Abstract
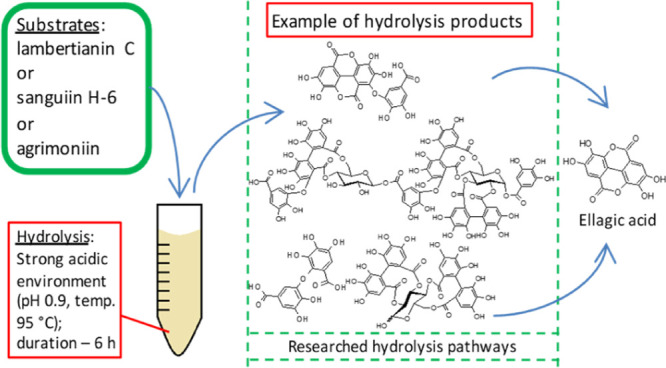
The strong acid hydrolysis analysis of galloyl-O-digalloyl-type ellagitannins (ETs), lambertianin C (LC) and sanguiin H-6, and dehydrodigalloyl-type ET, agrimoniin (AM), was performed. A quantitative and qualitative analysis of the degradation products of individual ETs was conducted using high-performance liquid chromatography–diode array detecto–electrospray ionization interface–mass spectrometry (HPLC–DAD–ESI–MS). The data indicate that ETs undergo multidirectional changes in a strongly acidic environment, where the process of successive hydrolysis of ester bonds to form ellagic acid (EA) is the dominant phenomenon in the initial phase of the reaction, followed by the depolymerization process and the formation of low-molecular ETs. Characteristic products of ET hydrolysis were distinguished: for LC: dimeric ET plus one galloyl moiety without one EA moiety (M = 1736 Da), for all analyzed ETs: sanguisorbic acid dilactone (M = 470 Da), and for AM: dehydrodigallic acid (M = 338 Da). The research carried out has allowed to create a database of possible products and routes of transformation of individual ETs, which should facilitate future research on the transformation of ETs, including potential prohealth properties of its breakdown products, under conditions occurring during food processing or digestion.
Keywords: lambertianin C, sanguiin H-6, agrimoniin, acidic hydrolysis, galloyl, sanguisorbic acid, dehydrodigallic acid, HPLC−MS
Introduction
In recent years, the interest in the beneficial activity of molecules found in unprocessed foods has increased. A polyphenol group with exceptional structures and documented health benefits are ellagitannins (ETs). One of the main sources of ETs in the diet are fruits such as pomegranates, black raspberries, raspberries, strawberries, walnuts, and almonds.1,2 Blackberry fruits are also a rich source of ETs.3−5 Sanguiin H-6 (SH-6), lambertianin C (LC), and agrimoniin (AM) are the most commonly consumed oligomeric ellagitannins (ETOMs) by humans because they are the main ETs of blackberries and raspberries3,5 as well as the strawberry fruit.6
Many of the physicochemical and biological properties of ETOMs known so far, such as anti-inflammatory and anticancer properties,7,8 result from their complex structure. In turn, the metabolism of ET in mammalian organisms and their health beneficial effects are often attributed to ellagic acid (EA),9 which is the result of the hydrolysis of native ETs. However, the EA metabolites, urolithins,10,11 resulting from the action of enzymes and microorganisms of the gastrointestinal tract, are also attributed to health beneficial effects.12 The consequence of ET hydrolysis in the gastrointestinal tract is a prolonged release of EA and its gradual degradation to urolithins, followed by absorption into the bloodstream.13,14 In addition to the aforementioned EA and its bacterial metabolites, low molecular weight ETs have a more favorable health beneficial effect compared to ETOMs as they are a better substrate for the bacterial microflora of the digestive tract to produce easily absorbed nasutins and urolithins.15
The literature to date on intermediate ET transformation products under the influence of chemical rather than biological factors is very poor. A lot of work has already been done on the transformation of ETs under the influence of biological conditions, that is, explaining their pathway during digestion by mammals, as well as by humans under the influence of enzymes and conditions in the gastrointestinal tract (native and produced by the intestinal microflora).10,13,14,16−23 In some articles, the need to supplement knowledge about ET behavior under the influence of various chemical agents is indirectly pointed out.24 It is only known that the appearance of EA, 3,4,5,3′,4′,5′-hexahydroxydiphenic acid α-d-glucoside (HHDP-glu), di- and trimers deprived of EA residues and sanguisorbic acid, resulting from ET tetramers, was recorded in a boiling environment of pure water after 24 h25 It has also been observed that ETs in a strongly alkaline medium degrade rapidly to EA.26 According to results obtained by Daniel et al.,27 the optimal environment for releasing EA from ETs found in raspberries is a medium at pH alkaline, that is, at pH 8.
As a result of acid or alkaline hydrolysis of ETs, their ester bonds break, leading to the release of 3,4,5,3′,4′,5′-hexahydroxydiphenic acid (HHDP) moieties, which then undergo spontaneous rearrangement (lactonization) to EA.28 In fruit processing, many ETs are being depolymerized or lost.29,30 However, the pathway leading from ETs to EA is far unknown. Our study was aiming to expand the knowledge on ET products that may occur during processing. The conditions in this work were stronger than normally occurring in food processing, causing higher quantity production of byproducts, which enabled us to monitor the whole process of ET transformation and properly characterize arising molecular structures. An advantage of this work, in relation to other research studies which treat on environmental factors1,31 and earlier mentioned biological factors10,13,14,21−23 affecting breakdown of ETs, is that pure ET preparations were used as substrates of hydrolysis instead of extracts containing a mixture of different ETs and other substances. Additionally, having a comprehensive database of ET hydrolysis products and their hydrolysis pathways, which this work intends to be, it will be easier to research their breakdown products during food processing or digestion in the gastrointestinal tract (regarding its different parts).
According to the terminology used in the science of polymers, three basic processes of polymer destruction are known, that is, depolymerization, degradation, and destruction. Depolymerization is understood as the process of thermal decomposition of a polymer into a monomer, which is the opposite of polymerization. Degradation is the partial breakdown of a polymer into intermediate molecular weight fragments. Destruction consists of breaking down polymer chains into low-molecular compounds other than the monomer. Polymer degradation and destruction are caused by physical factors (heat and light),32 chemical factors (redox potential),33 as well as biological factors (enzymes).34
This study investigates the depolymerization and degradation processes of ETs as natural polymers under conditions that reflect the environmental pH and temperature typical for strong hydrolysis. The additional reason to study the degree of degradation of ET polymers was that health beneficial properties of ET are attributed not only to the smallest fragments, such as EA,35 but also to monomeric ETs.15
Materials and Methods
Chemicals and Standards
For acid hydrolysis, 99% trifluoroacetic acid (TFA) ReagentPlus purchased from Sigma-Aldrich (Steinheim, Germany) was used. For ET analysis, all solvents used were of high-performance liquid chromatography (HPLC) grade. Acetonitrile, methanol, and formic acid were purchased from Sigma-Aldrich Chemie (Steinheim, Germany), and orthophosphoric acid was from Avantor Performance Materials B.V. (Deventer, Holland). Ultrapure water for HPLC–diode array detector (DAD)–electrospray ionization interface (ESI)–mass spectrometry (MS) analysis was obtained from a Hydrolab HLP5 System (Straszyn, Poland). LC and SH-6 standards from raspberries and AM standard from strawberries were prepared in our laboratory according to the procedure described in previous papers.36,37 The HPLC purity (210 nm) of ET used in these experiments was of a minimum 90%. The purity was checked at 210 nm because this wavelength is well absorbed by procyanidins, which potentially have the greatest influence on the purity of the obtained ET preparations.
