–14d and 1–21d (Fig. 3) and there was no significant difference between groups at other periods.
Fig. 1.
Feed Intake (FI, grams) mean and standard error of treatment at different time periods. Treatments were negative control (NC) –and uninfected, untreated and Challenge (CH) – where animals were infected with 10x the manufactured recommended dose. Different superscript letters indicate significant difference with Tukey test (P < 0.05); * sign on letters indicate a significant level of P<0.07.
Fig. 2.
Body weight gain (BWG, grams) mean and standard error of treatment at different time periods. Treatments are negative control (NC) and uninfected, untreated and Challenge (CH), where animals were infected with 10x the manufactured recommended dose. Different supercript letters indicate significant difference with Tukey test (P < 0.05).
Fig. 3.
Mean Feed Conversion Ratio (FCR) of treatment at different time periods. Treatments are negative control (NC) and uninfected, untreated and Challenge (CH), where animals were infected with 10x the manufactured recommended dose. Different superscript letters indicate a significant difference with Tukey test (P < 0.10).
3.2. Macroscopic and histological analyses
The CH group presented higher score of coccidiosis macroscopic lesions in comparison to the NC group at time 14d (P < 0.05) (CH ISI score1.8 ± 0.7; NC ISI score: 0.0 ± 0.0) and 21 days (NC ISI score 0.0 ± 0.0 and CH ISI score 2.7 ± 0.7). There was no difference for the other macroscopic parameters of ISI between groups at the other periods.
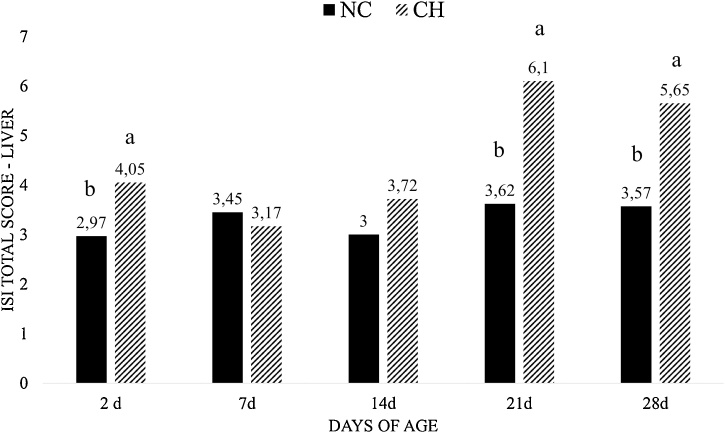
For microscopic results of liver, the CH group showed higher ISI total score in comparison with the NC group at 2d, 21d and 28d of age (P < 0.05) (Fig. 4) and there was no significant difference at the other periods. The CH group presented higher ISI total score in comparison to other groups due to higher scores at lymphocyte infiltration and cell vacuolization (P < 0.05) (Fig. 5).
Fig. 4.
ISI total score of liver lesions at all periods. Data is the mean value of each treatment at different time intervals. Treatments are negative control (NC) and uninfected, untreated and Challenge (CH) – where individuals were infected with 10x the manufactured recommended dose. Different superscript letters indicate significant difference with Tukey test (P < 0.05).
Fig. 5.
