Abstract
Phenotype is the set of observable traits of an organism or condition. While advances in genetics, imaging, and molecular biology have improved our understanding of the underlying biology of Parkinson’s disease (PD), clinical phenotyping of PD still relies primarily on history and physical examination. These subjective, episodic, categorical assessments are valuable for diagnosis and care but have left gaps in our understanding of the PD phenotype. Sensors can provide objective, continuous, real-world data about the PD clinical phenotype, increase our knowledge of its pathology, enhance evaluation of therapies, and ultimately, improve patient care. In this paper, we explore the concept of deep phenotyping—the comprehensive assessment of a condition using multiple clinical, biological, genetic, imaging, and sensor-based tools—for PD. We discuss the rationale for, outline current approaches to, identify benefits and limitations of, and consider future directions for deep clinical phenotyping.
Keywords: Autonomic nervous system, gait, natural history, observational study, Parkinson’s disease, phenotype, real-world data, sleep, smartphone, social behavior
INTRODUCTION
Two centuries after its seminal description, Parkinson’s disease (PD) is now the world’s fastest growing major neurological disorder [1, 2]. Progress in genetics, imaging, and molecular biology have increased our understanding of the condition [3–5]. However, despite advances in technology, many basic clinical features of the disease remain elusive to specialists and researchers. This ignorance impairs our efforts to identify the etiologies of the disease, evaluate new therapies, and provide better care [6, 7].
In this paper, we examine the shortcomings in our current understanding of PD, introduce the concept of deep phenotyping, highlight existing efforts, introduce our own research, examine the benefits and limitations of this new approach, and discuss future directions.
SUPERFICIAL UNDERSTANDING OF PARKINSON’S DISEASE
Today, the principal means for assessing PD are categorical, episodic assessments conducted in the clinic [8]. The 99.9% of the time during which individuals with PD are not directly observed by clinicians, until recently, has not been assessed [9, 10]. Diaries completed by patients concerning their response to medications provide a window into how individuals are affected by the disease in their natural environment, have helped evaluate the benefits of new treatments, and have been the basis for new approvals [11–13]. However, these diaries are episodic, burdensome, primarily focus on motor function, and often require individuals to evaluate their disease using unfamiliar clinical terms.
In the 19th century, Drs. Parkinson, Charcot, and Gowers provided detailed descriptions of PD based on observation and examination [14–16]. However, basic characteristics, such as what proportion of the day an individual with PD has tremor, are known only to those affected by the disease. These measures, which extend beyond the time that individuals are directly observed in clinic, are poorly characterized in the scientific literature by wide-ranging estimates.
For the past decade, accelerometers have helped quantify motor features of PD [17–22]. However, most of these studies (Table 1) have limited their assessments to tasks performed in the clinic [18]. A 2018 systematic review of 24 wearable sensor studies in PD found only seven that assessed the disease in a “free-living environment” [18].
Table 1.
Large wearable sensor studies in Parkinson’s disease, 2015–2019
| Study | Year | Sample size | Sensor location | Number of sensors | Assessment | Country | Assessment setting | Findings |
| Van Wamelen et al., [131] | 2019 | 108 | Wrist | 1 | Bradykinesia, dyskinesia | UK | In clinic | Wearable sensors detected bradykinesia and dyskinesia, which were associated with non-motor symptoms. |
| Hasegawa et al., [132] | 2019 | 223 | Both feet, shins, wrists, sternum, lumbar region | 8 | Sway, gait, posture, balance | USA | In clinic | Objective measures of balance correlated significantly with disease severity and patient-reported outcomes. |
| Haji Ghassemi et al., [133] | 2019 | 150 | Both shoes | 2 | Gait, turning | Germany | In clinic | Number of steps per turn differed between control participants and those with even early stage PD. |
| Nguyen et al., [134] | 2019 | 119 | Both shoes | 2 | Gait, turning | Germany | In clinic | Combining gait and turning assessments improved classification of individuals with and without PD. |
| Buckley et al., [135] | 2019 | 134 | Lumbar region, back of head | 2 | Upper body accelerations | UK | In clinic | Upper body motion was moderately better at classifying PD gait than lower body motion. |
| Khoury et al., [136] | 2019 | 165 | Both feet | 16 | Vertical foot force | France | In clinic | Force data from wearable foot sensors distinguished between control participants and those with PD. |
| Bertoli et al., [137] | 2018 | 236 | Both ankles | 2 | Gait | Belgium, Israel, Italy, UK | In clinic | Wearable sensors effectively measured aspects of gait, which differed significantly between control participants and those with PD. |
| Silva de Lima et al., [138] | 2018 | 304 | Wrist | 1 | Gait | Netherlands | Anywhere | Higher age and severity of motor symptoms were associated with less time spent walking. |
| Schlachetzki et al., [139] | 2017 | 291 | Both shoes | 2 | Gait | Germany | In clinic | Wearable sensor-based gait analysis differentiated gait characteristics of individuals with and without PD and sensitively tracked changes in gait over time. |
Source: PubMed search of wearable sensors to assess neurological conditions, filtered by sample size greater than 100, on 11/15/2019 (“parkinson disease” [MeSH Terms] OR (“parkinson” [All Fields] AND “disease” [All Fields]) OR “parkinson disease” [All Fields] OR (“parkinson’s” [All Fields] AND “disease” [All Fields]) OR “parkinson’s disease” [All Fields]) AND (wearable[All Fields] AND (“Sensors (Basel)” [Journal] OR “sensors” [All Fields])).
Wearable sensors and smartphone embedded sensors are providing glimpses into how PD affects individuals in everyday life [23]. A recent ambulatory monitoring study of individuals with newly diagnosed PD employed a wrist-worn sensor (Global Kinetics; Parkville, Australia) and detected clinically meaningful tremor among a few participants for over half the day. Studies of smartphone embedded sensors, some large-scale, have captured remote, objective assessments of PD (Table 2). In general, though, these studies are limited by small sample size and short duration, rely on participants to conduct active tasks (e.g., tapping on the screen), and lack companion sensors which can paint a more complete picture of the disease [24].
Table 2.
Large smartphone studies in Parkinson’s disease, 2015–2019
| Study | Year | Sample size | Smartphone tasks | Country | Assessment setting | Findings |
| Lo et al., [140] | 2019 | 237 | Voice, balance, gait, reaction time, finger tapping, rest tremor, postural tremor | UK | Anywhere | Smartphone tests and machine learning algorithms were shown to predict future clinical outcomes in early PD with sensitivity greater than 90%. |
| Prince et al., [141] | 2019 | 1,513 | Voice, gait, finger tapping, memory | UK | Anywhere | Machine learning inferred missing data in smartphone assessments of individuals with PD, increasing classification accuracy from 73% to 82%. |
| Singh et al., [142] | 2019 | 5,826 | Voice | USA | Anywhere | Smartphone apps detected PD using voice data from 10-second audio clips with 99% accuracy. |
| Rusz et al., [143] | 2018 | 110 | Voice | Czech Republic | In clinic | Acoustic information from smartphones differentiated individuals with and without PD with a sensitivity of 85%. |
| Zhan et al., [26] | 2018 | 129 | Voice, finger tapping, gait, balance, reaction time | USA | Anywhere | Smartphone assessments detected intraday symptom fluctuations and the effects of dopaminergic medications. The assessments correlated to standard clinic measures of PD. |
| Prince et al., [144] | 2018 | 548 | Finger tapping | UK | Anywhere | Smartphone tests correlated to self-assigned severity labels in individuals with severe PD. |
| Arora et al., [83] | 2018 | 522 | Voice, balance, gait, reaction time, finger tapping, rest tremor, postural tremor | Italy | In clinic | Smartphone tasks distinguished between individuals with either PD or idiopathic REM sleep behavior disorder and healthy controls with mean sensitivity and specificity values of 85% to 92%. |
| Bot et al., [44] | 2016 | 9,520 | Voice, gait, finger tapping, memory | USA | Anywhere | Smartphone app enrolled participants nationally and assessed multiple motor features of PD. |
Source: PubMed search of smartphones using sensors to assess neurological conditions, filtered by sample size greater than 100, on 11/15/2019 (“parkinson disease” [MeSH Terms] OR (“parkinson” [All Fields] AND “disease” [All Fields]) OR “parkinson disease” [All Fields] OR (“parkinson’s” [All Fields] AND “disease” [All Fields]) OR “parkinson’s disease” [All Fields]) AND (“smartphone” [MeSH Terms] OR “smartphone” [All Fields] OR “smartphones” [All Fields]).
Despite advances in our ability to assess motor features using technology, details, like when the tremor occurs, for how long, and at what amplitude and frequency, are still lacking. In addition, most sensor studies only assess tremor in one limb, even though its presence in multiple limbs has been known for centuries [14, 15, 25].
Similarly, the extent of intra- and inter-day disease variability remains largely unassessed in the 21st century. A recent smartphone study found that the magnitude of inter-day variability of motor impairment was far greater than the overall average progression of motor features over six months [26]. Variability in non-motor features like sleep, neuropsychiatric, and autonomic dysfunction is even less well characterized. Moreover, assessing the impact of these features on the lives of individuals with PD is almost exclusively limited to pen-and-paper surveys [27–29].
