Abstract
Context
Reduced bone material strength index (BMSi) and increased cortical porosity (CtPo) have emerged as potentially contributing to fracture risk in type 2 diabetes mellitus (T2DM) patients.
Objective
To determine whether BMSi or CtPo are related to other diabetic complications.
Design
Cross-sectional observational study.
Setting
Subjects recruited from a random sample of southeast Minnesota residents.
Participants
A total of 171 T2DM patients (mean age, 68.8 years) and 108 age-matched nondiabetic controls (mean age, 67.3 years).
Main Measures
Bone material strength index was measured using microindentation, skin advanced glycation end-products (AGEs) measured using autofluorescence, high-resolution peripheral quantitative computed tomography at the distal radius and tibia, assessment of diabetic microvascular complications including urine microalbuminuria, retinopathy, neuropathy, and vascular disease (ankle brachial index and transcutaneous oxygen tension [TcPO2]). All analyses were adjusted for age, sex, and body mass index.
Results
Skin AGEs were negatively correlated with the BMSi in both T2DM (r = -0.30, P < 0.001) and control (r = -0.23, P = 0.020) subjects. In relating diabetic complications to CtPo, we found that T2DM patients with clinically significant peripheral vascular disease (TcPO2 ≤ 40 mm Hg) had higher (+21.0%, P = 0.031) CtPo at the distal tibia as compared to controls; in these subjects, CtPo was negatively correlated with TcPO2 at both the distal tibia (r = -0.39, P = 0.041) and radius (r = -0.41, P = 0.029).
Conclusions
Our findings demonstrate that bone material properties are related to AGE accumulation regardless of diabetes status, while CtPo in T2DM patients is linked to TcPO2, a measure of microvascular blood flow.
Keywords: bone, osteoporosis, diabetes, vascular disease, AGEs
Type 2 diabetes mellitus (T2DM) is an enormous and growing public health problem (1). In addition to the well-recognized complications of T2DM (retinopathy, nephropathy, neuropathy, and vascular disease), these patients are at an elevated fracture risk (2) despite having preserved or even increased areal bone mineral density (aBMD), as assessed by dual-energy x-ray absorptiometry (DXA) (3, 4). Moreover, fractures in T2DM patients result in substantially higher morbidity as compared to nondiabetic fracture patients (5). Epidemiologic data indicate that the Fracture Risk Assessment Tool (FRAX) and aBMD underestimate the risk of fracture in T2DM patients, suggesting that other factors are responsible for explaining the increased fracture risk (4, 6, 7).
Of all the skeletal parameters evaluated in patients with T2DM, the 2 that have emerged as potential mediators of skeletal fragility in this disorder are reductions in bone material properties and increases in cortical porosity (CtPo). Our group initially reported that postmenopausal women with longstanding (> 10 years) T2DM had reductions in the bone material strength index (BMSi) using in vivo microindentation (OsteoProbe®) (8). Similar findings were reported by 2 other independent groups (9, 10), although a more recent study found reductions in the BMSi in black, but not white, patients with T2DM (11).
Similarly, increased CtPo has emerged as a potential risk factor contributing to fragility fractures in T2DM patients (12, 13). Interestingly, recent studies have demonstrated that CtPo independently predicts forearm fracture risk in nondiabetic, osteopenic postmenopausal women regardless of the World Health Organization-FRAX (WHO-FRAX) score or aBMD (14, 15). Moreover, bisphosphonate treatment could attenuate the increase in CtPo in osteopenic postmenopausal women (15, 16). These findings suggest that CtPo may be a promising target for osteoporosis treatment (17), particularly in T2DM patients who have normal aBMD but increased CtPo.
Although increased CtPo has emerged as a potential skeletal abnormality in T2DM patients (12, 18, 19), as with the BMSi, the findings have been inconsistent. Indeed, although some studies have shown higher CtPo in T2DM patients compared with nondiabetic controls (18–21), others found no differences (8, 22). Further, it is at present unclear whether increased CtPo in T2DM patients is associated with 1 or more diabetic complication(s). Using clinical evaluations, Shanbhogue et al (21) found that T2DM patients with evidence of retinopathy, nephropathy, or neuropathy had higher CtPo at the radius and a trend towards higher CtPo at the tibia as compared to nondiabetic controls. However, the presence or absence of vascular disease (macro- and/or microvascular) was not assessed in these patients, and it is not clear which specific diabetic complication(s) may be most related to the development of increased CtPo.
In the present study, we performed a comprehensive assessment of diabetic complications in a T2DM cohort of men ≥ 50 years of age and postmenopausal women. Specifically, we evaluated urine microalbuminuria, retinopathy, and neuropathy (touch, temperature, and vibration sensation), as well as transcutaneous oxygen tension (TcPO2, a noninvasive method to evaluate microvascular blood flow) and the ankle brachial index (a measure of macrovascular blood flow). We then related the presence or absence of these diabetic complications to bone material properties (BMSi), skin advanced glycation end-products (AGEs), and bone microarchitecture assessed by high-resolution peripheral quantitative computed tomography (HRpQCT) imaging at the distal radius and tibia in the T2DM patients, and we also compared the bone microarchitecture findings in the T2DM patients to a cohort of nondiabetic control subjects.