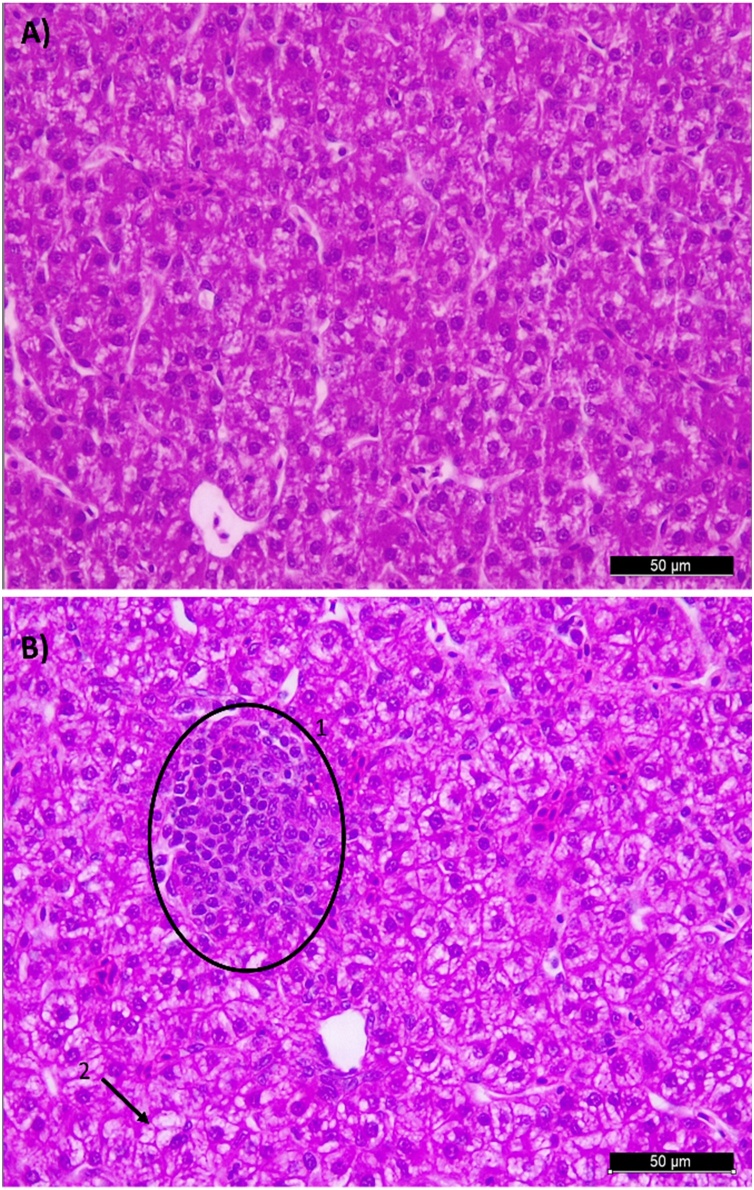
Photomicrographs chicken liver sections stained with hematoxylin and eosin. A) normal liver histological structure of non-challenge (NC) group (400X); B) 1. Inflammatory cell infiltration (circle) and 2. Presence of vacuolization (arrow) in liver of an individual from the Challenged group (CH) (400X). These changes contributed to the highest (P< 0.05) ISI index in Eimeria sp. challenge group at 21 days of age.
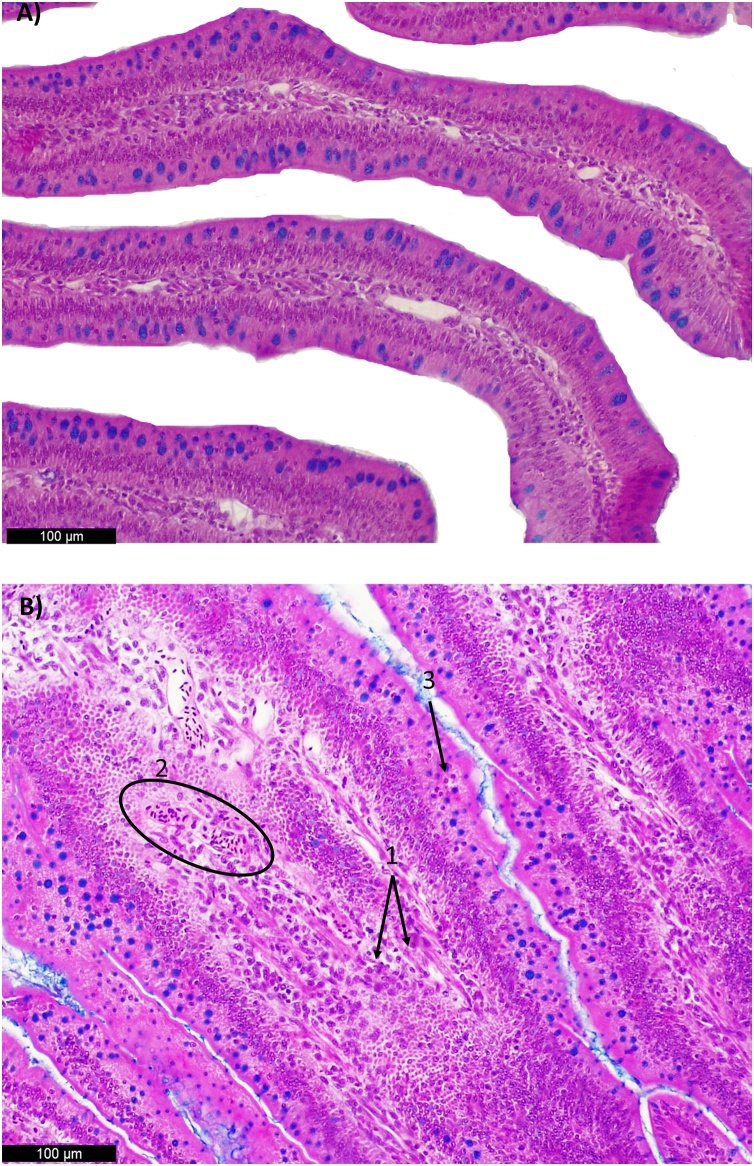
In the duodenum, the CH group showed higher ISI total score compared with NC group (P < 0.05) (CH ISI score 16.6 ± 0.9; NC ISI score: 12.6 ± 1.0) at 28d, but no significant statistical difference was observed at the other periods. These results were due to higher scores of inflammatory cell infiltration in the lamina propria and epithelium and congestion in the CH group when compared to the NC group (Fig. 6).
Fig. 6.