DEEP PHENOTYPING OF PARKINSON’S DISEASE
What PD needs is deep phenotyping. Phenotype is a set of observable traits of an organism or condition [30]. In medicine, phenotyping comes from a medical history, physical examination, imaging, and blood tests. Deep phenotyping “shows the different dimensions of a disease” and leverages all available data sources to gather details specific to the individual [30]. Current phenotyping efforts that rely on subjective characterizations about an individual’s walking ability, for example, have been described as “sloppy or imprecise” [30]. In a 2015 Nature piece, deep phenotyping is described as gathering “details about disease manifestations in a more individual and finer-grained way, and uses sophisticated algorithms to integrate the resulting wealth of data with other kinds of information” [31]. This approach results in large data sets that require data fusion algorithms to integrate the results [30, 32].
Many deep phenotyping efforts have used molecular tools, such as genomics, proteomics, and metabolomics, or electrophysiological tools, like electroencephalography, to develop new insights [33, 34]. Such studies have advanced our understanding of pathophysiology in diseases ranging from diabetes to osteoarthritis [35, 36]. In addition to evaluating those with a disease, this approach can track individuals as they transition from a healthy to a diseased state [35].
In this paper, we instead focus on deep clinical phenotyping, which can be integrated with molecular approaches. Deep clinical phenotyping has four principal characteristics. First, the effort begins with the history and examination as in a traditional clinical appointment. Second, deep clinical phenotyping uses sensors or other tools to provide objective measurement of the individual or the condition. Third, multiple domains (e.g., activity and respiratory function) of the disease of interest are assessed. Fourth, these assessments extend to real-world settings like the home. The depth of the phenotype is a function of the quality of the assessment of each domain, the number of areas evaluated, and the duration of observation.
New sensors can generate large volumes of data that are measured in number of observations per person per second [37]. These tools can thus provide high-definition descriptions of previously unobservable clinical features. For example, a recent study used digital sensors, including a smartphone, smartwatch, a digital assessment application, and a bed sensor, to provide a “minute-level behaviorgram” of individuals with either Alzheimer’s disease, mild cognitive impairment, or no evidence of cognitive deficit [38]. This behaviorgram combined assessments of physical activity (e.g., steps taken), sleep (e.g., duration of sleep), and phone usage (e.g., text messages received) to a paint a picture of how cognitive impairment affects individuals in their daily lives.
CURRENT DEEP PHENOTYPING STUDIES IN PARKINSON’S DISEASE
Deep clinical phenotyping studies in PD have begun (Table 3) [39, 40]. For over a decade, the Parkinson’s Progression Markers Initiative has sought to identify biological markers of disease progression and in so doing has collected a wealth of clinical, genetic, imaging, and biological data [41]. Recently, it added sensors, including a smartwatch and a smartphone application, to the current clinical assessments. The expectation is that the sensor data will provide more objective and frequent assessments of PD both in and outside the clinic. Together with the biological assessments, a more complete picture of cohorts with and at risk for PD can be created.
Table 3.
Current studies of deep phenotyping in Parkinson’s disease
| Study | Primary funder(s) | Design | Sample size | Controls | Duration | Frequency of visits | Clinical measures | Digital tools | Biological sample(s) | Imaging |
| Parkinson’s Progression Markers Initiative [41] | Michael J Fox Foundation | Multi-center | 2000 | Yes | 10+ years | 3-, 6-, and 12-month intervals | Yes | Smartwatch | Blood | DaTscan MRI |
| Smartphone application | CSF | |||||||||
| Personalized Parkinson Project [39] | Verily Life Sciences LLC | Single-center | 650 | No | 2 years | Annual | Yes | Smartwatch | Blood | MRI |
| CSF | ||||||||||
| Stool | ||||||||||
| Luxembourg Parkinson’s Study [43] | Luxembourg National Research Fund | Multi-center | 1600 | Yes | 4 years | Annual | Yes | Smartphone application Gait sensor | Blood | None |
| Urine | ||||||||||
| Saliva | ||||||||||
| WATCH-PD [42] | Biogen and Takeda | Multi-center | 100 | No | 1 year | 1- and 3-month intervals | Yes | Smartwatch | None | DaTscan |
| Smartphone application | ||||||||||
| Wearable sensors | ||||||||||
| University of Rochester Udall Center | National Institute of Neurological Disorders and Stroke | Single-center | 50 | Yes | 2 years | 6- and 12-month intervals | Yes | Smartphone application Wearable sensors | Blood | None |
| In-home radio wave device | ||||||||||
| Video analytics |
Additional studies are using smartwatch embedded sensors to assess PD. The 650-person Personalized Parkinson Project in the Netherlands has started enrolling individuals with PD who will be followed annually in clinic and with a smartwatch [39]. In addition, the study is collecting multiple biological samples, including blood, cerebrospinal fluid, and stool, and capturing structural and functional MRIs from research participants. The study aims to address the stagnation in our understanding of the etiology, pathophysiology, and progression of PD by developing “deep and repeated multi-dimensional phenotyping” of PD enabled by continuous monitoring with a wearable device for two years [39].
A separate U.S. study called Watch-PD is using a different smartwatch along with wearable sensors in the clinic to assess PD. In contrast to the Personalized Parkinson Project, which has broad inclusion criteria, Watch-PD is focused on developing novel digital assessments of individuals with early, untreated PD [42]. The hope is that the resulting digital assessments could be used as outcome measures or endpoints in future clinical trials in this population.
The Luxembourg Parkinson’s Study is another deep phenotyping study that is addressing the “substantial gaps in our understanding of the underlying mechanisms and the complex clinical presentation” of PD [43]. To do so, the Luxembourg study is utilizing a smartphone application and a shoe sensor to develop “rater-independent” measures of the clinical disease features. These will be combined with detailed biological assessments from at least blood, saliva, and urine to develop a “data driven approach” to evaluate the disease [43].
A NEW EFFORT
To complement these efforts, we launched a study, Super-PD, that uses multiple sensors to enable deep clinical phenotyping of individuals with and without PD (Fig. 1). We are enrolling fifty individuals, thirty-five with PD, and fifteen age- and sex-matched controls in a two-year prospective cohort study.
Fig. 1.
Picture of the digital devices that will be used in our deep clinical phenotyping study.
Compared to prior work, this study combines multiple sensors that significantly increase the depth and breadth of signals collected. The first measure is the second generation of the mPower smartphone application originally released on Apple’s open-source ResearchKit platform in March 2015 [44]. The initial study enrolled over 15,000 participants with and without PD throughout the U.S. and used the smartphone’s sensors to assess voice, finger tapping, gait, and balance [44]. The second- generation application is much like the first but has additional tremor assessments and allows for passive monitoring using the Global Positioning System (GPS). Analysis of results from a similar application with similar assessments generated a “mobile Parkinson’s disease score,” which allows a frequent, objective assessment of disease severity by anyone with a smartphone anywhere in the world [26, 45].
The second measure is a set of wearable sensors (MC10; Lexington, MA) that can be placed almost anywhere on the body. In an initial study, five sensors (one on each limb and one on the chest) were used to quantify what proportion of a day individuals with PD are lying, sitting, standing, or walking [25]. These sensors can also assess tremor, gait, sleep, and potentially dyskinesias. The second generation of the sensor adds electrocardiogram (ECG) capabilities. Compared to the smartphone assessment, the wearable sensors, which will be applied using double-sided adhesives to the chest and most affected arm and leg, provide a more accurate measure of movement for the body parts where they are placed. However, over the course of the study, participants will likely wear the sensors less often than they carry their smartphone.
The third assessment is a video analytics tool, broadly available via computer browser, which uses machine learning rather than raters to assess movements in PD. Participants are asked to perform elements of the motor portion of the Movement Disorder Society—Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) in front of a web camera on a laptop computer [8]. Using machine vision, a computer algorithm can automatically and precisely identify subtle movement of the facial muscles, hands and fingers and characteristics of spoken words and syllables and correlate these features with manually annotated MDS-UPDRS scores. Based on a task that asks participants to maintain a neutral face, the computer algorithm has identified facial microexpressions around the lips and eyebrows that can differentiate those with PD from those without [46].
Finally, the Super-PD study utilizes a radio wave sensor called Emerald (Cambridge, MA) that is installed in an individual’s home and uses electromagnetic waves to assess individuals in their natural environment [47, 48]. Low power radio waves transmitted by the device reflect off non-stationary objects, most notably people and pets, and are used to detect location, movement, gait trajectories, sleep, and other activities in the home. An initial study quantified gait speed and tracked sleep patterns, including the time, duration, and interruptions when individuals were in bed [47]. The entirely passive sensor monitors up to a range to of 1,200 square feet (110 square meters) and works best when only 2–3 individuals regularly occupy the space.
Participants will also undergo in-clinic assessments with traditional rating scales to assess motor function, mood, sleep, and cognition and provide a blood sample to be stored for analysis as part of the Parkinson’s Disease Biomarker Program [8, 49–54]. The scales will be augmented by the recently developed, online Patient-Reports of Problem (PROP) assessment. The PROP assessment, which was developed to give participants the opportunity to express themselves in their own words, covers three domains: general health, psychosocial wellbeing, and Parkinson’s disease. Each PROP survey asks individuals what bothers them, how the problems affect daily functioning, the severity of the problems, and what they do to alleviate each problem [55]. Natural language processing, standardized clinical curation, and machine learning are used to quantify and analyze responses [56]. Together, the PROP and digital measures will give a robust portrayal of what individuals with PD say and do.
BENEFITS OF DEEP CLINICAL PHENOTYPING
Deep clinical phenotyping can provide new insights into various aspects of PD (Table 4). This approach uses sensors to examine how PD affects people in their daily lives and extends beyond biological markers to give a more complete characterization of the disease.
Table 4.