Materials and Methods
Study subjects
The study was approved by the Mayo Clinic Institutional Review Board and written, informed consent was obtained from all participants. Eligible T2DM patients were identified using the resources of the Rochester Epidemiology Project (23). Nondiabetic controls were recruited from the local population. The subjects were studied between January 2017 and September 2019. We recruited 171 T2DM patients, including 75 postmenopausal women (serum follicle-stimulating hormone > 20 IU/L and/or age > 55 years and no menses for at least a year) and 96 men ≥ 50 years. Type 2 diabetes mellitus patients were defined as having a median HbA1c ≥ 6.5% over the previous 5 years. Matched nondiabetic controls (n = 108; 63 women and 45 men) were defined as having a HbA1c < 6.5% at the screening visit. Of the 108 controls, 88 had HbA1c values < 5.7% and 20 had HbA1c values between 5.7% and 6.1%. Exclusion criteria included: (1) any disorders associated with altered skeletal structure and function, including presence of stage IV or V chronic kidney disease, chronic liver disease, unstable cardiovascular disease, malignancy, chronic gastrointestinal disease, autoimmune rheumatologic conditions (eg, rheumatoid arthritis), hypo- or hyperparathyroidism, Cushing’s syndrome, severe chronic obstructive pulmonary disease, alcoholism, or type 1 diabetes; and (2) treatment with oral corticosteroids (inhaled corticosteroids did not preclude inclusion) > 3 months at any time or > 10 days within the previous year and treatment within the past year with either anticonvulsants (except gabapentin), pharmacological doses of thyroid hormone (causing thyroid stimulating hormone decline below normal), adrenal or anabolic steroids, aromatase inhibitors, calcitonin, estrogen, selective estrogen receptor modulators, parathyroid hormone, denosumab, or thiazolidinediones. We also excluded subjects who had been exposed to a bisphosphonate within the past 5 years. All subjects were required to have sufficient levels of vitamin D (serum 25-hydroxyvitamin D [25-(OH)D] ≥ 15 ng/ml) in order to exclude patients who may have osteomalacia.
Study protocol
All studies were performed and samples were collected in the Mayo Clinical Research and Trials Unit. Study subjects had a health interview and medical record review by the study coordinator. Weight was obtained using an electronic scale (Model 5002; Tronic, Inc., White Plains, New York) and height was measured using a customized stadiometer (Mayo Section of Engineering). Current use of diabetes medications, classified as metformin, DPP-4 inhibitors, GLP-1 receptor agonists, sulfonylureas, thiazolidinediones, or insulin was recorded. All subjects had aBMD of the hip, radius, lumbar spine (L1–L4), and total body regions measured by DXA; cortical and trabecular bone microarchitecture was assessed from HRpQCT images of the nondominant distal radius and tibia. All T2DM patients had a baseline assessment of diabetic complications.
DXA and high-resolution peripheral quantitative computed tomography
Dual-energy x-ray absorptiometry (Lunar iDXA; GE Medical Systems, Chicago, Illinois) was used to evaluate aBMD measurements at the total body, hip, L1-L4 AP spine, and forearm (24–26). HRpQCT scans (Scanco Medical AG, Bruttisellen, Switzerland) were acquired for all subjects using a validated protocol to obtain high resolution in vivo images of the radius and tibia with the XtremeCT, as previously described from our group (27–29), with the caveat that we used the newest generation scanner (XtremeCT II) that offers an even higher resolution (61 µm versus 82 µm on the XtremeCT I). In addition, here we report the results based on the more proximal scans for cortical measures, as described by Patsch et al (12), since this proximal site seems to provide better information on cortical microstructure and may therefore be more sensitive to changes in patients with T2DM. We scanned the nondominant forearm and ankle, except in subjects with a prior local fracture. Image quality was graded by a trained technician according to the manufacturer-suggested image grading system (grade 1 [no visible motion artifacts] to grade 5 [severe motion artifacts]). Scans were repeated for images with a score of 3, 4, or 5, and no analyses were done on scans unless they had scores of 1 or 2. Trabecular bone volume fraction (BV/TV), trabecular number (TbN), trabecular thickness (TbTh), and trabecular separation (TbSp) were derived, as previously described (27–29). For the cortical parameters, we used the extended cortical analysis for XtremeCT II to obtain CtPo, cortical pore diameter, cortical thickness, cortical volumetric BMD, and cortical area. Microfinite element analysis (µFEA) was performed using the manufacturer’s software.
Assessment of bone material properties
Reference point indentation was performed using the OsteoProbe®, as previously described (8–11). Briefly, following local anesthesia, the OsteoProbe® needle was inserted through the skin and periosteum of the anterior midtibia. While keeping the device perpendicular to the bone surface (within 10°), the measurement was actuated by slowly compressing the device’s outer housing unit and compressing the internal primary spring until the trigger mechanism initiated an impact. The impact mechanism creates a force to drive the probe into the bone, while the displacement transducer measures indentation distance increase (IDI, µm) from impact. The IDI from the impact was converted by a computer algorithm to the BMSi, defined as 100 times the ratio of the harmonic mean IDI from 5 separate impacts into a polymethyl-methacrylate plastic calibration phantom relative to the IDI from the impact into the bone (30). For each subject, the BMSi was calculated as the average of 10 measurements at different midshaft tibial sites (separated by > 2 mm). In our laboratory, the within-subject (n = 10) precision error (coefficient of variation) of the OsteoProbe® was 1.65% for the BMSi.
Skin advanced glycation end-products
Skin AGEs were measured using the AGE Reader® (Diagnoptics), which measures an index of tissue accumulation of AGEs by means of skin autofluorescence (31) using an excitation light source with a wavelength between 300 and 420 nm. Previous studies have shown an error rate of ~5% when repeated skin autofluorescence measurements are made in control and T2DM patients (31).
Assessment of diabetic complications
These evaluations were done only in the T2DM patients. For the evaluation of diabetic retinopathy, the medical records were reviewed to determine if the subject had a funduscopic examination by an ophthalmologist within the past year. If so, then the findings were recorded as “no retinopathy,” “background diabetic retinopathy,” or “proliferative diabetic retinopathy” based on the findings in the medical record. Subjects without a funduscopic examination in the previous year were evaluated with dilated fundus photographs, which were obtained using a retinal camera in the Mayo Clinical Research and Trials Unit after the subject sat in a darkened room for approximately 20 minutes (to achieve pupillary dilatation). The images were then interpreted by an experienced opthalmologist and the subjects were classified as having no retinopathy, background diabetic retinopathy, or proliferative diabetic retinopathy. In situations where differentiation between background and proliferative retinopathy was unclear, patients were referred to an ophthalmologist for evaluation (and treatment, if necessary) (32). Patients with a history of laser photocoagulation or a history of macular edema were classified as having proliferative diabetic retinopathy. We classified patients as having diabetic retinopathy if they had either background or proliferative retinopathy.
The presence or absence of nephropathy was evaluated using urine microalbuminuria (mg/g creatinine), as assessed in a second-morning void specimen. Urine microalbumin testing was routinely performed by the Mayo Clinical Laboratory. A urine albumin/creatinine ratio of ≥ 30 mg/g was considered significant proteinuria consistent with diabetic nephropathy.
