Abstract
There is growing evidence that COVID-19 not only affects the lungs but beyond that the endothelial system. Recent studies showed that this can lead to microcirculatory impairments and in consequence to functional disorders of all inner organs. The combination of endothelial dysfunction with a generalized inflammatory state and complement elements may together contribute to the overall pro-coagulative state described in COVID-19 patients leading to venular as well as to arteriolar occlusions.
Keywords: COVID-19, endocytosis, endothelial cell dysfunction, endotheliitis
In December 2019, an outbreak of pneumonia due to a novel corona virus – the severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) – occurred in Wuhan, Hubei province, China. The virus causing the corona virus disease 2019 (COVID-19) has since spread to many countries resulting in a pandemic [1]. Symptoms most commonly reported include fever, cough, shortness of breath and loss of sense of taste and smell. Older patients with co-morbidities are more likely to develop respiratory failure due to severe alveolar damage [2]. In more severe cases, the disease can also show a rapid progression to organ failure, with complications, such as shock, acute respiratory distress syndrome (ARDS), acute cardiac injury, acute kidney injury, disseminated intravascular coagulopathy (DIC), which may ultimately prove fatal [3]. Recent observations suggest that respiratory failure in COVID-19 is not driven by the development of ARDS alone [4], but that macro-vascular as well as micro-vascular thrombotic processes may play a role [5, 6]. It is becoming apparent that severe cases of COVID-19 are characterized by hyper inflammation and a thrombotic phenomenon. Such major adverse clinical events seem to suggest that in advanced stages of this disease one target is the endothelium, one of the largest organs in the human body. Whether vascular derangements in COVID-19 are due to endothelial cell dysfunction is currently unknown.
The main transmission route is through virus containing droplet or aerosol but also smear transmission is possible. The virus replicates in the upper respiratory tract decent to lower respiratory tract. The spread of the virus in the host is thought to occur also via the vascular system and transgression into mucosal tissues of nose, throat and especially of the lungs, where the endothelium comes into contact with SARS CoV-2 at an early stage. The virus enters endothelial cells by endocytosis via the binding of its spike glycoprotein to a cellular receptor which facilitates viral attachment to the surface of target cells [7–9]. Angiotensin-converting enzyme 2 (ACE2) was identified as the main receptor for severe acute respiratory syndrome corona virus (SARS-CoV 2) [10], which is abundantly expressed in the lungs, the respiratory epithelium and alveolar monocytes [11] and may explain the many cases of rapidly occurring lung failure. However, ACE2 is also expressed by endothelial cells [12–14] in the heart and the macro vascular system, gut, kidneys, liver, central nervous system, and adipose tissue [15–17]. As the density of ACE2 differs in the various tissues – very high in the lungs – the receptor density may correlate with the severity of the disease in those tissue [18–20]. In addition, there are further receptors on the surface of human cells which can mediate the entry of SARS-CoV-2, including transmembrane serine protease 2 (TMPRSS2 [19]), sialic acid receptors [21], and extracellular matrix metalloproteinase inducer (CD147 [22]). These four receptors are known to be expressed by endothelial cells [23–26].
Whereas unperturbed endothelial cells provide very potent anti-coagulant properties [27], exposure to inflammatory stimuli can rapidly lead to a procoagulant behavior. Very recently, Varga et al. found evidence of direct SARS-CoV-2 infection of endothelial cells in several organs and diffuse endothelial inflammation associated with apoptosis [28]. They described this state as endotheliitis with viral elements within endothelial cells and accumulation of inflammatory cells, with evidence of endothelial and inflammatory cell death. Endothelial cell injury can strongly activate the coagulation system via exposure of tissue factor and other pathways. Endothelial dysfunction refers to a systemic condition in which the endothelium loses its physiological properties, including the tendency to promote vasodilation, fibrinolysis, and anti-aggregation [27]. Narrowing of organ supplying arteries as well as microcirculatory disturbances in liver, spleen and kidneys in patients with severe COVID-19 were already described recently [29].
