Abstract
The Wnt signalling pathways are of great importance in embryonic development and oncogenesis. Canonical and non-canonical Wnt signalling pathways are known, with the canonical (or β-catenin dependent) pathway being perhaps the best studied of these. While structural knowledge of proteins and interactions involved in canonical Wnt signalling has accumulated over the past 20 years, the pace of discovery has increased in recent years, with the structures of several key proteins and assemblies in the pathway being released. In this review, we provide a brief overview of canonical Wnt signalling, followed by a comprehensive overview of currently available X-ray, NMR and cryoEM data elaborating the structures of proteins and interactions involved in canonical Wnt signalling. While the volume of structures available is considerable, numerous gaps in knowledge remain, particularly a comprehensive understanding of the assembly of large multiprotein complexes mediating key aspects of pathway, as well as understanding the structure and activation of membrane receptors in the pathway. Nonetheless, the presently available data affords considerable opportunities for structure-based drug design efforts targeting canonical Wnt signalling.
Keywords: beta-catenin, cancer, crystallography, frizzled, glycogen synthase kinase, Wnt proteins
Brief overview of canonical Wnt signalling
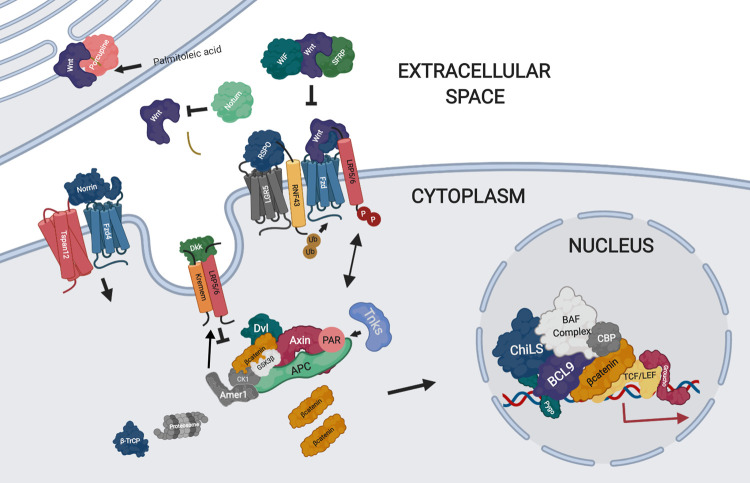
Wnt signalling involves a series of complex pathways and underpins developmental processes [1]. When dysregulated, it is synonymous with impaired regeneration and a variety of pathological states, including carcinogenesis [2–5]. Wnt signalling is primarily classed into canonical (β-catenin dependent) and non-canonical (β-catenin independent) pathways. Canonical Wnt signalling is primarily controlled through the regulation of three distinct multiprotein complexes: the signalosome, the degradosome and the nuclear enhanceosome, as well a variety of extracellular agonists and antagonists which precede these intracellular events [6] (Figure 1).
Figure 1. Graphical overview of canonical Wnt signalling.
Figure prepared using BioRender.
Wnt signalling can be initiated or enhanced by a variety of extracellular ligands, including Wnt and Norrin proteins, which bind to Frizzled (Fzd) receptors, and R-spondins (RSPOs), which bind to LGR family receptors. Wnt proteins are lipid-modified at a conserved serine by the O-acyltransferase Porcupine to facilitate secretion and receptor binding. Canonical Wnt signalling can be amplified following concomitant binding of Wnt and R-spondin (RSPO) ligands, which may function dependently or independently of LGR [7]. RSPOs prevents Fzd degradation by blocking the activity of the RING finger ubiquitin ligases, RNF43 and ZNRF3 [8,9]. Norrin is an atypical Wnt ligand that can bind specifically to Fzd4 and LRP5/6 [10], as well as the Fzd4–Tspan12 complex, to activate Wnt signalling [11]. Extracellular antagonists include Wnt inhibitory factor (WIF), secreted-Frizzled related proteins (sFRPs), Dickkopfs (DKKs) and Notum, each of which are diverse in structure and function (specific details of which will be elaborated later in the review). Wnt ligand binding to membrane-bound receptors and co-receptors results in the formation of multiprotein assemblies or ‘signalosomes’, composed of Fzd receptors and LRP5/6 co-receptors bound to Wnt ligands. These signalosomes are highly dynamic and can be negatively regulated by RNF43/ZNRF3, which, in turn, is balanced by R-spondin–LGR5 receptor interactions [12].
Intracellularly, Wnt signalling is controlled at the level of the β-catenin destruction complex, or the degradosome, which primarily consists of the scaffold protein Axin, glycogen synthase kinase 3β (GSK3β), casein kinase 1α (CK1α), protein phosphatase 2A (PP2A) and Adenomatous Polyposis Coli (APC). [13] In the absence of Wnt stimulation, β-catenin is sequentially phosphorylated by CK1α (Ser45) and GSK3β (Thr41, Ser37, Ser33), resulting in ubiquitin-mediated proteasomal degradation through a β-TrCP-dependent mechanism. Following Wnt stimulation, the degradosome is recruited to the membrane through a Dvl-Axin mediated mechanism, where phosphorylation of the co-receptor LRP5/6 on its cytoplasmic tail by GSK3β and CK1α/ε occurs [13]. The recruitment of GSK3β/CK1 and Axin can be mediated by adenomatous polyposis coli membrane recruitment 1 (Amer1) [14]. This, in turn, can result in the inhibition of GSK3β [15–18] and the translocation of β-catenin to the nucleus. Poly(ADP-ribosyl)ation of Axin by Tankyrase mediates its ubiquitination and subsequent degradation, destabilising the destruction complex, and thus activating Wnt signalling [19]. Once localised to the nucleus, β-catenin acts a co-factor for the initiation of the transcription of Wnt target genes [2]. This Wnt-driven transcriptional program is controlled by the Wnt enhanceosome, the core of which is made up of the ChiLS (Chip-SSDP/LIM-domain binding protein) complex, which binds Pygopus, Groucho/TLE and scaffold protein BCL9/legless [20,21]. In a ‘Wnt off’ context Groucho/TLE binds TCF/LEF and ChiLS to repress transcription, while in a ‘Wnt on’ environment, β-catenin induces an enhanceosome complex rearrangement to bind to TCF/LEF transcription factors, and other transcriptional co-activators (e.g. CREB-binding protein and BAF complex) to initiate target gene expression [22].
Structural knowledge of extracellular regulation of Wnt signalling
Wnts and related proteins
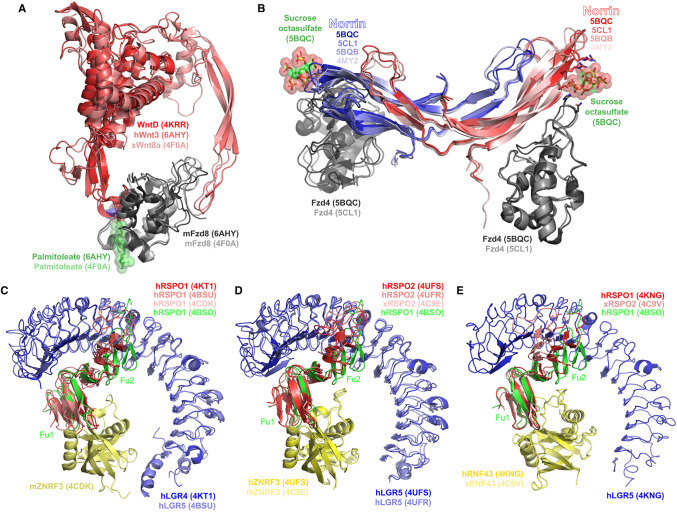
The structures of a relatively limited number of Wnt protein family members have been solved (Figure 2A). This is due in part to the presence of O-lipidation at a conserved serine that makes Wnt proteins highly hydrophobic and challenging to purify. The first structure of a Wnt protein solved was the Xenopus Wnt8 in complex with the mouse Fzd8 cysteine-rich domain (CRD), revealing a novel protein fold and the importance of lipidation for direct binding of Fzd [23]. This structure further illustrated that Wnts bind to Fzds at two distinct sites on opposite faces of the CRD. The Wnt protein family contains 19 members in mammals, however, the structure of only one mammalian Wnt has been solved experimentally [24]; further study of the Wnt family in mammals has been facilitated by computational approaches [25–27]. The structure of the N-terminal region of the Drosophila WntD protein revealed an overall similar fold to the N-terminal regions of other members of the Wnt family [28], although unlike other members of the family, this protein is not lipidated [29].
Figure 2. Structures related to interactions involving secreted positive regulators of Wnt signalling.
(A) Wnt-related proteins and interactions. Structures depicted include: WntD (PDB 4KRR); the human Wnt3 complex with the mouse Fzd8 cysteine-rich domain (PDB 6AHY); the Xenopus Wnt8a complex with the mouse Fzd8 cysteine-rich domain (PDB 4F0A). (B) Norrin and its interactions. Structures depicted include: unbound norrin-maltose binding protein fusion (PDB 4MY2), unbound norrin (PDB 5BQB), norrin-maltose binding protein fusion in complex with Fzd4 (PDB 5CL1), norrin-Fzd4-sucrose octasulfate ternary complex (PDB 5BQC). Maltose binding protein hidden. Key residues contacting sucrose octasulfate in PDB 5BQC are shown as sticks. (C) RSPO1–LGR–ZNRF3 complexes. Structure represented include: human RSPO1–LGR4 complex (PDB 4KT1), human RSPO1–LGR5 complex (PDB 4BSU), human RSPO1 in complex with mouse ZNRF3 (PDB 4CDK). The structure of native human RSPO1 (PDB 4BSO) is shown for reference. (D) RSPO2–LGR–ZNRF3 complexes. Structures represented include: human RSPO2–LGR5–ZNRF3 complex (PDB 4UFS), human RSPO2–LGR5 complex (PDB 4UFR), Xenopus RSPO2 in complex with mouse ZNRF3 (PDB 4C9E). The structure of native human RSPO1 (PDB 4BSO) is shown for reference. (E) R-spondin–LGR–RNF43 complexes. Structures represented include: human LGR5–RSPO1–RNF43 complex (PDB 4KNG); Xenopus RSPO2–RNF43 complex (PDB 4C9V). The native human RSPO1 (PDB 4BSO) is shown for reference.