Evaluation of diabetic neuropathy was as described in detail by the Mayo Clinic diabetic neuropathy research program (33–35). These sensory tests were used as quantitative measures of the occurrence and severity of diabetic polyneuropathy. Reference values for defined anatomical sites and the corrections for anthropomorphic variables are available (36). Three specific tests were performed: (1) a touch-pressure threshold test of the left dorsal terminal phalange of the first (great) toe using 9 graded monofilaments assessing significant sensory fiber function; (2) a test with a thermal disk with different cooling transfer characteristics to assess small fiber sensory function; and (3) a vibration sensation test by placing a small plastic disk connected to a vibrating instrument on the first (great) toe of the left foot. The subjects were asked whether they could feel the vibration when they saw “1” displayed on the monitor in front of them. The cooling sensation test was tested on the flat dorsal surface, overlying the distal half of the metatarsal bones. For each of the 3 tests, a score of 0–2 was defined as follows: a percentile < 95 was assigned a score of 0; a percentile of 95–98.9 was assigned a score of 1; and a percentile of ≥ 99 was assigned a score of 2 (a higher score indicates worse fiber function). Each of the 3 tests resulted in a score of 0–2. We added the 3 tests together so that the overall neuropathy score ranged from 0–6. A score of 0 indicates no abnormality, while a score of 6 would indicate the maximum number of abnormalities. Any subject who could not do the tests because they could not feel anything were assigned a value of 6. A composite test score of ≥ 1 was considered positive for diabetic neuropathy (33–35).
In order to obtain a quantitative measure of vascular disease, we assessed the ankle-brachial index (the ratio of blood pressure at the ankle to that at the arm), which provided a clinically relevant measure of peripheral vascular disease; 4 measurements were obtained, and the minimum value of these 4 measurements was used in the analyses (37). In order to assess the burden of ischemia in limbs with arterial occlusive disease, we used transcutaneous oximetry (TcPO2), a standard clinical test measuring transcutaneous oxygen tension in the foot; we used the minimum TcPO2 value following 3 minutes of leg elevation (38). This remains accurate in those with non- or poorly-compressible vessels and small vessel arterial disease.
Statistical analysis
Demographic, clinical characteristics, anthropometric, and biochemical parameters were compared between the T2DM and control groups using 2-sample t-tests, x2 tests, and Fisher’s exact tests as appropriate. Group comparisons of the bone parameters were obtained from linear regression models adjusted for age, sex, and body mass index (BMI). Relationships between variables were assessed using adjusted Spearman correlations. The test was performed at a significance level of P < 0.05 (2-tailed). In order to avoid issues related to multiple hypothesis testing, we were careful to prespecify our hypotheses and limit our primary comparisons to 2 group comparisons: either control versus T2DM subjects or, based on the correlation analysis and clinical thresholds for TcPO2 (38, 39), control subjects versus T2DM subjects with low TcPO2 values. In addition, we limited our primary analysis to examining differences between these groups in the BMSi and CtPo, with the other variables being part of the secondary, exploratory analyses.
Results
Patient characteristics
Clinical and key biochemical variables in the T2DM and control subjects are shown in Table 1. The groups were well matched for age, with a mean duration of T2DM in the patients of 15.4 years. As expected, the T2DM patients had a higher average BMI compared with the control group. HbA1c levels were significantly higher in subjects with T2DM versus the controls. The median HbA1c level over 5 years in the T2DM patients was 7.7%, indicating overall relatively good glycemic control. Table 1 also lists diabetes medication use in the T2DM patients; none of the patients were on a thiazolidinedione.
Table 1.
Characteristics of patients with T2DM and nondiabetic controls
| Characteristics | T2DM (N = 171) | Controls (N = 108) | P-Valuea |
|---|---|---|---|
| Demographic | |||
| Age (years) | 68.8 ± 7.6 | 67.3 ± 8.8 | 0.120 |
| Sex (men) | 96 (56.1%) | 45 (41.7%) | 0.018 |
| Race (white) | 166 (97.6%) | 106 (99.1%) | 0.652 |
| Duration of T2D (years) | 15.4 ± 6.4 | NA | NA |
| Anthropometry | |||
| Weight (kg) | 91.3 ± 16.4 | 79.1 ± 15.0 | < 0.001 |
| Body mass index (kg/m2) | 31.1 ± 4.2 | 27.9 ± 4.2 | < 0.001 |
| Biochemical | |||
| 25-hydroxyvitamin D, ng/mL | 35.3 ± 11.8 | 37.3 ± 11.4 | 0.169 |
| HbA1c, screen visit (%) |
7.8 ± 1.2 | 5.4 ± 0.3 | < 0.001 |
| HbA1c, 5-year median (%) |
7.7 ± 0.9 | NA | NA |
| Diabetes medications | NA | NA | |
| Metformin | 149 (87.1%) | NA | NA |
| DPP-4 inhibitors | 9 (5.3%) | NA | NA |
| GLP-1 receptor agonists | 14 (8.2%) | NA | NA |
| Sulfonylureas | 65 (38.0%) | NA | NA |
| Insulin | 71 (41.5%) | NA | NA |
Values are shown as means ± SD unless otherwise noted. Statistically significant P-values are shown in bold.
Abbreviations: NA, not applicable; T2DM, type 2 diabetes mellitus.
a P-values are shown for comparisons between the T2DM cases and non-diabetic controls using two‐sample t tests, x2 tests and Fisher’s Exact tests as appropriate.
Measured parameters
Table 2 shows the BMSi, skin AGEs, DXA aBMD, and HRpQCT parameters in the T2DM versus the control subjects. In this cohort, the BMSi did not differ between the T2DM versus control subjects, but skin AGEs were significantly higher (+17%, P < 0.001) in the T2DM patients. Even after adjusting for age, sex, and BMI, the T2DM patients had higher aBMD at all skeletal sites. Corticol porosity trended lower at the distal radius and higher at the distal tibia in the T2DM patients versus controls. In this cohort of T2DM patients, neither the BMSi (r = -0.02, P = 0.853) nor CtPo at the distal radius (r = 0.05, P = 0.544) or distal tibia (r = 0.03, P = 0.670 [all adjusted for age, sex, and BMI]) were correlated with the duration of diabetes. The cortical area was significantly greater at the radius but not different at the tibia in the T2DM versus control subjects. Trabecular parameters at the tibia, but not the radius, were significantly better (higher BV/TV and TbTh) in the T2DM versus the control subjects. Microfinite element analysis demonstrated better failure loads at both the distal radius and tibia in the T2DM subjects.
