Suprachoroidal gene transfer with nonviral nanoparticles provides noninvasive transgene expression in photoreceptors and RPE.
Abstract
Subretinal injections of viral vectors provide great benefits but have limited cargo capacity; they induce innate and adaptive immune responses, which may cause damage and preclude repeated injections; and they pose administration risks. As a new biotechnology, suprachoroidal injections of biodegradable nanoparticles (NPs) containing a reporter plasmid induce reporter expression in rat photoreceptors and RPE throughout the entire eye and maintain expression for at least 8 months. Multiple injections markedly increase expression. Suprachoroidal injection of NPs containing a VEGF expression plasmid caused severe subretinal neovascularization progressing to subretinal fibrosis, similar to what occurs in untreated patients with neovascular age-related macular degeneration, providing a new model and proof of concept for level and duration of expression. Suprachoroidal injection of NPs containing a VEGF-binding protein expression plasmid significantly suppressed VEGF-induced vascular leakage and neovascularization demonstrating therapeutic potential. These data suggest that nonviral NP suprachoroidal gene transfer may provide a noninvasive, repeatable alternative to subretinal injection of viral vectors.
INTRODUCTION
There has been great progress in ocular gene therapy for the treatment of inherited retinal degenerations. Leber congenital amaurosis (LCA) is a group of recessively inherited retinal degenerations in which there is profound vision loss in early childhood. A subgroup of patients with LCA has mutations in both copies of the Rpe65 gene, which encodes for an isomerase necessary for regeneration of rhodopsin. Visual function is poor early on, but retinal cell death occurs slowly, providing a window of opportunity for gene replacement. Subretinal injection of an adeno-associated virus serotype 2 (AAV2) vector expressing Rpe65 (LUXTURNA) improves mobility in some adult patients and has greater effects in young patients (1–3), which has led to approval of LUXTURNA by the U.S. Food and Drug Administration. Choroideremia is an X-linked disease due to a mutation in the CHM gene that results in degeneration of the retinal pigmented epithelium (RPE), retina, and choroid causing visual field constriction and reduction in central vision in young males (4). The CHM gene codes for Rab escort protein–1 (REP-1), and subretinal injection of an AAV2 vector expressing REP-1 resulted in improved visual acuity in the eyes of 14 patients compared with their untreated fellow eye (5). There have also been encouraging results using ocular gene therapy to provide long-term sustained delivery of antiangiogenic proteins for the treatment of neovascular age-related macular degeneration (AMD), the most prevalent cause of severe vision loss in patients over the age of 60 (6–9).
While the successes and future promise of ocular gene therapy are notable, they are balanced by potential pitfalls. One is that viral vectors are potent inducers of innate and adaptive immune responses that may cause damage to the retina and other ocular tissues and may compromise or preclude repeated vector administration (10, 11). If transgene expression level is insufficient to achieve a therapeutic effect, augmenting expression by a second vector injection may not be possible. A second pitfall is capacity limitation; AAV vectors have become the most commonly used vectors for ocular gene transfer and have many useful features but have limited cargo space preventing their use for transfer of large genes. The subretinal space has relative immune privilege, and thus, subretinal injection of viral vectors may reduce negative effects of preexistent immunity, but there are risks associated with subretinal injections, which is the third pitfall. Subretinal injections are done as part of a surgical procedure, vitrectomy, which carries a 1% risk of retinal detachment. Injection of viral vector under the fovea causes separation of central photoreceptors from the underlying RPE, which may damage the photoreceptors and cause permanent reduction in visual acuity (3). Occasionally, the pressure of a subretinal injection under the macula causes dehiscence through the thinnest part of the retina, the fovea, resulting in a macular hole (12). Even in the absence of a complication, there is an inherent disadvantage of subretinal injection of a viral vector, because transfection occurs only in the region of the retinal detachment caused by vector injection, which is usually 20% or less of the retina.
Intravitreous injection of AAV vectors is limited by binding of the vectors to the internal limiting membrane (ILM), which limits their penetration into the retina (13). After intravitreous injection of an AAV2 vector, transduction is limited to areas where the ILM is thin, ganglion cells around the fovea and adjacent to blood vessels and transitional epithelial cells in the pars plana. When AAV2 is injected into the vitreous cavity after vitrectomy and removal of the ILM, there is improved transduction of inner retinal cells, but this procedure must be done in the operating room and has a higher rate of complications than intravitreous injections without vitrectomy, and there is no transduction of photoreceptors, the target cells for most inherited retinal degenerations (14). In vivo–directed evolution has resulted in new AAV vectors with reduced binding to the ILM that penetrate further into the retina after intravitreous injection, resulting in widespread transduction of photoreceptors and no inflammation in rodents; however, in nonhuman primates, intravitreous injection of these vectors results in photoreceptor transduction in a limited area surrounding the fovea and significant inflammation (15).
The goal of this study was to develop a multipronged strategy to address the current pitfalls of ocular gene therapy. The first objective was to use nonviral polymeric vectors to eliminate problems from immune response and cargo size limitations. Previous attempts to use nonviral vectors have been limited by inefficient transfection (16), but recent advances in polymer chemistry and biomaterial engineering have helped to overcome extracellular and intracellular barriers and markedly improved nonviral transfection efficiency. Cationic poly(β-amino ester)s (PBAEs) with primary, secondary, and tertiary amines and hydrolytically cleavable ester bonds compact DNA into positively charged nanoparticles (NPs) that undergo endocytosis and endosomal escape (17, 18). Within minutes to hours, the DNA is released intracellularly and enters the nucleus allowing transgene expression. Modification of polymer end groups and linkages alters transfection efficiency in a cell type–specific manner, and screening of a combinatorial polymer library allows identification of optimal polymers for particular applications, including delivery to RPE cells (17, 19–21).
The second objective was to circumvent the potential problems of subretinal injections and determine whether polymeric vectors could provide efficient gene transfer to the retina and RPE when delivered noninvasively by suprachoroidal injection. The suprachoroidal space is a potential space along the inner surface of the sclera that can be expanded by injection of fluid just inside the sclera. Fluorescently labeled particles injected near the limbus flow circumferentially around the eye, resulting in a broad area of exposure (22). Suprachoroidal injection of triamcinolone acetonide results in sustained anti-inflammatory effects in the retina (22, 23) and prolonged improvements in macular edema in patients with uveitis or retinal vein occlusion (24, 25). We have recently demonstrated that suprachoroidal injection of AAV8.GFP, an AAV8-expressing green fluorescent protein (GFP), results in widespread expression of GFP in RPE cells and photoreceptors (26). This provides an advance over subretinal injection of viral vectors, but the suprachoroidal space has less immune privilege than the subretinal space, and therefore, preexistent neutralizing antibodies to AAV8 may limit transduction and preclude this approach. Also, we determined that neutralizing antibodies are induced after a single suprachoroidal injection of AAV8, and therefore, while it might be possible to do repeated injections in the same eye or the fellow eye within a 2-week window, it may not be possible at longer intervals between injections. In this study, we sought to circumvent the problems of subretinal or suprachoroidal injection of viral vectors by developing nonviral suprachoroidal gene transfer to the retina.
RESULTS
NP preparation and characterization
PBAEs have been used in formulating NPs for gene delivery because they are biodegradable to reduce cytotoxicity, are positively charged to bind DNA and condense it into an NP, and contain titratable secondary and tertiary amines to promote endosomal escape (17, 27). PBAE 457 (Fig. 1A) NPs formulated at a 30 (w/w) polymer:DNA mass ratio were used on the basis of our previous findings from a high-throughput transfection screen using human RPE cells and human retinal endothelial cells (28). PBAE 457 [molecular weight of 14.9 kDa (Mn) and 35.3 kDa (Mw), 2.37 polydispersity index (PD)] was found to be safe and effective for in vitro transfection.