Photomicrographs of chicken duodenum sections stained with hematoxylin and eosin. Alcian Blue was used to stain goblet cells. A) Normal histological duodenum structure of non-challenge treatment (NC) (200X); B) Challenged treatment (CH) (200x) 1. Inflammatory cell infiltration in the lamina propria; 2. Congestion and 3. Inflammatory cell infiltration in the epithelium; These alterations contributed to the highest ISI index (P< 0.05) in coccidiosis challenged group at 28 days in comparison to the non-challenged group.
In the jejunum at 21d, the CH group showed higher ISI total score when compared with the NC group (P < 0.05) (CH ISI score 16.6 ± 0.6; NC ISI score 10.8 ± 0.6). There was no significant difference between groups at the other periods. The CH group presented higher ISI total score in comparison to other groups due to higher scores (P < 0.05) caused by inflammatory cell infiltration in the lamina propria and epithelium, increase of lamina propria thickness and presence of oocysts (Fig. 7).
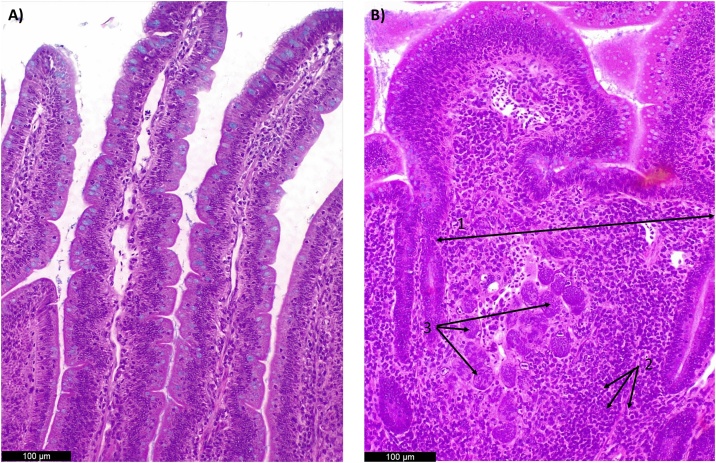
Fig. 7.