Acid Hydrolysis of ETs
In the present work, a series of galloyl-O-digalloyl (GOD)-type ET (Figure S1) hydrolysis, that is, trimeric LC (Figure S2) and dimeric SH-6 (Figure S3) and dimeric dehydrodigalloyl (GOG)-type ET (Figure S1), that is, AM (Figure S4), were conducted. The first two constitute the majority of the total ET pool found in raspberries and blackberries and the last in strawberries. Hydrolysis was conducted in a 0.2 M TFA environment at pH 0.9 and a temperature of 95 °C to mimic the initial phase of the so-called strong hydrolysis using strong acid. In such conditions, depolymerization and intensive release of HHDP acid and formation of EA could be expected. Standard hydrolysis of ET for the total determination based on EA is conducted in 2 M TFA medium, 95 °C, and for at least 6 h;38 so when using 10 times lower the concentration of this acid, it was assumed that many intermediate ET hydrolysis products will be formed.
For hydrolysis, aqueous ET solutions were prepared, respectively: LC, SH-6, and AM at a concentration of about 200 mg/L. Then, concentrated TFA was added to the solutions in such an amount that its final concentration in the reaction mixture was 0.2 M (pH 0.9). The mixture prepared in an amount of 170 μL was placed with an automatic pipette in chromatographic vials with inserts and sealed with a screw cap. The samples were then placed in an incubator with a thermostat set at 95 °C. After the set time, that is, after 1, 2, 3, 4, 5, and 6 h, samples were taken out sequentially, cooled to 4 °C in an ice bath, and subjected to chromatographic analysis. Identification of ET hydrolysis products was performed by means of HPLC coupled to MS using the ESI. Quantification of the initial concentration of each ET and later, their residue after the specified time of reaction and their products of hydrolysis and/or other transformations, was carried out using a DAD coupled to the above-mentioned instrument. Analytical parameters used for quantitative analysis are available in the Supporting Information (Table S1).
HPLC–DAD–ESI–MS Analysis of ETs and Its Hydrolysis Products
The method described is the modification of the one used by Sójka et al.31 A Dionex Ultimate 3000 HPLC coupled with a DAD and Q Exactive Orbitrap MS (Thermo Fisher Scientific, Waltham, MA) was used for the identification of ET and their hydrolysis products. The solvents used for separations were as follows: solvent A: 0.4% (v/v) formic acid in water and solvent B: 90:9.6:0.4 (v/v/v) acetonitrile/water/formic acid solution. The following gradient was used: 0–4 min, 5% (v/v) B; 4–8 min, 5–15% (v/v) B; 8–29 min, 15–40% (v/v) B; 29–33 min, 40–73% (v/v) B; 33–37 min, 73% (v/v) B; 37–38 min, 73–5% (v/v) B; and 38–45 min, 5% (v/v) B. The column used was a 150 mm × 4.6 mm i.d., 3 μm, Gemini NX C18 110 Å, with a 4 mm × 3 mm i.d. guard column of the same material (Phenomenex, Torrance, CA). The column temperature was set to 35 °C, the flow rate was 0.9 mL/min, and the injection volume was 10 μL. Chromatographic data were collected using Xcalibur software (Thermo Fisher Scientific, Waltham, MA, USA). The MS system coupled to the HPLC was an Orbitrap mass spectrometer equipped with an H-ESI probe used in the negative mode. The source parameters were as follows: vaporizer temperature of 500 °C, ion spray voltage of 4 kV, capillary temperature of 400 °C, and sheath gas and auxiliary gas flow rates of 75 and 15 units, respectively. The detector was operated in either the full MS or full MS/dd-MS2 scan modes. In the full MS mode, a scan range of m/z 200–3000 was used. To generate MS2 data, the full MS/dd-MS2 scan mode was used. In this mode, the selected precursor ions entered into an HDC collision cell, where they were fragmented with normalized collision energy (NCE) to obtain product ion spectra (MS2). In these experiments, the NCE used to generate MS2 spectra was set to 30.
ETs were quantified at wavelength 250 nm, using calibration curves made by analysis of water solutions of self-prepared standards of LC, SH-6, and AM. To simplify calculations, all hydrolysis product peak areas were equated to initial ET (explained precisely in the following chapter —), and for this purpose, molar extinction coefficients from the literature were used.3 It is noticeable that the value of the molar extinction coefficient of ETs relies on a number of aromatic rings which are part of it. In case of LC, all peak areas of hydrolysis products with a nominal mass (M) of 2200 Da and higher were treated as LC; the peak area of the M = 1736 Da product was multiplied by 1.5, and peak areas of products with M lower than 1736 Da were multiplied by 2 and in the case of M = 784 Da and lower—by 3. In case of SH-6, all peak areas of hydrolysis products with M = 1266 Da and higher were treated as SH-6; peak areas of M lower than 1266 Da were multiplied by 2 and in case of M = 784 Da—by 3. In the case of AM, the same calculation method was used as in the case of SH-6. EA was quantified at wavelength 360 nm, using a calibration curve made by analysis of water–methanol solutions (30:70 v/v) of EA Sigma-Aldrich, the purchased standard of a minimum purity of 95%.
Calculation in the Process of Hydrolysis
The course of the hydrolysis process of the ETs tested was expressed as a percentage share of products formed during the hydrolysis period, calculated on the amount of initial ET reacted at a given time. This way of presenting the results was to highlight the trends occurring during the hydrolysis reaction of the ETs studied. The following formula was used for calculations, eq 1
| 1 |
where LETx is the percentage share of the particular product in the loss of initial ET. AETx is the the peak area of a product on chromatogram. AET0 is the the peak area of initial ET at zero time of hydrolysis. AET0τ is the the peak area of initial ET at a particular time of hydrolysis.
The calculations of half-life (τ1/2) for individual ETs were made based on the equations of the trend lines of their loss relative to the initial amount (%; vertical axis) in time (h; horizontal axis). The trend equations used were of third degree polynomials, and the value of the loss in time was assumed to be 50% (value substituted for y in the equations below). Calculated values of τ1/2 are shown in Table 1. The following polynomials represent the loss of individual tested ETs during their hydrolysis, respectively for LC, eq 2
| 2 |
Table 1. Most Important Parameters Characterizing the Hydrolysis of the Researched ETs in the Environment of pH 0.9 and Temperature of 95 °Ca.
| ET | Τ1/2 (h) | loss of initial ET after 6 h(%) | share of EA in loss of initial amount of ET after 6 h(%) |
|---|---|---|---|
| LC | 1.9ab ± 0.3 | 95.0a ± 1.8 | 26.6c ± 2.4 |
| SH-6 | 2.2b ± 0.3 | 85.0b ± 2.4 | 11.3a ± 0.8 |
| AM | 1.3a ± 0.1 | 95.0a ± 3.7 | 17.9b ± 1.1 |
Values in the column marked with the same letter do not differ statistically significantly at the level of p ≤ 0,05; τ1/2- half-life; the value τ1/2 was calculated on the basis of the graphs depicting the loss of the researched ETs during their hydrolysis reaction.
| 3 |
| 4 |
Statistics
All analyzes and experiments were performed in duplicates. Data included in Table 1 were statistically analyzed using one-way analysis of variance and Duncan’s test at significance level p ≤ 0.05. Calculations were made in Statistica 9 software (Statsoft, Kraków, Poland).
Results and Discussion
General Hydrolysis Parameters
The preliminary tests of hydrolysis of ETs in various conditions were carried out. Apart from pH 0.9 and 95 °C conditions, researched ETs were also exposed to pH 3 and temperature 95 °C and pH 7 and 37 °C environments. It was found that under the conditions of pH 0.9 and 95 °C, the greatest amount of individual hydrolysis products of the researched ETs was identified, that is, for LC, SH-6, and AM 25, 17, and 21, respectively (Table S2). On the basis of the collected results, it was decided to describe the results of the experiments at pH 0.9 and temp. 95 °C.