Fig. 1. Polymer structure and NP characterization.
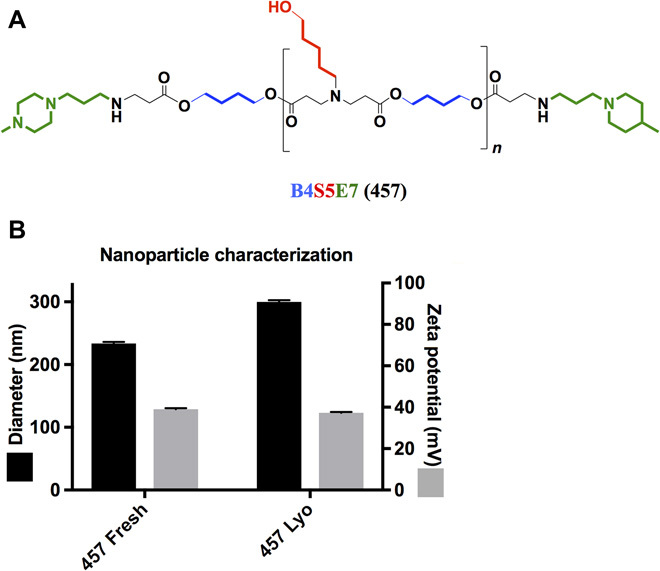
(A) Cationic 1-(3-aminopropyl)-4-methyl-piperazine endcapped poly(1,4-butanediol-diacrylate-co-5-amino-1-pentanol) (named PBAE 457) is used to electrostatically self-assemble with plasmid DNA (pDNA) to form nonviral gene delivery NPs. (B) Hydrodynamic diameter and zeta potential of 457/DNA NPs before and after lyophilization are similar (n = 3 independent batches; mean ± SD).
Tzeng and Green (29) showed that PBAE NP analogs to PBAE 457 can undergo lyophilization and long-term storage at −20°C without losing transfection efficacy (20). From a clinical translation perspective, it is critical to be able to store PBAE NPs frozen in the absence of water, as the polymer is hydrolytically cleavable and PBAE NPs can become unstable. Comparison of the PBAE 457/DNA NP physical properties before and after lyophilization shows that the NPs maintain similar biophysical properties (small size and positive zeta potential) (Fig. 1B). These NP properties can aid in cellular uptake and intracellular delivery following local suprachoroidal injection.
Suprachoroidal injection of NPs containing a GFP expression plasmid
Two months after suprachoroidal injection in Brown Norway rats of PBAE NPs containing 1 μg of pEGFP-N1, an expression plasmid in which a cytomegalovirus promoter drives expression of GFP (CMV-GFP), transverse frozen sections were cut through the entire globe. Figure 2A illustrates the location of the injection site, 1 mm posterior to the limbus in the 6:30 meridian, and provides a schematic map to illustrate the location in the eye from which sections originated. There was strong fluorescence that colocalized with anti-GFP staining indicating GFP expression in photoreceptor inner and outer segments and RPE in all four quadrants 600 μm posterior to the limbus (Fig. 2, B to E). This indicates production of GFP throughout the anterior retina around the entire circumference of the eye. The findings were similar in all four quadrants 1800 μm posterior to the limbus (Fig. 2, F to I). This is the most posterior location that transverse sections can be cut, because beyond this point the sections become oblique due to the curvature of the eye; this location is about halfway between the equator of the eye and the optic nerve. GFP expression was seen in some cells in the inner retina (arrows), but most of the GFP fluorescence and staining was in the photoreceptors and RPE.
Fig. 2. Widespread expression of GFP in retina and RPE 2 months after suprachoroidal injection of NPs containing a CMV-GFP expression plasmid.
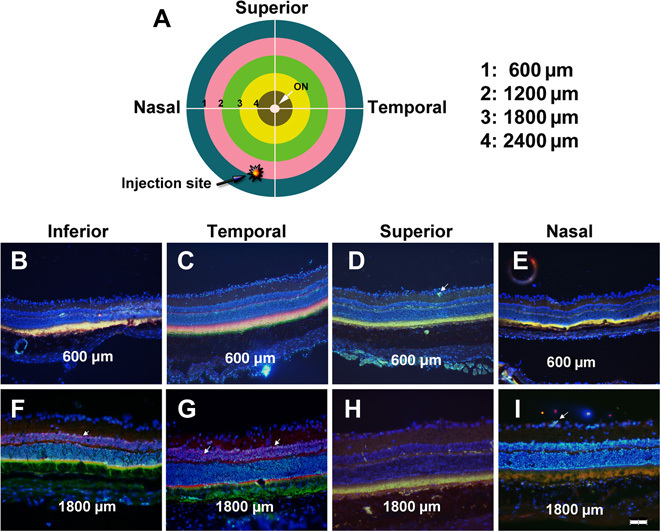
Brown Norway rats were given a 3-μl suprachoroidal injection of PBAE NPs containing 1 μg of CMV-GFP expression plasmid 1 mm posterior to the limbus in the inferonasal quadrant near the vertical meridian (A). Two months after injection, rats were euthanized, eyes were frozen in embedding medium, and transverse sections were immunohistochemically stained for GFP, counterstained with Hoechst, and merged with its fluorescent image. In the inferior (B), temporal (C), superior (D), and nasal (E) meridians of a section 600 μm posterior to the limbus through anterior retina [intersections of inner surface of blue ring and white lines in (A)], there was colocalization of fluorescence with anti-GFP staining in RPE and photoreceptor inner and outer segments. At the inferior (F), temporal (G), superior (H), and nasal (I) meridians of a section 1800 μm posterior to the limbus through posterior retina, staining for GFP in RPE and photoreceptor inner and outer segments was similar to that seen in anterior retina. Occasional weak expression of GFP was seen in cells of the inner retina (arrows). Scale bar, 100 μm.
Suprachoroidal injection of 2.85 × 1010 genome copies (GC) of AAV8.GFP results in very strong expression of GFP in photoreceptors and RPE (26). Very strong fluorescence is needed for it to be seen on a flat mount, and the expression of GFP after subretinal injection of AAV8.GFP is sufficiently strong to visualize fluorescence in about 25% of an RPE/choroid flat mount. After suprachoroidal injection of 1 μg of GFP expression plasmid, the level of expression of GFP in RPE and photoreceptors is less than that seen after suprachoroidal injection of 2.85 × 1010 GC of AAV8.GFP, and the fluorescence is not strong enough to be well visualized on flat mounts. However, it is possible to increase the expression of GFP by performing multiple suprachoroidal injections of NPs containing GFP expression plasmid. Rats were given three suprachoroidal injections of NPs containing 1 μg of GFP expression plasmid 3 days apart, and after 3 weeks, RPE/choroid flat mounts were examined by fluorescence microscopy. Nonuniform GFP fluorescence was observed in about 20% of the RPE/choroid flat mounts, and high magnification showed that the fluorescence was in hexagonal RPE cells (fig. S1).