Photomicrographs of chicken jejunum sections stained with hematoxylin and eosin. Alcian Blue was used to stain goblet cells. A) Normal histological structure of non-challenge treatment (NC) in jejunum (200X); B) Challenged treatment (CH) (200x) 1. Increase of lamina propria thickness; 2. Inflammatory cell infiltration in the lamina propria; 3 Inflammatory cell infiltration in the epithelium and 4. Presence of oocysts; These alterations contributed to the highest ISI index (P< 0.05) in coccidiosis challenged group at 21 days in comparison to the non-challenged treatment.
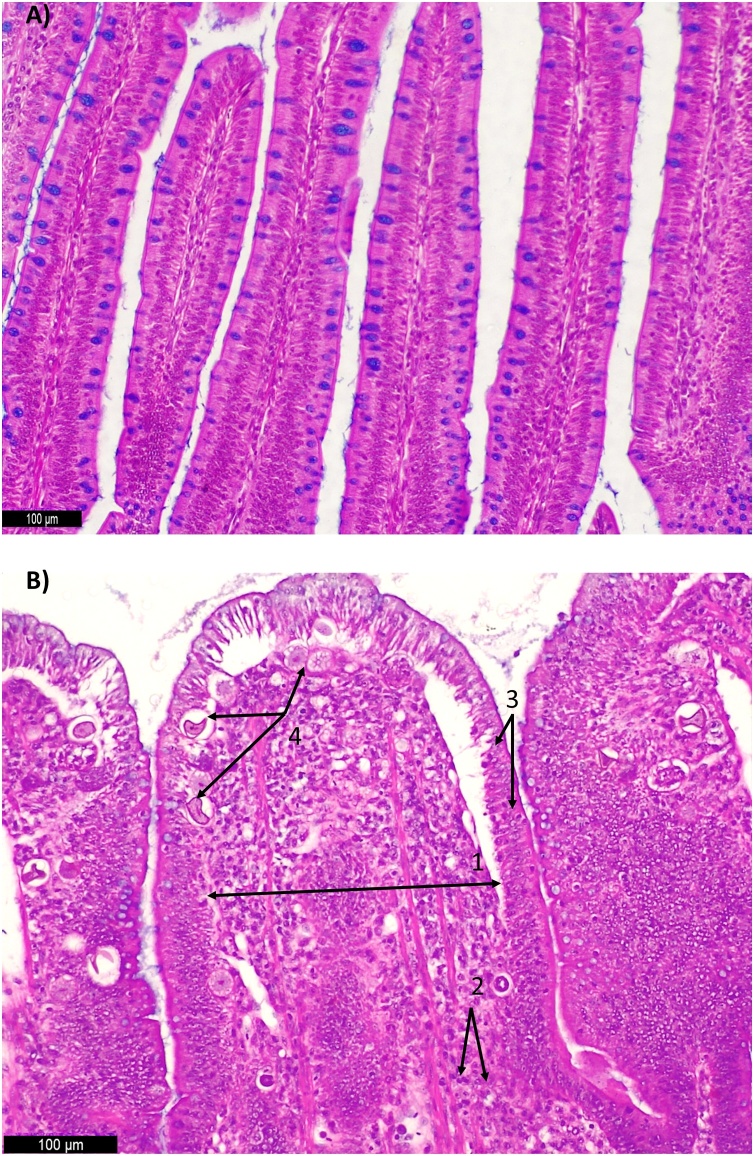
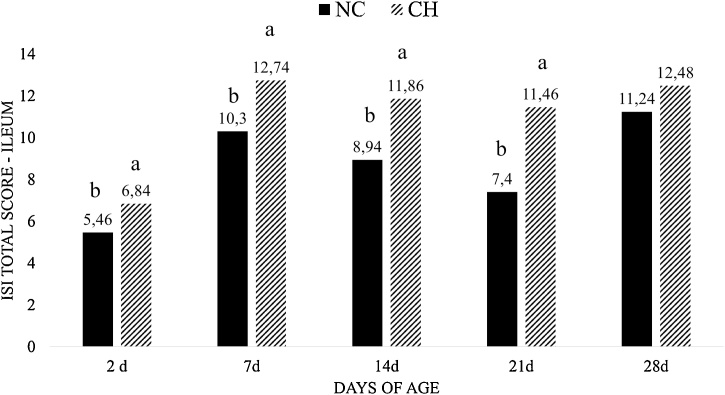
In the ileum, the CH group presented higher ISI total score when compared with the NC group (P < 0.05) (Fig. 8) at times 2d, 7d, 14d and 21d. No significant difference was observed between groups at 28d. At 2d, the higher ISI total score in the CH group was due to inflammatory cell infiltration in the lamina propria. At 7d, the CH group showed higher ISI total score when compared to the NC group due to higher scores (P < 0.05) of inflammatory cell infiltration in the epithelium. At 14d and 21d, the CH group presented higher ISI total score when compared to the NC group due to higher scores(P < 0.05) of lamina propria thickness, inflammatory cell infiltration in the lamina propria and presence of oocysts (Fig. 9).