Norrin
Norrin is an atypical Wnt signalling activator displaying a distinct fold to Wnt, achieving its activity through forming a ternary complex with a Fzd and an LGR (Figure 2B). The first structure of Norrin obtained was that of its fusion with maltose binding protein [10]; a complex of this fusion protein with the Fzd4 CRD was subsequently obtained [30]. The structures of the unfused Norrin, its complex with Fzd4, and the Norrin-Fzd4 ternary complex with the heparin mimic sucrose octasulfate have also been determined [31], revealing the potential for glycosaminoglycans to bridge the Norrin-Fzd4 interaction. Specifically, norrin residues Lys58, Arg107, Arg109 and Arg115 and Fzd4 residues His154 and Asn155 interact directly with sucrose octasulfate in the crystal structure (Figure 2B).
R-spondins (RSPOs)
RSPOs feature two furin repeat domains (Fu1 and Fu2), as illuminated in the structures of the human RSPO1 and the Xenopus RSPO2 [32,33], and between which considerable flexibility is observed (Figure 2C–E). Numerous structures of RSPO1 bound to the leucine-rich repeat (LRR) ectodomains of LGR4 and LGR5 have been reported [33–35]; a structure of RSPO2 bound to the LGR5 ectodomain has also been reported [36]. In all cases, these structures feature the LRRs of the LGR ectodomain curving around Fu2 of the RSPO. Fu1 of RSPOs mediates the interaction with ZNRF3 [32–37] and RNF43 [32]. Due to the complementary utilisation of the Fu1 and Fu2 domains, ternary complexes of RSPOs, LGRs and RING finger ubiquitin ligases are possible and have been structurally characterised [36–38]. Subtle variations in how ZNRF3 and RNF43 are recognised by LGRs are observed in the crystal structures; specifically, the LRR does not appear to directly bind ZNRF3 (although in one structure, a helix immediately after the LRR is observed to bind to ZNRF3) (Figure 2C,D), while RNF43 is directly bound by the LRR, albeit weakly (Figure 2E).
Secreted Frizzled-related proteins (sFRPs)
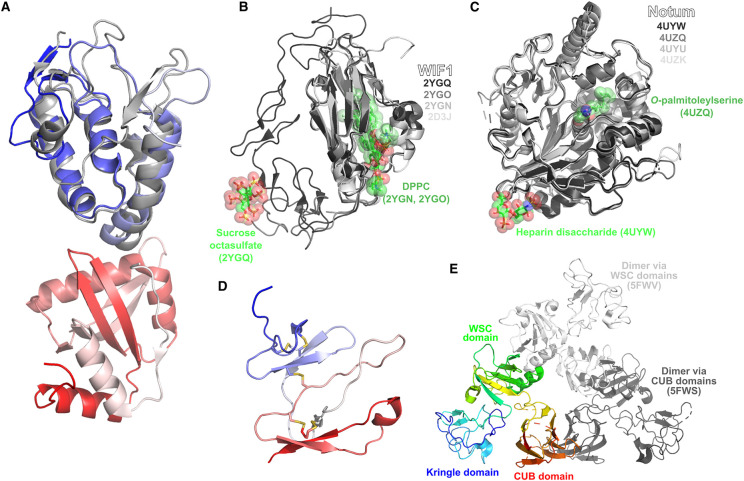
In mammals, five sFRPs are known (sFRP1–5). These proteins feature a two-domain structure, containing an N-terminal Fzd-type CRD Frizzled-type cysteine-rich domain (CRD), and a C-terminal netrin-like domain (NLD) [39]. The exact mechanism by which sFRPs function as Wnt signalling inhibitors is still under investigation (in particular, the importance of the NLD in inhibition), but it is widely believed they act as inhibitors by binding Wnt proteins using their CRD, thus preventing the ability of Wnts to bind Fzds and initiate Wnt signalling [40]. Structural knowledge of sFRPs and related proteins is presently limited, with only two such structures reported (Figure 3): the mouse sFRP3 CRD [41] and the Xenopus Sizzled protein [42] (Figure 3A). The mouse sFRP3 CRD — along with the mouse Fzd8 CRD — were the first structures of Fzd-type cysteine-rich domains to be characterised. The Sizzled structure is of particular interest as it is the only structure of an sFRP-related protein to feature both the CRD and NLD, providing insight into how the two domains may co-ordinate to modulate Wnt signalling.
Figure 3. Structures related to interactions involving secreted negative regulators of Wnt signalling.
(A) Secreted Frizzled-related proteins. Legend: grey — mouse sFRP3 cysteine-rich domain (PDB 1IJX); blue-white-red N-to-C-terminal — Xenopus Sizzled (PDB 5XGP). (B) Wnt Inhibitory Factors (WIFs). Structures depicted include: NMR structure of WIF domain of native human WIF1 (PDB 2D3J); WIF domain of human WIF1 bound to dipalmitoylphosphatidylcholine (DPPC) (PDB 2YGN); WIF domain and epidermal growth factor-like (EGF-like) domain 1 of human WIF1 (PDB 2YGO); WIF domain and EGF-like domains 1–3 of human WIF bound to DPPC and sucrose octasulfate (PDB 2YGQ). (C) Notum. Structures depicted include: native Drosophila Notum (PDB 4UZK); native human Notum (PDB 4UYU); human Notum bound to O-palmitoleylserine (PDB 4UZQ); human Notum bound to heparin disaccharide (ΔUA(2S)α1-4GlcNS(6S) (PDB 4UYW). (D) NMR structure of mouse Dickkopf-2 (PDB 2JTK). Structure coloured from N-to-C-terminal by blue-white-red gradient. (E) Dimer formation by Kremen1. Monomeric Kremen1 (PDB 5FWU) is depicted as red-to-blue N-to-C terminal rainbow, with positions of second molecule in dimeric Kremen1 forms (PDB 5FWV, 5FWS) show as white/grey relative to monomeric form.
Wnt inhibitory factors (WIFs)
WIFs inhibit Wnt signalling by directly binding the Wnt lipid moiety, to prevent Fzd receptor binding, and prevent Wnt signalling [43]. The structure of the WIF domain of WIF-1 was initially determined by NMR, revealing an immunoglobulin-like fold and the location of the putative lipid-binding site [44] (Figure 3B). The site was subsequently confirmed by X-ray crystallography, as well as the involvement of WIF epidermal growth factor-like domains in binding glycosaminoglycans [45].
Notum
Notum is an extracellular deacetylase that removes O-lipidation from Wnt proteins, thus deactivating them. The structural biology of Notum has primarily been elaborated by a single extensive study [46], wherein structures of human and Drosophila Notum bound to O-palmitoleylserine, a heparin disaccharide, and the heparin analogue sucrose octasulfate were determined (Figure 3C). O-palmitoleylserine is bound by Notum at a hydrophobic cavity deep in the structure. Although a complex with full length Wnt was not determined, the study suggested that the formation of a Wnt-Notum complex is facilitated by heparin binding.
Dickkopfs (DKKs)
Four mammalian DKKs are known (DKK1-4). These proteins feature two CRDs of a distinct type to that found in sFRPs and Fzds, and primarily act to block canonical Wnt signalling by binding to LRP family co-receptors [47]. DKK also facilitates the Kremen-mediated endocytosis of LRP5/6 [48]. The majority of DKK structures have been determined in complex with LRPs, which will be covered later in the review. Only one structure of an isolated DKK CRD has been determined, that of the second CRD of the mouse DKK2 (Figure 3D) [49].
Kremens
In conjunction with DKKs, Kremens facilitate blocking of canonical Wnt signalling by promoting the endocytosis of LRPs. The structure of the Kremen1 ectodomain revealed a triangular arrangement of its Kringle, WSC and CUB domains [50] (Figure 3E). The Kringle and WSC domains bind DKK at the opposite face to its LRP-binding interface, while the CUB domain mediates Kremen1 dimerisation in a structure obtained from one of the crystal forms [50]. The WSC domain can also mediate dimerisation (Figure 3E).
Structure knowledge of Wnt receptors and co-receptors
Frizzleds (Fzds)
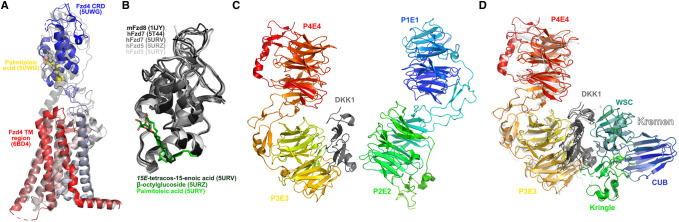
Together with the related Smoothened receptor, which mediates Hedgehog signalling, the Frizzleds form a class of G protein-coupled receptors that feature a seven-helical transmembrane domain (as per other GPCRs) and a distinctive cysteine-rich ectodomain (CRD) used to bind ligands. The structures of the CRDs of Fzd2 [51], Fzd4 [30,31,52,53], Fzd5 [54,55], Fzd7 [52,54–56] and Fzd8 [41,52,57] are presently represented in the Protein Data Bank; only a single Fzd transmembrane domain structure, that of Fzd4 [58], is presently known (Figure 4A,B). No structures featuring both the CRD and TM regions of Fzds are presently available. However, the structural biology of the Fzds can be inferred from that of the more extensively structurally characterised Smoothened [59–66]. Of particular note is the recent determination of Smoothened in an active conformation bound to a G protein, revealing a similar opening of the intracellular regions of the receptor to that observed in other classes of GPCRs; such opening was earlier inferred to occurred in Fzds [67].