To investigate the duration of expression, we assessed retinas for GFP expression at several time points after suprachoroidal injection of PBAE NPs containing 1 μg of pEGFP-N1. In this time course experiment, we also further evaluated the posterior extent of GFP expression by cutting sagittal ocular sections through the center of the cornea and posterior retina and merging fluorescence with anti-GFP staining. Two weeks after NP injection, there was moderate GFP expression in photoreceptor inner and outer segments and RPE (Fig. 3A). Strong GFP expression was seen in photoreceptor inner and outer segments and RPE at 1, 2, 4, and 6 months after injection of NPs (Fig. 3, B to E). A composite of sections through the center of an eye 4 months after NP injection showed strong GFP fluorescence in photoreceptors and RPE around the circumference of the eye, with slightly less fluorescence in the anterior-most retina opposite the injection site (Fig. 3F). While the images in Figs. 2 and 3 show GFP expression in photoreceptors and RPE, this is better appreciated in high-magnification images (fig. S2). Mean GFP levels measured by enzyme-linked immunosorbent assay (ELISA) were similar in homogenates of RPE/choroid and retina and were maintained through 8 months after suprachoroidal injection of NPs, the longest time point measured (Fig. 3G). Expression of GFP started early and was maintained without substantial decline at least through 8 months (there was no significant difference among time points for retina or RPE/choroid by Dunn’s test of multiple comparisons with Kruskal-Wallis rank test and Sidak multiple comparisons adjustment method). There was no detectable GFP in retinal or RPE/choroid homogenates 2 weeks after intravitreous injection of PBAE NPs containing 1 μg of pEGFP-N1, but mean GFP levels in retina and RPE/choroid were high 2 and 4 weeks after subretinal injection of NPs containing 1 μg of pEGFP-N1 (Fig. 3H). To quantitatively test whether transgene expression could be increased by multiple vector injections, rats were given a suprachoroidal injection of NPs containing 1 μg of pEGFP-N1 in each eye followed by a second injection of the same amount of NPs 2 days later in one eye and a third injection in the same eye after another 2 days; thus, one eye received one injection, and the other eye received three injections. Two weeks later, rats were euthanized and GFP levels were measured in retinal and RPE/choroid homogenates. The mean level of GFP was significantly higher in the retina of eyes given three injections of NPs compared to those given one injection (Fig. 3H). There was no significant difference in RPE/choroid.
Fig. 3. Time course of GFP expression after suprachoroidal injection of NPs containing a CMV-GFP expression plasmid.
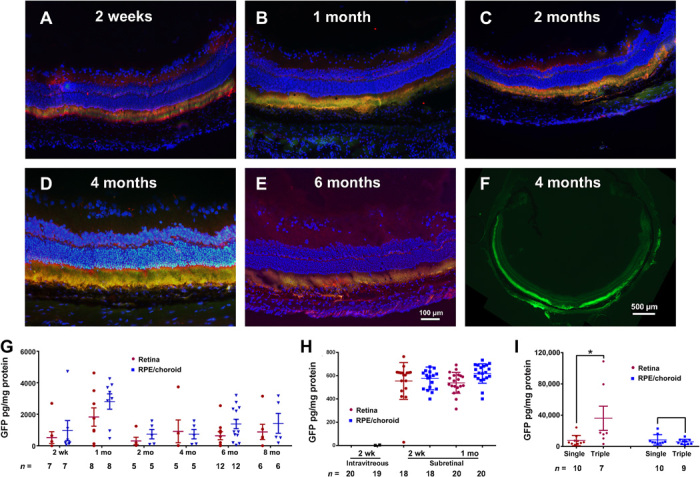
Brown Norway rats were given suprachoroidal, intravitreous, or subretinal injection of PBAE NPs containing 1 μg of CMV-GFP expression plasmid. Ocular sections immunohistochemically stained for GFP (Hoechst counterstain) merged with fluorescent image showed GFP in photoreceptor inner and outer segments and RPE 2 weeks (A), 1 month (B), 2 months (C), 4 months (D), and 6 months (E) after vector injection. Low magnification showed GFP fluorescence around the entire circumference of the eye (F). ELISA showed high levels of GFP in retina and RPE/choroid from 2 weeks to 8 months (last time point measured). (G). GFP was undetectable in retina and RPE/choroid 2 weeks after intravitreous injection of PBAE NPs, but there were high levels 2 weeks and 1 month after subretinal injection (H). Compared with eyes given a single suprachoroidal injection of PBAE NPs containing 1 μg of CMV-GFP, those given three injections had significantly higher levels of GFP in retina, but not RPE/choroid (I). *P = 0.018 by two-sample t test with equal variance for comparison with corresponding single injection control.
Suprachoroidal injection of NPs containing a VEGF expression plasmid
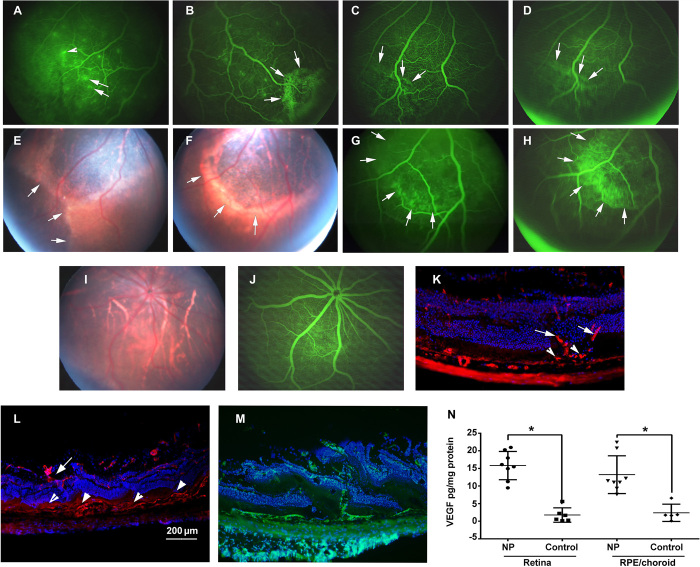
Expression of GFP provides valuable information to assess the localization and level of expression, but it is difficult to put that into context, because GFP has no biological activity. Therefore, we sought to express a protein with well-characterized biological activity in the retina. We have a good understanding of the effects of high levels of vascular endothelial growth factor (VEGF) in the retina, because we previously generated transgenic mice in which the rhodopsin promoter drives expression of VEGF165 in photoreceptors and characterized the effects over time (30). A complementary DNA (cDNA) for VEGF165 was substituted for GFP in pEGFP-N1 to generate a VEGF expression plasmid, pVEGF, which was incorporated into PBAE NPs. Four weeks after suprachoroidal injection of PBAE NP containing 1 μg of pVEGF, fluorescein angiography (FA) in some rats showed small subretinal hyperfluorescent spots with fuzzy borders indicating leakage (Fig. 4A, arrows) and staining of the walls of some overlying retinal vessels, another indicator of vascular leakage (Fig. 4A, arrowhead). Larger subretinal hyperfluorescent lesions were seen in other rats 4 weeks after NP injection (Fig. 4B). Subretinal neovascularization (NV) is diagnosed in patients when the borders of a hyperfluorescent lesion seen on an image taken relatively soon after fluorescein injection show expansion and reduced sharpness of its borders on an image taken later, an indicator of spread of the dye from an intravascular to an extravascular space. This sign of subretinal NV was seen in sequential images obtained about a minute apart in an FA of a rat eye 8 weeks after pVEGF NP injection (Fig. 4, C and D, arrows). Five months after suprachoroidal injection of pVEGF NPs, there were large areas of subretinal fibrosis indicated by hypopigmented, irregular tissue with sharp borders passing under retinal vessels (Fig. 4, E and F, arrows). An FA of the eye shown in the fundus photograph in Fig. 4F showed hyperfluorescence within a small portion of the lightly pigmented tissue on an image taken early after fluorescein injection (Fig. 4G, arrows), with the remainder of the lesion becoming hyperfluorescent in an image taken about a minute later (Fig. 4H, arrows). Such slow filling with fluorescein dye is typical of subretinal fibrovascular tissue. Figure 4I shows an ocular section of the eye of a 4-month-old rho/VEGF transgenic mouse histochemically stained with Griffonia simplicifolia lectin (GSA), which selectively stains vascular cells. It shows vessels originating from the deep capillary bed of the retina, passing through the outer nuclear layer (arrows) resulting in extensive subretinal NV (arrowheads). The phenotype was identical in Brown Norway rats 4 months after suprachoroidal injection of PBAE NP containing 1 μg of pVEGF (Fig. 4J) with new vessels originating from the deep capillary bed, passing through the outer nuclear layer (arrow) to severe subretinal NV (arrowheads). In some eyes, the NV was so severe that it destroyed the outer nuclear layer in many locations because of the numerous vessels that traversed it (Fig. 4K). Two weeks after suprachoroidal injection of NPs containing 1 μg of pVEGF, VEGF protein levels were significantly elevated in retinal and RPE/choroid homogenates (Fig. 4L).