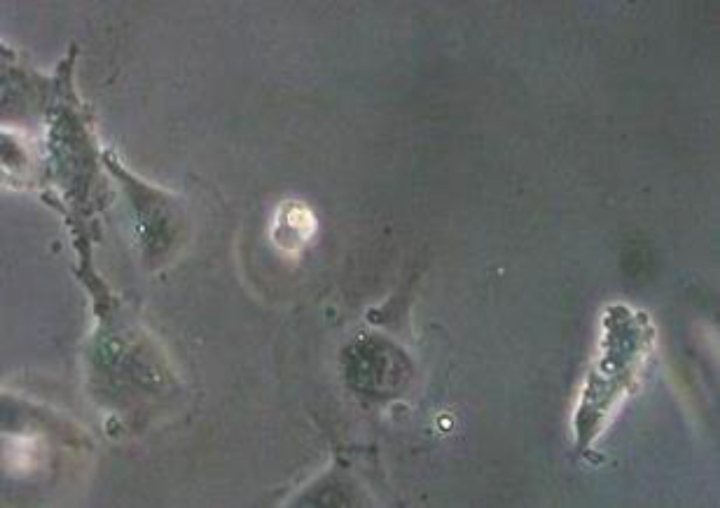
COVID-19-endotheliitis could explain the impaired microcirculation in different vascular beds [30] and their clinical sequelae in patients with COVID-19. During inflammatory activation or apoptosis, endothelial cells become pro-coagulant [31] and release microvesicles (MV) which can affect the function of target cells through surface interaction and receptor activation, cellular fusion and the delivery of intra-vesicular cargo [31]. Among others, endothelial MV have been described to affect hemostasis [27]. Figure 1 shows a picture with apoptotic human umbilical venous endothelial cells with massive signs of blebbing and detached microvesicles.
Fig.1.
Apoptotic endothelial cells with signs of blebbing and detached microvesicles (cLSM, objective ×40).
Pathological investigations and also imaging studies confirmed the COVID-19 disease as a thrombo-inflammatory process that initially affects the lungs and in consequence the perfusion, which consecutively can affect all organs of the body. It is well known that a variety of viruses can affect the coagulation system including HIV, Dengue virus, and Ebola virus [32, 33]. Very recently, Spiezia et al. reported that SARS-CoV-2 may predispose patients to thrombotic disease, both in the venous and arterial circulations [34]. Excessive inflammation, platelet activation, endothelial dysfunction, and stasis related to the infection were described [35], which can result in severe hypercoagulability and predispose to thrombosis. That has been related to deaths in critically ill COVID-19 cases [36]. A single-center retrospective cohort study of 183 patients with confirmed COVID-19 evaluated coagulation abnormalities that mimic disseminated intravascular coagulation (DIC) [37]. According to the International Society on Thrombosis and Hemostasis definition of DIC, 15 of 21 non-survivors (71%) were classified as having overt-DIC (≥5 points) at any time during follow-up, whereas only 1 of 162 survivors (0.6%) met these criteria (P < 0.001). Likewise, Tang et al. reported that 71.4% of non-survivors and 0.6% of survivors of COVID-19 showed evidence of overt DIC [38]. This is in line with another study, in which a clear correlation between D-dimer levels, disease progression and chest CT features suggesting venous thrombosis were reported [39]. Also, pulmonary embolism is with 30% significantly more frequent in COVID-19 patients [40] than usually occurring in critically ill patients without COVID-19 infection (1.3%, [41]) or in emergency department patients (3 to 10% [42]). Tee et al. were able show that in the lung a vascular areas occur which most likely represent 3–5 mm micro infarcts [43] which confirms the micro vascular involvement in the course of the disease.
In conclusion, it seems that COVID-19 is a disease affecting the lungs and, beyond that, the endothelial system. Recent studies show that this can lead to microcirculatory impairments, and in consequence to functional disorders of all inner organs. The combination of endothelial dysfunction with a generalized inflammatory state and complement elements may together contribute to the overall pro-coagulative state described in COVID-19 patients.
