Abstract
Graft-versus-host disease (GVHD) is the primary cause of non-relapse-related morbidity and mortality in patients who undergo hematopoietic stem cell transplantation. Dermatologic manifestations are common in both acute and chronic GVHD. In the acute setting, skin involvement often provides the first indication of GVHD and may progress to erythroderma and even skin necrolysis. In the chronic phase, skin involvement is extraordinarily polymorphic, potentially involves all layers of the skin and subcutaneous tissue, and presents in sclerotic and nonsclerotic forms. Management of cutaneous disease is challenging and ideally employs a multi-disciplinary approach and an understanding of the multiple medical issues facing patients with GVHD. The dermatologist plays a key role in caring for the patient with GVHD by providing an accurate diagnosis, determination of disease activity and response to treatment, and appropriate consideration of all available treatment modalities, including topical, systemic, and physical interventions (e.g. phototherapy, extracorporeal photopheresis). This chapter describes the cutaneous manifestations of acute and chronic GVHD and provides an evidence- based review of current treatment interventions for patients with GVHD skin disease.
Graft-versus-host disease (GVHD) affects approximately 50% of patients who undergo allogeneic hematopoietic stem cell transplantation (HSCT) and is the leading cause of non-relapse-related mortality. GVHD has been traditionally divided into acute and chronic manifestations based on clinical presentation and time of disease onset (i.e. before or after 100 days after transplant); however, evolving HSCT regimens, including non-myeloablative conditioning and donor lymphocyte infusion (DLI), the transfer of additional hematopoietic cells after transplant, have altered the timing of classic acute and chronic disease manifestations. Careful clinical classification is now the predominant factor in disease staging [1].
Acute Graft-versus-Host Disease
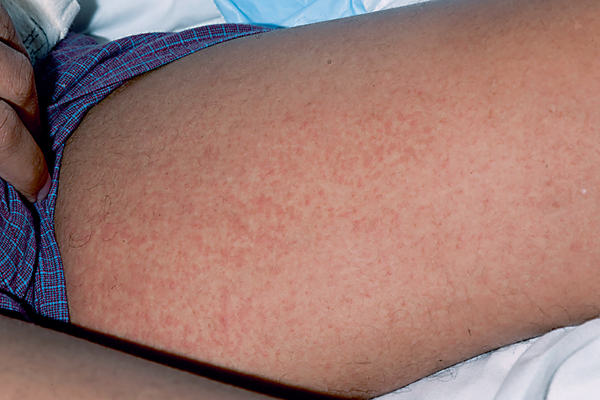
Acute GVHD (aGVHD) occurs in approximately 40% of patients who undergo allogeneic HSCT [2, 3], typically 2–4 weeks after transplant. Late-onset aGVHD (after 100 days) may also occur following reduced intensity or nonmyeloablative preparatory regimens and following DLI. aGVHD primarily affects the skin, gastrointestinal tract, and liver. Frequently, skin involvement is the first sign of aGVHD, and most commonly presents as a rapid-onset, symmetric, morbilliform exanthem (fig. 1) or folliculocentric erythematous papules [4]. Initially, aGVHD may be most prominent on the acral surfaces, particularly the palms, and then extend over the upper trunk and lateral neck, face, and ears. Eruption of lymphocyte recovery and toxic erythema of chemotherapy may also occur in the first several weeks following HSCT, and can be difficult to distinguish from aGVHD. Rarely, aGVHD may progress to erythroderma, bullae formation, or necrolysis, resembling Stevens-Johnson syndrome/toxic epidermal necrolysis.
Fig. 1.
aGVHD. Morbilliform eruption on the medial thigh.
The characteristic histologic finding of aGVHD of the skin is an interface dermatitis with vacuolar change, particularly at the tips of the rete ridges. Similar changes may be present at the upper portions of eccrine and follicular structures. Histologic differentiation of aGVHD from viral exanthem and eruption of lymphocyte recovery can be challenging. In histologic grade I aGVHD, subtle vacuolization of the basal layer is seen. Grade II aGVHD is distinguished by keratinocyte apoptosis and satellitosis. Grades III and IV are characterized by subepidermal clefting and dermal-epidermal separation, respectively [3].
Clinical correlation is integral to the rapid and accurate diagnosis of aGVHD (table 1). Voluminous, watery diarrhea is suggestive of acute gastrointestinal involvement. Associated symptoms include abdominal pain, vomiting, or anorexia. Elevated total bilirubin is a marker of hepatic involvement, but may be difficult to differentiate from other causes of liver dysfunction, including drug hypersensitivity and viral infection. The prognosis for patients with aGVHD is determined by the overall severity of skin, hepatic, and gastrointestinal involvement. Acute skin GVHD is staged as follows: (1) <25% body surface area (BSA); (2) 25–50% BSA; (3) >50% BSA or erythroderma, and (4) erythroderma with bullae. Similar staging of hepatic and gastrointestinal aGVHD based on bilirubin elevation, quantity of diarrhea and presence of pain/ileus is used to determine an overall aGVHD grade. Grade III and IV aGVHD is associated with approximately 31% and 9% 5-year survival, respectively [3, 5].
Table 1.