Fig. 4. Nonviral suprachoroidal gene transfer of Vegf causes subretinal NV progressing to subretinal fibrosis.
Rats were given suprachoroidal injection of PBAE NPs containing 1 μg of pVEGF. After 4 weeks, FA showed subretinal hyperfluorescence (A and B) (arrows) and leakage from retinal vessels (arrowhead). After 8 weeks, subretinal hyperfluorescence (C) (arrows) spread over a minute (D) (arrows), indicating leakage from subretinal NV. After 5 months, there was subretinal fibrosis (E and F) that stained with fluorescein (G and H) (arrows). An eye injected with control vector showed normal fundus (I) and FA (J). Four-month-old rho/VEGF mouse (K) showed new vessels from deep capillaries (arrows) to subretinal space (arrowheads; DyLight 594–conjugated GSA). Four months after suprachoroidal injection of NPs containing 1 μg of pVEGF, new vessels extended from deep capillaries to severe subretinal NV (L) and, in another eye, showed severe NV with disrupted retina (M) (fluorescein isothiocyanate GSA). Two weeks after suprachoroidal injection of 1 μg of pVEGF in NP, ELISA showed significantly higher VEGF in retina or RPE/choroid versus controls (N) (n = 8 NPs and n = 6 controls). *P < 0.0001 for retina and for RPE/choroid by two-sample t test with unequal variance for comparison with control. Scale bar, 200 μm.
Effects of suprachoroidal or subretinal injection of NPs containing an expression plasmid for a VEGF-neutralizing protein
To explore the therapeutic potential of our nonviral gene transfer platform, we substituted a cDNA for a novel VEGF-neutralizing protein consisting of three repeats of domains 1 to 3 of Flt1 coupled together and to the coding sequence of immunoglobulin 1 (IgG1) Fc by GGGGS linkers (3sFlt1Fc) for GFP in pEGFP-N1 to generate p3sFlt1Fc, which was incorporated into PBAE NPs. Two weeks after suprachoroidal injection of PBAE NP containing 1 μg of p3sFlt1Fc in Brown Norway rats, high levels of 3sFlt1Fc protein were measured in retinal homogenates (Fig. 5A). Two weeks after suprachoroidal injection of PBAE NP containing 1 μg of p3sFlt1Fc or 1 μg of a CMV-Luciferase expression plasmid (pCMV-Luc) as control, rats were given an intravitreous injection of 100 ng of recombinant VEGF165, and 24 hours later, retinal vascular permeability was assessed by measurement of the amount of intravenously injected Evans Blue dye that had leaked into the retina. The mean vitreous level of Evans Blue dye per milligram of retina was significantly less in p3sFlt1Fc NP-injected eyes than in those that had been injected with NPs containing control pCMV-Luc (Fig. 5B).
Fig. 5. Effect of suprachoroidal or subretinal injection of NPs containing a VEGF-neutralizing protein expression plasmid.
There was a significant increase in sFlt1 retinal protein 1 month after suprachoroidal injection of PBAE NPs containing 1 μg of p3sFlt1Fc (A) (n = 5; *P = 0.043 by Wilcoxon signed-rank test). Two weeks after suprachoroidal injection of 1 μg of p3sFlt1Fc in NPs (one eye) and 1 μg of pCMV-Luc in NP (other eye), 100 ng of VEGF165 was injected into both eyes. After 12 hours, Evans Blue assay showed significantly less dye leakage in p3sFlt1Fc-injected eyes (B) (n = 7; *P = 0.011 by paired t test). At P14, rho/VEGF mice were given subretinal injection of 1 μg of p3sFlt1Fc in NP (one eye) and 1 μg of pCMV-Luc in NP (other eye). At P35 (n = 8), RPE/choroid flat mounts stained with DyLight 594–labeled GSA showed red dots throughout control flat mounts (C) seen to be subretinal neovascularization (NV) by magnification (C) (upper right), which was decreased in sFlt1 NP-injected eyes (D) (magnified box, lower left). The mean area of NV per flat mount was significantly less in the p3sFlt1Fc NP group (E) (*P = 0.025 by Wilcoxon signed-rank test). This difference was maintained at P70 (F to H) (n = 6; *P = 0.046 by Wilcoxon signed-rank test).
The efficacy of NPs containing p3sFlt1Fc was also tested in the rho/VEGF transgenic mouse model of subretinal NV. At postnatal day 14, rho/VEGF mice were given a subretinal injection of PBAE NPs containing 1 μg of p3sFlt1Fc or pCMV-Luc. At P35, retinal flat mounts showed significantly less mean area of subretinal NV per retina in eyes injected with p3sFlt1Fc NP versus those injected with pCMV-Luc NPs (Fig. 5, C to E). This difference was maintained at P70 (Fig. 5, F to H).
DISCUSSION
Nonviral gene transfer with polymeric vectors provides a means to avoid issues related to immune response but has been hampered by poor transfection efficiency. In this study, we demonstrate a PBAE polymeric vector that has excellent transfection efficiency in vivo. The specific structure of PBAE polymer (Fig. 1) used in this study was selected on the basis of initial in vitro transfection screening results in human retinal endothelial cells and RPE cells (28) and expression of a reporter gene in retina 72 hours after subretinal injection of PBAE NP carrying plasmid DNA (pDNA) (31). We now show that suprachoroidal injection of PBAE NPs results in transfection of photoreceptors and RPE throughout the entire rat eye including regions remote from the injection site. While diffusion of polymeric NPs in tissue has been previously documented (32), the lateral and radial penetration of PBAE NPs from the suprachoroidal space is most likely due to a transient pressure increase that is induced by the introduced volume into a confined space (33). Expression was quite strong 2 weeks after injection and was maintained for at least 8 months, the longest time point examined. Compared to a single suprachoroidal injection of NPs containing pEGFP-N1, three injections resulted in a fivefold increase in ocular expression of GFP, demonstrating the feasibility of boosting expression by repeated injections.