During the hydrolysis of the ET tested, the decrease in the primary substance and the gain in the products of the reaction were monitored. Throughout, the half-life was calculated for each of the ETs tested (Table 1). The data in Table 1 show that LC and AM were almost completely hydrolyzed (95% decrease after 6 h), and for SH-6, the decrease was 85%. AM because of the lowest half-life, that is, 1.3 h, proved to be the least stable substance and SH-6 the most durable (τ1/2 = 2.2 h), which further confirms its smallest loss after 6 h of hydrolysis. The degradation of all ETs tested in an environment with a pH of 0.9 and a temperature of 95 °C is associated with their structure as esters of HHDP acid, which are unstable in strongly acidic environments,4 and therefore, the loss of the HHDP residue is the same as the loss of the rest of EA.
In the conducted experiment, it was assumed that EA is the final product of transformation of the studied ETs. However, the greater part of the ETs tested was broken down to various intermediates than EA. This is confirmed by the data in Table 1, estimating the percentage EA share in the decrease of the original amount of the ET after 6 h of hydrolysis. These data indicate that only 26% of the initial amount of LC was hydrolyzed to EA, while for SH-6 and AM, these values were smaller and were 11.3 and 17.9%, respectively. In the studied reaction environment, a similar effect was achieved to that obtained by Japanese researchers25 but in a shorter time (6 h instead of 27 h), that is, the appearance of various intermediates of ET hydrolysis was noted. As expected, a ten-fold decrease in the concentration of TFA acid resulted in the conversion of almost the entire amount of ET used but not only to EA, as in the case of the classical method of ET determination.38 Hydrolysis of ester bonds predominated in the strongly acidic environment with a pH of 0.9 and a temperature of 95 °C. This can be seen in the graphs of the individual ET hydrolysis (Figure 1), which are expressed as a percentage of individual products formed during the hydrolysis, calculated on the reacted amount of the initial ET.
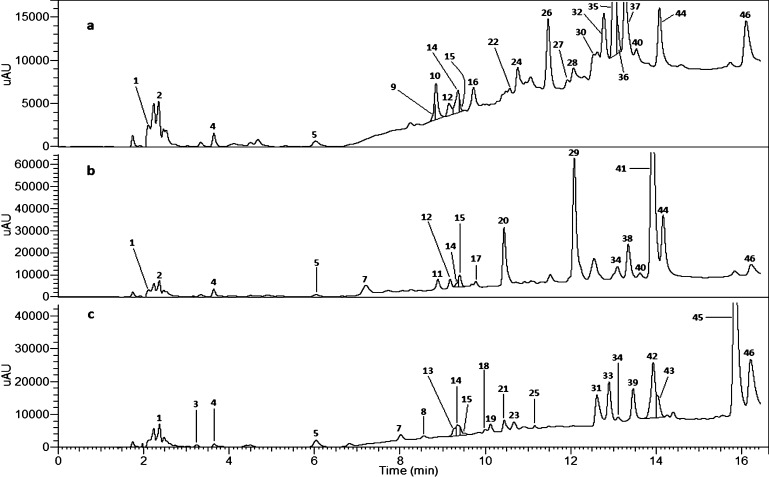
Figure 1.
Chromatograms at wavelength 250 nm of solutions obtained after 4 h of hydrolysis of (a) LC, (b) SH-6 and, (c) AM, respectively, in the condition of pH 0.9 and temperature 95 °C. Number of peak corresponds to numbers in Table 2 and on Figures 3 and 4.
The quality identification of hydrolysis products is given in Table 2. The products formed during hydrolysis were divided into primary and secondary (Figure 2). Primary names were given to those products that were formed first and then became substrates for the formation of secondary products. Most often they can be distinguished by the nature of the course of the curves plotted on the basis of the share of individual intermediate ETs calculated on the original quantity of the ET tested. The curves showing primary products are characterized by a step increase in the share of initial ET loss in the first hour of the reaction. Secondary products, on the other hand, usually show slow growth, often close to linear. For each of the ETs tested, the main primary products were substances formed after the loss of one EA residue, that is, in the case of LC products with M = 2502 Da (24, 30, 32, and 36), in the case of SH-6 products with M = 1568 Da (20, 29, 38), and in the case of AM products with M = 1568 Da (31, 39, and 42).
Table 2. Characterization of Hydrolysis Products of LC, SH-6, and AM, Respectively, Obtained after 4 h of Hydrolysisa.
| . | occurrence |
|||||||
|---|---|---|---|---|---|---|---|---|
| peak no | tR (min) | tentative structural assignment | nominal mass (Da) | MS data (m/z) | MS/MS data (m/z) | LC | SH-6 | AM |
| 1 | 2.11 | sanguisorbic acid α-d-glucoside | 650 | [649]− | 315, 301a,275, 169b | + | + | |
| 2 | 2.37 | HHDP-glu | 482 | [481]− | 385, 301a, 275 | + | + | + |
| 3 | 3.24 | dehydrodigallic acid | 338 | [337]− | 169b, 125c | + | ||
| 4 | 3.64 | gallic acid | 170 | + | + | + | ||
| 5 | 6.03 | bis-HHDP-α-d-glucose | 784 | [783]−, [391]−2 | 639, 515, 475, 391, 301a, 275, 249 | + | + | + |
| 6 | 7.20 | SH-6 without two ellagic moieties isomer | 1266 | [1265]−, [632]−2 | 613, 445, 315, 301a, 287, 241 | + | ||
| 7 | 8.01 | galloyl-O-galloyl-HHDP-α-d-glucose | 802 | [801]−, [400]−2 | 647, 445, 331, 319,301a, 275, 169b, 125c | + | ||
| 8 | 8.54 | galloyl-O-galloyl-HHDP-α-d-glucose | 802 | [801]−, [400]−2 | 632, 577, 460, 301a, 275, 169b | + | ||
| 9 | 8.76 | galloyl-HHDP-α-d-glucose | 634 | [633]− | 392, 301a, 275, 169b | + | ||
| 10 | 8.84 | dimeric ET plus one galloyl moiety without one ellagic moiety | 1736 | [1735]−, [867]−2 | 1691d, 1127, 730, 633f, 469e, 315, 301a, 286 | + | ||
| 11 | 8.88 | SH-6 without two ellagic moieties isomer | 1266 | [1265]−, [632]−2 | 469e, 445, 315, 301a, 287 | + | ||
| 12 | 9.17 | SH-2 isomer without ellagic moiety | 802 | [801]−, [400]−2 | 315, 301a, 275 | + | + | |
| 13 | 9.26 | galloyl-O-galloyl-HHDP-α-d-glucose | 802 | [801]−, [400]−2 | 635, 462, 301a, 275, 145 | + | ||
| 14 | 9.34 | bis-HHDP-α-d-glucose | 784 | [783]−, [391]−2 | 301a, 275, 249, 231 | + | + | + |
| 15 | 9.40 | galloyl-HHDP-α-d-glucose | 634 | [633]− | 543, 506, 401, 301a, 275, 169b | + | + | + |
| 16 | 9.72 | LC without two ellagic moieties isomer | 2200 | [1099]−2 | 1627, 1004, 633f, 469e, 445, 315, 301a, 287, 275 | + | ||
| 17 | 9.76 | hydrated SH-2 isomer | 1122 | [1121]−, [560]−2 | 902, 867, 730, 633f, 496, 366, 315, 301a, 275, 245, 169b, 125c | + | ||
| 18 | 9.98 | galloyl-O-galloyl-HHDP-α-d-glucose | 802 | [801]−, [400]−2 | 422, 379, 331, 301a,211, 169b | + | ||
| 19 | 10.11 | AM without two ellagic moieties | 1266 | [1265]−, [632]−2 | 867, 633f, 613, 574, 461, 301a, 275, 169b | + | ||