Figure 4. Structures related to transmembrane proteins.
(A) Frizzled-4 model generated by overlay of Frizzled-4 cysteine-rich domain (CRD) bound to palmitoleic acid (PDB 5UWG) and Frizzled-4 transmembrane (TM) region (PDB 6BD4) to Smoothened bound to cholesterol (PDB 5L7D; transparent grey). (B) Representative Frizzled CRD structures and complexes. Structures depicted include: native mouse Fzd8 CRD (PDB 1IJY), native human Fzd7 (PDB 5T44), human Fzd7 bound to 15E-tetracos-15-enoic acid (PDB 5URV), human Fzd5 bound to β-octylglucoside (PDB 5URZ), human Fzd5 bound to palmitoleic acid (PDB 5URY). (C) Atomic structure of LRP6 ectodomain constructed from fitting X-ray structures of P1E1 and P2E2 regions (PDB 4DG6) and the complex of DKK1 with P3E3 and P4E4 regions (PDB 3S2K) to the electron microscopy structure (PDB 5GJE). Legend: red-to-blue rainbow — LRP6 N-to-C-terminal; grey — DKK1. (D) LRP6–DKK-Kremen interactions. LRP6–DKK1–Kremen1 complex (PDB 5FWW) depicted in colours; LRP6–DKK1 complex (PDB 3S2K), unbound DKK1 (PDB 2JTK) and unbound Kremen1 (PDB 5FWT) overlaid to PDB 5FWW and depicted in transparent grey.
Low-density lipoprotein receptor-related proteins 5 and 6 (LRP5/6)
LRP5 and LRP6 function as Wnt ligand co-receptors in canonical Wnt signalling, and are antagonised by DKKs and Kremens. The ectodomain of LRP6 has been extensively structurally characterised and is primarily defined by the presence of four 7-bladed β-propellers followed by EGF-like domains (P1E1, P2E2, P3E3, P4E4) (Figure 4C). The structure of the combination of the LRP6 P1E1 and P2E2 regions [68], as well as that of the P3E3 and P4E4 regions [68–70], have been determined by X-ray crystallography, while the structure of the complete ectodomain has been determined by electron microscopy [69,71]. DKK1 has been structurally demonstrated to bind at the P1E1 [72] and P3E3 [50,68,70] regions, with Kremen binding on the opposite face of DKK to LRP, allowing formation of a ternary complex (Figure 4D).
Structural knowledge of intracellular proteins and complexes mediating Wnt signalling
Dishevelleds (Dvls)
Dvls feature three ordered domains: an N-terminal DIX domain, promoting Dvl oligomerisation into signalosomes, as well as mediating Axin binding; a PDZ domain, facilitating interactions with various proteins as well as weakly contributing to Fzd binding (although demonstrated to be dispensable for canonical Wnt signalling [73]); and a DEP domain, facilitating high affinity interaction with Fzds [74], as well as Fzd endocytosis [75]. These domains of Dvl1 and Dvl2 have been the focus of all currently published structures.
Structural characterisation of wildtype and mutant DIX domains of human Dvl2, mouse Dvl1 and mouse Dvl2 reveal in all instances the formation of a superhelical oligomeric structure [76–79]; the pitch of the superhelix varies appears to vary according to the specific DIX domain being examined, as well as through the introduction of interface mutants (Figure 5A). The variety of Dvl PDZ structures determined illustrate the flexibility of the domain's binding pocket to accommodate various ligands (Figure 5B) [80–83]. In particular, a series of structures of the human Dvl2 PDZ in complex with several peptides derived from phage display suggests how Dvl PDZ domains may recognise the C-terminal KTxxxW motifs contained in Fzds [81]. The structure of the mouse Dvl1 DEP domain was the first of any Dvl domain to be solved, demonstrating a fold exhibiting a strong electric dipole suggested to facilitate membrane targeting [84]. While the Dvl DEP domain has been illustrated to afford a key role in directly binding Fzds [74], the most recent structural evidence for any Dvl DEP domain — that of the human Dvl2 in a domain-swapped dimeric configuration — illustrate a potential role for the DEP domain in assembling Wnt signalosomes, as well as in mediating signal directionality [85] (Figure 5C).
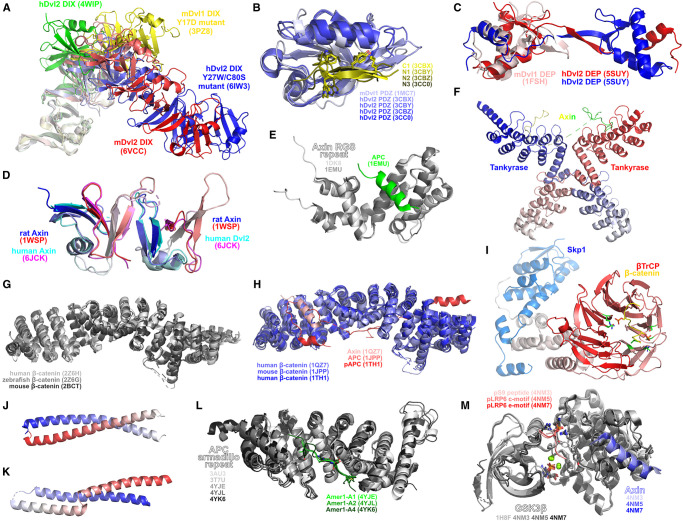
Figure 5. Structures related to cytoplasmic proteins and interactions.
(A) Dvl DIX domain oligomeric structures. (B) Dvl PDZ domain. Structures represented include: native mouse Dvl1 PDZ (PDB 1MC7), human Dvl2 PDZ in complex with C1 inhibitory peptide (PDB 3CBX), human Dvl2 PDZ in complex with N1 inhibitory peptide (PDB 3CBY), human Dvl2 PDZ in complex with N2 inhibitory peptide (PDB 3CBZ), human Dvl2 PDZ in complex with N3 inhibitory peptide (PDB 3CC0). The structure of the N2 complex with Dvl2 PDZ was inferred via the generation of a symmetry-related dimer. Selected residues with similar chemical functionality across multiple peptides — loosely highlighting how Fzd KTxxxW motifs may be recognised by Dvl PDZ domains — are shown as sticks. (C) Dvl DEP domain. Structures represented include: native mouse Dvl1 DEP domain (PDB 1FSH); human Dvl2 DEP domain dimer crystallised from dimeric fraction (PDB 5SUY). (D) Axin DIX domain. Structures represented include: rat Axin homodimer (PDB 1WSP); human Axin-Dvl2 heterodimer (PDB 6JCK). Each molecule coloured from N-to-C-terminal in blue-to-red/cyan-to-magenta gradient. (E) Axin RGS repeat in unbound (PDB 1DKS) and APC-bound (PDB 1EMU) states. (F) Mouse tankyrase-axin complex (PDB 3UTM). Each tankyrase monomer is coloured from N-to-C terminal as blue/red to white gradient. Dashes indicate missing portions of the axin structure. (G) Native β-catenin structures. (H) Complexes of interactions of cytoplasmic β-catenin. Structures represented include: human β-catenin bound to Xenopus Axin (PDB 1QZ7); mouse β-catenin bound to an APC fragment (PDB 1JPP); human β-catenin bound to a phosphorylated human APC fragment (PDB 1TH1). (I) APC N-terminal coiled-coil region (residues 2–55) (PDB 1DEB). Each chain coloured from N-to-C terminal as blue/red to white gradient. (J) APC N-terminal helical region (residues 126–250) (PDB 1M51). Coloured from N-to-C terminal as blue-white-red gradient. (K) APC armadillo repeat. Structures represented include: native APC (PDB 3AU3 and 3T7U); APC in complex with Amer1-A1 (PDB 4YJE); APC in complex with Amer1-A2 (PDB 4YJL); APC in complex with Amer1-A4 (PDB 4YK6). (L) β-TrCP-Skp1-β-catenin complex (PDB 1P22). Phosphate-contacting residues in β-TrCP are shown green. Phosphorylated β-catenin residues and their contacts in β-TrCP shown as sticks. (M) GSK3β complexes elaborating Wnt signalling. Structures represented include: apo-GSK3β (PDB 1H8F); GSK3β bound to N-terminal autoinhibitory phosphopeptide (pS9) and Axin (PDB 4NM3); GSK3β bound to phosphorylated LRP6 c-motif and Axin (PDB 4NM5); GSK3β bound to phosphorylated LRP6 e-motif and Axin (PDB 4NM7). Bound ADP, phosphorylated residues on peptides and Arg96, Arg180, Lys205 and Tyr216 shown as sticks. Magnesium shown as green spheres.
Axin and tankyrase
Axin, like Dvl, contains a DIX domain which can undergo head-to-tail oligomerisation (Figure 5D). Recently, a complex between the Axin DIX domain and the Dvl2 DIX domain has been determined [86], revealing a similar structure of the Axin-Dvl heterodimer compared with both the Axin DIX homodimer [87] and Dvl homomer structures [76–79]. Although extended heterooligomer structures have not been demonstrated, these are presumed to form a superhelical structure with a varied pitch compared to the currently determined Dvl DIX homooligomer structures. Axin also directly interacts and has been structurally characterised with APC [88], GSK3β [89], β-catenin [90] and tankyrase. With the exception of its interaction with APC (Figure 5E), Axin utilises short segments to interact with these proteins (Figure 5F,H,L). The complex of Axin with the tankyrase ankyrin repeat reveals that the N-terminal of Axin binds to tankyrase in a 1:2 fashion (Figure 5F) [91].