Fig. 8.
ISI total score of ileum at all periods. Data is the mean of treatments in all time intervals. Treatments are negative control (NC) and uninfected, untreated and Challenge (CH) – where animals were infected with 10x the manufactured recommended dose. Different superscript letters indicate a significant difference with Tukey test (P < 0.05).
Fig. 9.
Photomicrographs of chicken ileum sections stained with hematoxylin and eosin. Alcian Blue was used to stain the goblet cells. A) Normal ileum histological structure of non-challenge treatment (NC) (200X); B) Challenged treatment (CH) (200x) 1. Higher lamina propria thickness; 2. Inflammatory cell infiltration in the lamina propria; 3. Presence of oocysts; These alterations contributed to the highest ISI index (P< 0.05) in coccidiosis challenged group at 21 days in comparison to the non-challenged group.
3.3. Pearson’s correlation
The results obtained with Pearson’s correlation demonstrates how the damages to gut, evaluated by ISI methodology, could negatively affect the performance of broilers (P < 0.05). It was observed that the higher the ISI total score, the worse the zootechnical performance results of broilers.
In the duodenum, the inflammatory cell infiltration in the lamina propria observed at 21d exhibited negative correlation with the BWG (r= −0.91) and FI (r=−0.83) at the next time interval (28d). The ISI total score measured in the duodenum at 28d was negatively correlated to the BWG (r=−0.93) and FI (r= −0.97) at the same time.
The presence of oocysts observed in the microscopic analysis of the jejunum at 7d negatively affected the BWG (r=−0.88), FI (r=−0.70) and FCR (r = 0.75) at 14d and the BWG (r=−0.80), FI (r=−0.69) and FCR (r = 0.81) at 21d. At 14d, the presence of oocysts in the jejunum negatively affected the BWG (r=−0.85), FI (r=−0.73) and FCR (r = 0.70) at 28d. The presence of oocysts observed in the jejunum at 21d negatively affected the BWG (r=−0.81) and FI (r=−0.84) at the same time.
The increase of lamina propria thickness observed in the jejunum at 2d had a negative correlation with the FI at 7d (r=− 0.68), 14d (r=−0.86), 21d (r=−0.81) and 28d (r=−0.88) and the BWG at 21d (r=−0.73) and 28d (r=−0.76). The increase of lamina propria thickness observed in the jejunum at 21d was negatively correlated with BWG (r=−0.74) at 28d.
The inflammatory cell infiltration in the epithelium of the jejunum at 14d presented a negative correlation with BWG (r=−0.88), FI (r=−0.65) and FCR (r = 0.92) at 28d. In the same intestinal portion, the inflammatory cell infiltration in the lamina propria at 21d showed a negative influence over the BWG (r=−0.87), FI(r=−0.75) and FCR (r = 0.69) at 28d. The ISI total score measured in the jejunum at 21d negatively affected the BWG (r=−0.92), FI (r=−0.89) and FCR (r = 0.63) at 28d.