VEGF is a critical stimulus for the development of choroidal NV in patients with neovascular AMD (30, 34–36). Because of the importance of VEGF in human retinal disease, the effects of prolonged high-level production of VEGF in the retina have been extensively studied. Transgenic mice, in which the very strong rhodopsin promoter drives expression of VEGF in photoreceptors, develop NV that originates from the deep capillary bed of the retina and grows through the outer nuclear layer containing the cell bodies of photoreceptors into the subretinal space. Suprachoroidal injection of PBAE NPs containing a VEGF expression plasmid caused a similar, but more severe, phenotype with new vessels originating from the deep capillary bed of the retina and growing into the subretinal space. The NV started in the periphery near the injection site and, over time, extended posteriorly to involve a large part of the eye. In some areas, the NV growing through the outer nuclear layer was so severe that the outer nuclear layer was fragmented into small remaining islands of cells (Fig. 4K). In patients with neovascular AMD, long-standing choroidal NV results in subretinal fibrosis, which has historically been termed a disciform scar (37). Extensive subretinal fibrosis that is typical of disciform scars was seen 5 months after suprachoroidal injection of NPs containing pVEGF (Fig. 4, E to H), demonstrating long-term high expression of VEGF that phenocopies a late stage of a human disease process that, to our knowledge, has not previously been seen in other animal models of choroidal NV. These experiments serve two purposes. They illustrate the capacity of the suprachoroidal PBAE vector platform to provide high-level, long-term expression of a biologically active protein in the retina, causing more severe consequences than transgenic expression of the same protein with a strong retina-specific promoter. They also provide a new animal model that mimics the chronicity of pathology seen in patients with neovascular AMD culminating in the late stage of the disease not seen in previous models.
Subretinal injection of an AAV8 vector expressing a VEGF-neutralizing protein suppresses subretinal NV in the well-characterized rho/VEGF transgenic mouse model of subretinal NV (38). Subretinal injection of PBAE NPs containing p3sFlt1Fc coding for a VEGF-binding protein also suppressed subretinal NV in rho/VEGF mice. In rats, suprachoroidal injection of p3sFlt1Fc NPs resulted in high levels of the VEGF-binding protein in the eye and suppressed VEGF-induced retinal vascular leakage. This provides proof of concept for the therapeutic potential of suprachoroidal injection of PBAE vectors. The potential clinical impact of this therapy is high because suprachoroidal injections of PBAE vectors can be done in an outpatient setting, does not require an invasive surgical procedure that carries risk, does not require separation of the photoreceptors from the RPE that carries additional risk, and can be repeated to boost expression multiple times without activating immune response. The utilization of this technology to provide tunable, sustained suppression of a VEGF-neutralizing protein could benefit millions of patients with retinal/choroidal vascular diseases including neovascular AMD, diabetic retinopathy, and retinal vein occlusion (36).
Another application of suprachoroidal gene transfer that will be explored in future studies is gene replacement for inherited retinal degenerations. The characteristics of the platform discussed above including its excellent safety profile that does not require a surgical procedure or separation of photoreceptors and RPE and ability to perform it in an outpatient setting make it particularly appealing for this application, which, for best outcomes, should be done in very young patients who, with many types of degeneration, will have normal central vision and a lot to lose if things do not go well. The large capacity of PBAE vectors allowing incorporation of large genes such as the Stargardt disease gene, Abca4, is another major advantage.
In summary, we have described a new nonviral vector with high transfection efficiency combined with a noninvasive mode of delivery that provides widespread gene transfer throughout the eye. In vivo transfection efficacy is shown in both mouse and rat models, with the nonviral delivery of plasmids encoding GFP, VEGF, and a novel VEGF-neutralizing protein. The potential impact on the field is substantial because (i) PBAE vectors, unlike viral vectors, are nonimmunogenic, which can enhance safety and allow repeated injections to boost expression when needed; (ii) PBAE vectors have greater carrying capacity than AAV vectors, allowing transfer of very large genes; and (iii) suprachoroidal injection of PBAE vectors allows gene therapy in an outpatient setting without the risks associated with vitrectomy and separating the retina from the RPE. Gene therapy for inherited retinal degenerations is best done in children in whom there are still a large number of surviving photoreceptors to be rescued, and vitrectomy is particularly problematic in children. While the potential is clearly seen in these studies, additional studies are needed to optimize the PBAE polymer, the expression plasmid, promoter and enhancer elements, and transgenes for a wide variety of translational applications. The potential for nonviral long-term expression of therapeutic proteins in the eye and replacement of dysfunctional and/or toxic mutated proteins is exciting.
MATERIALS AND METHODS
Experimental design
The initial objective was to determine whether the cellular location, extent, and level of expression could be achieved after suprachoroidal injection of PBAE vector NPs containing a GFP reporter gene. The extent of GFP expression was assessed by fluorescence microscopy of retinal and RPE/choroid flat mounts and of serial ocular sections through the entire globe. Immunohistochemical staining for GFP was used to confirm that fluorescence was due to GFP. The cellular localization of GFP was determined on high-magnification views of fluorescent and anti-GFP images. The level of GFP was quantified by ELISA.
The second objective was to assess the effect of expressing a protein with biologic activity by suprachoroidal nonviral gene transfer. Our laboratory has a great deal of experience with transgenic expression of VEGF165 in the retina, and we are knowledgeable regarding the range of phenotypes caused by overexpression of VEGF. Therefore, we sought to express VEGF165 by nonviral suprachoroidal gene transfer and characterized the resulting phenotype by in vivo imaging over time, including FA and fundus photography, and histopathology using vascular-specific stains.
The third objective was to explore the therapeutic potential of nonviral gene transfer. We packaged an expression plasmid for VEGF-neutralizing protein in PBAE NP vector and performed short- and long-term studies. For short-term studies, we did suprachoroidal injection of the PBAE NPs containing the anti-VEGF expression plasmid, and after 2 weeks, we measured the expression level of the anti-VEGF protein and tested its effect on VEGF-induced vascular leakage. For the long-term studies, we used a well-characterized transgenic mouse model in which VEGF is expressed in photoreceptors driven by the rhodopsin promoter. These mice develop subretinal NV that worsens over time so we performed injections with anti-VEGF or control vector at P14 and measured the amount of subretinal NV at P35 and P70.
Polymer synthesis
1,4-Butanediol diacrylate (B4), 5-amino-1-pentanol (S5), and 1-(3-aminopropyl)-4-methyl-piperazine (E7) were purchased from Alfa Aesar (Ward Hill, MA). PBAE polymer was synthesized by a two-step reaction. First, an acrylate-terminated base polymer was first synthesized by Michael addition reaction of 1,4-butanediol diacrylate (B4) with 5-amino-1-pentanol (S5) at 1.05:1 acrylate:amine monomer molar ratio in the dark under magnetic stirring for 24 hours at 90°C. In the second step, the acrylate-terminated base polymer was endcapped through another Michael addition reaction in the presence of excess of primary amine-containing small-molecule 1-(3-aminopropyl)-4-methyl-piperazine (E7). For example, 625 mg of polymer in 4 ml of tetrahydrofuran (THF) was mixed with 4 ml of 0.5 M end-capping amine solution in THF and stirred at 500 rpm overnight at 23°C. The final polymer (termed “PBAE 457”) was purified by precipitation into diethyl ether and stored in dimethyl sulfoxide (DMSO) at 100 mg/ml with desiccant at −20°C until use. Molecular weight was determined by dissolving PBAE 457 at 5 mg/ml in 94% THF, 5% DMSO, and 1% piperidine. The polymer solution was filtered through a 0.2-μm polytetrafluoroethylene (PTFE) syringe filter and then analyzed by gel permeation chromatography using polystyrene standards.