Clinical manifestations of aGVHD
| Skin | Erythema of palms, soles, ears Perifollicular erythema Generalized exanthem Bullae/necrolysis |
| Liver | Endothelialitis Pericholangitis Cholestasis (elevation of total bilirubin ≥2 mg/dl) Lymphocytic infiltration of portal system |
| Gastrointestinal tract | Upper: mucositis, vomiting, abdominal pain, anorexia Lower: secretory diarrhea (≥500 ml) |
Chronic Graft-versus-Host Disease
Chronic GVHD (cGVHD) develops in approximately 30–70% of patients who undergo allogeneic HSCT. The median onset is 4–6 months following HSCT and symptoms usually present within 3 years of transplantation [1, 6]. cGVHD may evolve from aGVHD (progressive), occur following resolution of aGVHD (quiescent), or arise de novo. Similar to aGVHD, chronic disease may result in significant morbidity and mortality [6]. Unlike aGVHD, however, the pathophysiology behind cGHVD is less well understood. Patients often demonstrate features of autoimmunity with symptoms similar to well-characterized autoimmune diseases such as scleroderma and Sjögren’s syndrome. Recently reported autoantibodies found in cGVHD patients provide further evidence for dysfunctional humoral immunity [7, 8]. Risk factors associated with cGVHD include history of aGVHD, older age at time of HSCT, HLA-antigen mismatch, female donor and male recipient, DLIs, and peripheral blood as the stem cell source [9].
Nearly any organ system may be affected by cGVHD, and 50% of patients will have involvement of three or more organ systems [6]. The skin is the most common target for cGVHD, but it varies markedly in clinical presentation. A National Institutes of Health consensus effort led to a set of proposed cGVHD disease definitions [1]. Diagnostic manifestations are those that are sufficiently unique to cGVHD to render the diagnosis, whereas distinctive manifestations require additional histologic or laboratory confirmation (table 2). According to the National Institutes of Health criteria, diagnosis of cGVHD requires: (1) at least one diagnostic sign of cGVHD, or the presence of at least 1 distinctive manifestation confirmed by biopsy or relevant tests, and (2) exclusion of other diagnoses, including aGVHD.
Table 2.
Common clinical manifestations of cGVHD
| Organ/system | Sign/symptom |
|---|---|
| Skin |
Poikiloderma Lichen planus-like disease Lichen sclerosus-like disease Superficial morphea-like disease Scleroderma-like disease Subcutaneous fibrosis/fasciitis Erythema Hypo-/hyperpigmentation Ichthyosis Keratosis pilaris-like eruption Maculopapular rash Pruritus Sweat impairment |
| Scalp and body hair | Alopecia (scarring or nonscarring) |
| Nails | Pterygium unguis Splitting, ridging, brittle nails Onycholysis |
| Eyes | Dry eyes Cicatricial conjunctivitis |
| Oral | Xerostomia White reticulated plaques Restriction of mouth opening Ulceration |
| Genitalia |
Lichen planus-like disease Vaginal sclerosis Erosions |
| Lung | Bronchiolitis obliterans |
| Gastrointestinal | Dysphagia Esophageal webbing Esophageal stenosis or strictures, upper to mid-third |
| Liver | Elevated total bilirubin, alkaline phosphatase, ALT, AST |
| Musculoskeletal |
Fasciitis Joint stiffness, contractures Edema |
| Hematologic | Thrombocytopenia Venous thromboembolism |
Diagnostic criteria (sufficient to establish diagnosis of cGVHD) in bold. Adapted from Filipovich et al. [1].
Chronic Graft-versus-Host Disease Skin and Mucosal Manifestations
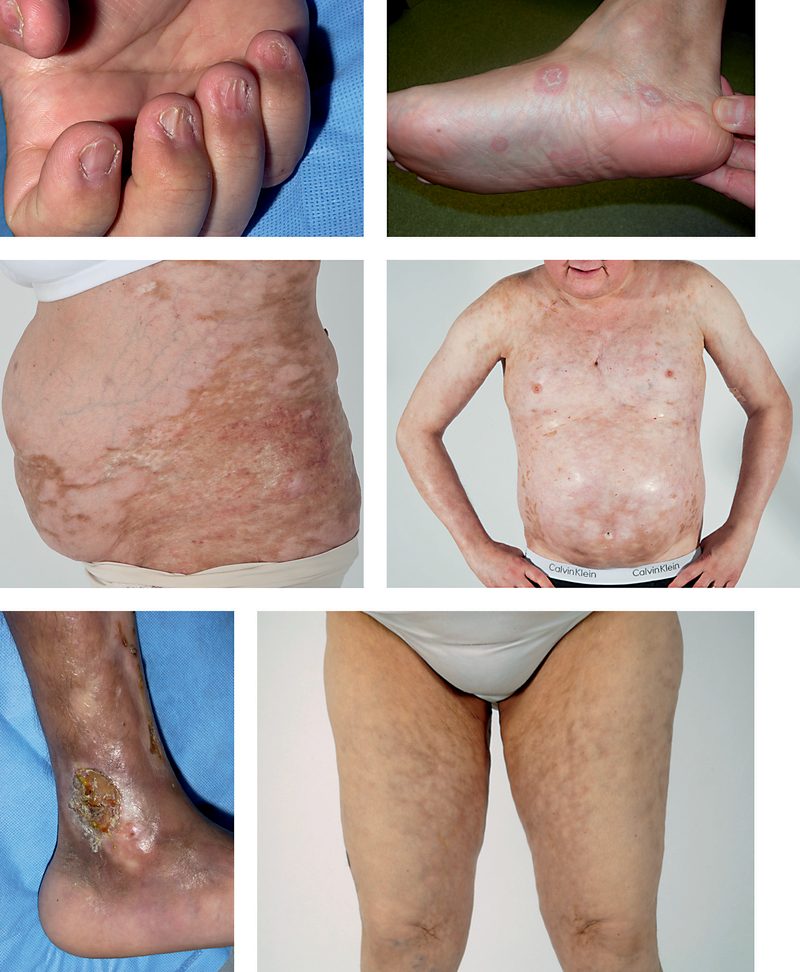