The inflammatory cell infiltration in the epithelium of the ileum at 2d negatively affected the BWG (r=−0.72) and FI (r=−0.70) at 21d and BWG (r=−0.71) at 28d. The inflammatory cell infiltration in the lamina propria observed at 14d showed a negative influence over the BWG (r=−0.90), FI (r=−0.77) and FCR (r = 0.66) at 14d, BWG (r=−0.86), FI (r=−0.80) and FCR (r = 0.72) at 21d and BWG (r=−0.81) and FI (r=−0.81) at 28d.
The presence of oocysts in the ileum at 7d negatively influenced the BWG and FI at 14d (r=−0.74 and r=−0.68, respectively) and at 21d (r=−0.74 and r=−0.78, respectively). At 14d, the presence of oocysts in the ileum presented a negative influence over the BWG (r=−0.76), FI(r=−0.70) and FCR(r = 0.76) at 21d.
The increase of lamina propria thickness in the ileum at 14d showed a negative effect over the BWG (r=−0.85), FCR (r = 0.87) at 14d, BWG (r=−0.66) and FCR (r = 0.68) at 21d, and BWG (r=−0.62), FI (r=−0.61) at 28d.
The ISI total score was measure in the ileum at 14d could strongly affect negatively the performance at other periods: BWG (r=−0.95), FI (=−0.84) and FCR (r = 0.71) at 14d, BWG (r=−0.93), FI (r=−0.83) and FCR (r = 0.85) at 21d, and BWG (r=−0.83) and FI (r=−0.87) at 28d. The ISI total score measured in the ileum at 21d was observed to affect negatively the BWG (r=−0.85), FI (r=−0.81) and FCR (r = 0.79) at 21d and, BWG (r=−0.75) and FI (r=−0.79) at 28d.
4. Discussion
The consequences of self-maintenance include a decrease in animal productivity (Klasing, 2007). The damage in the intestinal mucosa caused by Eimeria leads to a decrease in digestion and absorption of feed (Hofacre et al., 2003) and it is also associated with inflammation, which reduces FI and increases energy demands (Kogut and Klasing, 2009). In the present study, it was observed a reduction of FI of 13% at 14d, 21d and 28d and a reduction of BWG of 18% at 7d and of 19% at 21d and 28d. The biggest reduction was observed for BWG at 14d, with a decrease of 24% in CH group when compared with NC group. We also observed a decrease of 13% and 7% on FCR between groups at 14d and 21d, respectively. According to Jiang et al. (2010), the induction of an acute inflammatory reaction reduces the BWG in 22%. From this decrease, 59% comes from the reduction on FI and the other 41% would be consequence of the immune response. The histological parameters result of liver, showed higher ISI total score in the CH group in comparison with the NC group due to higher scores at lymphocyte infiltration (P < 0.05). In fact, liver in birds has a role as ectopic lymphoid tissue, mainly in the beginning of life (Oláh et al., 2013). The founds in liver reinforce its importance as lymphoid organ of broilers that was activated in order to combat the Eimeria infection in the gut.
The histological paramenters in the ileum showed an increase of lamina propria thickness and of inflammatory cell infiltration in the lamina propria observed at 14d presented a strong negative influence over the performance at 14d, 21d and 28d. Both these parameters were described by Belote et al. (2018) as good parameters to compare intestinal health between different treatments.