Deep phenotyping measures of Parkinson’s disease
| Domain | Motor | Non-motor | Functional | Social | |||||
| Activity | Tremor | Other | Automatic | sleep | Cognition | ||||
| Measures | •Number of steps | •Portion of day with tremor | •Portion of day with dyskinesias | •Heart rate range | •Duration of sleep | •Variability in activities | •Time in bed | •Time at home | |
| •Gait speed | •Location, amplitude, and frequency of tremor | •Facial movements | •Heart rate variability | •Number of turns in bed and arousals | •Time in bathroom | •Time at home alone | |||
| •Portion of day sitting, standing, walking, and lying down | •Response to therapy | •Respiratory rate | •Frequency and duration of naps | •Frequency of bathroom use | •Lifespace | ||||
| •Presence of REM sleep behavior disorder | |||||||||
| “DO” | Smartphone application | ✓ | ✓ | ✓ | |||||
| Wearable sensors | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Passive in-home monitor | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Video analytics | ✓ | ✓ | |||||||
| Standard clinical assessment | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| “SAY” | Standard patient-reported outcomes | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| PROP assessment | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
Smartphone applications can detect subtle tremor that is missed by trained raters [57]. In addition, smartphone embedded sensors can readily assess gait and global motor function. In one study, a smartphone application detected that individuals with PD walked about 25% less than those without the disease [57].
Wearable sensors can also quantify gait speed, which is reduced in PD and fluctuates in response to levodopa and deep brain stimulation [58–62]. In geriatric medicine, “gait speed” is the “sixth vital sign” [63], and reductions are associated with increased mortality [64, 65]. For the past decade, Dr. Jeffrey Kaye at Oregon Health Sciences University and colleagues have outfitted homes of older adults with multiple sensors to assess motor, functional, and social behavior [66]. They have quantified the gait speed of individuals in their natural environment, and they and others have found that declines in walking speed predict cognitive impairment and dementia [66–68]. Similarly, reductions in daily physical activity are associated with an increased risk of Alzheimer’s disease and potentially PD [69–71].
Multiple non-motor features, including autonomic function and sleep, can also be evaluated. The physiologic oscillation in the time interval between heartbeats, known as heart rate variability, is reduced in PD [72–74]. Prior studies of heart rate variability in PD have collected observations on up to 150 participants for 24 hours with an ambulatory ECG, generating around 3600 hours of data [72, 75]. In contrast, deep phenotyping studies such as ours, can generate data volumes that dwarf more traditional studies with serial ECG assessments over longer periods of time (e.g., weekly intervals). In addition, as with motor features, the effect of exercise, medication, walking, and sleep on heart rate response can be assessed.
Sleep has also been inadequately assessed in PD. Most studies use polysomnography for single night observations in artificial sleep labs [76–78]. Accelerometers have helped measure different aspects of sleep at home [17, 18]. These studies have quantified various aspects of sleep and have found that individuals with PD turn fewer times but leave the bed more often [79, 80]. In our study, the number and duration of naps, duration of sleep, number of sleep interruptions, and large movements during sleep will all be quantified. The latter could be used to assess rapid eye movement (REM) sleep behavioral disorder, which is an early feature of the disease that can be identified by multiple digital devices [81–83].
Sensor technology also enables functional assessments in the home. The Emerald device can quantify number of visits to the bathroom and time spent in the bathroom. Such assessments can provide new insights into the impact of urinary urgency and constipation and supplement current measures of these symptoms, which rely on diaries, surveys, and patient-reported outcomes [84].
Finally, this study will provide novel insights into how PD disease affects an individual’s interaction with the environment or social function. A small smartphone study used GPS to measure the “lifespace,” or the “geographic area in which a person lives and conducts their activities,” of individuals with PD [85]. The lifespace can be mapped in a de-identified way that measures time and distance away from home without giving the specific location of participants. Such assessments could reflect both motor (e.g., mobility) and non-motor (e.g., depression) features of PD. Inside the home, the radio-wave sensor can quantify how much time is spent at home, where it is spent (e.g., bedroom), and what proportion of time an individual is alone. In older adults, increased time outside the home is associated with superior physical ability and improved emotional state [86]. Conversely, loneliness is associated with decreased time out of the home, motor slowing, functional decline, and death [87–89]. Loneliness not only affects individuals with PD but also their caregivers [90, 91].
In essence, sensors increase the precision of established measures to capture greater variability in symptoms and enable more frequent, seamless data collection. Within the context of deep clinical phenotyping studies, sensors can identify new outcome measures of PD and generate composite digital biomarkers that holistically measure disease symptoms and progression.
LIMITATIONS OF DEEP CLINICAL PHENOTYPING
New efforts confront many barriers and limitations. Among them are who participates in such research studies, their privacy, the ability to capture their data, the validity of the data, and their subsequent analysis and generalizability. Thus far, participants in deep phenotyping studies have not been representative of the general population. They are overwhelmingly white, well educated, and likely have more trust in clinicians and health care institutions than other groups [92]. Social and economic factors also contribute to the digital divide, with differential access to the internet and digital devices [93]. Engaging in outreach, reducing financial and travel burdens, and bringing research tools to participants (e.g., direct-to-consumer genetic testing, smartphone applications, online research platforms) can address some of this selection bias, which plagues both deep phenotyping and traditional research studies [94].
The privacy of participants is another concern. Deep phenotyping requires extensive monitoring of individuals, including continuous passive location assessment and monitoring in their homes. Despite the intrusion, studies that have assessed privacy concerns have generally found only modest concerns [23, 47]. That said, these responses come from those who are willing to share de-identified data. Privacy concerns may also be less among those with PD, who often are either retired or disabled, have guaranteed health insurance (e.g., Medicare in the U.S.), and are invested in clinical research. Privacy views may differ for those without or at risk for PD, who are likely to be younger, employed, and less secure in their health insurance [95].
Some sensors inform researchers not only about the behavior of research participants but also about those that live with them or come in close contact to them. Sensors that allow for easy pausing of data collection could help protect privacy. In addition, the Emerald device, for example, filters all movement patterns to isolate those from just the study participant. In other cases, sensors may collect information about all activity in its range. In these cases, researchers need to ignore such data where feasible and discard it where not. To assist with the conduct and oversight of such studies, ethicists, consent specialists, and voices of those affected by the disease are also valuable, if not essential.
Interest in studies utilizing sensors is high, including among older individuals [18, 39, 96, 97]. However, maintaining that interest is difficult as participation often wanes quickly [44, 98]. Supporting and engaging participants throughout a study’s course is thus important. In addition, capturing data from participants in uncontrolled (real-world) environments is not easy. Poor connectivity (e.g., to Wi-Fi) and limited storage capacity of sensors are potential obstacles that need to be overcome.
While these digital devices are appealing, additional work is required to ensure their validity. Validation is the “process of ensuring that the digital measurement tool is meeting its intended use by generating objective data that accurately represents the concept of interest... that it purports to be measuring” [99]. Validation seeks to answer whether researchers built the right tool to assess measures of how someone feels, functions, or survives, such as gait. Validity is divided into two components, analytic validation and clinical validation [99, 100]. The former assesses whether algorithms accurately process the data (i.e., is the calculation of gait speed from accelerometry data true?). The latter evaluates whether the measurement (e.g., gait speed) reflects an important health domain. Large scale public-private partnerships, such as Mobilise-D, are seeking to develop clinically valid digital mobile assessments that can be applied to PD and other chronic medical conditions. These efforts in multiple sclerosis, for example, employed digital measures alongside traditional measures to assess ambulation in the clinic and in a real-world setting [101]. While larger scale studies and rigorous validation of novel digital measures is needed for PD, the field should recognize that comparison against traditional measures may not be the best approach for such validation, particularly because many of the digital measures can be more accurate and sensitive than traditional “gold” standards. After confirming their reliability and correlation with clinical scales, digital measures may be directly validated against pathophysiological biomarkers in PD.
Finally, analysis of data from deep phenotyping studies requires new methodologic and analytic approaches and new collaborations. Deep phenotyping studies generate large datasets that are characterized by high volume, variety, velocity, veracity, and value [102]. Each of these characteristics comes with its own challenges that require multi-disciplinary teams to address. Such data is often highly contaminated with irrelevant factors such as idiosyncratic behaviors, environmental settings and changes, and effects of comorbid conditions. Analysis needs to account for this substantial noise in the data [103]. Hybrid approaches that combine techniques from multiple fields will be essential. In addition to clinicians, research teams often need computer scientists (artificial intelligence and machine learning), data scientists (high dimensional data), electrical engineers (signal processing), and persons with PD to manage and interpret the data.
FUTURE DIRECTIONS
Deep phenotyping offers at least three great potential advances. First, deep clinical phenotyping will enhance our understanding of the nature of the disease, including its expression, pathophysiology, and potential modulators [7, 104]. Detailed, frequent, real-world assessments of PD will provide a more complete picture of the disease’s manifestations, their sequence of development, their impact on individuals’ everyday life, and their relationship to pathology. Current PD phenotypes are based on traditional scales and likely incomplete, if not inaccurate [105]. What we see in our clinic today is an arbitrary snapshot that may not reflect the real-world. New tools can monitor features of the disease inside and outside the clinic. They will allow us to identify new potential modulators of Parkinson’s course, such as weather, altitude, diurnal patterns, sleep, activity, and other factors that have either not been considered or are difficult to measure.