| 20 | 10.43 | SH-10 isomer | 1568 | [1567]−, [783]−2 | 821, 613, 445, 315, 301a, 287, 231, 193 | + | ||
| 21 | 10.44 | AM without two ellagic moieties | 1266 | [1265]−, [632]−2 | 1259, 633f, 613, 563, 481g, 463, 445, 377, 354, 331, 301a, 275, 169b | + | ||
| 22 | 10.57 | LC without two ellagic moieties isomer | 2200 | [1099]−2 | 1721, 633f, 469e, 445, 315, 301a, 275 | + | ||
| 23 | 10.67 | AM without two ellagic moieties | 1266 | [1265]−, [632]−2 | 1178, 633f, 613, 461,463, 455, 445, 397, 331, 301a, 275, 169b | + | ||
| 24 | 10.74 | LC without ellagic moiety isomer | 2502 | [1250]−2 | 1389, 633f, 445, 315, 301a, 287, 275 | + | ||
| 25 | 11.14 | galloyl-HHDP-α-d-glucose | 634 | [633]− | 301a, 275,169b | + | ||
| 26 | 11.47 | dimeric ET plus one galloyl moiety without one ellagic moiety | 1736 | [1735]−, [867]−2 | + | |||
| 27 | 11.92 | LC without two ellagic moieties isomer | 2200 | [1099]−2 | 1610, 1475, 1042, 633f, 469e, 445, 315, 301a, 275 | + | ||
| 28 | 12.06 | LC without two ellagic moieties isomer | 2200 | [1099]−2 | 1204, 1006, 897, 633f, 469e, 445, 315, 301a, 275 | + | ||
| 29 | 12.07 | SH-10 isomer | 1568 | [1567]−, [783]−2 | 827, 633f, 469e, 315, 301a, 287, 241 | + | + | |
| 30 | 12.53 | LC without ellagic moiety isomer | 2502 | [1250]−2 | 2239, 897, 769, 633f, 469e, 315, 301a, 275 | + | ||
| 31 | 12.61 | AM without one ellagic moiety | 1568 | [1567]−, [783]−2 | 1522, 1025, 897, 633f, 613,461, 301a, 275 | + | ||
| 32 | 12.77 | LC without ellagic moiety isomer | 2502 | [1250]−2 | 1744, 897, 745, 633f, 469e, 315, 301a, 275 | + | ||
| 33 | 12.88 | agrimonic acid A/B | 1104 | [1103]−, [551]−2 | 601, 495, 353, 301a, 275, 169b, 125c | + | ||
| 34 | 13.09 | sanguisorbic acid dilactone | 470 | [469]− | 315, 301a, 290, 175 | + | + | + |
| 35 | 13.00 | dimeric ET plus one galloyl moiety | 2038 | [1018]−2, [469]− | 1282, 897, 633f, 469e, 445, 315, 301a, 275 | + | ||
| 36 | 13.11 | LC without ellagic moiety isomer | 2502 | [1250]−2 | 1998, 1218, 897, 745, 469e, 315, 301a, 275 | + | ||
| 37 | 13.27 | LC residue | 2804 | [1401]−2 | 897, 633f, 542, 483, 315, 301a,275 | + | ||
| 38 | 13.33 | SH-10 isomer | 1568 | [1567]−, [783]−2 | 1410, 1170, 897, 633f, 469e, 443, 315, 301a, 287, 275 | + | + | |
| 39 | 13.46 | AM without one ellagic moiety | 1568 | [1567]−, [783]−2 | 897,633f, 613, 301a, 275 | + | ||
| 40 | 13.61 | galloyl-bis-HHDP-α-d-glucose (casuarictin) | 936 | [935]−, [467]−2 | 301a, 275 | + | + | |
| 41 | 13.91 | SH-6 residue | 1870 | [1869]−, [934]−2 | 1532, 1479, 1374, 452, 421, 315, 301a,287 | + | ||
| 42 | 13.93 | AM without one ellagic moiety | 1568 | [1567]−, [783]−2 | 1322, 898, 633f, 613, 445, 301a, 275 | + | ||
| 43 | 14.05 | agrimonic acid A/B | 1104 | [1103]−, [551]−2 | 935h, 697, 633f,597, [458]−2,[382]−2, 301a, 275, 169b, 125c | + | ||
| 44 | 14.15 | SH-2 isomer | 1104 | [1103]−, [551]−2 | 925, 853, 777, 589i, 469e, 315, 301a, 287, 275 | + | + | |
| 45 | 15.84 | AM residue | 1870 | [1869]−, [934]−2 | 935j, 897, 633f, 301a, 275 | + | ||
| 46 | 16.21 | EA | 302 | [301]− | + | + | + | |
Masses pointed with a bold font have occurred with intensity greater than 103 counts per second; m/z marked with a letter are characteristic and indicate: a—EA moiety, b—GA moiety, c—GA moiety without the carboxyl group (−COO), d—m/z = 1735 without −COO, e—sanguisorbic acid moiety, f—galloyl-HHDP-α-d-glucose moiety, g—HHDP-glu moiety, h—agrimonic acid moiety without galloyl moiety, i—gal-HHDP-glu moiety without −COO, and j—potentillin moiety (AM monomer).
Figure 2.
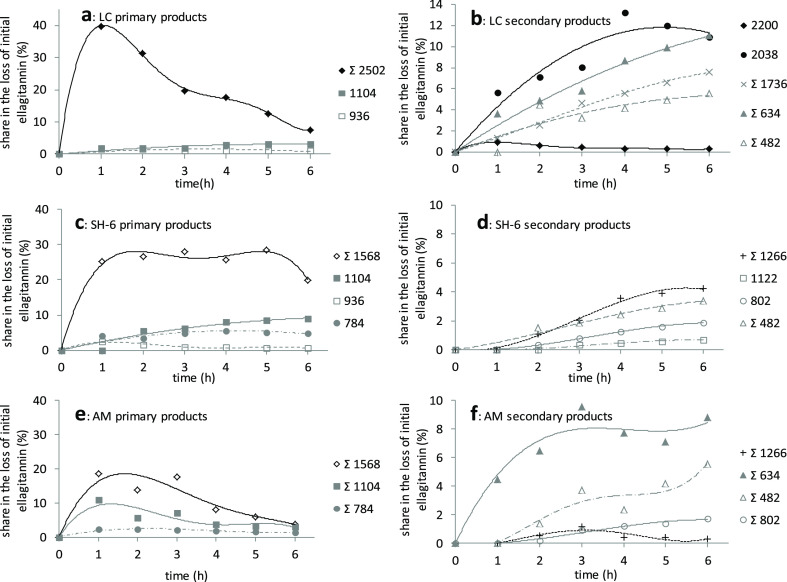
Kinetics of hydrolysis of LC (a,b), SH-6 (c,d), and AM (e,f) in pH 0.9 (TFA) and temp. 95 °C environment. Numbers in legend are the molecular weights of particular hydrolysis products. The sum symbol (Σ) in legend indicates that the line on the graph refers to the kinetics of summarized hydrolysis products with the same molecular weight.
LC Transformations
The dominant LC conversion under the conditions described is the hydrolysis of one EA residue to form LC without one EA moiety (Figure 3a). An LC molecule deprived of one EA residue has also been reported in the literature as native to the raspberry fruit.3 Theoretically, there are four ways to detach the rest of EA from the LC molecule, generating the possibility of the formation of four isomers with M = 2502 Da, which is shown in Figure 3a—all of them were identified (24, 30, 32, and 36), and so far, the formation of two isomers under milder conditions has been described in the literature.31 LC without one EA moiety as a typical primary substance is present in the reaction mixture in large quantities, that is, 40% of the sum of transformation products after 1 h of hydrolysis, and then its content gradually decreases to a level of about 7% after 6 h of reaction time. The resulting LC without one EA moiety molecule, in the next stage (Figure 3a), lost the next EA residue to form, in this case, LC without two EA moieties (16, 22, 27, and 28), but in small amounts, that is, below 1% based on the loss of the original amount of LC (Figure 2b). When LC without two EA moieties is the result of the release of two EA residues from the third glucose residue of the LC molecule, it is an intermediate in the formation of dimeric ET plus one galloyl (GA) moiety (35; Figure 3a). The literature so far does not explain in detail the possible mechanism of ET with M = 2038 Da formation (35).31 Alternatively, dimeric ET plus one GA moiety is formed by the detachment of HHDP α-d-glucoside (HHDP-glu, 2) from LC without one EA moiety molecule (Figure 3a). It can be seen in Figure 2b that dimeric ET plus one GA moiety (M = 2038 Da) dominates among secondary reaction products.