The infection by Eimeria sp. changes the structure of the intestinal villi, decreasing the absorption capacity due to destruction of intestine epithelial cells (Shirley and Lillehoj, 2012). The Eimeria sp. biological cycle is very complex and comprises intracellular, extracellular, asexual and sexual stages. The species E. acervulina, E. maxima and E. tenella reach the crypt epithelium of the host (Shirley and Lillehoj, 2012). These protozoans induce a local inflammatory response due to intracellular development (Hong et al., 2006) and their replication leads to cellular damage in the epithelium (Shirley and Lillehoj, 2012). The highest number of oocysts of E. acervulina and E. maxima in the litter of commercial flocks are found on the period of 4–5 weeks after the infection (Long et al., 1975). In the duodenum, the highest ISI total scores of lesions were observed on the 4th week post-challenge, what could be related to oocysts release from the villi
During the 2nd and 3rd phase of the asexual replication, gut damage becomes more evident because of the high number of merozoites infecting enterocytes. This asexual life phase results in an explosion in parasite number (Chapman, 2003). Sporulated oocysts must excyst in the intestine after the ingestion, but not all of them really excyst. Up to 20% of ingested unsporulated oocysts can pass through undamaged and still sporulate later (Williams, 1995). This means that if a small proportion of the sporulated oocysts in an inoculum of a live vaccine fail to infect a chick, they can be re-ingested within a day of vaccination and infect a chick on a subsequent occasion (Williams, 1998). About 1 week after vaccination, a small number of the attenuated vaccine oocysts are observed multiplying with a small peak of production at 2–4 weeks, during which time, protective immunity is built up as the parasites recycle, and another peak appears at 4–6 weeks, decreasing thereafter (Chapman et al., 2016, Williams, 1998). The presence of oocysts in histological analysis of the jejunum and ileum at 7d indicate a negative effect on performance in the next 2 weeks (14d and 21d) (P < 0.05). The E. maxima oocyst sporulation in litter containing 5% moisture increasing to 16% during 106 h and in litter containing 60% moisture, increasing to 62% (Willian, 1998). It is noteworthy that specific E. maxima variants may be more frequent in broiler farms experiencing low performance of animals (Schwarz et al., 2012). When the ISI total score was measured in the ileum at 14d, we observed a strong negative effect on performance at following periods (14d, 21d and 28d) (P < 0.05), which suggests the use of this parameters as a good tool to control the Eimeria infection in broilers.
5. Conclusion
We observed that the challenge of birds with 10X dose of Eimeria vaccine leads to mild macroscopic lesion at the intestine and significant histological alterations as well as reduction on zootechnical performance;
The application of the ISI methodology allows a numeric translation of histologic lesion and its correlation with losses in animal performance;
The most expressive correlation was the total ISI score of the ileum at 14d that shows a strong negatively effect on performance at following periods (14d, 21d and 28d) (P < 0.05), which suggests the use of this methodology could be a good tool to control Eimeria infection in broilers.
Conflict of interest
All authors supported the design, interpretation of the data and manuscript review of this study. There were no conflicting interests that could have influenced the study.
Acknowledgments
The authors wish to acknowledge the LABMOR team who were responsible for much of the work behind this study. Bruna Belote and Adrien Sanches are thankful to CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for the scholarship support.
References
- Belote B.L., Tujimoto-Silva A., Hümmelgen P.H., Sanches A.W.D., Wammes J.C.S., Hayashi R.M., Santin E. Histological parameters to evaluate intestinal health on broilers challenged with Eimeria and Clostridium perfringens with or without enramycin as growth promoter. Poult. Sci. 2018;97:2287–2294. doi: 10.3382/ps/pey064. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Origins of coccidiosis research in the fowl— The first fifty years. Avian Dis. 2003;47:1–20. doi: 10.1637/0005-2086(2003)047[0001:OOCRIT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Barta J.R., Hafeez M.A., Matsler P., Rathinam T., Raccoursier M. The epizootiology of Eimeria infections in commercial broiler chickens where anticoccidial drug programs were employed in six successive flocks to control coccidiosis. Poult. Sci. 2016;95:1774–1778. doi: 10.3382/ps/pew091. [DOI] [PubMed] [Google Scholar]