Second, deep phenotyping could help us evaluate new treatments objectively and efficiently. Current PD clinical trials are large, long, expensive, and prone to fail [106, 107]. In this century, the U.S. Food and Drug Administration (FDA) has approved only three new classes of medications for PD (adrenergic agonists, atypical antipsychotics, and adenosine A2A receptor antagonists), each of which benefits a small subset of patients. Moreover, despite extensive discussion and large efforts [108–110], no therapies that target the underlying pathology have emerged. A new approach is needed, and deep phenotyping could generate the objective, sensitive, and high-definition assessments required to evaluate a new generation of therapies [17].
Such approaches are gaining traction. In the U.S., the 21st Century Cures Act introduced the use of real-world data, including sensor data, to support regulatory decision making [111]. The inclusion of real-world data and the resulting real-world evidence reflects the rapid acceleration in the “use of computers, mobile devices, wearables and other biosensors to gather huge amounts of health-related data” [111]. These data can, according to the FDA, “allow us to better design and conduct clinical trials and studies in the health care setting to answer questions previously thought infeasible” [111]. In 2019, the FDA issued a guidance document that indicated that real-world evidence could be used to “provide evidence in support of the effectiveness or safety of a new drug approval” [112] and a similar guidance for devices [113]. Also in 2019, the European Medicines Agency deemed top stride velocity (95th percentile measured at the ankle) as “an appropriate endpoint in studies to support regulatory decision making on medicines for the treatment of Duchenne Muscular Dystrophy” [114]. The FDA has already approved one treatment (dalfampridine) for multiple sclerosis based on gait speed [115, 116]. Such measures could be readily applied to PD.
Third, a prerequisite for personalized care for PD is individual data [117]. These data can be patient-reported, genetic, biological, or clinical. In a 2013 review, Dr. Walter Maetzler and colleagues predicted such an application. They wrote, “Measuring (Parkinson’s) disease-related outcomes objectively ... , continuously ... , unobtrusively ... , and with high ecological validity ... could boost the efficiency and relevance of (patient) visits, and improve patient care. This clinical wish appears to [be] coming within reach with the advent of new, wearable technology that can quantitatively collect, analyze, and deliver data to both the patient and the doctor” [17].
Personalized medicine for PD is just beginning. Current treatment decisions are largely based on ad hoc assessments [118, 119]. Other diseases are further ahead. Individuals with diabetes are no longer dependent on in-clinic assessments to dose their insulin. Now, blood glucose levels are measured continuously and dosing of medication is tailored to these data in real time, resulting in greater potential for optimal disease control. Future objective assessments of PD (e.g., of gait) could enable tailoring of medical or surgical treatments to the individual [120, 121]. Deep brain stimulation parameters, for example, could be automatically adjusted based on continuous, real-world assessments from implanted, sensing leads, a process that is underway [122–124]. Patients could customize their activities and treatments based on sensor data and foster self-efficacy [125].
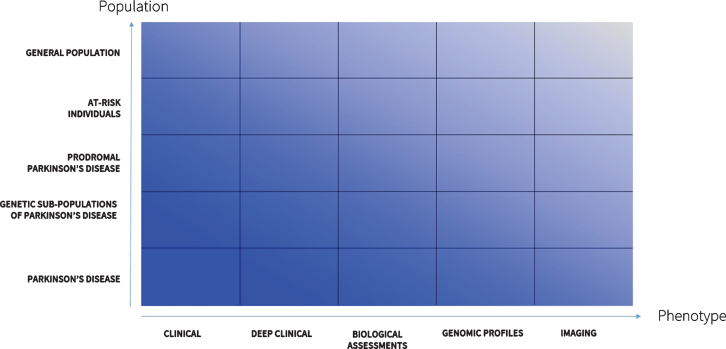
Fourth, deep phenotyping will enable the discovery of new disease subtypes [126]. Clustering individuals into groups with similar progression or traits can power new studies that seek to understand the biological differences across these groups. The future (Fig. 2) will see expanded deep phenotyping efforts. These may include generating phenotypes specific to genetic sub-types of PD (e.g., due to LRRK2 or GBA mutations) that could inform gene-directed clinical trials [127]. Previous studies using traditional rating scales have suggested that these genetic sub-populations have different features and progression rates [128, 129]. Deeper phenotyping will likely be able to confirm or refute such findings and identify many other hidden differences. Deep phenotyping of individuals at risk for or with prodromal PD will permit better understanding of the nature of PD and its evolution. This will be especially important for assessing the effectiveness of novel interventions aimed at these groups.
Fig. 2.
Landscape of deep phenotyping of Parkinson’s disease.
The mathematician Dr. Freeman Dyson said “New directions in science are launched by new tools much more often than by new concepts. The effect of the concept-driven revolution is to explain old things in new ways [6]. The effect of a tool-driven revolution is to discover new things that have to be explained” [130]. We now have these new tools. New insights await us.
CONFLICTS OF INTEREST
Ray Dorsey has served as a consultant to 23andMe, Abbott, Abbvie, American Well, Biogen, Clintrex, DeciBio, Denali Therapeutics, GlaxoSmithKline, Grand Rounds, Karger, Lundbeck, MC10, MedAvante, Medical-legal services, Mednick Associates, National Institute of Neurological Disorders and Stroke, Olson Research Group, Optio, Origent Data Sciences, Inc., Prilenia, Putnam Associates, Roche, Sanofi, Shire, Sunovion Pharmaceuticals, Teva, UCB, and Voyager Therapeutics. He has received honoraria from the American Academy of Neurology, American Neurological Association, and University of Michigan. He has received research support from Abbvie, Acadia Pharmaceuticals, AMC Health, BioSensics, Burroughs Wellcome Fund, Davis Phinney Foundation, Duke University, Food and Drug Administration, GlaxoSmithKline, Greater Rochester Health Foundation, Huntington Study Group, Michael J. Fox Foundation, National Institutes of Health/National Institute of Neurological Disorders and Stroke, National Science Foundation, Nuredis, Inc., Patient-Centered Outcomes Research Institute, Pfizer, Prana Biotechnology, Raptor Pharmaceuticals, Roche, Safra Foundation, Teva Pharmaceuticals, and University of California Irvine. Dr. Dorsey provides editorial services for Karger Publications has ownership interests in Blackfynn, a data integration company, and Grand Rounds, an online second opinion service.
Larsson Omberg has received research support from the National Institutes of Health/National Institute of Neurological Disorders and Stroke.
Jamie Adams has received research support from Biogen, BioSensics, Empire Clinical Research Investigator Program, Michael J. Fox Foundation, National Institutes of Health/National Institute of Neurological Disorders and Stroke, Pfizer, and Safra Foundation.
Katherine Amodeo serves as Co-Investigator for the Lewy Body Dementia Association Research Center of Excellence (LBDA-RCOE) at the University of Rochester Medical Center. She has served as PI and Co-I for PD and DLB clinical trials funded by Acadia Pharmaceuticals, EIP Pharma, Michael J. Fox Foundation, National Institutes of Health/National Institute of Neurological Disorders and Stroke, and Roche. She serves as medical monitor for a PD trial funded by Biogen. Dr. Amodeo’s fellowship in Movement Disorders was funded by the Safra Foundation and Michael J. Fox Foundation.
Erika Augustine has served as a consultant to Biomarin Pharma, Neurogene, Regenxbio, and Beyond Batten Disease Foundation. She has received research support from Abeona Therapeutics, Neurogene, and National Institutes of Health/National Institute of Neurological Disorders and Stroke. Dr. Augustine has served as central rater for a clinical trial funded by Signant Health.
Zachary Kabelac is co-founder of Emerald Innovations, Inc., which produces similar radio wave devices to those used in the reported research. Dr. Kabelac has been issued two patents: US9753131B2 and US20170042432A1.
Dina Katabi is co-founder of Emerald Innovations, Inc., which produces similar radio wave devices to those used in the reported research. Dr. Katabi has five patents, either pending or issued: WO2015102713A3, US20190188533A1, WO2018183106A1, WO2018013192A2, and US20170042432A1.
Karl Kieburtz has served as a consultant to Blackfynn, Clintrex, Genentech/Roche, and Novartis. He has received research support from National Institutes of Health (National Institute of Neurological Disorders and Stroke and National Center for Advancing Translational Sciences) and Michael J. Fox Foundation. Dr. Kieburtz has ownership interests in Clintrex, Hoover Brown LLC, and Safe Therapuetics.
Max Little has received an academic grant from the Michael J. Fox Foundation.
Suchi Saria has received research support from American Heart Association, Child Health Imprints, Defense Advanced Research Projects Agency, National Institutes of Health/National Institute of Neurological Disorders and Stroke, and National Science Foundation.
Giovanni Schifitto has received research support from the National Institutes of Health/National Institute of Neurological Disorders and Stroke.
Ruth Schneider has received research support from Acadia Pharmaceuticals, Biohaven Pharmaceuticals, Canadian Institute of Health, Massachusetts General Hospital, Michael J. Fox Foundation, National Institutes of Health/National Institute of Neurological Disorders and Stroke, Nuredis, Inc., and Pfizer.
Gaurav Sharma has received research support from MC10.
Ira Shoulson is founder and principal manager of Grey Matter Technologies LLC and is compensated by the company for work related to the reported research.
Christopher Tarolli has received research support from American Academy of Neurology Institute, BioSensics, Greater Rochester Health Foundation, Michael J. Fox Foundation, and National Institutes of Health/National Institute of Neurological Disorders and Stroke. He has received honoraria from the American Academy of Neurology and Davis Phinney Foundation. Dr. Tarolli has received clinical training support from Medtronic.