Diagnostic skin manifestations of cGVHD include poikiloderma, lichen planus-like, lichen-sclerosus-like, morphea-like eruptions, as well as scleroderma-like disease and subcutaneous fibrosis/fasciitis (fig. 2b–f). Approximately 10% of patients with cGVHD develop sclerotic skin involvement [10]. Sclerotic changes in the skin may occur at any level from the upper dermis to the muscle fascia. Involvement of the upper dermis results in well-circumscribed hyperpigmented plaques of superficial morphea or gray-white atrophic plaques of lichen sclerosus. Diffuse sclerotic involvement resembles scleroderma [10]. Fibrosis of the subcutaneous fat and/or fascia causes skin, particularly of the medial arms or thighs, to ‘ripple’ and ‘dimple,’ resembling eosinophilic fasciitis. Fascial disease can also manifest as a ‘groove sign’ – linear depressions between muscle groups or along the course of superficial blood vessels. New-onset limb edema may portend sclerotic skin involvement, or fascial involvement may occur insidiously, leading to range of motion deficits and joint contractures without skin changes. If sclerotic changes are suspected, evaluation of joint range of motion in the affected area is recommended to follow progression of disease. Magnetic resonance imaging may aid in the diagnosis of fascial involvement [11].
Fig. 2.
The spectrum of chronic cutaneous GVHD. a Nail thinning, ridging and micronychia. b Lichen planus-like plaques. c Morphea-like sclerotic changes on the abdomen and flank. d Extensive sclerosis of the chest and abdomen with dyspigmentation. e Scleroderma-like skin fibrosis of the distal ankle with ulceration. f Subcutaneous/fascial cGVHD, rippling of the skin of the medial thighs with overlying hyperpigmentation.
Other less specific cGVHD skin changes include pruritus, decreased sweating, erythema and maculopapular rash. Nail changes include longitudinal ridging, splitting, brittle features, pterygium and partial or complete nail loss (fig. 2a). In the setting of non-diagnostic cutaneous signs or symptoms after HSCT, alternative dermatologic diagnoses should be considered. Drug eruptions and viral exanthems may mimic cGVHD, or may also induce a flare of cutaneous cGVHD. Phototoxicity from voriconazole, an antifungal agent commonly employed in the HSCT setting, should also be considered in the differential diagnosis of a cGVHD flare [12]. Dermatologic exam should also include evaluation for genital and oral mucosal cGVHD. In a prospective study, 49% of women manifested vulvo-vaginal involvement 2 years after transplant [13]. Women may describe dryness, vulvodynia, pruritus, or dyspareunia. Diagnostic findings include vaginal scarring/stenosis and lichen planus-like features. Erosions, fissures and ulcers are also common. Involvement of the oral cavity is also common in cGVHD and manifests as erythema, lichenoid changes, xerostomia, ulcers and/or mucoceles [14]. Oral cGVHD may resemble oral lichen planus with lacy white reticular plaques. Perioral sclerosis may result in a restricted ability to open the mouth. Candidiasis may result from salivary gland dysfunction.
Histologic findings of cGVHD reflect the variable clinical presentation of the disease. Lichen planus-like cGVHD displays satellitosis and vacuolization of the epidermal basal layer, reminiscent of aGVHD [15]. Lichen sclerosus-like GVHD, morphea-like cGVHD, and cGVHD fascial involvement are reflected by thickening, homogenization and compaction of collagen in the papillary dermis, reticular dermis, or fascial tissue, respectively.
Weight loss, diarrhea, dyspnea, ocular sicca, and fatigue are other features suggestive of active cGVHD. Nausea, vomiting, anorexia, or dysphagia, from esophageal webbing and strictures may indicate cGVHD gastrointestinal disease. In the lungs, cGVHD can manifest as bronchiolitis obliterans syndrome, with dry cough, wheezing, and dyspnea. Hematologic abnormalities include lymphopenia, eosinophilia, and hyper- or hypogammaglobulinemia.
Therapy
The following section provides an overview of therapies for GVHD that might be utilized under a dermatologist’s care. Phototherapy and extracorporeal photopheresis will be reviewed separately in the chapter by Greinix and Tanew [this vol., pp. 116–131]. Treatment for cGVHD of the skin can be divided into skin-directed (including phototherapy) and systemic interventions. Systemic intervention may be chosen based on the severity of skin involvement, multiple organ involvement, or lack of response to skin-directed therapy. Other important considerations are risk of malignancy relapse, risk of infection, and rate of cGVHD disease progression.