Plasmid preparation
pEGFP-N1, a plasmid in which a CMV promoter drives expression of GFP, was obtained from Addgene (6085-1, Watertown, MA), and pCMV-Luc, a plasmid in which the CMV promoter drives expression of firefly luciferase, was obtained from Elim Biopharmaceuticals Inc. (San Francisco, CA). An expression plasmid for VEGF (pVEGF) was generated by excising EGFP (enhanced GFP) from pEGFP-N1 and replacing it with the coding sequence for VEGF165 excised from pRho-hVEGF (30). A cDNA consisting of three repeats of domains 1 to 3 of Flt1, which are critical for VEGF binding, coupled together with GGGGS linker was synthesized by Integrated DNA Technologies (Coralville, IA). The 3sFlt1 cDNA was packaged in pUCIDT, and sequence was confirmed. The 3sFlt1 cDNA was excised from pUCIDT and inserted into pFUSE-hIgG1-FC (pfuse-hg1fc1, InvivoGen, San Diego, CA). The 3sFlt1Fc fragment was then excised and inserted into pEGFP-N1 after EGFP was removed to produce p3sFlt1Fc, the expression plasmid for the VEGF-binding protein. All plasmids were prepared using a Qiagen HiSpeed endotoxin-free column (Germantown, MD). Final DNA concentrations were determined by NanoDrop. pDNA was concentrated with a vacuum drier to 800 to 1000 ng/μl for further use.
NP formulation
pDNA-carrying NPs were formulated by electrostatic binding of positively charged PBAE polymer and negatively charged expression plasmids (pEGFP-N1, pVEGF, p3sFlt1Fc, or pCMV-Luc). PBAE 457 in DMSO at 100 mg/ml and pDNA in water or 1× Tris EDTA (TE) buffer at 1 mg/ml were both diluted with 25 mM sodium acetate buffer (pH 5, NaAc) to 5.55 and 0.28 mg/ml, respectively. Then, polymer and pDNA solutions were mixed at 3:2 (v/v) ratio to achieve a 30 (w/w) ratio of polymer to DNA and then incubated for 10 min to allow particle complexation. To lyophilize the NPs, final NP solution was mixed with sucrose as a cryoprotectant to a final 3% sucrose solution and then aliquoted such that each aliquot had 17.4 μg of pDNA and 5 mg of sucrose. Aliquoted NPs were lyophilized and stored with desiccant at −20°C until use. Particle size and zeta potential before and after lyophilization were measured by dynamic light scattering using Zetasizer Nano ZS (Malvern Instruments). Freshly prepared NPs were diluted with 25 mM NaAc and 10 mM NaCl for particle size and zeta potential measurements, respectively. Lyophilized particles were reconstituted with water first to sucrose (100 mg/ml) and then diluted with 10% sucrose solution and 10 mM NaCl for particle size and zeta potential measurements, respectively.
Experimental animals
All experimental animals were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for Use of Animals in Ophthalmic and Vision Research, and protocols were reviewed and approved by the Johns Hopkins University Animal Care and Use Committee. Norway Brown rats were purchased from Charles River (Frederick, MD, USA). Rho/VEGF transgenic mice in which the rhodopsin promoter drives expression of VEGF165 in photoreceptors have been described (30).
Suprachoroidal injection of NPs in rats
Rats were anesthetized by ketamine and xylazine, and 0.5% proparacaine eye drops were applied. The eyes were visualized with the Zeiss Stereo Dissecting Microscope (Zeiss, Oberkochen, Germany). A 30-gauge needle on a 1-ml syringe was used to make a partial thickness (4/5 way through sclera) circumferential opening in the sclera 1 mm posterior to the limbus, and a 34-gauge needle with a blunt 45° bevel connected to a 5-μl Hamilton syringe (Hamilton Company, Reno, NV) containing vector was inserted into the scleral opening, with the bevel facing downward and slowly advanced through the remaining scleral fibers into the suprachoroidal space. The plunger of the syringe was slowly advanced to expand the suprachoroidal space and inject 3 μl containing PBAE NPs. After 30 s, a cotton swab was applied to the injection site as the needle was removed to prevent reflux.
Subretinal injection of NPs in mice
Mice were anesthetized, pupils were dilated, and eyes were visualized with the Stereo Dissecting Microscope. A 30-gauge needle on a 1-ml syringe was used to make a partial thickness opening in the sclera 1 mm posterior to the limbus, and a 34-gauge needle with a blunt 45° bevel connected to a 5-μl Hamilton syringe containing NPs was inserted into the scleral opening and slowly advanced through the remaining scleral fibers into the subretinal space. One microliter of vehicle containing NPs was injected, and the needle was withdrawn while holding a cotton-tipped applicator over the injection site.
ELISAs for EGFP, VEGF, or sFlt1
Mice were euthanized, and the entire retina or the entire eyecup, consisting of RPE, choroid, and sclera, was placed in phosphate-buffered saline (PBS) containing protease inhibitor cocktail (11836170001, Roche, Mannheim, Germany) and sonicated for 5 s. Samples were centrifuged at 20,000g for 5 min, and the protein concentrations were determined by a CCB-G250 binding assay (#5000006, Bio-Rad, Hercules, CA). GFP protein levels were measured using a GFP SimpleStep ELISA kit (ab171581, Abcam, Cambridge, MA). Briefly, 50 μl of sample or GFP standard dilutions was added to duplicate wells of 96-well plates, followed by 50 μl of anti-GFP antibody. Plates were incubated at 23°C for 1 hour and washed five times with rinse buffer, and after addition of 100 μl of 3,3’,5,5’-tetramethylbenzidine (TMB) substrate solution, they were incubated at 23°C in the dark for 10 min. After addition of 100 μl of stop solution, absorption was measured at 450 nm with the SpectraMax Plus 384 Microplate Reader. Measurement of VEGF levels in homogenates of retina or RPE/choroid was done with a VEGF ELISA kit (DVE00, R&H, Minneapolis, MN). ELISA to measure Flt1 extracellular domain (amino acids 1 to 328) was done with an sFlt1 ELISA kit (MBS2516240, MyBioSource, San Diego, CA) using the manufacturer’s instructions.
Fundus photography and FA
Rats were anesthetized with an intramuscular injection of ketamine (80 mg/kg) and xylazine (10 mg/kg), and pupils were dilated with 1% tropicamide (Akorn, Lake Forest, IL). A drop of Goniotaire (Altaire Pharmaceuticals Inc., Aquebogue, NY) was placed in the eyes, and retinal images were obtained with a Micron III biomicroscope (Phoenix Research Laboratories, Pleasanton, CA). For FA, the appropriate filters were inserted, an intraperitoneal injection of 60 μl of 10% sodium fluorescein (Akorn) was given, and retinal images were obtained at 30 s and, in some instances, at subsequent time points.
Immunohistochemistry
At several time points after suprachoroidal injection of 3 μl of NPs containing 1 μg of pEGFP-N1, rats were euthanized and eyes were frozen in optimal cutting temperature embedding compound (Miles Diagnostics, Elkhart, IN). Frozen sections (10 μm) were fixed in 4% paraformaldehyde, and nonspecific binding was blocked by a 30-min incubation in 8% normal rabbit serum at 25°C. The sections were incubated with a polyclonal antibody (1:300) against EGFP conjugated with Alexa Fluor 594 (A-21312, ThermoFisher, Waltham, MA) at 23°C for 2 hours. After washing with PBS containing 0.05% Tween 20, slides were counterstained with Hoechst 33258 (861405, Sigma-Aldrich, St. Louis, MO) and viewed with a Nikon fluorescence microscope. Ocular sections from rats that had been given a suprachoroidal injection of NPs containing VEGF expression plasmid or RPE whole mounts from rho/VEGF mice were stained with fluorescein isothiocyanate–labeled (FL-1201, Vector Laboratories, Burlingame, CA) or DyLight 594–labeled Griffonia simplicifolia lectin (DL-1207, Vector Laboratories), which selectively stains vascular cells as previously described (38).