- Dalloul R.A., Lillehoj H.S. Poultry coccidiosis. Recent advancements in control measures and vaccine development. Expert Rev. Vaccines. 2006;5:143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- Hofacre C.L., Beacorn T., Collett S. Using competitive exclusion, mannan-oligosaccharide and other necrotic enteritis. Poult. Sci. 2003;12:60–64. [Google Scholar]
- Hong Y.H., Lillehoj H.S., Lillehoj E.P., Lee S.H. Changes in immune-related gene expression and intestinal lymphocyte sub- populations following Eimeria maxima infection of chickens. Vet. Immunol. Immunopathol. 2006;114:259–272. doi: 10.1016/j.vetimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Schatzmayr G., Mohnl M., Applegate T.J. Net effect of an acute phase response--partial alleviation with probiotic supplementation. Poult. Sci. 2010;89:28–33. doi: 10.3382/ps.2009-00464. [DOI] [PubMed] [Google Scholar]
- Klasing K.C. Nutrition and the immune system. Br. Poult. Sci. 2007;48:525–537. doi: 10.1080/00071660701671336. [DOI] [PubMed] [Google Scholar]
- Kogut M.H., Klasing K. An immunologist’s perspective on nutrition, immunity, and infectious diseases: introduction and overview. J. Appl. Poult. Res. 2009;18:103–110. [Google Scholar]
- Kraieski A.L., Hayashi R.M., Sanches A., Almeida G.C., Santin E. Effect of aflatoxin experimental ingestion and Eimeria vaccine challenges on intestinal histopathology and immune cellular dynamic of broilers: applying an Intestinal Health Index. Poult. Sci. 2017;95:1078–1087. doi: 10.3382/ps/pew397. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Lillehoj H.S., Jang S.I., Lee S.H., Bautista D.A., Donald Ritter G., Lillehoj E.P., Siragusa G.R. Comparison of live Eimeria vaccination with in-feed salinomycin on growth and immune status in broiler chickens. Res. Vet. Sci. 2013;95:110–114. doi: 10.1016/j.rvsc.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Long P.L., Tompkins R.V., Millard B.J. Coccidiosis in broilers: evaluation of infection by the examination of broiler house litter for Oocysts. Avian Pathol. 1975;4:287–294. doi: 10.1080/03079457509353877. [DOI] [PubMed] [Google Scholar]
- Min W., Kim W.H., Lillehoj E.P., Lillehoj H.S. Recent progress in host immunity to avian coccidiosis: IL-17 family cytokines as sentinels of the intestinal mucosa. Dev. Comp. Immunol. 2013;41:418–428. doi: 10.1016/j.dci.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Oláh I., Nagy N., Vervelde L. Structure of the avian lymphoid system. Avian Immunol. 2013;2:11–44. [Google Scholar]
- Rapp W., Wurster K. Alcian blue staining intestinal goblet cell antigen (GOA): A Marker for gastric signet ring cell and colonic colloidal carcinoma. Wien Klin Wochenschr. 1978;56:1185–1187. doi: 10.1007/BF01476863. [DOI] [PubMed] [Google Scholar]
- Rostagno H.S., albino L.F.T., Donzele J.L., Gomes P.C., Oliveira R.F., Lopes D.C., Ferreira A.S., Barreto S.L.T., Euclides R.F. 3rd edition. Universidade Federal de Viçosa; Viçosa - MG: 2011. Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais de aves e suínos; p. 252. [Google Scholar]
- Schwarz R.S., Jenkins M.C., Klopp S., Miska K.B. Genomic analysis of Eimeria spp. populations in relation to performance levels of broiler chicken farms in Arkansas and North Carolina. J. Parasitol. 2012;95:871–880. doi: 10.1645/GE-1898.1. [DOI] [PubMed] [Google Scholar]
- Shirley M.W., Lillehoj H.S. The long view: a selective review of 40 years of coccidiosis research. Avian Pathol. 2012;41:111–121. doi: 10.1080/03079457.2012.666338. [DOI] [PubMed] [Google Scholar]
- Vermeulen A.N., Schaap D.C., Schetters T.P.M. Control of coccidiosis in chickens by vaccination. Vet. Parasitol. 2001;100:13–20. doi: 10.1016/s0304-4017(01)00479-4. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Epidemiological studies of coccidiosis in the domesticated fowl (Gallus gallus): I. The fate of ingested oocysts of Eimeria tenella during the prepatent period in susceptible chicks. Appl. Parasitol. 1995;36:83–89. [PubMed] [Google Scholar]
- Williams R.B. Epidemiological aspects of the use of live anticoccidial vaccines for chickens. Int. J. Parasitol. 1998;28:1089–1098. doi: 10.1016/s0020-7519(98)00066-6. [DOI] [PubMed] [Google Scholar]