Table 2.
Measured parameters in patients with T2DM and nondiabetic controls
| Parameter | T2DM Cases (N = 171) |
Controls (N = 108) | Difference (%) | P-Valuea |
|---|---|---|---|---|
| BMSi | 74.8 ± 0.8 | 75.0 ± 1.0 | -0.3 | 0.843 |
| Skin AGEs (AU) | 2.88 ± 0.05 | 2.46 ± 0.06 | 17.4 | <0.001 |
| DXA aBMD | ||||
| Femoral neck (g/cm2) | 0.99 ± 0.01 | 0.94 ± 0.01 | 5.7 | 0.004 |
| Total hip (g/cm2) | 1.08 ± 0.01 | 1.02 ± 0.01 | 5.5 | 0.003 |
| Ultradistal radius (g/cm2) | 0.48 ± 0.01 | 0.47 ± 0.01 | 4.0 | 0.039 |
| Total radius (g/cm2) | 0.70 ± 0.01 | 0.68 ± 0.01 | 3.2 | 0.029 |
| Lumbar spine (L1–L4) (g/cm2) | 1.34 ± 0.01 | 1.26 ± 0.02 | 6.2 | 0.002 |
| Total body (g/cm2) | 1.25 ± 0.01 | 1.21 ± 0.01 | 3.2 | 0.008 |
| Distal radius HRpQCT | ||||
| CtPo (%) | 1.30 ± 0.07 | 1.53 ± 0.09 | -15.2 | 0.050 |
| CtPo diameter (mm) | 0.187 ± 0.003 | 0.188 ± 0.004 | -0.5 | 0.868 |
| Ct vBMD (mg/cm3) | 997 ± 3.2 | 1002 ± 4.0 | -0.4 | 0.414 |
| CtTh (mm) | 2.02 ± 0.02 | 1.96 ± 0.03 | 3.3 | 0.074 |
| Ct area (mm2) | 86.7 ± 0.83 | 82.8 ± 1.06 | 4.7 | 0.006 |
| Tb vBMD (mg/cm3) | 124 ± 3.9 | 117 ± 4.9 | 6.5 | 0.244 |
| Trabecular BV/TV | 0.17 ± 0.01 | 0.15 ± 0.01 | 7.4 | 0.214 |
| TbN (1/mm) | 1.16 ± 0.03 | 1.12 ± 0.03 | 3.3 | 0.402 |
| TbTh (mm) | 0.229 ± 0.002 | 0.225 ± 0.003 | 1.5 | 0.336 |
| TbSp (mm) | 0.98 ± 0.03 | 0.98 ± 0.04 | -0.6 | 0.920 |
| µFEA failure load (N) | 5127 ± 51 | 4897 ± 64 | 4.7 | 0.007 |
| Distal tibia HRpQCT | ||||
| CtPo (%) | 3.11 ± 0.11 | 2.78 ± 0.14 | 11.8 | 0.074 |
| CtPo diameter (mm) | 0.206 ± 0.003 | 0.204 ± 0.004 | 1.2 | 0.641 |
| Ct vBMD (mg/cm3) | 925 ± 3.8 | 930 ± 4.9 | -0.6 | 0.398 |
| CtTh (mm) | 2.47 ± 0.03 | 2.43 ± 0.04 | 1.4 | 0.510 |
| Ct area (mm2) | 184 ± 1.6 | 180 ± 2.0 | 2.2 | 0.140 |
| Tb vBMD (mg/cm3) | 143 ± 3.1 | 133 ± 3.9 | 7.5 | 0.054 |
| Trabecular BV/TV | 0.211 ± 0.004 | 0.196 ± 0.005 | 7.7 | 0.031 |
| TbN (1/mm) | 1.25 ± 0.02 | 1.24 ± 0.03 | 1.2 | 0.706 |
| TbTh (mm) | 0.266 ± 0.002 | 0.256 ± 0.002 | 3.6 | 0.004 |
| TbSp (mm) | 0.89 ± 0.02 | 0.88 ± 0.03 | 1.0 | 0.832 |
| µFEA failure load (N) | 11723 ± 109 | 11281 ± 139 | 3.9 | 0.017 |
Values are presented as adjusted means ± SEM or adjusted percent difference.Statistically significant P-values are shown in bold.
Abbreviations: AU, arbitrary units; microFEA, micro finite element analysis; AGEs, advanced glycation endproducts; BMSi, bone material strength index; BV/TV, bone volume fraction; CtPo, cortical porosity; Ct area, cortical area; CtTh, cortical thickness; Ct vBMD, cortical volumetric bone mineral density; DXA, dual-energy x-ray absorptiometry; aBMD, areal bone mineral density; HRpQCT, high resolution peripheral quantitative computed tomography; TbN, trabecular number; TbSp, trabecular separation; TbTh, trabecular thickness; Tb vBMD, trabecular volumetric bone mineral density.
a Adjusted for age, sex, and BMI.
Evaluation of diabetic complications
Table 3 shows the distribution of diabetic complications (retinopathy, nephropathy, neuropathy, vascular disease) in the T2DM patients as directly assessed during the study. Of the T2DM patients, 71.3% had at least 1 diabetic complication, with the most prevalent being peripheral neuropathy.
Table 3.