Topical Therapy
Topical corticosteroids are first-line therapy for limited chronic cutaneous, oral and mucosal cGVHD, and may also be useful for symptomatic relief of mild cutaneous aGVHD. Medium-high potency topical corticosteroids provide relief for superficial skin changes such as a lichen planus-like rash or pruritus symptoms and avoid interference with graft-versus-malignancy effects. Unfortunately, topical corticosteroids may provide only short-term relief, and may lead to poor wound healing, skin atrophy, striae formation, and increased risk of local infection. Therefore it is recommended that topical medications only be used for short periods and withdrawn or tapered following symptom relief [16].
The nonsteroidal topical immunomodulators, tacrolimus and pimicrolimus are particularly beneficial for sites at risk of skin atrophy with topical steroids. Choi and Nghiem [17] described a cGVHD response to tacrolimus 0.01% in 13/18 (72%) patients. Topical tacrolimus has also been used to treat ocular and oral GVHD symptoms [18–23]. Pimecrolimus has similarly been reported in several case reports [23, 24]. Topical calcineurin inhibitor therapy is generally well tolerated; however, systemic absorption has been reported in GVHD patients following use on mucosal surfaces and in the pediatric setting [21].
Systemic Therapy
Corticosteroids
Corticosteroids are first-line therapy for both aGVHD and cGVHD. Prednisone 1 mg/kg/day is commonly used for aGVHD [25]. Higher-dose steroid regimens (e.g. 2 mg/kg/day) were not found to provide additional benefit in grade I–II aGVHD [26]. Doses less than 1 mg/kg/day are sometimes used in practice, but evidence is lacking in clinical trials [25]. Corticosteroids are effective for approximately 50% of patients with aGVHD, particularly those with skin-limited disease [27]. For cGVHD, a common regimen is 1 mg/kg/day for 2 weeks, followed by reducing dosage over a period of 6–8 weeks [25]. Most patients who will respond to steroid therapy will do so within 3 months [28]. Alternate day steroid dosing is commonly employed to minimize steroid-induced adverse effects; however, there have been no randomized studies comparing daily versus alternate day dosing. Side effects of systemic corticosteroids in the transplant setting include bone density loss, avascular necrosis of bone, and diabetes mellitus. Because prolonged treatment is often required for control of GVHD, steroid-sparing agents are commonly employed. However, there is no single steroid alternative therapy that has demonstrated proven superiority.
Tacrolimus and Cyclosporine
The systemic calcineurin inhibitors tacrolimus and cyclosporine are used for GVHD prophylaxis as well as for active aGVHD [27]. Although they are frequently employed with systemic steroids for cGVHD, the evidence supporting their additive benefit in this setting is limited [25]. In a nonrandomized trial, complete response and 4-year survival rates of 33 and 51%, respectively, were observed in the CsA/prednisone group, compared to 16 and 26% with prednisone alone [29]. In contrast, a randomized trial of 287 patients found no difference in overall survival [30]. However, treatment-related mortality, recurrent malignancy, need for secondary therapy and/or immunosuppression, avascular necrosis, and disease-free survival were significantly improved in the CsA/prednisone cohort. Important complications of calcineurin inhibitor treatment include renal insufficiency, hypertension and infection.
Mycophenolate Mofetil
Mycophenolate mofetil (MMF) is an inhibitor of purine synthesis via the inosine monophosphate dehydrogenase enzyme, and preferentially suppresses proliferation of B and T cells. It is associated with dose-dependent cytopenias and gastrointestinal adverse effects such as upper and lower enteritis. Small retrospective trials for aGVHD and cGVHD have demonstrated partial or complete response in the majority of patients [31–34]. However, in a multicenter, double-blind, trial comparing MMF to standard immunosuppressive therapy for initial treatment of cGVHD, no difference was found in treatment success (p = 0.22) between the two arms, and in fact, the cumulative incidence of treatment failure appeared to be higher in the MMF group (p = 0.03) [35].
Sirolimus
Sirolimus, or rapamycin, is a bacterial macrolide antibiotic with immunosuppressive properties and antiproliferative effects on fibroblasts and smooth muscle cells. It inhibits the mammalian target of rapamycin, which leads to downregulation of DNA transcription, translation and protein synthesis [36]. Sirolimus may also decrease the risk of cutaneous malignancy [37, 38]. In a prospective randomized controlled trial of sirolimus versus standard immunosuppressive therapy in the renal allograft setting, 12/17 patients in the standard treatment group had progression of actinic keratoses or verruca vulgaris at 12 months, whereas no patients (0/16) in the sirolimus group had progression of skin lesions [39]. In a retrospective review of sirolimus for steroid-refractory aGVHD, improvement was seen in 20/22 patients [36]. Response rates for cGVHD range between 56 and 81% [16]. Jedlickova et al. [40] reported a 76% response in a series of 34 patients with scleroderma-like GVHD. Adverse effects include cytopenias, impaired wound healing and transplant-associated microangiopathy, which is increased with concomitant calcineurin inhibitor therapy.