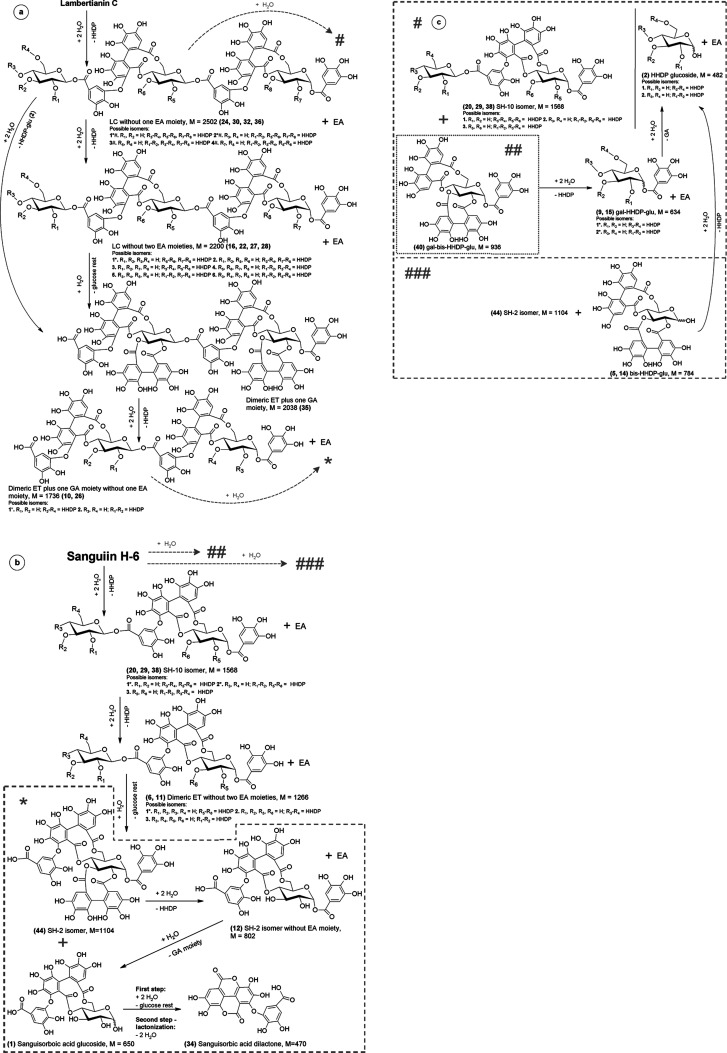
Figure 3.
Possible LC (a–c) and SH-6 (b,c) hydrolysis paths in strongly acidic environment. Explanations: letter in circle, located in the upper left corner refers to figure division; the asterisk/hashtag-labeled isomer is a substrate in the next step; gray arrows lead to a section of the path, separated by a dashed or dotted line.
It is interesting that dimeric ET plus one GA moiety, by disconnecting one EA residue, is a source of dimeric ET plus one GA moiety without one EA moiety (10 and 26), subsequent secondary LC hydrolysis products whose share in the reaction mixture is 7.5% after 6 h. Monomers GA-bis-HHDP-α-d-glucose (casuarictin, 40) and sanguiin H-2 isomer (SH-2 isomer, 44) are present in small amounts, and the course of their generation trend line does not clearly indicate their original character (Figure 2a). It can be assumed, however, that with the complexity of the studied system, the formation of these monomers is conditioned by quite different transformations than simple hydrolysis of the ether bond in the external sanguisorboyl group of LC. Detachment of one EA residue from the SH-2 isomer results in the SH-2 isomer without the EA moiety (Figure 3b; 12). Further decay (12) is one of the possibilities of sanguisorbic acid dilactone formation (34), which is characteristic for both LC and SH-6 degradation and also for AM.1 In the case of LC and SH-6, one of the possibilities of sanguisorbic acid dilactone formation is the breakdown of the intermediate, that is, sanguisorbic acid glucoside (Figure 3b; 1). The mechanism of sanguisorbic acid dilactone formation (34), in the case of AM, given the lack of the sanguisorboyl group in its structure, remains unknown. Dimeric ET without one EA moiety, sanguiin H-10 isomers (SH-10 isomers, 29, and 38), were marked in the reaction mixture only qualitatively because their peaks overlapped with other substances, among others with the residue LC, so that they could not be quantified. The formation of SH-10 isomers (29 and 38) is conditioned by the depolymerization of LC without one EA moiety to form an additional monomer LC and SH-6—casuarictin (40). One of the reasons for the low amount of casuarictin monomer in the postreaction mixture is that under strong hydrolysis conditions in which the detachment of EA residues is promoted, casuarictin undergoes rapid further hydrolysis (Figure 3c) to form galloyl-HHDP-α-d-glucose (gal-HHDP-glu; Figure 2a; 9 and 15). Gal-HHDP-glu as a secondary product of LC destruction after 6 h of hydrolysis accounts for approximately 11% of the total hydrolysis products (Figure 2b). The postreaction mixture was also rich in many other secondary LC decomposition products. It is presumed that the bis-HHDP-α-d-glucose molecule (bis-HHDP-glu; 5 and 14) formed indirectly from LC undergoes a rapid disconnection of the rest of the EA under these conditions to form HHDP-glu (Figure 3c; 2), whose share in the total products after 6 h is about 5.5% (Figure 2b). The loss of LC after 6 h of hydrolysis at pH 0.9 and at 95 °C is as much as 95.5%, while 61.2% of these products have been quantified.
SH-6 Transformations
The main products of SH-6 hydrolysis were SH-6 without one EA moiety (SH-10 isomers; Figure 2b; 20, 29, and 38), that is, as in the case of LC, the tested ET loses one EA acid residue first (Figure 3b). Molecules with this structure are also found natively in raspberry fruits3,31 and in pomegranate fruits.1 After the first hour of hydrolysis, the equilibrium of this product was established at the level of about 25% of the sum of products and after 6 h to fall to the level of about 20%. In the TFA acid environment, another EA residue is detached from ET with M = 1568 Da to form SH-6 without two EA moieties (Figure 3b; 6 and 11) as a secondary product, whose share in the total products increases to 4% after 6 h (Figure 2d). While the hydrolysis of the first EA residue from SH-6 is the dominant phenomenon, the hydrolysis of the next EA residue is more than 4 times less frequent. In similar studies,31 the presence of one isomer of SH-6 without two EA moieties has been demonstrated. Probably, after disconnection of the first and second EA moieties from the second glucose molecule in SH-6, hydrolysis of the glycosidic bond occurs, resulting in the formation of the SH-2 isomer (Figure 3b; 44). The second pathway of SH-2 isomer formation and simultaneously bis-HHDP-glu (5 and 14) formation is the hydrolysis of the glycosidic bond in the SH-6 molecule. This possibility is confirmed by the occurrence of HHDP-glu (2) among secondary products as a sign of successive hydrolysis of the ester bond in the bis-HHDP-glu molecule. High concentration of SH-2 and bis-HHDP-glu can be found in blackberries.39
The presence of the SH-2 isomer without the EA moiety (12) indicates further degradation of the SH-2 isomer by disconnecting one EA residue (Figure 3b). The accumulation of the SH-2 isomer without the EA moiety in the reaction environment is probably due to the greater resistance of 4,6-sanguisorbic moiety to hydrolysis compared to the EA moiety. As with LC hydrolysis, further degradation of the SH-2 isomer without the EA moiety can lead to the formation of sanguisorbic acid dilactone (34) with an intermediate stage of sanguisorbic acid glucoside (1), as shown in Figure 3b. The low concentration of SH-6 monomer–casuarictin (40)–in the postreaction mixture, which was also noted in the case of LC, is conditioned by its rapid degradation to gal-HHDP-glu (9 and 15), which is promoted by the reaction environment (Figure 3c). The research presented in the literature31 where the course of hydrolysis in the SH-6 and LC mixture was studied but under milder conditions (pH 6) showed a similar tendency of casuarictin behavior, that is, its disappearance over time. On the other hand, the occurrence, after 6 h in the reaction mixture, of about 3.5% of HHDP-glu (2) can be considered as a result of the loss of the EA residue by bis-HHDP-glu with M = 784 Da (5 and 14; probably at fourth and sixth carbon of the glucose ring). Under conditions of strong hydrolysis, about 85% of the initial amount of SH-6 was transformed after 6 h, and about 64% of the products were identified on the basis of the starting material. Figure 2d shows that the main secondary hydrolysis products are SH-6 without two EA moieties, SH-2 isomer without EA moiety, and HHDP-glu which are formed by disconnecting the HHDP residue from SH-10 isomers, SH-2 isomers, and bis-HHDP-glu, respectively. In addition, a product with M = 1122 Da appeared in small amounts not exceeding 1%, which can be hydrated SH-2 isomer (17).