Distribution of directly assessed diabetic complications in patients with T2DM
| T2DM (N = 171) | N (% with complication)a | |
|---|---|---|
| Retinopathy, N (%) | 33 (19.3%) | |
| None | 138 (80.7%) | |
| Background | 24 (14.0%) | |
| Proliferative | 9 (5.3%) | |
| Nephropathy, median (IQR) | 10 (5–29) | 42 (24.6%) |
| Neuropathy, N (%) | 70 (41.7%) | |
| 0 | 98 (58.3%) | |
| 1 | 36 (21.4%) | |
| 2 | 20 (11.9%) | |
| 3–6 | 14 (8.3%) | |
| Missing | 3 | |
| Vascular | ||
| ABI, median (IQR) | 1.04 (0.93–1.13) | 31 (18.1%) |
| TcPO2, median (IQR) | 52.3 (44.4–62.4) | 30 (17.8%) |
| Any complication | 122 (71.3%) | |
| 0 | 49 (28.7%) | |
| 1 | 58 (33.9%) | |
| 2 | 50 (29.2%) | |
| 3–5 | 14 (8.2%) |
a Presence of diabetic complication defined as follows: retinopathy = background or proliferative retinopathy; nephropathy = urine albumin/creatinine ≥ 30 mg/g (median [IQR] is for urine albumin/creatinine); neuropathy = neuropathy test score ≥ 1; macrovascular = ABI ≤ 0.9; microvascular = TcPO2 ≤ 40 mm Hg.
Relationship of measured parameters to diabetes control and directly assessed diabetic complications
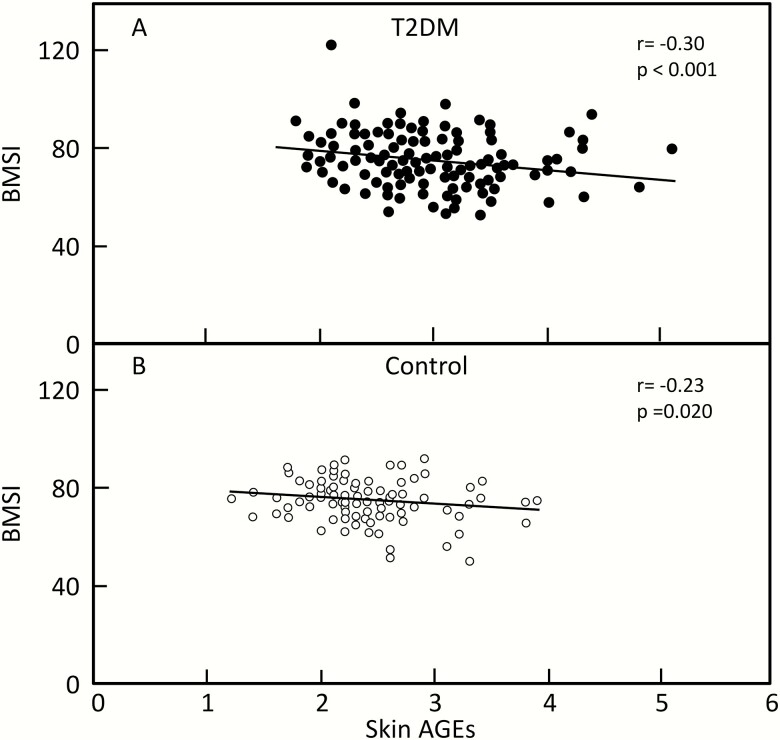
Table 4 shows the Spearman correlation coefficients between the measured parameters, HbA1c, and diabetic complications. Neither the BMSi nor skin AGEs were correlated with the mean HbA1c over the previous 5 years. When we examined potential relationships between the BMSi and skin AGEs, these variables were significantly correlated both in the T2DM (r = -0.30, P < 0.001; Fig. 1A) and control (r = -0.23, P < 0.001; Fig. 1B) subjects.
Table 4.
Spearman correlation coefficients (rp-value) between the measured parameters, 5-year median HbA1c, and directly assessed diabetic complications in patients with T2DMa (correlations are adjusted for age, sex, and BMI)
| Vascular | ||||||
|---|---|---|---|---|---|---|
| Parameter | Median HbA1c | Retin-opathy | Microalb-uminuria | Neuro-pathy | ABI | TcPO2 |
| BMSi | 0.000.961 | 0.010.892 | 0.030.711 | -0.16 0.047 | -0.060.436 | -0.20 0.018 |
| Skin AGEs | 0.010.930 | 0.120.136 | 0.040.633 | 0.090.233 | -0.100.191 | -0.010.903 |
| DXA aBMD | ||||||
| Femoral neck (g/cm2) | 0.080.311 | -0.010.873 | -0.100.177 | 0.050.536 | -0.18 0.017 | -0.120.124 |
| Total hip (g/cm2) | 0.110.154 | 0.010.888 | -0.090.235 | 0.040.632 | -0.110.144 | -0.090.231 |
| Ultradistal radius (g/cm2) | 0.020.768 | 0.040.608 | 0.060.419 | -0.010.853 | 0.060.410 | 0.100.186 |
| Total radius (g/cm2) | 0.050.502 | 0.030.691 | 0.040.568 | 0.010.892 | 0.000.997 | 0.120.116 |
| Lumbar spine (L1–L4) (g/cm2) | -0.110.147 | 0.010.863 | -0.090.252 | 0.030.670 | -0.17 0.034 | -0.010.868 |
| Total body (g/cm2) | -0.020.832 | -0.010.858 | -0.040.618 | -0.030.724 | -0.090.251 | -0.010.889 |
| Distal radius HRpQCT | ||||||
| CtPo (%) | -0.010.883 | 0.120.127 | 0.030.701 | -0.020.818 | -0.050.543 | -0.17 0.029 |
| CtPo diameter (mm) | -0.020.816 | 0.020.808 | 0.010.863 | 0.000.957 | 0.000.961 | -0.010.857 |
| Ct vBMD (mg/cm3) | -0.010.908 | 0.020.841 | 0.010.900 | 0.020.756 | 0.050.546 | 0.150.051 |
| CtTh (mm) | 0.080.286 | 0.100.224 | 0.100.225 | 0.120.120 | -0.040.627 | 0.070.371 |
| Ct area (mm2) | -0.050.521 | -0.030.687 | 0.090.236 | -0.030.665 | -0.070.386 | -0.090.254 |
| Tb vBMD (mg/cm3) | 0.18 0.020 | 0.050.494 | -0.020.779 | 0.000.985 | 0.040.610 | 0.010.941 |
| Trabecular BV/TV | 0.19 0.017 | 0.040.577 | 0.000.961 | 0.010.862 | 0.030.675 | 0.000.956 |
| TbN (1/mm) | 0.17 0.034 | 0.000.999 | -0.040.636 | -0.080.342 | 0.050.530 | 0.000.965 |
| TbTh (mm) | 0.18 0.024 | 0.100.220 | 0.060.455 | 0.070.382 | -0.050.566 | -0.020.782 |
| TbSp (mm) | -0.16 0.038 | 0.000.992 | 0.050.560 | 0.070.390 | -0.050.566 | -0.010.915 |