Michael McDermott has received research support from National Institutes of Health/National Institute of Neurological Disorders and Stroke and Parkinson Study Group. Dr. McDermott has been compensated by Cynapsus Therapetuics, Prilenia, and Voyager Therapeutics for service on a Data Safety and Monitoring Board.
Emma Waddell, Roy Adams, Rafayet Ali, Abigail Arky, Karthik Dinesh, Ehsan Hoque, Alistair Glidden, Estella Jensen-Roberts, Daniel Kinel, Karlo Lizarraga, Taylor Myers, Sara Riggare, Spencer Rosero, Anna Stevenson, and Jiebo Luo have no disclosures.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number P50NS108676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- [1]. GBD 2016 Neurology Collaborators (2019) Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18, 459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. GBD 2016 Parkinson’s Disease Collaborators (2018) Global, regional, and national burden of Parkinson’s disease, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 17, 939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Klein C, Westenberger A (2012) Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med 2, a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Politis M, Pagano G, Niccolini F (2017) Imaging in Parkinson’s disease. Int Rev Neurobiol 132, 233–274. [DOI] [PubMed] [Google Scholar]
- [5]. Antony PMA, Diederich NJ, Krüger R, Balling R (2013) The hallmarks of Parkinson’s disease. FEBS J 280, 5981–5993. [DOI] [PubMed] [Google Scholar]
- [6]. Espay AJ, Lang AE (2018) Parkinson diseases in the 2020s and beyond: Replacing clinico-pathologic convergence with systems biology divergence. J Parkinsons Dis 8, S59–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Weiner WJ (2008) There is no Parkinson disease. Arch Neurol 65, 705–708. [DOI] [PubMed] [Google Scholar]
- [8]. Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord 23, 2129–2170. [DOI] [PubMed] [Google Scholar]
- [9]. Riggare S, Höglund PJ, Hvitfeldt Forsberg H, Eftimovska E, Svenningsson P, Hägglund M (2019) Patients are doing it for themselves: A survey on disease-specific knowledge acquisition among people with Parkinson’s disease in Sweden. Health Informatics J 25, 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Palfreman J, Profile: Sara Riggare, Journal of Parkinson’s Disease, https://www.journalofparkinsonsdisease.com/blog/patient_perspective/profile-sara-riggare, Posted March 20, 2014, Accessed November 15, 2019.
- [11]. Hauser RA, Friedlander J, Zesiewicz TA, Adler CH, Seeberger LC, O’Brien CF, Molho ES, Factor SA (2000) A home diary to assess functional status in patients with Parkinson’s disease with motor fluctuations and dyskinesia. Clin Neuropharmacol 23, 75–81. [DOI] [PubMed] [Google Scholar]
- [12]. Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, Vanagunas A, Othman AA, Widnell KL, Robieson WZ, Pritchett Y, Chatamra K, Benesh J, Lenz RA, Antonini A (2014) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: A randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13, 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. LeWitt PA, Guttman M, Tetrud JW, Tuite PJ, Mori A, Chaikin P, Sussman NM, 6002-US-005 U. S. Study Group (2008) Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: A double-blind, randomized, multicenter clinical trial (6002-US-005). Ann Neurol 63, 295–302. [DOI] [PubMed] [Google Scholar]
- [14]. Goetz CG (2011) The history of Parkinson’s disease: Early clinical descriptions and neurological therapies. Cold Spring Harb Perspect Med 1, a008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Gowers W (1886) A Manual of Diseases of the Nervous System, J & A Churchill, London. [Google Scholar]
- [16]. Parkinson J (2002) An essay on the shaking palsy. J Neuropsychiatry Clin Neurosci 14, 223–236. [DOI] [PubMed] [Google Scholar]
- [17]. Maetzler W, Domingos J, Srulijes K, Ferreira JJ, Bloem BR (2013) Quantitative wearable sensors for objective assessment of Parkinson’s disease. Mov Disord 28, 1628–1637. [DOI] [PubMed] [Google Scholar]
- [18]. Johansson D, Malmgren K, Alt Murphy M (2018) Wearable sensors for clinical applications in epilepsy, Parkinson’s disease, and stroke: A mixed-methods systematic review. J Neurol 265, 1740–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Chen B, Patel S, Buckley T, Rednic R, McClure DJ, Shih L, Tarsy D, Welsh M, Bonato P (2011) A web-based system for home monitoring of patients with Parkinson’s disease using wearable sensors. IEEE Trans Biomed Eng 58, 831–836. [DOI] [PubMed] [Google Scholar]
- [20]. Pardoel S, Kofman J, Nantel J, Lemaire ED (2019) Wearable-sensor-based detection and prediction of freezing of gait in Parkinson’s disease: A review. Sensors 19, 5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Rovini E, Maremmani C, Cavallo F (2017) How wearable sensors can support Parkinson’s disease diagnosis and treatment: A systematic review. Front Neurosci 11, 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Brognara L, Palumbo P, Grimm B, Palmerini L (2019) Assessing gait in Parkinson’s disease using wearable motion sensors: A systematic review. Diseases 7, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Dorsey ER, Glidden AM, Holloway MR, Birbeck GL, Schwamm LH (2018) Teleneurology and mobile technologies: The future of neurological care. Nat Rev Neurol 14, 285. [DOI] [PubMed] [Google Scholar]
- [24]. Ferreira JJ, Godinho C, Santos AT, Domingos J, Abreu D, Lobo R, Gonçalves N, Barra M, Larsen F, Fagerbakke Ø, Akeren I, Wangen H, Serrano JA, Weber P, Thoms A, Meckler S, Sollinger S, van Uem J, Hobert MA, Maier KS, Matthew H, Isaacs T, Duffen J, Graessner H, Maetzler W (2015) Quantitative home-based assessment of Parkinson’s symptoms: The SENSE-PARK feasibility and usability study. BMC Neurol 15, 89–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Adams JL, Dinesh K, Xiong M, Tarolli CG, Sharma S, Sheth N, Aranyosi AJ, Zhu W, Goldenthal S, Biglan K, Dorsey ER, Sharma G (2017) Multiple wearable sensors in Parkinson and Huntington disease individuals: A pilot study in clinic and at home. Digit Biomark 1, 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Zhan A, Mohan S, Tarolli C, Schneider RB, Adams JL, Sharma S, Elson MJ, Spear KL, Glidden AM, Little MA, Terzis A, Dorsey ER, Saria S (2018) Using smartphones and machine learning to quantify Parkinson disease severity: The mobile Parkinson disease score. JAMA Neurol 75, 876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Hanna KK, Cronin-Golomb A (2012) Impact of anxiety on quality of life in Parkinson’s disease. Parkinsons Dis 2012, 640707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Havlikova E, van Dijk JP, Nagyova I, Rosenberger J, Middel B, Dubayova T, Gdovinova Z, Groothoff JW (2011) The impact of sleep and mood disorders on quality of life in Parkinson’s disease patients. J Neurol 258, 2222–2229. [DOI] [PubMed] [Google Scholar]
- [29]. Schrag A (2006) Quality of life and depression in Parkinson’s disease. J Neurol Sci 248, 151–157. [DOI] [PubMed] [Google Scholar]
- [30]. Robinson PN (2012) Deep phenotyping for precision medicine. Hum Mutat 33, 777–780. [DOI] [PubMed] [Google Scholar]
- [31]. Delude CM (2015) Deep phenotyping: The details of disease. Nature 527, S14–S15. [DOI] [PubMed] [Google Scholar]
- [32]. Robinson PN, Mungall CJ, Haendel M (2015) Capturing phenotypes for precision medicine. Cold Spring Harb Mol Case Stud 1, a000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Tracy RP (2008) ‘Deep phenotyping’: Characterizing populations in the era of genomics and systems biology. Curr Opin Lipidol 19, 151–157. [DOI] [PubMed] [Google Scholar]
- [34]. FitzGerald G, Botstein D, Califf R, Collins R, Peters K, Van Bruggen N, Rader D (2018) The future of humans as model organisms. Science 361, 552. [DOI] [PubMed] [Google Scholar]
- [35]. Chen R, Mias GI, Li-Pook-Than J, Jiang L, Lam HY, Chen R, Miriami E, Karczewski KJ, Hariharan M, Dewey FE, Cheng Y, Clark MJ, Im H, Habegger L, Balasubramanian S, O’Huallachain M, Dudley JT, Hillenmeyer S, Haraksingh R, Sharon D, Euskirchen G, Lacroute P, Bettinger K, Boyle AP, Kasowski M, Grubert F, Seki S, Garcia M, Whirl-Carrillo M, Gallardo M, Blasco MA, Greenberg PL, Snyder P, Klein TE, Altman RB, Butte AJ, Ashley EA, Gerstein M, Nadeau KC, Tang H, Snyder M (2012) Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell 148, 1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Berenbaum F (2019) Deep phenotyping of osteoarthritis: A step forward. Ann Rheum Dis 78, 3–5. [DOI] [PubMed] [Google Scholar]
- [37]. Pentland A (2015) Social Physics: How Social Networks Can Make Us Smarter, Penguin Books, New York. [Google Scholar]
- [38]. Chen R, Jankovic F, Marinsek N, Foschini L, Kourtis L, Signorini A, Pugh M, Shen J, Yaari R, Maljkovic V, Sunga M, Song HH, Jung HJ, Tseng B, Trister A (2019) Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams in Proceedings of the 25th ACMSIGKDD International Conference on Knowledge Discovery & Data Mining, ACM, Anchorage, AK, USA, pp. 2145-2155.