β-catenin
The structures of the armadillo repeat regions of human [92], mouse [93] and zebrafish [92] β-catenin have been characterised, revealing a relatively conserved and remarkably rigid structure (Figure 5G). Complexes of this region of β-catenin with APC [90,94,95] and axin [88,96,97] have also been determined (Figure 5H). Axin utilises a short helical fragment to bind to armadillo repeats 3 and 4 of β-catenin, while APC uses an extended region to interact with approximately the entire length of β-catenin. Although part of APC binds to β-catenin at an overlapping region to Axin, APC does not share Axin's helical secondary structure in this location, indicating β-catenin's ability to bind peptides distinct in sequence and structure. N-terminal phosphorylation of β-catenin facilitates its destruction, and complexes of the phosphorylated N-terminal of β-catenin with the SCF ubiquitin ligase β-TrCP-Skp1 have been determined. These indicate that the phosphorylated N-terminal of β-catenin interacts with β-TrCP at the opposite face of the β-propeller to Skp1, with pSer33 bound by the first and second blades of the β-propeller and pSer37 bound by the fifth blade (Figure 5I) [98,99].
Adenomatous polyposis coli protein (APC)
APC is a very large protein comprising, in simplest terms, an N-terminal leucine-rich region (residues 1–730) and a C-terminal serine-rich region (residues 731–2832). Short fragments from the C-terminal serine-rich region have been structurally demonstrated in complex with a range of proteins, including axin (Figure 5E), β-catenin (Figure 5H), the Src-homology 3 domain of DDEF1 [100], and the PDZ1 [101] and PDZ2 [102] domains of DLG1. The N-terminal leucine-rich region contains at least three helical regions that have been structurally characterised: an N-terminal dimeric coiled-coil domain (residues 2–55) [103] (Figure 5J), an helical region forming a monomeric coiled-coil (residues 126–250) [104] (Figure 5K) and a series of armadillo repeats (residues 453–767) [105–109]. The N-terminal dimeric coiled-coil is poorly stable in isolation, suggesting that the dimerisation motif may extend beyond the first 55 amino acids of APC, although it is unclear whether the monomeric coiled-coil that follows contributes to dimerisation. The APC armadillo repeat region has been structurally characterised with several fragments of Amer1 (Figure 5L). These structures reveal that Amer1 fragments use a relatively functionally conserved motif to bind APC, consisting of Ser/Thr/Tyr to bind armadillo repeats 4–6 and a small polar amino acid (Gly/Ser/Cys) followed immediately by a glycine and a negatively charged amino acid to bind repeats 2–4. Hydrophobic amino acids (typically a longer chain aliphatic amino acid followed by alanine) bind repeats 1–3.
Glycogen synthase kinase 3β (GSK3β)
While GSK3β has been extensively structurally characterised as part of many medicinal chemistry research programs, a small selection of structures provide specific insight into its role in modulating Wnt signalling (Figure 5M). X-ray crystal structures of GSK3β in complex with the minimal binding segment of Axin [89] illustrate that Axin utilises an α-helical segment to bind GSK3β. The structures of GSK3β bound to its phosphorylated autoinhibitory peptide and phosphorylated LRP6 motifs illustrate the importance of conformational changes in regulating GSK3β function and how primed substrates are recognised by GSK3β [15]. Specifically, the loop from residues 89-95 moves from the open conformation observed in the unbound state [110] to clamp onto the peptide, the phosphorylated residue is bound by three positively charged residues — Arg96, Arg180 and Lys205 — and Tyr216 rotates to facilitate peptide access to the active site.
Structural knowledge of intranuclear proteins and complexes mediating Wnt signalling
Nuclear β-catenin
Structures of TCFs [111–114] and LEF-1 [115] in complex with β-catenin (Figure 6A) reveal that β-catenin wraps around the N-terminal of these proteins, utilising approximately the full length of the armadillo repeats to bind the transcription factors, similar to how APC is bound by β-catenin (Figure 5H). Bcl9 binds to the first armadillo repeat of β-catenin using a short helix located between proline-rich stretches of its sequence, while the β-catenin inhibitor ICAT uses a small N-terminal helical domain to bind the final armadillo repeats of β-catenin, and a C-terminal extension that overlaps with much of the TCF/LEF binding site, thus blocking TCF/LEF binding [97,116]. TCFs and LEFs utilise a high mobility group (HMG) box domain to bind DNA; the structure of the mouse LEF-1 HMG box domain bound to DNA was one of the earliest structures of such a domain, as well as a DNA–HMG box complex, and illustrates the bending of the DNA double helix characteristic of DNA–HMG box interactions (Figure 6B) [117].
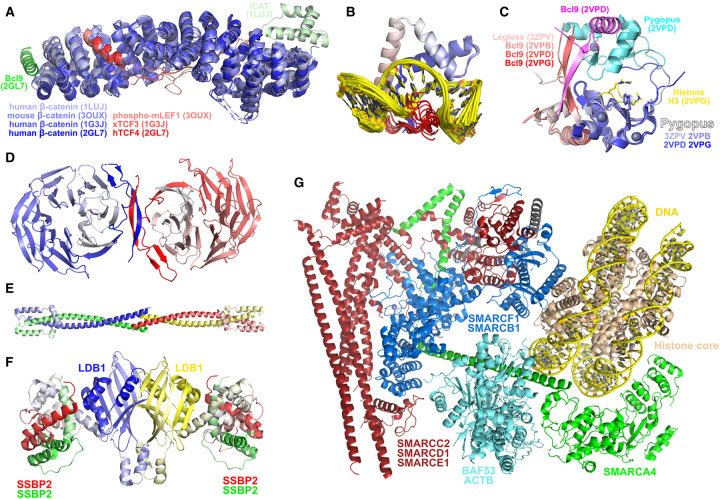
Figure 6. Structures related to intranuclear proteins and interactions.
(A) Nuclear β-catenin interactions. Structures represented include: human β-catenin bound to mouse ICAT (PDB 1LUJ), mouse β-catenin bound to phosphorylated mouse LEF1 (PDB 3OUX), human β-catenin bound to Xenopus TCF3 (PDB 1G3J), ternary complex of human β-catenin, human TCF4 and human Bcl9 (PDB 2GL7). (B) Mouse LEF1 high mobility group domain (blue–red N-to-C-terminal) bound to DNA (yellow) (PDB 2LEF). (C) Pygopus–Bcl9 interactions. Structure represented include: Drosophila Pygopus–Legless complex (PDB 3ZPV), human Pygopus1–Bcl9 complex (PDB 2VPB), human Pygopus1–Bcl9 dimer of dimers (PDB 2VPD), human Pygopus1–Bcl9-histone H3 tail ternary complex (PDB 2VPG). (D) TLE1 WD repeat dimer (PDB 1GXR). Legend: blue-white — molecule 1 N-to-C terminal; red-white — molecule 2 N-to-C terminal. (E) TLE1 Q domain tetramer (PDB 4OM2). Each chain coloured from N-to-C terminal in blue/red/yellow/green to white. (F) Assembly of the ChiLS complex in 4:2 stoichiometry based on available structures (PDB 6TYD and PDB 6S9S). (G) Structure of nucleosome-bound human BAF complex (PDB 6LTJ).
B-cell CLL/lymphoma 9 protein (Bcl9) and Pygopus
Bcl9 forms a ternary complex with β-catenin and TCF transcription factors, binding at a distinct site on β-catenin to TCF, as well as other β-catenin-interacting proteins [111,118]. The function of Bcl9 is enhanced via binding to Pygopus proteins and their homologues, wherein a helical segment of Bcl9 interacts with the PHD-type zinc finger of Pygopus [119,120]; the Bcl9-like protein (BCL9L) forms a similar complex with Pygopus [121,122]. The human Bcl9–Pygopus heterodimer has been characterised in complex with a methylated histone fragment, illustrating the importance of Trp366 in Pygopus in interacting with methylated arginine and lysine; this residue is substituted for a phenylalanine in Drosophila Pygopus and likely facilitates similar interactions. Additionally, Bcl9 and Pygopus have been demonstrated to form a dimer of heterodimers; such an arrangement appears compatible with the binding of methylated histones (Figure 6C).
Groucho family proteins
Structures of the human Groucho family protein TLE1 have been obtained for its C-terminal WD repeat region, a seven-bladed β-propeller forming a dimer mediated by its N-terminal segment [123,124] (Figure 6D). The N-terminal Q domain, which mediates TCF binding, forms a dimeric coiled coil which in turn dimerises in a head-to-head fashion to give the active tetrameric species [125] (Figure 6E).
ChiLS complex
The biological assembly of the LUFS domain of the human SSDP2 revealed a tightly packed tetramer formed by dimerisation of dimers [126]. The biological assembly of the Xenopus LDB1 bound to darpin 10 illustrates the dimerisation of LDB proteins [21]. The biological assembly of the human SSDP2 in complex with the human LDB1 illustrates a 2:1 stoichiometry between SSDP2 and LDB1, with LDB1 binding at the tetramerization interface of SSDP2 [127]; this in turn suggests that the SSDP2 tetramer previously determined may represent an inactive state. Judicious overlay of the presently determined structures allows the development of a structural model of the ChiLS complex, displaying the determined 4 : 2 stoichiometry between the SSDP and LDB components [21] (Figure 6F).
BAF complex
The BAF complex is a very large complex comprised of numerous subunits that functions as a Wnt transcriptional co-activator. The SWI/SNF-related matrix-associated actin-dependent regulator of chromatin (SMARC) subfamily members, which are key components of this complex, have been the subject of numerous structural biology [128–130] and medicinal chemistry [131–133] efforts. Very recently, the structure of a nucleosome-bound human BAF complex has been determined [134] (Figure 6G). This structure reveals that SMARCC2 forms a dimeric coiled-coil, with which helical regions of SMARCD1 and SMARCE1 interact and which likely forms a scaffold for the complex. SMARCF1 contains an armadillo repeat-like region that interacts with this helical scaffold on one face and with the N-terminal domain of SMARCB1 with its opposing face. The SMARCB1 C-terminal domains interact with the SWIRM domains of both SMARCC1 molecules, an interaction that appears further stabilised by BAF45D. This assembly positions SMARCB1 to interact directly with histones H2A and H2B on one face of the nucleosome. SMARCA4 adopts a highly extended conformation, interacting with almost all subunits of the complex, cradling the opposite face of the nucleosome to SMARCB1 with its helicase domains. The extended conformation and nucleosome-binding by SMARCA4 appear to be supported through interaction with actin-like protein 6A (BAF53) and cytoplasmic actin 1 (ACTB).