AM Transformations
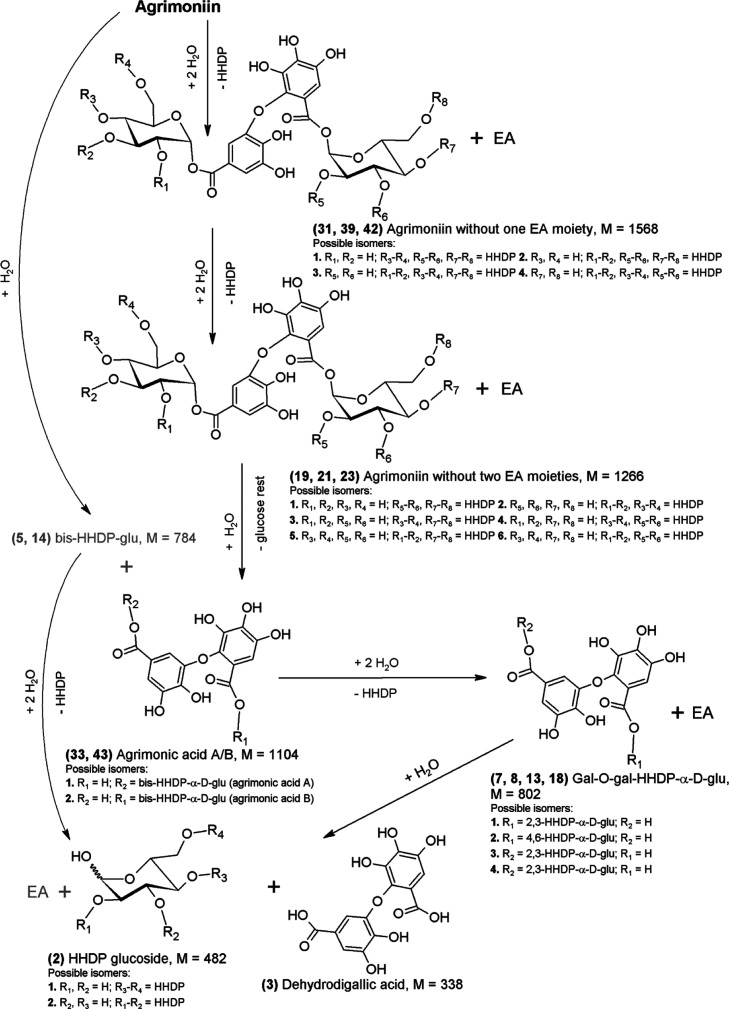
The main products of AM hydrolysis, resulting from the detachment of one EA moiety, were AM without one EA moiety (Figure 4; 31, 39, and 42), and three of the four possible isomers of this substance were qualitatively determined (Figure 2c). The share of AM without one EA moiety in the total hydrolysis products increases rapidly after 1 h to about 20% and then slowly decreases to the level of about 4% (Figure 2e). As in the case of the hydrolysis of two previously discussed starting substances in pH 0.9 environment, AM without two EA moieties appear as secondary products (19, 21, and 23)—three out of six possible isomers were qualitatively determined, which is the result of the detachment of another EA moiety from AM without one EA moiety. Parallel to this process, in AM without one EA moiety, the glycosidic bond at the first carbon of the glucose moiety is hydrolyzed resulting in the formation of bis-HHDP-glu (5 and 14) or HHDP-glu and ET with M = 1104 Da (Figure 1c; 33, 43), whose share in the total products after 1 h was 3% (concerns bis-HHDP-glu only) and 11%, respectively. After 6 h, a balance for bis-HHDP-glu of about 2% was established. A detachment of the bis-HHDP-glu moiety from the AM molecule will also lead to formation of ET with M = 1104 Da (Figure 4; 33 and 43). It should be noted at this point that, while bis-HHDP-glu, which arose from AM, is identical to those that arose from SH-6, ETs with M = 1104 Da arising from AM due to the difference in binding between the individual monomers of the studied ET dimers are different substances than in the case of SH-6, namely agrimonic acid A and B isomers (33 and 43).40 According to Figure 2f, gal-HHDP-glu (9 and 15) is the main secondary product. Their occurrence and successive increase in the share of total products to the level of approx. 9% can be seen in depolymerization of AM without one EA moiety and high instability ET with M = 936 Da, that is, the AM monomer—potentillin.40 The degradation of the potentillin monomer to gal-HHDP-glu and further to HHDP glucoside (2) occurs analogously to the casuarictin (40), as shown in Figure 3c. The characteristic secondary products of AM hydrolysis are HHDP-glu and galloyl-O-galloyl-HHDP-α-d-glucose (7, 8, 13, and 18), formed from agrimonic acid by successive hydrolysis of ester bonds and release of EA (Figure 4).
Figure 4.
Possible AM hydrolysis path in strongly acidic environment. Gray color indicates products of the alternative route of the hydrolysis pathway.
In this case, galloyl-O-galloyl-HHDP-α-d-glucose is composed of the dehydrodigallic acid moiety (3),41 attached to α-glycosidic bond in 2,3-HHDP-glu or 4,6-HHDP-glu molecules, which gives the possibility of formation of four isomers of this substance. Dehydrodigallic acid was determined only in the case of AM, so it can be considered a characteristic breakdown product of AM. During the hydrolysis of AM, sanguisorbic acid dilactone was also determined (34), as in the case of LC and SH-6, but the mechanism of formation of this substance indirectly from AM is unclear. The structure of the AM molecule theoretically excludes the formation of sanguisorbic acid dilactone. When the sanguisorbyl group is characteristic for LC and SH-6, the dehydrodigalloyl group is characteristic of the AM molecule. One possibility is polymerization and oxidation reactions,42 which lead to the formation of sanguisorbic acid dilactone from AM. In the studied reaction environment, in addition to hydrolysis of ester and ether bonds, there must be other transformations that require further research.
Summing up the research, it can be stated that for each of the three ETs tested in an environment with pH 0.9 and the temperature of 95 °C, the process of biphasic, successive hydrolysis of ester bonds between HHDP molecules and glucose hydroxyl groups dominates, especially at the beginning, leading to a release of EA molecules.
The next step is hydrolysis of the glycosidic bond with the depolymerization nature, as well as further release of EA moieties from the newly formed ET, resulting in the formation of HHDP-glu derivatives. Many intermediate products have been identified in postreaction mixtures. For LC, SH-6, and AM, there were 22, 17, and 21 different substances, respectively, which were formed during hydrolysis under the tested conditions. If the quantity of decomposition products formed is the criterion of stability, then the most stable turned out to be SH-6 while AM, having the same molecular weight as SH-6, almost matched the result obtained for a larger LC molecule. Many of the tested decomposition products of the ETs tested are the same, even compared to AM, which has a slightly different molecular structure than that of LC and SH-6.