- [39]. Bloem BR, Marks WJ Jr, Silva de Lima AL, Kuijf ML, van Laar T, Jacobs BPF, Verbeek MM, Helmich RC, van de Warrenburg BP, Evers LJW, intHout J, van de Zande T, Snyder TM, Kapur R, Meinders MJ (2019) The Personalized Parkinson Project: Examining disease progression through broad biomarkers in early Parkinson’s disease. BMC Neurol 19, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Apple Watch for Parkinson’s - WATCH-PD, https://watchpdstudy.org, Accessed October 30, 2019.
- [41]. Marek K, Jennings D, Lasch S, Siderowf A, Tanner C, Simuni T, Coffey C, Kieburtz K, Flagg E, Chowdhury S, Poewe W, Mollenhauer B, Klinik P-E, Sherer T, Frasier M, Meunier C, Rudolph A, Casaceli C, Seibyl J, Mendick S, Schuff N, Zhang Y, Toga A, Crawford K, Ansbach A, De Blasio P, Piovella M, Trojanowski J, Shaw L, Singleton A, Hawkins K, Eberling J, Brooks D, Russell D, Leary L, Factor S, Sommerfeld B, Hogarth P, Pighetti E, Williams K, Standaert D, Guthrie S, Hauser R, Delgado H, Jankovic J, Hunter C, Stern M, Tran B, Leverenz J, Baca M, Frank S, Thomas C-A, Richard I, Deeley C, Rees L, Sprenger F, Lang E, Shill H, Obradov S, Fernandez H, Winters A, Berg D, Gauss K, Galasko D, Fontaine D, Mari Z, Gerstenhaber M, Brooks D, Malloy S, Barone P, Longo K, Comery T, Ravina B, Grachev I, Gallagher K, Collins M, Widnell KL, Ostrowizki S, Fontoura P, Ho T, Luthman J, Brug Mvd, Reith AD, Taylor P (2011) The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 95, 629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wearable Assessments in the Clinic and Home in PD (WATCH-PD), U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/NCT03681015, Posted October 8, 2019, Accessed January 16, 2020.
- [43]. Hipp G, Vaillant M, Diederich NJ, Roomp K, Satagopam VP, Banda P, Sandt E, Mommaerts K, Schmitz SK, Longhino L, Schweicher A, Hanff A-M, Nicolai B, Kolber P, Reiter D, Pavelka L, Binck S, Pauly C, Geffers L, Betsou F, Gantenbein M, Klucken J, Gasser T, Hu MT, Balling R, Krüger R (2018) The Luxembourg Parkinson’s study: A comprehensive approach for stratification and early diagnosis. Front Aging Neurosci 10, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Bot BM, Suver C, Neto EC, Kellen M, Klein A, Bare C, Doerr M, Pratap A, Wilbanks J, Dorsey ER, Friend SH, Trister AD (2016) The mPower study, Parkinson disease mobile data collected using ResearchKit. Sci Data 3, 160011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Zhan A, Little M, Harris D, Abiola S, Dorsey E, Saria S, Terzis A (2016) High frequency remote monitoring of Parkinson’s disease via smartphone: Platform overview and medication response detection, arXiv:1601.00960.
- [46]. Langevin R, Ali MR, Sen T, Snyder C, Myers T, Dorsey ER, Hoque ME (2019) The PARK framework for automated analysis of Parkinson’s disease characteristics. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies 3, 1–22. [Google Scholar]
- [47]. Kabelac Z, Tarolli CG, Snyder C, Feldman B, Glidden A, Hsu CY, Hristov R, Dorsey ER, Katabi D (2019) Passive monitoring at home: A pilot study in Parkinson disease. Digit Biomark 3, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Adib F, Kabelac Z, Katabi D, Miller RC (2014) 3D tracking via body radio reflections in 11th USENIX Symposium on Networked Systems Design and Implementation (NSDI ’14), USENIX Association, Seattle, WA, pp. 317-329.
- [49]. Jenkinson C, Fitzpatrick RAY, Peto VIV, Greenhall R, Hyman N (1997) The Parkinson’s Disease Questionnaire (PDQ-39): Development and validation of a Parkinson’s disease summary index score. Age Ageing 26, 353–357. [DOI] [PubMed] [Google Scholar]
- [50]. Chaudhuri KR, Pal S, DiMarco A, Whately-Smith C, Bridgman K, Mathew R, Pezzela FR, Forbes A, Högl B, Trenkwalder C (2002) The Parkinson’s disease sleep scale: A new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J Neurol Neurosurg Psychiatry 73, 629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Leentjens AFG, Dujardin K, Pontone GM, Starkstein SE, Weintraub D, Martinez-Martin P (2014) The Parkinson Anxiety Scale (PAS): Development and validation of a new anxiety scale. Mov Disord 29, 1035–1043. [DOI] [PubMed] [Google Scholar]
- [52]. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- [53]. Sheikh JI, Yesavage JA (1986) Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol 5, 165–173. [Google Scholar]
- [54].Parkinson’s Disease Biomarkers Program, National Institutes of Health, https://pdbp.ninds.nih.gov, Accessed February 26, 2020.
- [55]. Ofri D (2017) What Patients Say, What Doctors Hear, Beacon Press, Boston. [Google Scholar]
- [56].(2019) Thirty Second Annual Symposium on Etiology, Pathogenesis, and Treatment of Parkinson Disease and Other Movement Disorders. Mov Disord 34, S16. [DOI] [PubMed] [Google Scholar]
- [57]. Lipsmeier F, Taylor KI, Kilchenmann T, Wolf D, Scotland A, Schjodt-Eriksen J, Cheng W-Y, Fernandez-Garcia I, Siebourg-Polster J, Jin L, Soto J, Verselis L, Boess F, Koller M, Grundman M, Monsch AU, Postuma RB, Ghosh A, Kremer T, Czech C, Gossens C, Lindemann M (2018) Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson’s disease clinical trial. Mov Disord 33, 1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Yang Y-R, Lee Y-Y, Cheng S-J, Lin P-Y, Wang R-Y (2008) Relationships between gait and dynamic balance in early Parkinson’s disease. Gait Posture 27, 611–615. [DOI] [PubMed] [Google Scholar]
- [59]. Curtze C, Nutt JG, Carlson-Kuhta P, Mancini M, Horak FB (2016) Objective gait and balance impairments relate to balance confidence and perceived mobility in people with Parkinson disease. Phys Ther 96, 1734–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Bryant MS, Rintala DH, Hou JG, Lai EC, Protas EJ (2011) Effects of levodopa on forward and backward gait patterns in persons with Parkinson’s disease. Neurorehabilitation 29, 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Nutt JG, Rufener SL, Carter JH, Anderson VC, Pahwa R, Hammerstad JP, Burchiel KJ (2001) Interactions between deep brain stimulation and levodopa in Parkinson’s disease. Neurology 57, 1835–1842. [DOI] [PubMed] [Google Scholar]
- [62]. Hausdorff JM, Gruendlinger L, Scollins L, O’Herron S, Tarsy D (2009) Deep brain stimulation effects on gait variability in Parkinson’s disease. Mov Disord 24, 1688–1692. [DOI] [PubMed] [Google Scholar]
- [63]. Fritz S, Lusardi M (2009) White Paper: “Walking Speed: The Sixth Vital Sign”. J Geriatr Phys Ther 32, 2–5. [PubMed] [Google Scholar]
- [64]. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J (2011) Gait speed and survival in older adults. JAMA 305, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. White DK, Neogi T, Nevitt MC, Peloquin CE, Zhu Y, Boudreau RM, Cauley JA, Ferrucci L, Harris TB, Satterfield SM, Simonsick EM, Strotmeyer ES, Zhang Y (2012) Trajectories of gait speed predict mortality in well-functioning older adults: The health, aging and body composition study. J Gerontol A Biol Sci Med Sci 68, 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Kaye JA, Maxwell SA, Mattek N, Hayes TL, Dodge H, Pavel M, Jimison HB, Wild K, Boise L, Zitzelberger TA (2011) Intelligent systems for assessing aging changes: Home-based, unobtrusive, and continuous assessment of aging. J Gerontol B Psychol Sci Soc Sci 66B, i180–i190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Dodge HH, Mattek NC, Austin D, Hayes TL, Kaye JA (2012) In-home walking speeds and variability trajectories associated with mild cognitive impairment. Neurology 78, 1946–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Grande G, Triolo F, Nuara A, Welmer A-K, Fratiglioni L, Vetrano DL (2019) Measuring gait speed to better identify prodromal dementia. Exp Gerontol 124, 110625. [DOI] [PubMed] [Google Scholar]
- [69]. Maraki MI, Stefanis L, Yannakoulia M, Kosmidis MH, Xiromerisiou G, Dardiotis E, Hadjigeorgiou GM, Sakka P, Scarmeas N, Stamelou M (2019) Motor function and the probability of prodromal Parkinson’s disease in older adults. Mov Disord 34, 1345–1353. [DOI] [PubMed] [Google Scholar]
- [70]. Chastan N, Bair W-N, Resnick SM, Studenski SA, Decker LM (2019) Prediagnostic markers of idiopathic Parkinson’s disease: Gait, visuospatial ability and executive function. Gait Posture 68, 500–505. [DOI] [PubMed] [Google Scholar]
- [71]. Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA (2012) Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 78, 1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72]. Haapaniemi TH, Pursiainen V, Korpelainen JT, Huikuri HV, Sotaniemi KA, Myllylä VV (2001) Ambulatory ECG and analysis of heart rate variability in Parkinson’s disease. J Neurol Neurosurg Psychiatry 70, 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Niwa F, Kuriyama N, Nakagawa M, Imanishi J (2011) Circadian rhythm of rest activity and autonomic nervous system activity at different stages in Parkinson’s disease. Auton Neurosci 165, 195–200. [DOI] [PubMed] [Google Scholar]
- [74]. Maetzler W, Karam M, Berger MF, Heger T, Maetzler C, Ruediger H, Bronzova J, Lobo PP, Ferreira JJ, Ziemssen T, Berg D (2015) Time- and frequency-domain parameters of heart rate variability and sympathetic skin response in Parkinson’s disease. J Neural Transm 122, 419–425. [DOI] [PubMed] [Google Scholar]
- [75]. Yalcin A, Atmis V, Cengiz OK, Cinar E, Aras S, Varli M, Atli T (2016) Evaluation of cardiac autonomic functions in older Parkinson’s disease patients: A cross-sectional study. Aging Dis 7, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76]. Martinez-Ramirez D, De Jesus S, Walz R, Cervantes-Arriaga A, Peng-Chen Z, Okun MS, Alatriste-Booth V, Rodriguez-Violante M (2015) A polysomnographic study of Parkinson’s disease sleep architecture. Parkinsons Dis 2015, 570375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77]. Mantovani S, Smith SS, Gordon R, O’Sullivan JD (2018) An overview of sleep and circadian dysfunction in Parkinson’s disease. J Sleep Res 27, e12673. [DOI] [PubMed] [Google Scholar]
- [78]. Yong M-H, Fook-Chong S, Pavanni R, Lim L-L, Tan E-K (2011) Case control polysomnographic studies of sleep disorders in Parkinson’s disease. PLoS One 6, e22511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79]. Sringean J, Taechalertpaisarn P, Thanawattano C, Bhidayasiri R (2016) How well do Parkinson’s disease patients turn in bed? Quantitative analysis of nocturnal hypokinesia using multisite wearable inertial sensors. Parkinsonism Relat Disord 23, 10–16. [DOI] [PubMed] [Google Scholar]
- [80]. Uchino K, Shiraishi M, Tanaka K, Akamatsu M, Hasegawa Y (2017) Impact of inability to turn in bed assessed by a wearable three-axis accelerometer on patients with Parkinson’s disease. PLoS One 12, e0187616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Zhao M, Yue S, Katabi D, Jaakkola TS, Bianchi MT (2017) Learning sleep stages from radio signals: A conditional adversarial architecture in Proceedings of the 34th International Conference on Machine Learning, eds. Doina P, Yee Whye T, PMLR, pp. 4100-4109.