Future challenges in the structural biology of Wnt signalling
Wnt structural biology has considerably grown in the past 20 years, however, there are still a number of notable gaps in knowledge. These include the structure of an active Wnt signalosome and/or components thereof (e.g. full length Fzd, Fzd in an active conformation, Fzd bound to Dvl), an overall view of the Wnt degradosome, and a comprehensive understanding of the structure of the Wnt enhanceosome. Cryoelectron microscopy, which has facilitated the structural determination of many targets that were typically challenging or thought impossible by X-ray crystallography (including very large protein complexes and membrane-bound proteins in various states), has the potential to fill these gaps in structural knowledge of Wnt signalling. Nonetheless, significant protein engineering is likely to be required to achieve constructs sufficiently stable for structure determination, as has facilitated the elaboration of membrane protein structure and pharmacology. Computational approaches may also be valuable to fill some of these gaps — in particular, the combinatorial range of potential protein–protein interactions regulating the earlier stages of the pathway. The present structural data on canonical Wnt signalling affords numerous opportunities for structure-based drug design, with the recent growth allowing further dissection and effective targeting of this fascinating pathway.
Perspectives
Canonical/β-catenin-dependent Wnt signalling is a pathway of enormous interest as a potential target in cancer treatment, as well as being crucial in the early stages of development.
Structural knowledge of proteins and interactions involved in facilitating and antagonising canonical Wnt signalling has grown considerably over the past 20 years.
Major frontiers to conquer relate primarily to understanding the assembly of large multiprotein complexes mediating Wnt signalling — in particular, the structure, activation and interactions of membrane receptors, as well as the assembly of nuclear proteins.
Acknowledgements
Molecular structures presented in this work were identified by surveying UniProt and the Protein Data Bank for relevant proteins. All molecular structure figures were prepared using open-source PyMOL.
Abbreviations
- APC
Adenomatous Polyposis Coli
- BCL9
B-cell CLL/lymphoma 9 protein
- ChiLS
Chip-SSDP/LIM-domain binding protein
- CK1α
Casein kinase 1α
- CRD
Cysteine-rich domain
- CUB
Complement C1r/C1s, Uegf, Bmp1
- Dkk
Dickkopf proteins
- Dvl
Dishevelled
- Fzd
Frizzled receptor
- GSK3β
Glycogen synthase kinase 3β
- HMG
High mobility group
- LGR
Leucine-rich repeat-containing G-protein coupled receptor
- LRP
low-density lipoprotein receptor
- LRR
leucine-rich repeat
- NLD
Netrin-like domain
- PP2A
Protein phosphatase 2A
- RSPO
R-spondin
- SCF
Skp-Cullin-F-box
- sFRP
secreted-Frizzled related protein
- SMARC
SWI/SNF-related matrix-associated actin-dependent regulator of chromatin
- TCF/LEF
T-cell factor/lymphoid enhancer factor
- WIF
Wnt inhibitory factor
- WSC
Cell wall integrity and stress response components
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
M.A. is a recipient of a Curtin Research Fellowship. S.Ö-G.P. is a recipient of an Institute of Genetics and Molecular Medicine Early Career Award.
Open Access
Open access for this article was enabled by the participation of University of Edinburgh in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with JISC.
Author Contribution
M.A. conceived the topic, identified relevant structures, prepared molecular structural figures and accompanying text. S.Ö-G.P. prepared the figure and text describing signalling background. Both authors critically reviewed and revised the manuscript.
References
- 1.Olsen J.J., Pohl S.O., Deshmukh A., Visweswaran M., Ward N.C., Arfuso F. et al. (2017) The role of Wnt signalling in angiogenesis. Clin. Biochem. Rev. 38, 131–142 PMID: [PMC free article] [PubMed] [Google Scholar]
- 2.Nusse R. and Clevers H. (2017) Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 10.1016/j.cell.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 3.Pohl S.G., Brook N., Agostino M., Arfuso F., Kumar A.P. and Dharmarajan A. (2017) Wnt signaling in triple-negative breast cancer. Oncogenesis 6, e310 10.1038/oncsis.2017.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clevers H. and Nusse R. (2012) Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 5.Clevers H., Loh K.M. and Nusse R. (2014) Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346, 1248012 10.1126/science.1248012 [DOI] [PubMed] [Google Scholar]
- 6.Gammons M. and Bienz M. (2018) Multiprotein complexes governing Wnt signal transduction. Curr. Opin. Cell Biol. 51, 42–49 10.1016/j.ceb.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 7.Lebensohn A.M. and Rohatgi R. (2018) R-spondins can potentiate WNT signaling without LGRs. eLife. 7, e33126 10.7554/eLife.33126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao H.X., Xie Y., Zhang Y., Charlat O., Oster E., Avello M. et al. (2012) ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485, 195–200 10.1038/nature11019 [DOI] [PubMed] [Google Scholar]
- 9.Koo B.K., Spit M., Jordens I., Low T.Y., Stange D.E., van de Wetering M. et al. (2012) Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488, 665–669 10.1038/nature11308 [DOI] [PubMed] [Google Scholar]
- 10.Ke J., Harikumar K.G., Erice C., Chen C., Gu X., Wang L. et al. (2013) Structure and function of Norrin in assembly and activation of a Frizzled 4-Lrp5/6 complex. Genes Dev. 27, 2305–2319 10.1101/gad.228544.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai M.B., Zhang C., Shi J., Johnson V., Khandan L., McVey J. et al. (2017) TSPAN12 is a Norrin co-receptor that amplifies Frizzled4 ligand selectivity and signaling. Cell Rep. 19, 2809–2822 10.1016/j.celrep.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmon K.S., Gong X., Lin Q., Thomas A. and Liu Q. (2011) R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl Acad. Sci. U.S.A. 108, 11452–7 10.1073/pnas.1106083108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamos J.L. and Weis W.I. (2013) The beta-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 5, a007898 10.1101/cshperspect.a007898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanneberger K., Pfister A.S., Brauburger K., Schneikert J., Hadjihannas M.V., Kriz V. et al. (2011) Amer1/WTX couples Wnt-induced formation of PtdIns(4,5)P2 to LRP6 phosphorylation. EMBO J. 30, 1433–1443 10.1038/emboj.2011.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamos J.L., Chu M.L., Enos M.D., Shah N. and Weis W.I. (2014) Structural basis of GSK-3 inhibition by N-terminal phosphorylation and by the Wnt receptor LRP6. eLife. 3, e01998 10.7554/eLife.01998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu G., Huang H., Garcia Abreu J. and He X. (2009) Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS ONE 4, e4926 10.1371/journal.pone.0004926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piao S., Lee S.H., Kim H., Yum S., Stamos J.L., Xu Y. et al. (2008) Direct inhibition of GSK3beta by the phosphorylated cytoplasmic domain of LRP6 in Wnt/beta-catenin signaling. PLoS ONE 3, e4046 10.1371/journal.pone.0004046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cselenyi C.S., Jernigan K.K., Tahinci E., Thorne C.A., Lee L.A. and Lee E. (2008) LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of beta-catenin. Proc. Natl Acad. Sci. U.S.A. 105, 8032–8037 10.1073/pnas.0803025105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariotti L., Pollock K. and Guettler S. (2017) Regulation of Wnt/beta-catenin signalling by tankyrase-dependent poly(ADP-ribosyl)ation and scaffolding. Br. J. Pharmacol. 174, 4611–4636 10.1111/bph.14038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiedler M., Graeb M., Mieszczanek J., Rutherford T.J., Johnson C.M. and Bienz M. (2015) An ancient Pygo-dependent Wnt enhanceosome integrated by chip/LDB-SSDP. eLife 4, e09073 10.7554/eLife.09073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renko M., Fiedler M., Rutherford T.J., Schaefer J.V., Pluckthun A. and Bienz M. (2019) Rotational symmetry of the structured Chip/LDB-SSDP core module of the Wnt enhanceosome. Proc. Natl Acad. Sci. U.S.A. 116, 20977–20983 10.1073/pnas.1912705116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Tienen L.M., Mieszczanek J., Fiedler M., Rutherford T.J. and Bienz M. (2017) Constitutive scaffolding of multiple Wnt enhanceosome components by Legless/BCL9. eLife 6, e20882 10.7554/eLife.20882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janda C.Y., Waghray D., Levin A.M., Thomas C. and Garcia K.C. (2012) Structural basis of Wnt recognition by Frizzled. Science 337, 59–64 10.1126/science.1222879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirai H., Matoba K., Mihara E., Arimori T. and Takagi J. (2019) Crystal structure of a mammalian Wnt-frizzled complex. Nat. Struct. Mol. Biol. 26, 372–379 10.1038/s41594-019-0216-z [DOI] [PubMed] [Google Scholar]