The characteristic products of ETs hydrolysis were distinguished for the ETs tested, that is, dimeric ET plus one GA moiety without one EA moiety (M = 1736 Da) for LC, sanguisorbic acid dilactone (M = 470 Da) for all researched ETs, and dehydrodigallic acid (M = 338 Da) for AM. The results of the research also show that during the acid hydrolysis, the ETs undergo far-reaching changes and the multitude of identified intermediates of the hydrolysis of the ETs tested shows its complicated nature.
As the beneficial effect of ETs is attributed to themselves as well as to their degradation products, for example, arising during digestion, expanding knowledge about the transformation of ETs under acid hydrolysis is important to study the possible mechanism of their health beneficial properties and possible breakdown pathways occurring in gastrointestinal tract and during food processing.
Glossary
Abbreviations
- AM
agrimoniin
- bis-HHDP-glu
bis-HHDP-α-d-glucose
- EA
ellagic acid
- ET
ellagitannin
- ETOM
oligomeric ellagitannin
- GA
galloyl
- gal-HHDP-glu
galloyl-HHDP-α-d-glucose
- HHDP
hexahydroxydiphenic acid
- HHDP-glu
HHDP α-d-glucoside
- LC
lambertianin C
- M
nominal mass
- SH-2
sanguiin H-2
- SH-6
sanguiin H-6
- SH-10
sanguiin H-10
- TFA
trifluoroacetic acid
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.0c02674.
Structure of GOG and GOD bonds present in ETOMs; structure of LC; structure of SH-6; structure of AM; analytical parameters used for quantitative analysis; and the parameters characterizing the hydrolysis of the researched ETs in different environments (PDF)
Author Contributions
J.M. designed the research, supervised, performed the experiments, and wrote the manuscript; M.S., M.K., and E.K. edited the manuscript.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors declare no competing financial interest.
Supplementary Material
References
- García-Villalba R.; Espín J. C.; Aaby K.; Alasalvar C.; Heinonen M.; Jacobs G.; Voorspoels S.; Koivumäki T.; Kroon P. A.; Pelvan E.; Saha S.; Tomás-Barberán F. A. Validated Method for the Characterization and Quantification of Extractable and Nonextractable Ellagitannins after Acid Hydrolysis in Pomegranate Fruits, Juices, and Extracts. J. Agric. Food Chem. 2015, 63, 6555–6566. 10.1021/acs.jafc.5b02062. [DOI] [PubMed] [Google Scholar]
- Landete J. M. Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. 10.1016/j.foodres.2011.04.027. [DOI] [Google Scholar]
- Gasperotti M.; Masuero D.; Vrhovsek U.; Guella G.; Mattivi F. Profiling and Accurate Quantification ofRubusEllagitannins and Ellagic Acid Conjugates Using Direct UPLC-Q-TOF HDMS and HPLC-DAD Analysis. J. Agric. Food Chem. 2010, 58, 4602–4616. 10.1021/jf904543w. [DOI] [PubMed] [Google Scholar]
- Vrhovsek U.; Giongo L.; Mattivi F.; Viola R. A survey of ellagitannin content in raspberry and blackberry cultivars grown in Trentino (Italy). Eur. Food Res. Technol. 2008, 226, 817–824. 10.1007/s00217-007-0601-4. [DOI] [Google Scholar]
- Mertz C.; Cheynier V.; Günata Z.; Brat P. Analysis of phenolic compounds in two blackberry species (Rubus glaucus and Rubus adenotrichus) by high-performance liquid chromatography with diode array detection and electrospray ion trap mass spectrometry. J. Agric. Food Chem. 2007, 55, 8616–8624. 10.1021/jf071475d. [DOI] [PubMed] [Google Scholar]
- Klimczak E.; Rozpara E.; Król B. Distribution of ellagitannins in juice, flesh, and achenes as additional criterion for optimal utilization of strawberries. Zywn., Technol., Jakosc 2011, 6, 142–154. 10.15193/zntj/2011/79/142-154. [DOI] [Google Scholar]
- Ko H.; Hyelin J.; Dahae L.; Hyo-Kyoung C.; Ki S. K.; Kyung-Chul C. Sanguiin H6 suppresses TGF-b induction of the epithelial–mesenchymal transition and inhibits migration and invasion in A549 lung cancer. Bioorg. Med. Chem. Lett. 2015, 25, 5508–5513. 10.1016/j.bmcl.2015.10.067. [DOI] [PubMed] [Google Scholar]
- Fuhrman B.; Aviram M.. Protection against cardiovascular diseases. In Pomegranates: Ancient Roots to Modern Medicine, 1st ed.; Seeram N.P., Schulman R. N., Heber D., Eds.; CRC Press: Boca Raton, Florida, 2006; pp 63–80. [Google Scholar]
- Heber D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008, 269, 262–268. 10.1016/j.canlet.2008.03.043. [DOI] [PubMed] [Google Scholar]
- Espín J. C.; Larossa M.; García-Conesa M. T.; Tomás-Barberán F. A. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: the evidence so far. J. Evidence-Based Complementary Altern. Med. 2013, 2103, 270418. 10.1155/2013/270418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeram N. P.; Aronson W. J.; Zhang Y.; Henning S. M.; Moro A.; Lee R.-p.; Sartippour M.; Harris D. M.; Rettig M.; Suchard M. A.; Pantuck A. J.; Belldegrun A.; Heber D. Pomegranate Ellagitannin-Derived Metabolites Inhibit Prostate Cancer Growth and Localize to the Mouse Prostate Gland. J. Agric. Food Chem. 2007, 55, 7732–7737. 10.1021/jf071303g. [DOI] [PubMed] [Google Scholar]
- Tomás-Barberán F. A.; González-Sarrías A.; García-Villalba R.; Núñez-Sánchez M. A.; Selma M. V.; García-Conesa M. T.; Espín J. C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2016, 61, 1500901. 10.1002/mnfr.201500901. [DOI] [PubMed] [Google Scholar]
- Kosmala M.; Zduńczyk Z.; Juśkiewicz J.; Jurgoński A.; Karlińska E.; Macierzyński J.; Jańczak R.; Rój E. Chemical composition of defatted strawberry and raspberry seeds and the effect of these dietary ingredients on polyphenol metabolites, intestinal function, and selected serum parameters in rats. J. Agric. Food Chem. 2015, 63, 2989–2996. 10.1021/acs.jafc.5b00648. [DOI] [PubMed] [Google Scholar]
- Espín J. C.; González-Barrio R.; Cerdá B.; López-Bote C.; Rey A. I.; Tomás-Barberán F. A. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J. Agric. Food Chem. 2007, 55, 10476–10485. 10.1021/jf0723864. [DOI] [PubMed] [Google Scholar]
- Jurgoński A.; Juśkiewicz J.; Fotschki B.; Kołodziejczyk K.; Milala J.; Kosmala M.; Grzelak-Błaszczyk K.; Markiewicz L. Metabolism of strawberry mono- and dimeric ellagitannins in rats fed a diet containing fructo-oligosaccharides. Eur. J. Nutr. 2017, 56, 853–864. 10.1007/s00394-015-1133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Villalba R.; Selma M. V.; Espín J. C.; Tomás-Barberán F. A. Identification of novel urolithin metabolites in human feces and urine after the intake of a pomegranate extract. J. Agric. Food Chem. 2019, 67, 11099–11107. 10.1021/acs.jafc.9b04435. [DOI] [PubMed] [Google Scholar]