RPE/choroid flat mounts
Rats were given a suprachoroidal injection (3 μl) of NPs containing 1 μg of pEGFP-N1 and then, 3 days later, were given an identical repeat suprachoroidal injection in the same location followed by a third identical injection in another 3 days. After 3 weeks, the rats were euthanized and eyes were removed and fixed in 4% paraformaldehyde overnight. The anterior segments, vitreous, and retina were removed, and five radial cuts were made in the remaining eyecups containing RPE, choroid, and sclera. The eyecups were mounted on slides to form RPE/choroid flat mounts, which were examined by fluorescence microscopy.
Measurement of retinal vascular permeability
Two weeks after suprachoroidal injection of p3sFlt1Fc NPs in one eye and pCMV-Luc NPs in the fellow eye, rats were anesthetized, pupils were dilated, and eyes were visualized with the Stereo Dissecting Microscope. A 30-gauge needle on a 1-ml syringe was used to make a partial thickness opening in the sclera 1 mm posterior to the limbus, a 34-gauge needle connected to a 5-μl Hamilton syringe was used to penetrate the remaining fibers, the needle was inserted into the vitreous cavity, and 100 ng of VEGF165 (293-VE-001MG/CF, R&D Systems, Minneapolis, MN) was injected. After 12 hours, Evans Blue dye (50 mg/kg body weight) was injected in the tail vein, and after 2 hours, the chest cavity was opened and rats were perfused through the left ventricle with 10 ml of cold PBS. Retinas were dissected and weighed, and dye was extracted by incubating each retina in 150 μl of formamide overnight at 70°C with gentle shaking. The extract was centrifuged at 20,000g for 45 min at 4°C. The absorbance of supernatants (50 μl) was measured in a microplate reader at 620 nm. The concentration of the dye in the samples was calculated from a standard curve (100 to 5000 ng/ml) of Evans Blue dye in formamide and reported as nanograms of Evans Blue dye per milligram of retina.
Data analysis and statistical comparisons
Statistical analyses were performed using Stata version 14.2 (College Station, TX). Sample sizes were determined from previous experiments testing for the same outcomes, and experimental numbers are provided in each figure legend. Measurements were taken from distinct samples, and no data were excluded. The distribution of values in all datasets was evaluated by the Shapiro-Wilk normality test. The datasets for Figs. 2 (G and H), 3L, and 5 (A, E, and H) were not normally distributed, and therefore, nonparametric tests were used for statistical comparisons. The datasets for Figs. 2I, 4L, and 5B were normally distributed, and therefore, parametric tests were used for statistical comparisons. All the statistical tests were two-sided and conducted at 5% statistical significance.
Supplementary Material
Acknowledgments
Funding: This work was supported by R01EY031097, R21EY026148, R01EY028996, and R01EB022148 from the NIH and Biostatistics Core Grant EY01765 to the Wilmer Eye Institute from the NIH. It was also supported by the Dr. H. James and Carole Free Catalyst Award and unrestricted grant from Research to Prevent Blindness; the Louis B. Thalheimer Translational Fund; a grant from the Barth Foundation; a scholarship to J.K. from Samsung; and unrestricted grants from Conrad and Lois Aschenbach, Per Bang-Jensen, Andrew and Yvette Marriott, and Jean Lake. Author contributions: J.S. helped to design experiments, performed experiments, analyzed data, and edited and approved the manuscript. J.K. helped to design experiments, performed experiments, analyzed data, and edited and approved the manuscript. S.Y.T. helped to design experiments, performed experiments, analyzed data, and edited and approved the manuscript. K.D. helped to design experiments, performed experiments, analyzed data, and edited and approved the manuscript. Z.H. performed experiments, analyzed data, and edited and approved the manuscript. D.L. performed experiments, analyzed data, and edited and approved the manuscript. J.W. analyzed data, performed statistical analyses, and edited and approved the manuscript. J.J.G. developed PBAE vectors, designed experiments, analyzed data, and edited and approved the manuscript. P.A.C. designed experiments, analyzed data, wrote the first draft of manuscript, and approved the manuscript. Competing interests: P.A.C., J.J.G., J.K., and J.S. are inventors on a pending patent related to this work (no. 16/753,245, filed on 2 April 2020). The authors declare no other competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors. The gene delivery polymer can be provided by JHU pending scientific review and a completed material transfer agreement. Requests for polymer should be submitted to green@jhu.edu. No unique computer code was generated for use in this study.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/27/eaba1606/DC1
REFERENCES AND NOTES
- 1.Maguire A. M., Simonelli F., Pierce E. A., Pugh E. N. Jr., Mingozzi F., Bennicelli J., Banfi S., Marshall K. A., Testa F., Surace E. M., Rossi S., Lyubarsky A., Arruda V. R., Konkle B., Stone E., Sun J., Jacobs J., Dell'Osso L., Hertle R., Ma J.-x., Redmond T. M., Zhu X., Hauck B., Zelenaia O., Shindler K. S., Maguire M. G., Wright J. F., Volpe N. J., McDonnell J. W., Auricchio A., High K. A., Bennett J., Safety and eficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bainbridge J. W. B., Smith A. J., Barker S. S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G. E., Stockman A., Tyler N., Petersen-Jones S., Bhattacharya S. S., Thrasher A. J., Fitzke F. W., Carter B. J., Rubin G. S., Moore A. T., Ali R. R., Effect of gene therapy on visual function in Leber's Congenital Amaurosis. N. Engl. J. Med. 358, 2231–2239 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Hauswirth W. W., Aleman T. S., Kaushal S., Cideciyan A. V., Schwartz S. B., Wang L., Conlon T. J., Boye S. L., Flotte T. R., Byrne B. J., Jacobson S. G., Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum. Gene Ther. 19, 979–990 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cremers F. P., van de Pol D. J., van Kerkhoff L. P., Wieringa B., Ropers H. H., Cloning of a gene that is rearranged in patients with choroideraemia. Nature 347, 674–677 (1990). [DOI] [PubMed] [Google Scholar]
- 5.Xue K., Jolly J. K., Barnard A. R., Rudenko A., Salvetti A. P., Patrício M. I., Edwards T. L., Groppe M., Orlans H. O., Tolmachova T., Black G. C., Webster A. R., Lotery A. J., Holder G. E., Downes S. M., Seabra M. C., MacLaren R. E., Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia. Nat. Med. 24, 1507–1512 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campochiaro P. A., Nguyen Q. D., Shah S. M., Klein M. L., Holz E., Frank R. N., Saperstein D. A., Gupta A., Stout J. T., Macko J., Bartolomeo R. D., Wei L. L., Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: Results of a phase I clinical trial. Hum. Gene Ther. 17, 167–176 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Rakoczy E. P., Lai C.-M., Magno A. L., Wikstrom M. E., French M. A., Pierce C. M., Schwartz S. D., Blumenkranz M. S., Chalberg T. W., Degli-Esposti M. A., Constable I. J., Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomized clinical trial. Lancet 386, 2395–2403 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Campochiaro P. A., Lauer A. K., Sohn E. H., Mir T. A., Naylor S., Anderton M. C., Kelleher M., Harrop R., Ellis S., Mitrophanous K. A., Lentiviral vector gene transfer of endostatin/angiostatin for macular degeneration (GEM) study. Hum. Gene Ther. 28, 99–111 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heier J. S., Kherani S., Desai S., Dugel P., Kaushal S., Cheng S. H., Delacono C., Purvis A., Richards S., Le-Halpere A., Connelly J., Wadsworth S. C., Varona R., Buggage R., Scaria A., Campochiaro P. A., Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: A phase 1, open-label trial. Lancet 389, 50–61 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Li Q., Miller R., Han P.-Y., Pang J., Dinculescu A., Chiodo V., Hauswirth W. W., Intracoular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol. Vis. 14, 1760–1769 (2008). [PMC free article] [PubMed] [Google Scholar]