- [82]. Hsu C-Y, Ahuja A, Yue S, Hristov R, Kabelac Z, Katabi D (2017) Zero-effort in-home sleep and insomniamonitoring using radio signals in Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies, Association for Computing Machinery.
- [83]. Arora S, Baig F, Lo C, Barber TR, Lawton MA, Zhan A, Rolinski M, Ruffmann C, Klein JC, Rumbold J, Louvel A, Zaiwalla Z, Lennox G, Quinnell T, Dennis G, Wade-Martins R, Ben-Shlomo Y, Little MA, Hu MT (2018) Smartphone motor testing to distinguish idiopathic REM sleep behavior disorder, controls, and PD. Neurology 91, e1528–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84]. The Parkinson Study Group (2017) A randomized trial of relamorelin for constipation in Parkinson’s disease (MOVE-PD): Trial results and lessons learned. Parkinsonism Relat Disord 37, 101–105. [DOI] [PubMed] [Google Scholar]
- [85]. Liddle J, Ireland D, McBride SJ, Brauer SG, Hall LM, Ding H, Karunanithi M, Hodges PW, Theodoros D, Silburn PA, Chenery HJ (2014) Measuring the lifespace of people with Parkinson’s disease using smartphones: Proof of principle. JMIR Mhealth Uhealth 2, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86]. Petersen J, Austin D, Mattek N, Kaye J (2015) Time out-of-home and cognitive, physical, and emotional wellbeing of older adults: A longitudinal mixed effects model. PLoS One 10, e0139643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87]. Buchman AS, Boyle PA, Wilson RS, James BD, Leurgans SE, Arnold SE, Bennett DA (2010) Loneliness and the rate of motor decline in old age: The rush memory and aging project, a community-based cohort study. BMC Geriatrics 10, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88]. Perissinotto CM, Stijacic Cenzer I, Covinsky KE (2012) Loneliness in older persons: A predictor of functional decline and death. Arch Intern Med 172, 1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89]. Austin J, Dodge HH, Riley T, Jacobs PG, Thielke S, Kaye J (2016) A smart-home system to unobtrusively and continuously assess loneliness in older adults. IEEE J Transl Eng Health Med 4, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. McRae C, Fazio E, Hartsock G, Kelley L, Urbanski S, Russell D (2009) Predictors of loneliness in caregivers of persons with Parkinson’s disease. Parkinsonism Relat Disord 15, 554–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].When Parkinson’s Leads to Social Isolation, Parkinson’s Victoria, https://www.parkinsonsvic.org.au/about-us/media-release/when-parkinsons-leads-to-social-isolation, Posted November 13, 2017, Accessed November 15, 2019.
- [92]. Simuni T, Caspell-Garcia C, Coffey CS, Weintraub D, Mollenhauer B, Lasch S, Tanner CM, Jennings D, Kieburtz K, Chahine LM, Marek K (2018) Baseline prevalence and longitudinal evolution of non-motor symptoms in early Parkinson’s disease: The PPMI cohort. J Neurol Neurosurg Psychiatry 89, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93]. Norris P (2001) Digital Divide: Civic Engagement, Information Poverty, and the Internet Worldwide (Communication, Society and Politics), Cambridge University Press. [Google Scholar]
- [94]. Schneider MG, Swearingen CJ, Shulman LM, Ye J, Baumgarten M, Tilley BC (2009) Minority enrollment in Parkinson’s disease clinical trials. Parkinsonism Relat Disord 15, 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. Petersen C, DeMuro P (2015) Legal and regulatory considerations associated with use of patient-generated health data from social media and mobile health (mHealth) devices. Appl Clin Inform 6, 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96]. Silva de Lima AL, Hahn T, Evers LJW, de Vries NM, Cohen E, Afek M, Bataille L, Daeschler M, Claes K, Boroojerdi B, Terricabras D, Little MA, Baldus H, Bloem BR, Faber MJ (2017) Feasibility of large-scale deployment of multiple wearable sensors in Parkinson’s disease. PLoS One 12, e0189161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97]. Cohen S, Waks Z, Elm JJ, Gordon MF, Grachev ID, Navon-Perry L, Fine S, Grossman I, Papapetropoulos S, Savola J-M (2018) Characterizing patient compliance over six months in remote digital trials of Parkinson’s and Huntington disease. BMC Med Inform Decis Mak 18, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98]. Dorsey ER, “Yvonne” Chan Y-F, McConnell MV, Shaw SY, Trister AD, Friend SH (2017) The use of smartphones for health research. Acad Med 92, 157–160. [DOI] [PubMed] [Google Scholar]
- [99]. Coravos A, Goldsack JC, Karlin DR, Nebeker C, Perakslis E, Zimmerman N, Erb MK (2019) Digital medicine: A primer on measurement. Digit Biomark 3, 31–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100]. Goldsack JC, Coravos A, Bakker JP, Bent B, Dowling AV, Fitzer-Attas C, Godfrey A, Godino JG, Gujar N, Izmailova E, Manta C, Peterson B, Vandendriessche B, Wood WA, Wang KW, Dunn J (2020) Verification, analytical validation, and clinical validation (V3): The foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs). NPJ Digit Med 3, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101]. Shema-Shiratzky S, Hillel I, Mirelman A, Regev K, Hsieh KL, Karni A, Devos H, Sosnoff JJ, Hausdorff JM (2020) A wearable sensor identifies alterations in community ambulation in multiple sclerosis: Contributors to real-world gait quality and physical activity. J Neurol, doi: 10.1007/s00415-020-09759-7. [DOI] [PubMed]
- [102]. Elragal A (2014) ERP and big data: The inept couple. Procedia Technol 16, 242–249. [Google Scholar]
- [103]. Hernández-González J, Inza I, Lozano JA (2016) Weak supervision and other non-standard classification problems: A taxonomy. Pattern Recognit Lett 69, 49–55. [Google Scholar]
- [104]. Heilbron K, Noyce AJ, Fontanillas P, Alipanahi B, Nalls MA, Agee M, Auton A, Bell RK, Bryc K, Elson SL, Furlotte NA, Hinds DA, McCreight JC, Huber KE, Kleinman A, Litterman NK, McIntyre MH, Mountain JL, Noblin ES, Northover CAM, Pitts SJ, Sathirapongsasuti JF, Sazonova OV, Shelton JF, Shringarpure S, Tian C, Tung JY, Vacic V, Wilson CH, Cannon P, The 23andMe Research T (2019) The Parkinson’s phenome—traits associated with Parkinson’s disease in a broadly phenotyped cohort. NPJ Parkinsons Dis 5, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105]. Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I, Stern M, Tanner C, Weiner W (1990) Variable expression of Parkinson’s disease. Neurology 40, 1529. [DOI] [PubMed] [Google Scholar]
- [106]. Adams CP, Brantner VV (2006) Estimating the cost of new drug development: Is it really $802 million? Health Affairs 25, 420–428. [DOI] [PubMed] [Google Scholar]
- [107]. Dorsey ER, Papapetropoulos S, Xiong M, Kieburtz K (2017) The first frontier: Digital biomarkers for neurodegenerative disorders. Digit Biomark 1, 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108]. Writing Group for the NINDS Exploratory Trials in Parkinson Disease Investigators (2015) Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: A randomized clinical trial. JAMA 313, 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109]. Kalia LV, Kalia SK, Lang AE (2015) Disease-modifying strategies for Parkinson’s disease. Mov Disord 30, 1442–1450. [DOI] [PubMed] [Google Scholar]
- [110]. Parashos SA, Luo S, Biglan KM, Bodis-Wollner I, He B, Liang GS, Ross GW, Tilley BC, Shulman LM, Investigators N-P (2014) Measuring disease progression in early Parkinson disease: The National Institutes of Health Exploratory Trials in Parkinson Disease (NET-PD) experience. JAMA Neurol 71, 710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Real-World Evidence, U.S. Food and Drug Administration, https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence, Posted May 9, 2015, Accessed November 15, 2019.