- 25.Agostino M. and Pohl S.O. (2019) Wnt binding affinity prediction for putative Frizzled-type cysteine-rich domains. Int. J. Mol. Sci. 20, 4168 10.3390/ijms20174168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agostino M., Pohl S.O. and Dharmarajan A. (2017) Structure-based prediction of Wnt binding affinities for Frizzled-type cysteine-rich domains. J. Biol. Chem. 292, 11218–11229 10.1074/jbc.M117.786269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ain Q.U., Seemab U., Rashid S., Nawaz M.S. and Kamal M.A. (2013) Prediction of structure of human WNT-CRD (FZD) complex for computational drug repurposing. PLoS ONE 8, e54630 10.1371/journal.pone.0054630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu M.L., Ahn V.E., Choi H.J., Daniels D.L., Nusse R. and Weis W.I. (2013) Structural studies of Wnts and identification of an LRP6 binding site. Structure 21, 1235–1242 10.1016/j.str.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ching W., Hang H.C. and Nusse R. (2008) Lipid-independent secretion of a Drosophila Wnt protein. J. Biol. Chem. 283, 17092–8 10.1074/jbc.M802059200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen G., Ke J., Wang Z., Cheng Z., Gu X., Wei Y. et al. (2015) Structural basis of the Norrin-Frizzled 4 interaction. Cell Res. 25, 1078–1081 10.1038/cr.2015.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang T.H., Hsieh F.L., Zebisch M., Harlos K., Elegheert J. and Jones E.Y. (2015) Structure and functional properties of Norrin mimic Wnt for signalling with Frizzled4, Lrp5/6, and proteoglycan. eLife 4, e06554 10.7554/eLife.06554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zebisch M., Xu Y., Krastev C., MacDonald B.T., Chen M., Gilbert R.J. et al. (2013) Structural and molecular basis of ZNRF3/RNF43 transmembrane ubiquitin ligase inhibition by the Wnt agonist R-spondin. Nat. Commun. 4, 2787 10.1038/ncomms3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng W.C., de Lau W., Forneris F., Granneman J.C., Huch M., Clevers H. et al. (2013) Structure of stem cell growth factor R-spondin 1 in complex with the ectodomain of its receptor LGR5. Cell Rep. 3, 1885–1892 10.1016/j.celrep.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 34.Xu K., Xu Y., Rajashankar K.R., Robev D. and Nikolov D.B. (2013) Crystal structures of Lgr4 and its complex with R-spondin1. Structure 21, 1683–1689 10.1016/j.str.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D., Huang B., Zhang S., Yu X., Wu W. and Wang X. (2013) Structural basis for R-spondin recognition by LGR4/5/6 receptors. Genes Dev. 27, 1339–1344 10.1101/gad.219360.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zebisch M. and Jones E.Y. (2015) Crystal structure of R-spondin 2 in complex with the ectodomains of its receptors LGR5 and ZNRF3. J. Struct. Biol. 191, 149–155 10.1016/j.jsb.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng W.C., de Lau W., Madoori P.K., Forneris F., Granneman J.C., Clevers H. et al. (2013) Structures of Wnt-antagonist ZNRF3 and its complex with R-spondin 1 and implications for signaling. PLoS ONE 8, e83110 10.1371/journal.pone.0083110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen P.H., Chen X., Lin Z., Fang D. and He X. (2013) The structural basis of R-spondin recognition by LGR5 and RNF43. Genes Dev. 27, 1345–1350 10.1101/gad.219915.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pohl S., Scott R., Arfuso F., Perumal V. and Dharmarajan A. (2015) Secreted frizzled-related protein 4 and its implications in cancer and apoptosis. Tumour Biol. 36, 143–152 10.1007/s13277-014-2956-z [DOI] [PubMed] [Google Scholar]
- 40.Bovolenta P., Esteve P., Ruiz J.M., Cisneros E. and Lopez-Rios J. (2008) Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J. Cell Sci. 121, 737–746 10.1242/jcs.026096 [DOI] [PubMed] [Google Scholar]
- 41.Dann C.E., Hsieh J.C., Rattner A., Sharma D., Nathans J. and Leahy D.J. (2001) Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature 412, 86–90 10.1038/35083601 [DOI] [PubMed] [Google Scholar]
- 42.Bu Q., Li Z., Zhang J., Xu F., Liu J. and Liu H. (2017) The crystal structure of full-length sizzled from Xenopus laevis yields insights into Wnt-antagonistic function of secreted Frizzled-related proteins. J. Biol. Chem. 292, 16055–16069 10.1074/jbc.M117.791756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poggi L., Casarosa S. and Carl M. (2018) An eye on the Wnt inhibitory factor Wif1. Front. Cell Dev. Biol. 6, 167 10.3389/fcell.2018.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liepinsh E., Banyai L., Patthy L. and Otting G. (2006) NMR structure of the WIF domain of the human Wnt-inhibitory factor-1. J. Mol. Biol. 357, 942–950 10.1016/j.jmb.2006.01.047 [DOI] [PubMed] [Google Scholar]
- 45.Malinauskas T., Aricescu A.R., Lu W., Siebold C. and Jones E.Y. (2011) Modular mechanism of Wnt signaling inhibition by Wnt inhibitory factor 1. Nat. Struct. Mol. Biol. 18, 886–893 10.1038/nsmb.2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kakugawa S., Langton P.F., Zebisch M., Howell S., Chang T.H., Liu Y. et al. (2015) Notum deacylates Wnt proteins to suppress signalling activity. Nature 519, 187–192 10.1038/nature14259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bao J., Zheng J.J. and Wu D. (2012) The structural basis of DKK-mediated inhibition of Wnt/LRP signaling. Sci. Signal. 5, pe22 10.1126/scisignal.2003028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mao B., Wu W., Davidson G., Marhold J., Li M., Mechler B.M. et al. (2002) Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 417, 664–667 10.1038/nature756 [DOI] [PubMed] [Google Scholar]
- 49.Chen L., Wang K., Shao Y., Huang J., Li X., Shan J. et al. (2008) Structural insight into the mechanisms of Wnt signaling antagonism by Dkk. J. Biol. Chem. 283, 23364–23370 10.1074/jbc.M802375200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zebisch M., Jackson V.A., Zhao Y. and Jones E.Y. (2016) Structure of the dual-mode Wnt regulator Kremen1 and insight into ternary complex formation with LRP6 and Dickkopf. Structure 24, 1599–1605 10.1016/j.str.2016.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen P., Tao L., Wang T., Zhang J., He A., Lam K.H. et al. (2018) Structural basis for recognition of frizzled proteins by Clostridium difficile toxin B. Science 360, 664–669 10.1126/science.aar1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dang L.T., Miao Y., Ha A., Yuki K., Park K., Janda C.Y. et al. (2019) Receptor subtype discrimination using extensive shape complementary designed interfaces. Nat. Struct. Mol. Biol. 26, 407–414 10.1038/s41594-019-0224-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeBruine Z.J., Ke J., Harikumar K.G., Gu X., Borowsky P., Williams B.O. et al. (2017) Wnt5a promotes Frizzled-4 signalosome assembly by stabilizing cysteine-rich domain dimerization. Genes Dev. 31, 916–926 10.1101/gad.298331.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nile A.H., Mukund S., Stanger K., Wang W. and Hannoush R.N. (2017) Unsaturated fatty acyl recognition by Frizzled receptors mediates dimerization upon Wnt ligand binding. Proc. Natl Acad. Sci. U.S.A. 114, 4147–4152 10.1073/pnas.1618293114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raman S., Beilschmidt M., To M., Lin K., Lui F., Jmeian Y. et al. (2019) Structure-guided design fine-tunes pharmacokinetics, tolerability, and antitumor profile of multispecific frizzled antibodies. Proc. Natl Acad. Sci. U.S.A. 116, 6812–6817 10.1073/pnas.1817246116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nile A.H., de Sousa E.M.F., Mukund S., Piskol R., Hansen S., Zhou L. et al. (2018) A selective peptide inhibitor of Frizzled 7 receptors disrupts intestinal stem cells. Nat. Chem. Biol. 14, 582–590 10.1038/s41589-018-0035-2 [DOI] [PubMed] [Google Scholar]
- 57.Janda C.Y., Dang L.T., You C., Chang J., de Lau W., Zhong Z.A. et al. (2017) Surrogate Wnt agonists that phenocopy canonical Wnt and beta-catenin signalling. Nature 545, 234–237 10.1038/nature22306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang S., Wu Y., Xu T.H., de Waal P.W., He Y., Pu M. et al. (2018) Crystal structure of the Frizzled 4 receptor in a ligand-free state. Nature 560, 666–670 10.1038/s41586-018-0447-x [DOI] [PubMed] [Google Scholar]
- 59.Byrne E.F.X., Sircar R., Miller P.S., Hedger G., Luchetti G., Nachtergaele S. et al. (2016) Structural basis of smoothened regulation by its extracellular domains. Nature 535, 517–522 10.1038/nature18934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deshpande I., Liang J., Hedeen D., Roberts K.J., Zhang Y., Ha B. et al. (2019) Smoothened stimulation by membrane sterols drives Hedgehog pathway activity. Nature 571, 284–288 10.1038/s41586-019-1355-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hedger G., Koldso H., Chavent M., Siebold C., Rohatgi R. and Sansom M.S.P. (2019) Cholesterol interaction sites on the transmembrane domain of the hedgehog signal transducer and class F G protein-coupled receptor smoothened. Structure 27, 549–559 10.1016/j.str.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang P., Nedelcu D., Watanabe M., Jao C., Kim Y., Liu J. et al. (2016) Cellular cholesterol directly activates smoothened in hedgehog signaling. Cell 166, 1176–1187 10.1016/j.cell.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qi X., Liu H., Thompson B., McDonald J., Zhang C. and Li X. (2019) Cryo-EM structure of oxysterol-bound human smoothened coupled to a heterotrimeric Gi. Nature 571, 279–283 10.1038/s41586-019-1286-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang C., Wu H., Evron T., Vardy E., Han G.W., Huang X.P. et al. (2014) Structural basis for smoothened receptor modulation and chemoresistance to anticancer drugs. Nat. Commun. 5, 4355 10.1038/ncomms5355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang C., Wu H., Katritch V., Han G.W., Huang X.P., Liu W. et al. (2013) Structure of the human smoothened receptor bound to an antitumour agent. Nature 497, 338–343 10.1038/nature12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X., Zhao F., Wu Y., Yang J., Han G.W., Zhao S. et al. (2017) Crystal structure of a multi-domain human smoothened receptor in complex with a super stabilizing ligand. Nat. Commun. 8, 15383 10.1038/ncomms15383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wright S.C., Cañizal M.C.A., Benkel T., Simon K., Le Gouill C., Matricon P. et al. (2018) FZD5 is a Gαq-coupled receptor that exhibits the functional hallmarks of prototypical GPCRs. Sci. Signal. 11, eaar5536 10.1126/scisignal.aar5536 [DOI] [PubMed] [Google Scholar]