Hydroxychloroquine
Hydroxychloroquine (HCQ), a 4-aminoquinolone antimalarial drug, has also been employed in cGVHD with variable success. HCQ reduces proinflammatory cytokines such as IL-1, IL-6 and tumor necrosis factor-α, and has also been shown to interfere with antigen processing and presentation and to work synergistically with the calcineurin inhibitors to suppress proliferative T-cell responses in vitro [41, 42]. In a multi-institutional phase II study, 50% of patients with cGVHD treated with HCQ 800 mg daily in addition to their existing immunosuppressive therapy experienced improvement [41]. Responses were observed most commonly in skin, oral, and liver GVHD, and platelet count. Three cases of HCQ-related neuropathy or myopathy were reported. Other common side effects are gastrointestinal symptoms and retinopathy. More recently, a double-blind, placebo-controlled trial of 800 mg daily HCQ in newly diagnosed cGVHD (18 HCQ, 24 placebo) failed to demonstrate a difference between treatment groups [43].
Rituximab
Rituximab is a chimeric mouse/human anti-CD20 antibody, which has been used for the treatment of B-cell malignancies and autoimmune disease. Recent studies have implicated B cells in the pathogenesis of cGVHD [7, 8], and rituximab is thought to modulate both the Th2 and humoral components of the disease. Therapeutic response rates of 43–86% (median 67%) have been reported for steroid-refractory cGVHD [16, 44]. Responses have been reported most frequently for skin involvement, followed by oral mucosa, liver, and lung [45].
Imatinib Mesylate
Imatinib mesylate is a multikinase inhibitor originally used for treatment of BCR ABL-positive malignancies such as chronic myelogenous leukemia. It inhibits c-Abl, a downstream signaling molecule of transforming growth factor-β (TGFβ), as well as PDGF receptors [46]. TGFβ and PDGF have been implicated in fibroblast activation, leading to accumulation of extracellular matrix characteristic of sclerotic disease [47]. Patients with extensive cGVHD were found to produce stimulatory antibodies to the PDGF receptor in one study [48]. In preclinical murine studies, imatinib prevented fibrosis induced by bleomycin and in the TSK-1 model of scleroderma [49, 50]. Successful treatment of sclerotic-type cGVHD with imatinib 100 mg/day in the clinical setting was first reported in 2008 [51]. Olivieri et al. [52] subsequently reported 19 treatment-refractory cGVHD patients with sclerotic features treated with imatinib 50–200 mg/day. At median follow-up of 17 months, the overall response rate was 63%. In a second series, 14 patients with refractory sclerotic cGVHD treated with imatinib 400 mg/day (adults) or 100 mg/day (children) demonstrated an overall response rate of 50% [53]. Side effects of imatinib include fluid retention, muscle cramps, diarrhea, and bone marrow toxicity.
Thalidomide
Although its mechanisms of action are not completely understood, thalidomide is thought to inhibit expression of adhesion molecules, angiogenesis, NFκB activity, and cytokines, including tumor necrosis factor-α. It has shown some modest success in lichen planus-like cGVHD, but does not have efficacy in sclerotic disease [54, 55]. Vogelsang et al. [56] reported responses in 26/44 (59%) patients with refractory or high-risk cGVHD. In contrast, high-dose thalidomide at 200–800 mg/day resulted in an 11% response rate in a second study [55]. Another group found that thalidomide did not improve clinical response when added to a regimen of prednisone and cyclosporine [57], and use as cGVHD prophylaxis has been reported to increase risk of cGVHD [58]. Side effects of thalidomide include teratogenicity, sensory neuropathy, sedation, constipation, and granulocytopenia [27, 59].
Systemic Retinoids
Retinoids are vitamin A derivatives that induce normal differentiation of epithelial tissue, promote induction of T regulatory cells, block Th17 cell induction, and inhibit fibroblast proliferation as well as collagen production [16]. They have been used in various dermatologic contexts, including systemic sclerosis [60], but experience with GVHD is limited. A study of etretinate for sclerotic cGHVD in patients who had not responded to systemic steroids and/or cyclosporine described responses in 20/27 patients by 3 months [61]. However, ulceration of the skin and skin breakdown caused 6 patients to stop treatment. Other well-recognized side effects such as nail cracking, xerosis, hypercholesterolemia, eye irritation, chelitis, pruritus, and scaling were also common.
Mesenchymal Stem Cells
Mesenchymal stem cells (MSC) are multipotent progenitor cells found in the bone marrow and adipose tissue which are distinct from hematopoietic cells. They have generated considerable interest for their immunomodulating effects, which have been demonstrated in vivo and in vitro [62–64]. MSCs are thought to modulate T cell alloreactivity through a variety of mechanisms. Interestingly, the immunomodulatory effects of MSCs are similar regardless of whether the cells are derived from HLA-identical, partially matched or completely mismatched donors [65]. In a series of 55 patients with grade II–IV aGVHD treated with MSC, 30 (55%) had a complete response and 9 (16%) demonstrated a partial response [66]. Similarly, Weng et al. [67] demonstrated an overall skin response rate of 78% in patients with refractory cGVHD. More recently, Zhou et al. [68] demonstrated >70% improvement in Rodnan skin score, decreased symptoms, healing of ulcers and increased skin mobility in 4 patients with scleroderma-like cGVHD. To date, significant adverse events related to MSC infusion have not been reported. However, additional clinical data are needed to characterize optimal dosage, number of infusions and time intervals, MSC growth medium, origin of MSC cells (adipose versus bone marrow) and the value of HLA matching.