- 11.Reichel F. F., Dauletbekov D. L., Klein R., Peters T., Ochakovski G. A., Seitz I. P., Wilhelm B., Ueffing M., Biel M., Wissinger B., Michalakis S., Bartz-Schmidt K. U., Fischer M. D.; RD-eCURE Consortium , AAV8 can induce innate and adaptive immune response in the primate eye. Mol. Ther. 25, 2648–2660 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett J., Wellman J., Marshall K. A., Cague S. M., Ashtari M., Di Stefano-Pappas J., Elci O. U., Chung D. C., Sun J., Wright J. F., Cross D. R., Aravand P., Cyckowski L. L., Bennicelli J. L., Mingozzi F., Auricchio A., Pierce E. A., Ruggiero J., Leroy B. P., Simonelli F., High K. A., Maguire A. M., Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: A follow-on phase 1 trial. Lancet 388, 661–672 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalkara D., Kolstad K. D., Caporale N., Visel M., Klimczak R. R., Schaffer D. V., Flannery J. G., Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol. Ther. 17, 2096–2102 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi K., Igarashi T., Miyake K., Kobayashi M., Yaguchi C., Iijima O., Yamazaki Y., Katakai Y., Miyake N., Kameya S., Shimada T., Takahashi H., Okada T., Improved intravitreal AAV-mediated inner retinal gene transduction after surgical internal limiting membrane peeling in cynomologus monkeys. Mol. Ther. 25, 296–302 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalkara D., Byrne L. C., Klimczak R. R., Visel M., Yin L., Merigan W. H., Flannery J. G., Schaffer D. V., In vivo–directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 5, 189ra176 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Silvac I., Guay D., Mangion M., Champeil J., Baillet B., Non-viral nucleic acid delivery methods. Expert. Opin. Biol. Ther. 17, 105–118 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Green J. J., Langer R., Anderson D. G., A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc. Chem. Res. 41, 749–759 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhise N. S., Shmueli R. B., Gonzalez J., Green J. J., Assay for quantifying the number of plasmids encapsulated by polymer nanoparticles. Small 8, 367–373 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sunshine J. C., Peng D. Y., Green J. J., Uptake and transfection with polymeric nanoparticles are dependent on polymer end-group structure, but largely independent of nanoparticle physical and chemical properties. Mol. Pharm. 9, 3375–3383 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerrero-Cázares H., Tzeng S. Y., Young N. P., Abutaleb A. O., Quiñones-Hinojosa A., Green J. J., Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano 8, 5141–5153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangraviti A., Tzeng S. Y., Kozielski K. L., Wang Y., Jin Y., Gullotti D., Pedone M., Buaron N., Liu A., Wilson D. R., Hansen S. K., Rodriguez F. J., Gao G.-D., Meco F. D., Brem H., Olivi A., Tyler B., Green J. J., Polymeric nanoparticles for nonviral gene therapy extend brain tumor survival in vivo. ACS Nano 9, 1236–1249 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel S. R., Berezovsky D. E., McCarey B. E., Zarnitsyn V., Edelhauser H. F., Prausnitz M. R., Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest. Ophthalmol. Vis. Sci. 53, 4433–4441 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen M., Li X., Liu J., Han Y., Cheng L., Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model. J. Control. Release 203, 109–117 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Yeh S., Kurup S. K., Wang R. C., Foster C. S., Noronha G., Nguyen Q. D., Do D. V.; DOGWOOD Study Team , Suprachoroidal injection of triamcinolone acetonide, CLS-TA, for macular edema due to noninfectious uveitis: A randomized, Phase 2 Study (DOGWOOD). Retina 39, 1880–1888 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Campochiaro P. A., Wykoff C. C., Brown D. M., Boyer D. S., Barakat M., Taraborelli D., Noronha G.; Tanzanite Study Group , Suprachoroidal triamcinolone acetonide for retinal vein occlusion: Results of the Tanzanite Study. Ophthalmol. Retina 2, 320–328 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Ding K., Shen J., Hafiz Z., Hackett S. F., Silva R. L. E., Khan M., Lorenc V. E., Chen D., Chadha R., Zhang M., Van Everen S., Buss N., Fiscella M., Danos O., Campochiaro P. A., AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression. J. Clin. Invest. 130, 4901–4911 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J., Kang Y., Tzeng S. Y., Green J. J., Synthesis and application of poly(ethylene glycol)-co-poly(beta-amino ester) copolymers for small cell lung cancer gene therapy. Acta Biomater. 41, 293–301 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shmueli R. B., Sunshine J. C., Xu Z., Duh E. J., Green J. J., Gene delivery nanoparticles specific for human microvasculature and macrovasculature. Nanomedicine 8, 1200–1207 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzeng S. Y., Green J. J., Polymeric nucleic acid delivery for immunoengineering. Curr. Opin. Biomed. Eng. 7, 42–50 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto N., Tobe T., Hackett S. F., Ozaki H., Vinores M. A., La Rochelle W., Zack D. J., Campochiaro P. A., Transgenic mice with increased expression of vascular endothelial growth factor in the retina: A new model of intraretinal and subretinal neovascularization. Am. J. Pathol. 151, 281–291 (1997). [PMC free article] [PubMed] [Google Scholar]
- 31.Sunshine J. C., Sunshine S. B., Bhutto I., Handa J. T., Green J. J., Poly(B-amino ester)-nanoparticle mediated transfection of retinal pigmented epithelial cells in vitro and in vivo. PLOS ONE 7, e37453 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barua S., Mitragotri S., Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 9, 223–243 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim Y. C., Oh K. H., Edelhauser H. F., Prausnitz M. R., Formulation to target to the ciliary body and choroid via the suprachoroidal space of th eeye using microneedles. Eur. J. Pharm. Biopharm. 95, 398–406 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwak N., Okamoto N., Wood J. M., Campochiaro P. A., VEGF is an important stimulator in a model of choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 41, 3158–3164 (2000). [PubMed] [Google Scholar]
- 35.Rosenfeld P. J., Brown D. M., Heier J. S., Boyer D. S., Kaiser P. K., Chung C. Y., Kim R. Y.; MARINA Study Group , Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 355, 1419–1431 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Campochiaro P. A., Aiello L. P., Rosenfeld P. J., Anti-vascular endothelial growth factor agents in the treatment of retinal disease. From bench to bedside. Ophthalmology 123, S78–S88 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Chandra S. R., Gragoudas E. S., Friedman E., Van Buskirk E. M., Klein M. L., Natural history of disciform degeneration of the macula. Am. J. Ophthalmol. 78, 579–582 (1974). [DOI] [PubMed] [Google Scholar]
- 38.Liu Y., Fortmann S. D., Shen J., Wielechowski E., Tretiakova A., Yoo S., Kozarsky K., Wang J., Wilson J. M., Campochiaro P. A., AAV8-antiVEGFfab ocular gene transfer for neovascular age-related macular degeneration. Mol. Ther. 26, 542–549 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/27/eaba1606/DC1