| µFEA failure load (N) | -0.010.926 | 0.010.940 | 0.090.265 | -0.010.943 | -0.040.640 | -0.080.342 |
| Distal tibia HRpQCT | ||||||
| CtPo (%) | -0.040.591 | 0.020.791 | 0.000.992 | -0.040.620 | -0.070.388 | -0.120.143 |
| CtPo diameter (mm) | 0.020.829 | -0.060.477 | 0.060.467 | 0.010.877 | -0.110.165 | -0.050.550 |
| Ct vBMD (mg/cm3) | 0.060.440 | 0.020.833 | -0.010.876 | 0.020.846 | 0.120.137 | 0.090.240 |
| CtTh (mm) | 0.110.159 | 0.100.215 | 0.020.764 | 0.120.123 | 0.000.971 | 0.010.935 |
| Ct area (mm2) | 0.060.451 | 0.020.770 | 0.050.550 | -0.020.770 | -0.080.294 | -0.090.254 |
| Tb vBMD (mg/cm3) | 0.070.364 | 0.010.913 | -0.040.573 | -0.030.715 | -0.030.743 | 0.000.985 |
| Trabecular BV/TV | 0.090.279 | 0.010.877 | -0.040.590 | -0.010.893 | -0.030.732 | -0.010.854 |
| TbN (1/mm) | 0.050.513 | 0.050.561 | 0.050.512 | -0.080.306 | -0.050.563 | -0.010.093 |
| TbTh (mm) | 0.100.217 | -0.010.865 | 0.040.618 | 0.140.082 | 0.010.928 | -0.020.845 |
| TbSp (mm) | -0.050.565 | -0.050.498 | -0.030.697 | 0.100.189 | 0.060.483 | 0.010.871 |
| µFEA failure load (N) | 0.060.417 | -0.030.678 | -0.010.913 | -0.030.681 | -0.050.555 | -0.100.204 |
Abbreviations: microFEA, micro finite element analysis; AGEs, advanced glycation endproducts; BMSi, bone material strength index; BV/TV, bone volume fraction; CtPo, cortical porosity; Ct area, cortical area; CtTh, cortical thickness; Ct vBMD, cortical volumetric bone mineral density; DXA, dual-energy x-ray absorptiometry; aBMD, areal bone mineral density; HRpQCT, high resolution peripheral quantitative computed tomography; TbN, trabecular number; TbSp, trabecular separation; TbTh, trabecular thickness; Tb vBMD, trabecular volumetric bone mineral density.
a Continuous variables used except for retinopathy, which was dichotomized.
Figure 1.
A: BMSi versus skin AGEs in T2DM patients. B: BMSi versus skin AGEs in control subjects.
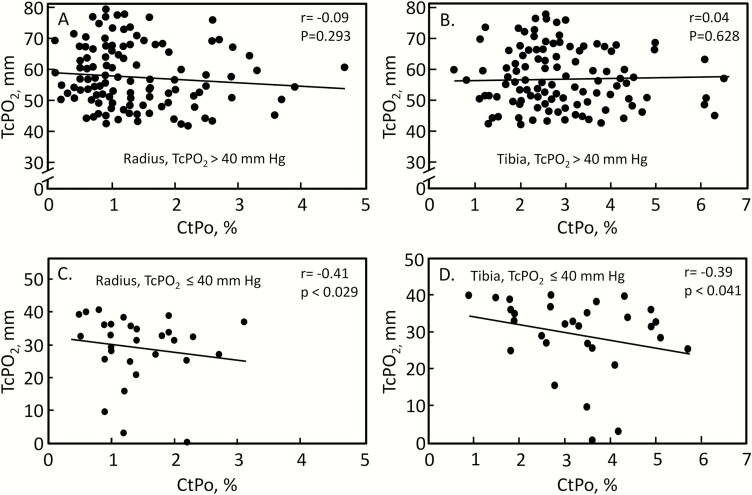
Although the BMSi was inversely associated with the neuropathy score (Table 4), the mean BMSi in the T2DM patients without peripheral neuropathy (score = 0; mean ± SEM BMSi, 75.9 ± 1.0) was not significantly different from those subjects with peripheral neuropathy (score ≥ 1; 73.1 ± 1.3, P = 0.107). The BMSi and distal radius CtPo were both inversely correlated with TcPO2 (measured at the dorsum of the foot; Table 4). As a TcPO2 above 40 mm Hg is generally considered to be representative of adequately oxygenated tissue (38, 39), we next stratified the T2DM patients into those with TcPO2 values above or below this level and re-evaluated these relationships. As shown in Fig. 2A and 2B, there was no significant correlation between CtPo at either the distal radius or tibia and TcPO2 in T2DM patients with TcPO2 values > 40 mm Hg. By contrast, in T2DM patients with TcPO2 values ≤ 40 mm Hg, there were significant inverse correlations between CtPo (distal radius and tibia) and TcPO2 (Fig. 2C and 2D). Since a low TcPO2 may be a consequence of long-term poor glycemic control, we also evaluated the relationship between the 5-year median HbA1c and CtPo. This was not significant in the overall group of T2DM patients at either the radius or the tibia (Table 4); nor was this relationship significant specifically in the subset of T2DM patients with TcPO2 values ≤ 40 mm Hg (r = -0.07, P = 0.737 for radius CtPo; and r = -0.14, P = 0.474 for tibial CtPo).
Figure 2.
A: Distal radius CtPo versus TcPO2 in T2DM patients with TcPO2 > 40. B: Distal tibia CtPo versus TcPO2 in T2DM patients with TcPO2 > 40. C: Distal radius CtPo versus TcPO2 in T2DM patients with TcPO2 ≤ 40. D: Distal tibia CtPo versus TcPO2 in T2DM patients with TcPO2 ≤ 40.