- Mena P.; Dall’Asta M.; Calani L.; Brighenti F.; Del Rio D. Gastrointestinal stability of urolithins: an in vitro approach. Eur. J. Nutr. 2017, 56, 99–106. 10.1007/s00394-015-1061-4. [DOI] [PubMed] [Google Scholar]
- Milala J.; Kosmala M.; Karlińska E.; Juśkiewicz J.; Zduńczyk Z.; Fotschki B. Ellagitannins from strawberries with different degrees of polymerization showed different metabolism through gastrointestinal tract of rats. J. Agric. Food Chem. 2017, 65, 10738–10748. 10.1021/acs.jafc.7b04120. [DOI] [PubMed] [Google Scholar]
- García-Villalba R.; Espín J. C.; Tomás-Barberán F. A. Chromatographic and spectroscopic characterization of urolithins for their determination in biological samples after the intake of foods containing ellagitannins and ellagic acid. J. Chromatogr. A 2016, 1428, 162–175. 10.1016/j.chroma.2015.08.044. [DOI] [PubMed] [Google Scholar]
- Piwowarski J.; Granica S.; Kiss A. Influence of Gut Microbiota-Derived Ellagitanninsʼ Metabolites Urolithins on Pro-Inflammatory Activities of Human Neutrophils. Planta Med. 2014, 80, 887–895. 10.1055/s-0034-1368615. [DOI] [PubMed] [Google Scholar]
- Goel G.; Puniya A. K.; Aguilar C. N.; Singh K. Interaction of gut microflora with tannins in feeds. Naturwissenschaften 2005, 92, 497–503. 10.1007/s00114-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Cerdá B.; Periago P.; Espín J. C.; Tomás-Barberán F. A. Identification of urolithin A as a metabolite produced by human colon microflora from ellagic acid and related compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. 10.1021/jf050384i. [DOI] [PubMed] [Google Scholar]
- Cerdá B.; Llorach R.; Cerón J. J.; Espín J. C.; Tomás-Barberán F. A. Evaluation of the bioavailability and metabolism in the rat of punicalagin and antioxidant polyphenol from pomegranate juice. Eur. J. Nutr. 2003, 42, 18–28. 10.1007/s00394-003-0396-4. [DOI] [PubMed] [Google Scholar]
- Lee J.-H.; Talcott S. T. Ellagic acid and ellagitannins affect on sedimentation in muscadine juice and wine. J. Agric. Food Chem. 2002, 50, 3971–3976. 10.1021/jf011587j. [DOI] [PubMed] [Google Scholar]
- Tanaka T.; Tachibana H.; Nonaka G.-i.; Nishioka I.; Hsu F.-L.; Kohda H.; Tanaka O. Tannins and Related Compounds. CXXII. New Dimeric, Trimeric and Tetrameric Ellagitannins, Lambertianins A-D, from Rubus lambertianus SERINGE. Chem. Pharmaceut. Bull. 1993, 41, 1214–1220. 10.1248/cpb.41.1214. [DOI] [PubMed] [Google Scholar]
- Hemingway R. W.; Hillis W. E. Behavior of ellagitannins, gallic acid, and ellagic acid under alkaline condition. Tappi 1971, 54, 933–936. [Google Scholar]
- Daniel E. M.; Ratnayake S.; Kinstle T.; Stoner G. D. The Effects of pH and Rat Intestinal Contents on the Liberation of Ellagic Acid from Purified and Crude Ellagitannins. J. Nat. Prod. 1991, 54, 946–952. 10.1021/np50076a004. [DOI] [PubMed] [Google Scholar]
- Sepúlveda L.; Ascacio A.; Rodríguez-Herrera R.; Aguilera-Carbó A.; Aguilar C. N. Ellagic acid: biological properties and biotechnological development for production processes. Afr. J. Biotechnol. 2012, 10, 4518–4523. 10.1002/chin.201250260. [DOI] [Google Scholar]
- Gancel A.-L.; Feneuil A.; Acosta O.; Pérez A. M.; Vaillant F. Impact of industrial processing and storage on major polyphenols and the antioxidant capacity of tropical highland blackberry (Rubus adenotrichus). Food Res. Int. 2011, 44, 2243–2251. 10.1016/j.foodres.2010.06.013. [DOI] [Google Scholar]
- Hager A. C.Processing and storage effects on black raspberry polyphenolics and antioxidant capacity. Master of Science Thesis, University of Arkansas, Fayetteville, 2008. [Google Scholar]
- Sójka M.; Janowski M.; Grzelak-Błaszczyk K. Stability and transformations of raspberry (Rubus idaeus L.) ellagitannins in aqueous solutions. Eur. Food Res. Technol. 2019, 245, 1113–1122. 10.1007/s00217-018-3212-3. [DOI] [Google Scholar]
- Jochum F. D.; Theato P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483. 10.1039/c2cs35191a. [DOI] [PubMed] [Google Scholar]
- Cho H.; Bae J.; Garripelli V. K.; Anderson J. M.; Jun H.-W.; Jo S. Redox-sensitive polymeric nanoparticles for drug delivery. Chem. Commun. 2012, 48, 6043–6045. 10.1039/c2cc31463k. [DOI] [PubMed] [Google Scholar]
- Chávez-González M.; Rodríguez-Durán L. V.; Balagurusamy N.; Prado-Barragán A.; Rodríguez R.; Contreras J. C.; Aguilar C. N. Biotechnological advances and challenges of tannase: An overview. Food Bioprocess Technol. 2012, 5, 445–459. 10.1007/s11947-011-0608-5. [DOI] [Google Scholar]
- Whitley A. C.; Stoner G. D.; Darby M. V.; Walle T. Intestinal epithelial cell accumulation of the cancer preventive polyphenol ellagic acid-extensive binding to protein and DNA. Biochem. Pharmacol. 2003, 66, 907–915. 10.1016/s0006-2952(03)00413-1. [DOI] [PubMed] [Google Scholar]
- Sójka M.; Macierzyński J.; Zaweracz W.; Buczek M. Transfer and mass balance of ellagitannins, anthocyanins, flavan-3-ols, and flavonols during the processing of red raspberries (Rubus idaeus L.) to juice. J. Agric. Food Chem. 2016, 64, 5549–5563. 10.1021/acs.jafc.6b01590. [DOI] [PubMed] [Google Scholar]
- Karlińska E.; Pecio Ł.; Macierzyński J.; Stochmal A.; Kosmala M. Structural elucidation of the ellagitannin with a molecular weight of 2038 isolated from strawberry fruit (Fragaria ananassa Duch.) and named fragariin A. Food Chem. 2019, 296, 109–115. 10.1016/j.foodchem.2019.05.191. [DOI] [PubMed] [Google Scholar]
- Klimczak E.; Król B. Macro- and microelements in determination of different forms of ellagic acid in by-products from strawberry processing. Zywn., Technol., Jakosc 2010, 17, 81–94. 10.15193/zntj/2010/71/081-094. [DOI] [Google Scholar]
- Kaume L.; Howard L. R.; Devareddy L. The blackberry fruit: A review on its composition and chemistry, metabolism and bioavailability, and health benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. 10.1021/jf203318p. [DOI] [PubMed] [Google Scholar]
- Okuda T.; Yoshida T.; Kuwahara M.; Memon M. U.; Shingu T. Tannins of Rosaceous medicinal plants. I. Structures of potentillin, agrimonic acids A and B, and agrimoniin, a dimeric ellagitannin. Chem. Pharm. Bull. 1984, 32, 2165–2173. 10.1248/cpb.32.2165. [DOI] [Google Scholar]
- Nawwar M. A. M.; Buddrus J.; Bauer H. Dimeric phenolic constituents from the roots of Tamarix nilotica. Phytochemistry 1982, 21, 1755–1758. 10.1016/s0031-9422(82)85054-1. [DOI] [Google Scholar]
- Tuominen A.; Sundman T. Stability and oxidation products of hydrolysable tannins in basic conditions detected by HPLC/DAD-ESI/QTOF/MS. Phytochem. Anal. 2013, 24, 424–435. 10.1002/pca.2456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.