- 68.Cheng Z., Biechele T., Wei Z., Morrone S., Moon R.T., Wang L. et al. (2011) Crystal structures of the extracellular domain of LRP6 and its complex with DKK1. Nat. Struct. Mol. Biol. 18, 1204–1210 10.1038/nsmb.2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen S., Bubeck D., MacDonald B.T., Liang W.X., Mao J.H., Malinauskas T. et al. (2011) Structural and functional studies of LRP6 ectodomain reveal a platform for Wnt signaling. Dev. Cell 21, 848–861 10.1016/j.devcel.2011.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahn V.E., Chu M.L., Choi H.J., Tran D., Abo A. and Weis W.I. (2011) Structural basis of Wnt signaling inhibition by Dickkopf binding to LRP5/6. Dev. Cell 21, 862–873 10.1016/j.devcel.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matoba K., Mihara E., Tamura-Kawakami K., Miyazaki N., Maeda S., Hirai H. et al. (2017) Conformational freedom of the LRP6 ectodomain is regulated by N-glycosylation and the binding of the Wnt antagonist Dkk1. Cell Rep. 18, 32–40 10.1016/j.celrep.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 72.Bourhis E., Wang W., Tam C., Hwang J., Zhang Y., Spittler D. et al. (2011) Wnt antagonists bind through a short peptide to the first beta-propeller domain of LRP5/6. Structure 19, 1433–1442 10.1016/j.str.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 73.Gammons M.V., Rutherford T.J., Steinhart Z., Angers S. and Bienz M. (2016) Essential role of the dishevelled DEP domain in a Wnt-dependent human-cell-based complementation assay. J. Cell Sci. 129, 3892–3902 10.1242/jcs.195685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tauriello D.V., Jordens I., Kirchner K., Slootstra J.W., Kruitwagen T., Bouwman B.A. et al. (2012) Wnt/beta-catenin signaling requires interaction of the dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc. Natl Acad. Sci. U.S.A. 109, E812–E820 10.1073/pnas.1114802109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu A., Xing Y., Harrison S.C. and Kirchhausen T. (2010) Structural analysis of the interaction between Dishevelled2 and clathrin AP-2 adaptor, a critical step in noncanonical Wnt signaling. Structure 18, 1311–1320 10.1016/j.str.2010.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kan W., Enos M.D., Korkmazhan E., Muennich S., Chen D.H., Gammons M.V. et al. (2020) Limited dishevelled/Axin oligomerization determines efficiency of Wnt/beta-catenin signal transduction. eLife 9, e55015 10.7554/eLife.55015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamanishi K., Sin Y., Terawaki S.I., Higuchi Y. and Shibata N. (2019) High-resolution structure of a Y27W mutant of the Dishevelled2 DIX domain. Acta Crystallogr. F Struct. Biol. Commun. 75, 116–122 10.1107/S2053230X18018290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Madrzak J., Fiedler M., Johnson C.M., Ewan R., Knebel A., Bienz M. et al. (2015) Ubiquitination of the dishevelled DIX domain blocks its head-to-tail polymerization. Nat. Commun. 6, 6718 10.1038/ncomms7718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Y.T., Dan Q.J., Wang J., Feng Y., Chen L., Liang J. et al. (2011) Molecular basis of Wnt activation via the DIX domain protein Ccd1. J. Biol. Chem. 286, 8597–8608 10.1074/jbc.M110.186742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheyette B.N., Waxman J.S., Miller J.R., Takemaru K., Sheldahl L.C., Khlebtsova N. et al. (2002) Dapper, a dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev. Cell 2, 449–461 10.1016/S1534-5807(02)00140-5 [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y., Appleton B.A., Wiesmann C., Lau T., Costa M., Hannoush R.N. et al. (2009) Inhibition of Wnt signaling by dishevelled PDZ peptides. Nat. Chem. Biol. 5, 217–219 10.1038/nchembio.152 [DOI] [PubMed] [Google Scholar]
- 82.Wong H.C., Bourdelas A., Krauss A., Lee H.J., Shao Y., Wu D. et al. (2003) Direct binding of the PDZ domain of dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol. Cell 12, 1251–1260 10.1016/S1097-2765(03)00427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee H.J., Wang N.X., Shi D.L. and Zheng J.J. (2009) Sulindac inhibits canonical Wnt signaling by blocking the PDZ domain of the protein dishevelled. Angew. Chem. Int. Ed. Engl. 48, 6448–6452 10.1002/anie.200902981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wong H.C., Mao J., Nguyen J.T., Srinivas S., Zhang W., Liu B. et al. (2000) Structural basis of the recognition of the dishevelled DEP domain in the Wnt signaling pathway. Nat. Struct. Biol. 7, 1178–1184 10.1038/82047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gammons M.V., Renko M., Johnson C.M., Rutherford T.J. and Bienz M. (2016) Wnt signalosome assembly by DEP domain swapping of dishevelled. Mol. Cell 64, 92–104 10.1016/j.molcel.2016.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamanishi K., Fiedler M., Terawaki S.I., Higuchi Y., Bienz M. and Shibata N. (2019) A direct heterotypic interaction between the DIX domains of dishevelled and axin mediates signaling to beta-catenin. Sci. Signal. 12, eaaw5505 10.1126/scisignal.aaw5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwarz-Romond T., Fiedler M., Shibata N., Butler P.J., Kikuchi A., Higuchi Y. et al. (2007) The DIX domain of dishevelled confers Wnt signaling by dynamic polymerization. Nat. Struct. Mol. Biol. 14, 484–492 10.1038/nsmb1247 [DOI] [PubMed] [Google Scholar]
- 88.Spink K.E., Polakis P. and Weis W.I. (2000) Structural basis of the axin-adenomatous polyposis coli interaction. EMBO J. 19, 2270–2279 10.1093/emboj/19.10.2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dajani R., Fraser E., Roe S.M., Yeo M., Good V.M., Thompson V. et al. (2003) Structural basis for recruitment of glycogen synthase kinase 3beta to the axin-APC scaffold complex. EMBO J. 22, 494–501 10.1093/emboj/cdg068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xing Y., Clements W.K., Le Trong I., Hinds T.R., Stenkamp R., Kimelman D. et al. (2004) Crystal structure of a beta-catenin/APC complex reveals a critical role for APC phosphorylation in APC function. Mol. Cell 15, 523–533 10.1016/j.molcel.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 91.Morrone S., Cheng Z., Moon R.T., Cong F. and Xu W. (2012) Crystal structure of a Tankyrase-Axin complex and its implications for axin turnover and Tankyrase substrate recruitment. Proc. Natl Acad. Sci. U.S.A. 109, 1500–1505 10.1073/pnas.1116618109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xing Y., Takemaru K., Liu J., Berndt J.D., Zheng J.J., Moon R.T. et al. (2008) Crystal structure of a full-length beta-catenin. Structure 16, 478–487 10.1016/j.str.2007.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huber A.H., Nelson W.J. and Weis W.I. (1997) Three-dimensional structure of the armadillo repeat region of beta-catenin. Cell 90, 871–882 10.1016/S0092-8674(00)80352-9 [DOI] [PubMed] [Google Scholar]
- 94.Ha N.C., Tonozuka T., Stamos J.L., Choi H.J. and Weis W.I. (2004) Mechanism of phosphorylation-dependent binding of APC to beta-catenin and its role in beta-catenin degradation. Mol. Cell 15, 511–521 10.1016/j.molcel.2004.08.010 [DOI] [PubMed] [Google Scholar]
- 95.Spink K E., Fridman S.G. and Weis W.I. (2001) Molecular mechanisms of beta-catenin recognition by adenomatous polyposis coli revealed by the structure of an APC-beta-catenin complex. EMBO J. 20, 6203–6212 10.1093/emboj/20.22.6203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xing Y., Clements W.K., Kimelman D. and Xu W. (2003) Crystal structure of a beta-catenin/axin complex suggests a mechanism for the beta-catenin destruction complex. Genes Dev. 17, 2753–2764 10.1101/gad.1142603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Daniels D.L. and Weis W.I. (2002) ICAT inhibits beta-catenin binding to Tcf/Lef-family transcription factors and the general coactivator p300 using independent structural modules. Mol. Cell 10, 573–584 10.1016/S1097-2765(02)00631-7 [DOI] [PubMed] [Google Scholar]
- 98.Wu G., Xu G., Schulman B.A., Jeffrey P.D., Harper J.W. and Pavletich N.P. (2003) Structure of a beta-TrCP1-Skp1-beta-catenin complex: destruction motif binding and lysine specificity of the SCF(beta-TrCP1) ubiquitin ligase. Mol. Cell 11, 1445–1456 10.1016/S1097-2765(03)00234-X [DOI] [PubMed] [Google Scholar]