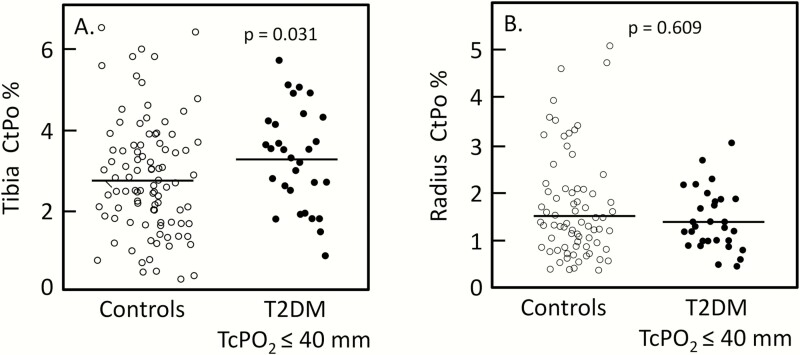
Based on these findings, we specifically focused on the group of T2DM patients with TcPO2 values ≤ 40 mm Hg. As shown in Fig. 3A, these patients had significantly higher CtPo (+21%, P = 0.031) at the distal tibia compared to controls, but not at the distal radius (Fig. 3B). Although our primary hypothesis based on the observed correlations (Fig. 2) and clinical TcPO2 threshold of 40 mm Hg (38, 39) was to evaluate CtPo in the T2DM patients with TcPO2 values ≤ 40 mm Hg, as a secondary analysis we also explored this in T2DM patients with TcPO2 values > 40 mm Hg. At the distal tibia, CtPo was similar in T2DM patients with TcPO2 values > 40 mm Hg (2.98 ± 0.12%) as compared with the control subjects (2.81 ± 0.13%; P = 0.375) and actually somewhat lower at the distal radius in T2DM patients with TcPO2 values > 40 mm Hg (1.28 ± 0.08 %) as compared with the control subjects (1.54 ± 0.09 %, P = 0.041).
Figure 3.
A: Distal tibia CtPo in T2DM patients with TcPO2 ≤ 40 versus control subjects. B: Distal radius CtPo in T2DM patients with TcPO2 ≤ 40 versus control subjects.
In contrast to these findings with CtPo, the BMSi did not differ in T2DM subjects with TcPO2 ≤ 40 mm Hg (77.1 ± 1.8) versus controls (75.2 ± 1.0, P = 0.328) or in T2DM subjects with TcPO2 > 40 mm Hg (74.0 ± 0.9) versus controls (P = 0.375). In addition, there was no correlation between the BMSi and CtPo in either the T2DM (r = 0.12, P = 0.162 at the radius; and r = -0.07, P = 0.401 at the tibia) or control (r = -0.09, P = 0.356 at the radius; and r = -0.07, P = 0.467 at the tibia) subjects.
Table 4 also shows that trabecular parameters (trabecular vBMD, BV/TV, TbN, TbTh) at the distal radius, but not distal tibia, were positively correlated with the 5-year median HbA1c level, even following the adjustment for age, sex, and BMI. Thus, not only did the T2DM subjects have better DXA aBMD measures at all sites (Table 3), but worse glycemic control was associated with better trabecular bone microarchitecture in these patients, at least at the distal radius (Table 4).
Impact of diabetes medication use on the BMSi and CtPo
Table 5 evaluates the impact of diabetes medication use on the 2 primary outcome variables, BMSi and CtPo. In this analysis, CtPo at the distal tibia was significantly lower in the T2DM patients on metformin (by 18.6%) as compared with those not on metformin, with the caveat being that the number of metformin nonusers (n = 22) was relatively small.
Table 5.
BMSi and CtPo in T2DM cases using current medications versus T2DM cases not currently using those same medications
| T2DM Patients Currently Using Medication |
T2DM Cases Not Currently Using Medication | Difference (%) | P-Valuea | |
|---|---|---|---|---|
| Parameter | Insulin (n = 71) |
Not Using Insulin (n = 100) | ||
| BMSi | 74.6 ± 1.4 | 75.6 ± 1.1 | -1,3 | 0.591 |
| CtPo (%) | ||||
| Distal radius | 1.32 ± 0.10 | 1.29 ± 0.08 | 2.1 | 0.835 |
| Distal tibia | 3.17 ± 0.17 | 2.99 ± 0.14 | 6.2 | 0.412 |
| Sulfonylurea (N = 65) | Not using sulfonylurea(N = 106) | |||
| BMSi | 75.4 ± 1.4 | 75.0 ± 1.1 | 0.6 | 0.814 |
| CtPo (%) | ||||
| Distal radius | 1.17 ± 0.10 | 1.38 ± 0.08 | -15.2 | 0.107 |
| Distal tibia | 2.88 ± 0.18 | 3.17 ± 0.14 | -9.3 | 0.194 |
|
Metformin
(N = 149) |
Not using metformin (N = 22) | |||
| BMSi | 74.6 ± 0.9 | 79.0 ± 2.4 | -5.5 | 0.092 |
| CtPo (%) | ||||
| Distal radius | 1.29 ± 0.07 | 1.40 ± 0.18 | -7.8 | 0.566 |
| Distal tibia | 2.98 ± 0.12 | 3.66 ± 0.30 | -18.6 | 0.038 |
|
DPP-4 inhibitor or GLP-1 receptor agonist
(N = 22) |
Not using DPP-4 inhibitor or GLP-1 receptor agonist
(N = 149) |
|||
| BMSi | 74.1 ± 2.4 | 75.3 ± 0.9 | -1.6 | 0.645 |
| CtPo (%) | ||||
| Distal radius | 1.59 ± 0.18 | 1.26 ± 0.07 | 26.2 | 0.089 |
| Distal tibia | 3.00 ± 0.32 | 3.07 ± 0.12 | -2.3 | 0.836 |
Values are presented as adjusted means ± SEM or adjusted percent difference. There were no T2DM patients on a thiazolidinedione and because of small number in the DPP-4 inhibitor and GLP-1 receptor agonist categories, these were combined. Statistically significant P-values are shown in bold.
Abbreviations: BMSi, bone material strength index; CtPo, cortical porosity.
aAdjusted for age, sex, and BMI.