Supportive Care Measures
Comprehensive management of the skin issues in the post-HSCT setting requires attention to the other risks intrinsic to this population, including drug exposures, infection and skin cancer. Ultraviolet radiation, drug eruptions and systemic infections all may induce a flare of GVHD. Sun protection with chemical and/or physical blockers should be emphasized, particularly in patients taking photosensitizing antimicrobial agents, including voriconazole, which has also been associated with increased risk of squamous cell carcinoma in the immunocompromised setting [12, 69]. Patients should be educated regarding skin cancer risk and require regular surveillance for skin cancer. In addition, prompt evaluation of new areas of skin breakdown and other possible areas of skin infection are warranted.
References
- 1.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. : National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11:945–956. [DOI] [PubMed] [Google Scholar]
- 2.Hari P, Carreras J, Zhang MJ, Gale RP, Bolwell BJ, Bredeson CN, et al. : Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol Blood Marrow Transplant 2008; 14:236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara JL, Levine JE, Reddy P, Holler E: Graft-versus-host disease. Lancet 2009;373:1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hausermann P, Walter RB, Halter J, Biedermann BC, Tichelli A, Itin P, et al. : Cutaneous graft-versus-host disease: a guide for the dermatologist. Dermatology 2008;216:287–304. [DOI] [PubMed] [Google Scholar]
- 5.Gratwohl A, Hermans J, Apperley J, Arcese W, Bacigalupo A, Bandini G, et al. : Acute graft-versus-host disease: grade and outcome in patients with chronic myelogenous leukemia. Working Party Chronic Leukemia of the European Group for Blood and Marrow Transplantation. Blood 1995;86:813–818. [PubMed] [Google Scholar]
- 6.Lee SJ: Have we made progress in the management of chronic graft-vs-host disease? Best Pract Res Clin Haematol 2010;23:529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, et al. : Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood 2005;105:2973–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miklos DB, Kim HT, Zorn E, Hochberg EP, Guo L, Mattes-Ritz A, et al. : Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood 2004;103:353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flowers ME, Inamoto Y, Carpenter PA, Lee SJ, Kiem HP, Petersdorf EW, et al. : Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 2011;117:3214–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kroft EB, Berkhof NJ, van de Kerkhof PC, Gerritsen RM, de Jong EM: Ultraviolet A phototherapy for sclerotic skin diseases: a systematic review. J Am Acad Dermatol 2008;59:1017–1030. [DOI] [PubMed] [Google Scholar]
- 11.Clark J, Yao L, Pavletic SZ, Krumlauf M, Mitchell S, Turner ML, et al. : Magnetic resonance imaging in sclerotic-type chronic graft-vs-host disease. Arch Dermatol 2009;145:918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel AR, Turner ML, Baird K, Gea-Banacloche J, Mitchell S, Pavletic SZ, et al. : Voriconazole-induced phototoxicity masquerading as chronic graft-versus-host disease of the skin in allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant 2009;15:370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zantomio D, Grigg AP, MacGregor L, Panek-Hudson Y, Szer J, Ayton R: Female genital tract graft-versus-host disease: incidence, risk factors and recommendations for management. Bone Marrow Transplant 2006;38:567–572. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter PA: How I conduct a comprehensive chronic graft-versus host disease assessment. Blood 2011;118:2679–2687. [DOI] [PubMed] [Google Scholar]
- 15.Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, et al. : Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. II. Pathology Working Group Report. Biol Blood Marrow Transplant 2006;12:31–47. [DOI] [PubMed] [Google Scholar]
- 16.Wolff D, Schleuning M, von Harsdorf S, Bacher U, Gerbitz A, Stadler M, et al. : Consensus conference on clinical practice in chronic GVHD: second-line treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant 2011;17:1–17. [DOI] [PubMed] [Google Scholar]
- 17.Choi CJ, Nghiem P: Tacrolimus ointment in the treatment of chronic cutaneous graft-vs-host disease: a case series of 18 patients. Arch Dermatol 2001;137:1202–1206. [DOI] [PubMed] [Google Scholar]
- 18.Riemens A, te Boome L, Imhof S, Kuball J, Rothova A: Current insights into ocular graft-versus-host disease. Curr Opin Ophthalmol 2010;21:485–494. [DOI] [PubMed] [Google Scholar]
- 19.Mawardi H, Stevenson K, Gokani B, Soiffer R, Treister N: Combined topical dexamethasone/tacrolimus therapy for management of oral chronic GVHD. Bone Marrow Transplant 2010;45:1062–1067. [DOI] [PubMed] [Google Scholar]
- 20.Tam PM, Young AL, Cheng LL, Lam PT: Topical 0.03% tacrolimus ointment in the management of ocular surface inflammation in chronic GVHD. Bone Marrow Transplant 2010;45:957–958. [DOI] [PubMed] [Google Scholar]
- 21.Prot-Labarthe S, Therrien R, Champagne MA, Duval M, Joubert C: Toxic serum levels of tacrolimus after topical administration in an infant with severe cutaneous graft-versus-host disease. Bone Marrow Transplant 2007;40:295–296. [DOI] [PubMed] [Google Scholar]
- 22.Administration FaD: Protopic (tacrolimus) ointment 0.03% Ointment 0.1%. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist.