References
- [1].World Health Organization. Coronavirus disease 2019 (COVID-19). Situation report – 70 [Internet]. Geneva: World Health Organization; 2020. Available at: https://apps.who.int/iris/bitstream/handle/10665/331683/nCoVsitrep30Mar2020-eng.pdf (Accessed: 26 April, 2020).
- [2]. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical Course and Outcomes of Critically Ill Patients With SARS-CoV-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study. Lancet Respir Med. 2020;8(5):475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retro-spective study. Chin Med J (Engl). 2020. DOI: 10.1097/CM9.0000000000000824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020. DOI: 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Jung F, Krieger V, Hufert FT, Küpper J-H. How we should respond to the Coronavirus SARS-CoV-2 outbreak: A German perspective. Clin Hemorheol Microcirc. 2020. DOI: 10.3233/CH-170277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Oudkerk M, Büller HR, Kuijpers D, van Es N, Oudkerk SF, McLoud TC, Gommers, van Dissel J, Ten Cate H, van Beek EJ. Diagnosis, Prevention, and Treatment of thromboembolic Complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;201629 DOI: 10.1148/radiol.2020201629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Letko M, Marzi A and Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacorona viruses. Nat Microbiol. 2020;5:562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Lu G, Qiao C, Hu Y, Yuen KY, Wang Q, Zhou H, Yan J, Qi J. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020. DOI: 10.1016/j.cell.2020.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2. Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Monteil V KH, Prado P, Hagelkrüys A, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020. https://www.cell.com/pbassets/products/coronavirus/CELL_CELL-D-20-00739.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Nicin L, Abplanalp WT, Mellentin H, Kattih B, Tombor L, John D, Schmitto JD, Heineke J, Emrich F, Arsalan M, Holubec T, Walther T, Zeiher AM, Dimmeler S. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. European Heart Journal. 2020. DOI: 10.1093/eurheartj/ehaa311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Sluimer JC, Gasc JM, Hamming I, van Goor H, Michaud A, van den Akker LH, Jutten B, Cleutjens J, Bijnens AP, Corvol P, Daemen MJ and Heeneman S. Angiotensin-converting enzyme 2 (ACE2) expression and activity in human carotid atherosclerotic lesions. J Pathol. 2008;215:273–9. [DOI] [PubMed] [Google Scholar]
- [14]. Lu R, Zhao X, Li J, Ni P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet. 2020;395(10224):565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–10. [DOI] [PubMed] [Google Scholar]
- [16]. Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong J-C, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System. Circ Res. 2020;126(10). DOI: 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Fan J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. BioRXiV preprint Server. DOI: 10.1101/2020.02.03.931766 [DOI] [Google Scholar]
- [18]. Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T and Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Perico L, Benigni A, Remuzzi G. Should COVID-19 Concern Nephrologists? Why and to What Extent? The Emerging Impasse of Angiotensin Blockade. Nephron. 2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Matsuyama S, Nao N, Shirato K, Kawase M, Saito S, Takayama I, Nagata N, Sekizuka T, Katoh H, Kato F, Sakata M, Tahara M, Kutsuna S, Ohmagari N, Kuroda M, Suzuki T, Kageyama T, Takeda M. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A. 2020;117:7001–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Tortorici MA, Walls AC, Lang Y, Wang C, Li Z, Koerhuis D, Boons GJ, Bosch BJ, Rey FA, de Groot RJ and Veesler D. Structural basis for human coronavirus attachment to sialic acid receptors. Nat Struct Mol Biol. 2019;26:481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Chen Z, Mi L, Xu J, Yu J, Wang X, Jiang J, Xing J, Shang P, Qian A, Li Y, Shaw PX, Wang J, Duan S, Ding J, Fan C, Zhang Y, Yang Y, Yu X, Feng Q, Li B, Yao X, Zhang Z, Li L, Xue X, Zhu P. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J Infect Dis. 2005;191:755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Yang J, Feng X, Zhou Q, Cheng W, Shang C, Han P, Lin CH, Chen HS, Quertermous T, Chang CP. Pathological Ace2-to-Ace enzyme switch in the stressed heart is transcriptionally controlled by the endothelial Brg1-FoxM1 complex. Proc Natl Acad Sci U S A. 2016;113:E5628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Aimes RT, Zijlstra A, Hooper JD, Ogbourne SM, Sit ML, Fuchs S, Gotley DC, Quigley JP, Antalis TM. Endothelial cell serine proteases expressed during vascular morphogenesis and angiogenesis. Thromb Haemost. 2003;89:561–72. [PubMed] [Google Scholar]
- [25]. Huang DT, Lu CY, Chi YH, Li WL, Chang LY, Lai MJ, Chen JS, Hsu WM, Huang LM. Adaptation of influenza A (H7N9) virus in primary human airway epithelial cells. Sci Rep. 2017;7:11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Vanarsdall AL, Pritchard SR, Wisner TW, Liu J, Jardetzky TS and Johnson DC. CD147 Promotes Entry of Pentamer-Expressing Human Cytomegalovirus into Epithelial and Endothelial Cells. mbio. 2018;9(3):e00781–18. DOI: 10.1128/mBio.00781-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Krüger-Genge A, Blocki A, Franke RP, Jung F. Vascular Endothelial Cell Biology: An Update. Int J Mol Sci. 2019;20.pii: E4411. 10.3390/ijms20184411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Holger Moch H. Endothelial cell infection and endotheliitis in COVID-19. The Lancet. 2020. DOI: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Jung EM, Stroszczinski C, Jung F. Contrast enhanced ultrasonography (CEUS)1to detect abdominal microcirculatory disorders in severe cases of COVID-19 infection: First experience. Clin Hemorheol Microcirc. 2020. DOI: 10.3233/CH-209003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Bombeli T, Karsan A, Tait JF, Harlan JM. Apoptotic Vascular Endothelial Cells Become Procoagulant. Blood. 1997;89:2429–42. [PubMed] [Google Scholar]
- [31]. Vítková V, Živný J, Janota J. Endothelial Cell-Derived Microvesicles: Potential Mediators and Biomarkers of Pathologic Processes. Biomark Med. 2018;12(2):161–75. [DOI] [PubMed] [Google Scholar]
- [32]. Antoniak S, Mackman N. Multiple roles of the coagulation protease cascade during virus infection. Blood. 2014;123:2605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Antoniak S. The coagulation system in host defense. Res Pract Thromb Haemost. 2018;2:549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Spiezia L, Boscolo A, Poletto F, et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020. pii: S0735-1097(20)35008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Zhai Z, Li C, Chen Y, et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020. DOI: 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Tang N, Li D, Wang X, Sun Z. Abnormal Coagulation Parameters Are Associated With Poor Prognosis in Patients With Novel Coronavirus Pneumonia. J Thromb Haemost. 2020;18(4):844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Leonard-Lorant I, Delabranche X, Severac F, Helms J, Pauzet C, Collange O, Schneider F, Labani A, Bilbault P, Moliere S, Leyendecker P, Roy C, Ohana M. Acute Pulmonary Embolism in COVID-19 Patients on CT Angiography and Relationship to D-Dimer Levels. Radiology. 2020:201561. DOI: 10.1148/radiol.2020201561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Lim W, Meade M, Lauzier F, et al. Failure of anticoagulant thrombo-prophylaxis: risk factors in medical-surgical critically ill patients. Crit Care Med. 2015;43:401–10. [DOI] [PubMed] [Google Scholar]
- [42]. Corrigan D, Prucnal C, Kabrhel C. Pulmonary embolism: the diagnosis, risk-stratification, treatment and disposition of emergency department patients. Clin Exp Emerg Med. 2016;3:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Tee A, Wong A, Yusuff T, Rao D, Sidhu P. Contrast-enhanced ultrasound (CEUS) of the lung reveals multiple areas of microthrombi in a COVID-19 patient. Intensive Care Med. DOI: 10.1007/s00134-020-06085-4 [DOI] [PMC free article] [PubMed] [Google Scholar]