- 99.Simonetta K.R., Taygerly J., Boyle K., Basham S.E., Padovani C., Lou Y. et al. (2019) Prospective discovery of small molecule enhancers of an E3 ligase-substrate interaction. Nat. Commun. 10, 1402 10.1038/s41467-019-09358-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaieda S., Matsui C., Mimori-Kiyosue Y. and Ikegami T. (2010) Structural basis of the recognition of the SAMP motif of adenomatous polyposis coli by the Src-homology 3 domain. Biochemistry 49, 5143–5153 10.1021/bi100563z [DOI] [PubMed] [Google Scholar]
- 101.Zhang Z., Li H., Chen L., Lu X., Zhang J., Xu P. et al. (2011) Molecular basis for the recognition of adenomatous polyposis coli by the discs large 1 protein. PLoS ONE 6, e23507 10.1371/journal.pone.0023507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Slep K.C. (2012) Structure of the human discs large 1 PDZ2- adenomatous polyposis coli cytoskeletal polarity complex: insight into peptide engagement and PDZ clustering. PLoS ONE 7, e50097 10.1371/journal.pone.0050097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Day C.L. and Alber T. (2000) Crystal structure of the amino-terminal coiled-coil domain of the APC tumor suppressor. J. Mol. Biol. 301, 147–156 10.1006/jmbi.2000.3895 [DOI] [PubMed] [Google Scholar]
- 104.Tickenbrock L., Cramer J., Vetter I.R. and Muller O. (2002) The coiled coil region (amino acids 129–250) of the tumor suppressor protein adenomatous polyposis coli (APC). Its structure and its interaction with chromosome maintenance region 1 (Crm-1). J. Biol. Chem. 277, 32332–8 10.1074/jbc.M203990200 [DOI] [PubMed] [Google Scholar]
- 105.Zhang Z., Akyildiz S., Xiao Y., Gai Z., An Y., Behrens J. et al. (2015) Structures of the APC-ARM domain in complexes with discrete Amer1/WTX fragments reveal that it uses a consensus mode to recognize its binding partners. Cell Discov. 1, 15016 10.1038/celldisc.2015.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Z., Lin K., Gao L., Chen L., Shi X. and Wu G. (2011) Crystal structure of the armadillo repeat domain of adenomatous polyposis coli which reveals its inherent flexibility. Biochem. Biophys. Res. Commun. 412, 732–736 10.1016/j.bbrc.2011.08.044 [DOI] [PubMed] [Google Scholar]
- 107.Morishita E.C., Murayama K., Kato-Murayama M., Ishizuka-Katsura Y., Tomabechi Y., Hayashi T. et al. (2011) Crystal structures of the armadillo repeat domain of adenomatous polyposis coli and its complex with the tyrosine-rich domain of Sam68. Structure 19, 1496–1508 10.1016/j.str.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 108.Jiang H., Deng R., Yang X., Shang J., Lu S., Zhao Y. et al. (2017) Peptidomimetic inhibitors of APC-Asef interaction block colorectal cancer migration. Nat. Chem. Biol. 13, 994–1001 10.1038/nchembio.2442 [DOI] [PubMed] [Google Scholar]
- 109.Zhang Z., Chen L., Gao L., Lin K., Zhu L., Lu Y. et al. (2012) Structural basis for the recognition of Asef by adenomatous polyposis coli. Cell Res. 22, 372–386 10.1038/cr.2011.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dajani R., Fraser E., Roe S.M., Young N., Good V., Dale T.C. et al. (2001) Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 105, 721–732 10.1016/S0092-8674(01)00374-9 [DOI] [PubMed] [Google Scholar]
- 111.Sampietro J., Dahlberg C.L., Cho U.S., Hinds T.R., Kimelman D. and Xu W. (2006) Crystal structure of a beta-catenin/BCL9/Tcf4 complex. Mol. Cell 24, 293–300 10.1016/j.molcel.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 112.Graham T.A., Ferkey D.M., Mao F., Kimelman D. and Xu W. (2001) Tcf4 can specifically recognize beta-catenin using alternative conformations. Nat. Struct. Biol. 8, 1048–1052 10.1038/nsb718 [DOI] [PubMed] [Google Scholar]
- 113.Poy F., Lepourcelet M., Shivdasani R.A. and Eck M.J. (2001) Structure of a human Tcf4-beta-catenin complex. Nat. Struct. Biol. 8, 1053–1057 10.1038/nsb720 [DOI] [PubMed] [Google Scholar]
- 114.Graham T.A., Weaver C., Mao F., Kimelman D. and Xu W. (2000) Crystal structure of a beta-catenin/Tcf complex. Cell 103, 885–896 10.1016/S0092-8674(00)00192-6 [DOI] [PubMed] [Google Scholar]
- 115.Sun J. and Weis W.I. (2011) Biochemical and structural characterization of beta-catenin interactions with nonphosphorylated and CK2-phosphorylated Lef-1. J. Mol. Biol. 405, 519–530 10.1016/j.jmb.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Graham T.A., Clements W.K., Kimelman D. and Xu W. (2002) The crystal structure of the beta-catenin/ICAT complex reveals the inhibitory mechanism of ICAT. Mol. Cell 10, 563–571 10.1016/S1097-2765(02)00637-8 [DOI] [PubMed] [Google Scholar]
- 117.Love J.J., Li X., Case D.A., Giese K., Grosschedl R. and Wright P.E. (1995) Structural basis for DNA bending by the architectural transcription factor LEF-1. Nature 376, 791–795 10.1038/376791a0 [DOI] [PubMed] [Google Scholar]
- 118.de la Roche M., Rutherford T.J., Gupta D., Veprintsev D.B., Saxty B., Freund S.M. et al. (2012) An intrinsically labile alpha-helix abutting the BCL9-binding site of beta-catenin is required for its inhibition by carnosic acid. Nat. Commun. 3, 680 10.1038/ncomms1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miller T.C., Mieszczanek J., Sanchez-Barrena M.J., Rutherford T.J., Fiedler M. and Bienz M. (2013) Evolutionary adaptation of the fly Pygo PHD finger toward recognizing histone H3 tail methylated at arginine 2. Structure 21, 2208–2220 10.1016/j.str.2013.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fiedler M., Sanchez-Barrena M.J., Nekrasov M., Mieszczanek J., Rybin V., Muller J. et al. (2008) Decoding of methylated histone H3 tail by the Pygo-BCL9 Wnt signaling complex. Mol. Cell 30, 507–518 10.1016/j.molcel.2008.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miller T.C., Rutherford T.J., Johnson C.M., Fiedler M. and Bienz M. (2010) Allosteric remodelling of the histone H3 binding pocket in the Pygo2 PHD finger triggered by its binding to the B9L/BCL9 co-factor. J. Mol. Biol. 401, 969–984 10.1016/j.jmb.2010.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miller T.C., Rutherford T.J., Birchall K., Chugh J., Fiedler M. and Bienz M. (2014) Competitive binding of a benzimidazole to the histone-binding pocket of the Pygo PHD finger. ACS Chem. Biol. 9, 2864–2874 10.1021/cb500585s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pickles L.M., Roe S.M., Hemingway E.J., Stifani S. and Pearl L.H. (2002) Crystal structure of the C-terminal WD40 repeat domain of the human Groucho/TLE1 transcriptional corepressor. Structure 10, 751–761 10.1016/S0969-2126(02)00768-2 [DOI] [PubMed] [Google Scholar]
- 124.Jennings B.H., Pickles L.M., Wainwright S.M., Roe S.M., Pearl L.H. and Ish-Horowicz D. (2006) Molecular recognition of transcriptional repressor motifs by the WD domain of the Groucho/TLE corepressor. Mol. Cell 22, 645–655 10.1016/j.molcel.2006.04.024 [DOI] [PubMed] [Google Scholar]
- 125.Chodaparambil J.V., Pate K.T., Hepler M.R., Tsai B.P., Muthurajan U.M., Luger K. et al. (2014) Molecular functions of the TLE tetramerization domain in Wnt target gene repression. EMBO J. 33, 719–731 10.1002/embj.201387188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang H., Wang Z., Tang Q., Yan X.X. and Xu W. (2019) Crystal structure of the LUFS domain of human single-stranded DNA binding protein 2 (SSBP2). Protein Sci. 28, 788–793 10.1002/pro.3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang H., Kim J., Wang Z., Yan X.X., Dean A. and Xu W. (2020) Crystal structure of human LDB1 in complex with SSBP2. Proc. Natl Acad. Sci. U.S.A. 117, 1042–1048 10.1073/pnas.1914181117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yan L., Xie S., Du Y. and Qian C. (2017) Structural insights into BAF47 and BAF155 complex formation. J. Mol. Biol. 429, 1650–1660 10.1016/j.jmb.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 129.Allen M.D., Bycroft M. and Zinzalla G. (2020) Structure of the BRK domain of the SWI/SNF chromatin remodeling complex subunit BRG1 reveals a potential role in protein-protein interactions. Protein Sci. 29, 1047–1053 10.1002/pro.3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Valencia A.M., Collings C.K., Dao H.T., St Pierre R., Cheng Y.C., Huang J. et al. (2019) Recurrent SMARCB1 mutations reveal a nucleosome acidic patch interaction site that potentiates mSWI/SNF complex chromatin remodeling. Cell 179, 1342–56.e23 10.1016/j.cell.2019.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Farnaby W., Koegl M., Roy M.J., Whitworth C., Diers E., Trainor N. et al. (2019) BAF complex vulnerabilities in cancer demonstrated via structure-based PROTAC design. Nat. Chem. Biol. 15, 672–680 10.1038/s41589-019-0294-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Papillon J.P.N., Nakajima K., Adair C.D., Hempel J., Jouk A.O., Karki R.G. et al. (2018) Discovery of orally active inhibitors of brahma homolog (BRM)/SMARCA2 ATPase activity for the treatment of brahma related gene 1 (BRG1)/SMARCA4-mutant cancers. J. Med. Chem. 61, 10155–10172 10.1021/acs.jmedchem.8b01318 [DOI] [PubMed] [Google Scholar]
- 133.Lolli G. and Caflisch A. (2016) High-Throughput fragment docking into the BAZ2B bromodomain: efficient in silico screening for X-ray crystallography. ACS Chem. Biol. 11, 800–807 10.1021/acschembio.5b00914 [DOI] [PubMed] [Google Scholar]
- 134.He S., Wu Z., Tian Y., Yu Z., Yu J., Wang X. et al. (2020) Structure of nucleosome-bound human BAF complex. Science 367, 875–881 10.1126/science.aaz9761 [DOI] [PubMed] [Google Scholar]