- 23.Ziemer M, Gruhn B, Thiele JJ, Elsner P: Treatment of extensive chronic cutaneous graft-versus-host disease in an infant with topical pimecrolimus. J Am Acad Dermatol 2004;50:946–948. [DOI] [PubMed] [Google Scholar]
- 24.Schmook T, Kraft J, Benninghoff B, Nindl I, Roewert J, Ulrich C, et al. : Treatment of cutaneous chronic graft-versus-host disease with topical pimecrolimus. Bone Marrow Transplant 2005;36:87–88. [DOI] [PubMed] [Google Scholar]
- 25.Wolff D, Gerbitz A, Ayuk F, Kiani A, Hildebrandt GC, Vogelsang GB, et al. : Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biol Blood Marrow Transplant 2010;16: 1611–1628. [DOI] [PubMed] [Google Scholar]
- 26.Mielcarek M, Storer BE, Boeckh M, Carpenter PA, McDonald GB, Deeg HJ, et al. : Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood 2009;113:2888–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penas PF, Fernandez-Herrera J, Garcia-Diez A: Dermatologic treatment of cutaneous graft versus host disease. Am J Clin Dermatol 2004;5:403–416. [DOI] [PubMed] [Google Scholar]
- 28.Vogelsang GB: How I treat chronic graft-versus-host disease. Blood 2001;97:1196–1201. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan KM, Witherspoon RP, Storb R, Deeg HJ, Dahlberg S, Sanders JE, et al. : Alternating-day cyclosporine and prednisone for treatment of high-risk chronic graft-v-host disease. Blood 1988;72: 555–561. [PubMed] [Google Scholar]
- 30.Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, et al. : Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood 2002;100:48–51. [DOI] [PubMed] [Google Scholar]
- 31.Vogelsang GB, Arai S: Mycophenolate mofetil for the prevention and treatment of graft-versus-host disease following stem cell transplantation: preliminary findings. Bone Marrow Transplant 2001;27: 1255–1262. [DOI] [PubMed] [Google Scholar]
- 32.Krejci M, Doubek M, Buchler T, Brychtova Y, Vorlicek J, Mayer J: Mycophenolate mofetil for the treatment of acute and chronic steroid-refractory graft-versus-host disease. Ann Hematol 2005;84: 681–685. [DOI] [PubMed] [Google Scholar]
- 33.Takami A, Mochizuki K, Okumura H, Ito S, Suga Y, Yamazaki H, et al. : Mycophenolate mofetil is effective and well tolerated in the treatment of refractory acute and chronic graft-versus-host disease. Int J Hematol 2006;83:80–85. [DOI] [PubMed] [Google Scholar]
- 34.Onishi C, Ohashi K, Sawada T, Nakano M, Kobayashi T, Yamashita T, et al. : A high risk of life-threatening infectious complications in mycophenolate mofetil treatment for acute or chronic graft-versus-host disease. Int J Hematol 2010;91: 464–470. [DOI] [PubMed] [Google Scholar]
- 35.Martin PJ, Storer BE, Rowley SD, Flowers ME, Lee SJ, Carpenter PA, et al. : Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood 2009;113:5074–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghez D, Rubio MT, Maillard N, Suarez F, Chandesris MO, Delarue R, et al. : Rapamycin for refractory acute graft-versus-host disease. Transplantation 2009;88:1081–1087. [DOI] [PubMed] [Google Scholar]
- 37.Alberu J, Pascoe MD, Campistol JM, Schena FP, Rial MD, Polinsky M, et al. : Lower malignancy rates in renal allograft recipients converted to sirolimusbased, calcineurin inhibitor-free immunotherapy: 24-month results from the CONVERT trial. Transplantation 2011;92:303–310. [DOI] [PubMed] [Google Scholar]
- 38.Mathew T, Kreis H, Friend P: Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant 2004;18:446–449. [DOI] [PubMed] [Google Scholar]
- 39.Salgo R, Gossmann J, Schofer H, Kachel HG, Kuck J, Geiger H, et al. : Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: reduced rate of (pre-)malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. Am J Transplant 2010;10:1385–1393. [DOI] [PubMed] [Google Scholar]
- 40.Jedlickova Z, Burlakova I, Bug G, Baurmann H, Schwerdtfeger R, Schleuning M: Therapy of sclerodermatous chronic graft-versus-host disease with mammalian target of rapamycin inhibitors. Biol Blood Marrow Transplant 2010;17:657–663. [DOI] [PubMed] [Google Scholar]
- 41.Gilman AL, Chan KW, Mogul A, Morris C, Goldman FD, Boyer M, et al. : Hydroxychloroquine for the treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant 2000;6:327–334. [DOI] [PubMed] [Google Scholar]
- 42.Ziegler HK, Unanue ER: Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci USA 1982;79:175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilman AL, Schultz KR, Goldman FD, Sale GE, Krailo MD, Chen Z, et al. : Randomized trial of hydroxychloroquine for newly diagnosed chronic graft-versus-host disease in children: A Children’s Oncology Group Study. Biol Blood Marrow Transplant 2011, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D, et al. : Rituximab for steroid-refractory chronic graft-versus-host disease. Blood 2006;108: 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, Cutler C, Mohty M, Kumar A: Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Marrow Transplant 2009;15: 1005–1013. [DOI] [PubMed] [Google Scholar]