- [112].Submitting Documents Using Real-World Data and Real-World Evidence to FDA for Drugs and Biologics Guidance for Industry, U.S. Department of Health and Human Services Food and Drug Administration, https://www.fda.gov/media/124795/download, Accessed November 15, 2019.
- [113].Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices: Guidance for Industry and Food and Drug Administration Staff, U.S. Food and Drug Administration, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-real-world-evidence-support-regulatory-decision-making-medical-devices, Accessed January 16, 2020.
- [114].Qualification Opinion, Stride velocity 95th centile as a secondary endpoint in Duchenne Muscular Dystrophy measured by a valid and suitable wearable device, European Medicines Agency, https://www.ema.europa.eu/en/documents/scientific-guideline/qualification-opinion-stride-velocity-95th-centile-secondary-endpoint-duchenne-muscular-dystrophy en.pdf, Posted April 26, 2019, Accessed November 15, 2019.
- [115]. Lamore R III, Jacob E, Jacob SC, Hilas O (2010) Dalfampridine (Ampyra): An aid to walking in patients with multiple sclerosis. Pharm Ther 35, 665–669. [Google Scholar]
- [116].Prescribing Information: AMPYRA, U.S. Food and Drug Administration, https://ampyra.com/prescribing-information.pdf, Accessed November 15, 2019.
- [117]. Tsiouris KM, Gatsios D, Rigas G, Miljkovic D, Seljak BK, Bohanec M, Arredondo MT, Antonini A, Konitsiotis S, Koutsouris DD, Fotiadis DI (2017) PD_Manager: An mHealth platform for Parkinson’s disease patient management. Healthc Technol Lett 4, 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118]. Riggare S, Hägglund M (2018) Precision medicine in Parkinson’s disease - exploring patient-initiated self-tracking. J Parkinsons Dis 8, 441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119]. Riggare S, Scott Duncan T, Hvitfeldt H, Hägglund M (2019) “You have to knowwhy you’re doing this”:Amixed methods study of the benefits and burdens of self-tracking in Parkinson’s disease. BMC Med Inform Decis Mak 19, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120]. Santiago A, Langston JW, Gandhy R, Dhall R, Brillman S, Rees L, Barlow C (2019) Qualitative evaluation of the personal KinetiGraphTM Movement Recording System in a Parkinson’s clinic. J Parkinsons Dis 9, 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121]. Heldman DA, Harris DA, Felong T, Andrzejewski KL, Dorsey ER, Giuffrida JP, Goldberg B, Burack MA (2017) Telehealth management of Parkinson’s disease using wearable sensors: An exploratory study. Digit Biomark 1, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122]. Chao-Hung K, Gabrielle AW-D, Andrew LK (2018) Approaches to closed-loop deep brain stimulation for movement disorders. Neurosurgical Focus FOC 45, E2. [DOI] [PubMed] [Google Scholar]
- [123]. Rosa M, Arlotti M, Ardolino G, Cogiamanian F, Marceglia S, Di Fonzo A, Cortese F, Rampini PM, Priori A (2015) Adaptive deep brain stimulation in a freely moving parkinsonian patient. Mov Disord 30, 1003–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124]. Shah SA, Tinkhauser G, Chen CC, Little S, Brown P (2018) Parkinsonian tremor detection from subthalamic nucleus local field potentials for closed-loop deep brain stimulation. In 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp. 2320-2324. [DOI] [PMC free article] [PubMed]
- [125]. Sarkar U, Fisher L, Schillinger D (2006) Is self-efficacy associated with diabetes self-management across race/ethnicity and health literacy? Diabetes Care 29, 823. [DOI] [PubMed] [Google Scholar]
- [126]. Saria S, Goldenberg A (2015) Subtyping: What it is and its role in precision medicine. IEEE Intell Syst 30, 70–75. [Google Scholar]
- [127]. Sardi SP, Cedarbaum JM, Brundin P (2018) Targeted therapies for Parkinson’s disease: From genetics to the clinic. Mov Disord 33, 684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128]. Saunders-Pullman R, Mirelman A, Alcalay RN, Wang C, Ortega RA, Raymond D, Mejia-Santana H, Orbe-Reilly M, Johannes BA, Thaler A, Ozelius L, Orr-Urtreger A, Marder KS, Giladi N, Bressman SB (2018) Progression in the LRRK2-associated Parkinson disease population. JAMA Neurol 75, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129]. Malek N, Weil RS, Bresner C, Lawton MA, Grosset KA, Tan M, Bajaj N, Barker RA, Burn DJ, Foltynie T, Hardy J, Wood NW, Ben-Shlomo Y, Williams NW, Grosset DG, Morris HR (2018) Features of GBA-associated Parkinson’s disease at presentation in the UK Tracking Parkinson’s study. J Neurol Neurosurg Psychiatry 89, 702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130]. Dyson F (1998) Imagined Worlds (The Jerusalem-Harvard Lectures), Harvard University Press, Cambridge. [Google Scholar]
- [131]. van Wamelen DJ, Hota S, Podlewska A, Leta V, Trivedi D, Rizos A, Parry M, Chaudhuri KR (2019) Non-motor correlates of wrist-worn wearable sensor use in Parkinson’s disease: An exploratory analysis. NPJ Parkinsons Dis 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132]. Hasegawa N, Shah VV, Carlson-Kuhta P, Nutt JG, Horak FB, Mancini M (2019) How to select balance measures sensitive to Parkinson’s disease from body-worn inertial sensors-separating the trees from the forest. Sensors 19, 3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133]. Haji Ghassemi N, Hannink J, Roth N, Gaßner H, Marxreiter F, Klucken J, Eskofier BM (2019) Turning analysis during standardized test using on-shoe wearable sensors in Parkinson’s disease. Sensors 19, 3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134]. Nguyen A, Roth N, Ghassemi NH, Hannink J, Seel T, Klucken J, Gassner H, Eskofier BM (2019) Development and clinical validation of inertial sensor-based gait-clustering methods in Parkinson’s disease. J Neuroeng Rehabil 16, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135]. Buckley C, Galna B, Rochester L, Mazzà C (2019) Upper body accelerations as a biomarker of gait impairment in the early stages of Parkinson’s disease. Gait Posture 71, 289–295. [DOI] [PubMed] [Google Scholar]
- [136]. Khoury N, Attal F, Amirat Y, Oukhellou L, Mohammed S (2019) Data-driven based approach to aid Parkinson’s disease diagnosis. Sensors 19, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137]. Bertoli M, Cereatti A, Trojaniello D, Avanzino L, Pelosin E, Del Din S, Rochester L, Ginis P, Bekkers EMJ, Mirelman A, Hausdorff JM, Della Croce U (2018) Estimation of spatio-temporal parameters of gait from magneto-inertial measurement units: Multicenter validation among Parkinson, mildly cognitively impaired and healthy older adults. Biomed Eng Online 17, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138]. Silva de Lima AL, Evers LJW, Hahn T, de Vries NM, Daeschler M, Boroojerdi B, Terricabras D, Little MA, Bloem BR, Faber MJ (2018) Impact of motor fluctuations on real-life gait in Parkinson’s patients. Gait Posture 62, 388–394. [DOI] [PubMed] [Google Scholar]
- [139]. Schlachetzki JCM, Barth J, Marxreiter F, Gossler J, Kohl Z, Reinfelder S, Gassner H, Aminian K, Eskofier BM, Winkler J, Klucken J (2017) Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS One 12, e0183989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140]. Lo C, Arora S, Baig F, Lawton MA, El Mouden C, Barber TR, Ruffmann C, Klein JC, Brown P, Ben-Shlomo Y, de Vos M, Hu MT (2019) Predicting motor, cognitive & functional impairment in Parkinson’s. Ann Clin Transl Neurol 6, 1498–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141]. Prince J, Andreotti F, De Vos M (2019) Multi-source ensemble learning for the remote prediction of Parkinson’s disease in the presence of source-wise missing data. IEEE Trans Biomed Eng 66, 1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142]. Singh S, Xu W (2020) Robust detection of Parkinson’s disease using harvested smartphone voice data: A telemedicine approach. Telemed J E Health 26, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143]. Rusz J, Hlavnička J, Tykalová T, Novotný M, Dušek P, Šonka K, Růžička E (2018) Smartphone allows capture of speech abnormalities associated with high risk of developing Parkinson’s disease. IEEE Trans Neural Syst Rehabil Eng 26, 1495–1507. [DOI] [PubMed] [Google Scholar]
- [144]. Prince J, Arora S, de Vos M (2018) Big data in Parkinson’s disease: Using smartphones to remotely detect longitudinal disease phenotypes. Physiol Meas 39, 044005. [DOI] [PubMed] [Google Scholar]