- 46.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, et al. : Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest 2004;114:1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varga J, Abraham D: Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest 2007;117: 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svegliati S, Olivieri A, Campelli N, Luchetti M, Poloni A, Trappolini S, et al. : Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood 2007;110: 237–241. [DOI] [PubMed] [Google Scholar]
- 49.Akhmetshina A, Venalis P, Dees C, Busch N, Zwerina J, Schett G, et al. : Treatment with imatinib prevents fibrosis in different preclinical models of systemic sclerosis and induces regression of established fibrosis. Arthritis Rheum 2009;60:219–224. [DOI] [PubMed] [Google Scholar]
- 50.Distler JH, Jungel A, Huber LC, Schulze-Horsel U, Zwerina J, Gay RE, et al. : Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum 2007;56:311–322. [DOI] [PubMed] [Google Scholar]
- 51.Moreno-Romero JA, Fernandez-Aviles F, Carreras E, Rovira M, Martinez C, Mascaro JM Jr: Imatinib as a potential treatment for sclerodermatous chronic graft-vs-host disease. Arch Dermatol 2008;144: 1106–1109. [DOI] [PubMed] [Google Scholar]
- 52.Olivieri A, Locatelli F, Zecca M, Sanna A, Cimminiello M, Raimondi R, et al. : Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood 2009;114:709–718. [DOI] [PubMed] [Google Scholar]
- 53.Magro L, Mohty M, Catteau B, Coiteux V, Chevallier P, Terriou L, et al. : Imatinib mesylate as salvage therapy for refractory sclerotic chronic graft-versus-host disease. Blood 2009;114:719–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Penas PF, Jones-Caballero M, Aragues M, Fernandez-Herrera J, Fraga J, Garcia-Diez A: Sclerodermatous graft-vs-host disease: clinical and pathological study of 17 patients. Arch Dermatol 2002;138:924–934. [DOI] [PubMed] [Google Scholar]
- 55.Parker PM, Chao N, Nademanee A, O’Donnell MR, Schmidt GM, Snyder DS, et al. : Thalidomide as salvage therapy for chronic graft-versus-host disease. Blood 1995;86:3604–3609. [PubMed] [Google Scholar]
- 56.Vogelsang GB, Farmer ER, Hess AD, Altamonte V, Beschorner WE, Jabs DA, et al. : Thalidomide for the treatment of chronic graft-versus-host disease. N Engl J Med 1992;326:1055–1058. [DOI] [PubMed] [Google Scholar]
- 57.Arora M, Wagner JE, Davies SM, Blazar BR, Defor T, Enright H, et al. : Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant 2001;7:265–273. [DOI] [PubMed] [Google Scholar]
- 58.Chao NJ, Parker PM, Niland JC, Wong RM, Dagis A, Long GD, et al. : Paradoxical effect of thalidomide prophylaxis on chronic graft-vs-host disease. Biol Blood Marrow Transplant 1996;2:86–92. [PubMed] [Google Scholar]
- 59.Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, et al. : Thalidomide for treatment of patients with chronic graft-versus-host disease. Blood 2000;96:3995–3996. [PubMed] [Google Scholar]
- 60.Maurice PD, Bunker CB, Dowd PM: Isotretinoin in the treatment of systemic sclerosis. Br J Dermatol 1989;121:367–374. [DOI] [PubMed] [Google Scholar]
- 61.Marcellus DC, Altomonte VL, Farmer ER, Horn TD, Freemer CS, Grant J, et al. : Etretinate therapy for refractory sclerodermatous chronic graft-versus-host disease. Blood 1999;93:66–70. [PubMed] [Google Scholar]
- 62.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. : Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30:42–48. [DOI] [PubMed] [Google Scholar]
- 63.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O: Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 2003;57:11–20. [DOI] [PubMed] [Google Scholar]
- 64.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC: Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003;75:389–397. [DOI] [PubMed] [Google Scholar]
- 65.Ringden O, Le Blanc K: Mesenchymal stem cells for treatment of acute and chronic graft-versus-host disease, tissue toxicity and hemorrhages. Best Pract Res Clin Haematol 2011;24:65–72. [DOI] [PubMed] [Google Scholar]
- 66.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. : Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008;371:1579–1586. [DOI] [PubMed] [Google Scholar]
- 67.Weng JY, Du X, Geng SX, Peng YW, Wang Z, Lu ZS, et al. : Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant 2010;45:1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou H, Guo M, Bian C, Sun Z, Yang Z, Zeng Y, et al. : Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: clinical report. Biol Blood Marrow Transplant 2010;16:403–412. [DOI] [PubMed] [Google Scholar]
- 69.Cowen EW, Nguyen JC, Miller DD, McShane D, Arron ST, Prose NS, et al. : Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J Am Acad Dermatol 2010;62:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]