Discussion
To our knowledge, our study is the first to perform a detailed, direct assessment of diabetic complications in patients with T2DM and relate these to the 2 major skeletal abnormalities described to date in these patients: impaired bone material properties and increased CtPo. Our data may also help explain some of the conflicting findings in the literature regarding the finding of a reduced BMSi or increased CtPo in T2DM patients.
We found that a higher burden of skin AGEs was associated with significantly worse bone material properties (BMSi) in both the T2DM and control subjects. These data are at least partly consistent with a previous, smaller study involving 16 T2DM subjects and 19 nondiabetic controls that also found a significant negative correlation between skin AGEs and the BMSi in the T2DM patients but not in the control subjects (9). Perhaps due to the larger number of nondiabetic controls (n = 108) in our study, we also found a significant negative correlation between the BMSi and skin AGEs in the nondiabetic subjects. Collectively, these data indicate that a lower BMSi is related to AGE accumulation, regardless of diabetes status, although patients with T2DM certainly have increased AGE accumulation (40), as also reflected by the significantly higher skin AGEs levels in our T2DM versus control subjects (Table 2). However, in contrast to our previous study (8) and the work of others (9, 10), the BMSi was not significantly different in this cohort of T2DM versus control subjects, which is consistent with the findings of Dawson-Hughes et al, at least in white T2DM patients (11). The reasons for these differing findings are unclear but may have to do with the degree of AGE accumulation in bone that may vary due to a number of factors in T2DM patients (40). Thus, a reduction in the BMSi may not be associated with T2DM per se but rather largely reflect AGE accumulation in bone and other tissues. Interestingly, although Dawson-Hughes et al did not find a reduction in the BMSi in white patients with T2DM, they did observe reduced BMSi in black T2DM patients (11). While skin AGEs cannot be assessed in dark-skinned individuals (31), it is noteworthy that black T2DM patients consistently have lower levels of the soluble circulating receptor for AGEs (sRAGE), a finding which is associated with greater tissue AGE accumulation (41). As such, black T2DM patients may be more susceptible to AGEs accumulation in skeletal tissues and thus have a more consistent reduction in the BMSi. In addition to these findings, Karim et al (42) also found an impairment in cortical bone material properties in surgical proximal femur specimens from T2DM patients as compared to nondiabetic controls using a related technique, cyclic reference point indentation; this impairment in bone material properties was accompanied by a trend towards increased bone AGEs accumulation. These human data are also consistent with studies in a mouse type I DM model showing that increased AGE accumulation in bone in the setting of chronic hyperglycemia was also associated with impaired bone material properties assessed by microindentation (43).
The other key finding of our study was that CtPo, particularly at the distal tibia, was correlated with tissue TcPO2 measured at the dorsum of the foot. TcPO2 is a noninvasive measure that reflects microvascular blood flow (44–46), and a TcPO2 > 40 mm Hg has been associated with adequate healing of lower extremity wounds in ischemic extremities (38, 39). Our findings would indicate that microvascular disease in T2DM may contribute to the development of CtPo in these patients and thereby increase fracture risk. The precise cellular mechanisms linking microvascular disease and increased CtPo remain to be defined, but this link is certainly plausible given the well-established coupling between angiogenesis and osteogenesis (47).
As in the case of the BMSi, our data may help explain some of the conflicting findings in the literature regarding increased CtPo in patients with T2DM, which has been observed in some (12, 18, 19) but not all studies (8, 10, 22). Although CtPo was not increased in our cohort of T2DM patients as a group, those patients with low TcPO2 values did have increased CtPo. Thus, depending on the prevalence of microvascular disease in a particular cohort of T2DM patients, CtPo may or may not be significantly different from nondiabetic controls.
In a previous study, Shanbhogue et al (21) studied patients with T2DM with retinopathy, nephropathy, or neuropathy, which they classified as microvascular disease (MVD)+ and compared skeletal parameters in these patients with those without any of these complications (MVD-). Although they did not evaluate TcPO2 or other parameters of vascular function, these investigators did find that the MVD+ T2DM patients had higher CtPo at the radius (P = 0.02), with a trend (P = 0.07) towards higher CtPo at the tibia. In our study, we found fairly similar inverse correlations between CtPo at the radius and tibia versus TcPO2 in T2DM patients with TcPO2 values ≤ 40 mm Hg (Fig. 2); however, CtPo was only reduced in these patients at the tibia and not the radius. Thus, although both the Shanbhogue study (21) and our study indicate a relationship between MVD and CtPo, there are differences between the 2 studies in terms of how MVD was evaluated (eg, using direct measurements of TcPO2 in our study) and potentially in other aspects of the study populations that may account for the somewhat differing findings. That said, the trend for differences in CtPo at the tibia in the Shanbhogue study (21), especially given the relatively small number of subjects in that study (n = 51), versus the significant differences in CtPo at the tibia in our much larger population certainly shows some consistent findings. In addition, because we measured TcPO2 in the lower extremity, this could also potentially explain why, in our study, T2DM patients with low TcPO2 values also had increased CtPo at the tibia but not at the radius, as the extent of vascular disease in a given patient may differ between the upper versus lower extremities.
Although CtPo at the radius and tibia was inversely correlated with TcPO2 in T2DM patients with TcPO2 values ≤ 40 mm Hg, CtPo at either site was not related to chronic glycemic control (5-year median HbA1c). This suggests that although poor glycemic control likely contributes to microvascular complications, it is the direct measure of that complication (ie, low TcPO2) rather than a measure of glycemic control that is relevant in predicting CtPo.
An intriguing observation in our study was that T2DM patients on metformin did have lower CtPo as compared to metformin nonusers, although we recognize the caveat that there were a relatively small number of metformin nonusers in this cohort. Nonetheless, if validated in larger cohorts, these data would be consistent with evolving data on potential beneficial effects of metformin on bone metabolism (48).
In contrast to cortical bone, we found that T2DM patients had better trabecular BV/TV and TbTh at the tibia and better µFEA-estimated failure loads at the radius and tibia, and the trabecular parameters at the radius were positively correlated with 5-year median HbA1c levels. These findings are generally consistent with previous studies showing preserved, or even better, trabecular bone microarchitecture in T2DM patients compared to nondiabetic subjects (18, 49). The preservation of trabecular bone parameters and the positive association with worse glycemic control may be related to the hyperinsulinemia in T2DM patients (50). Insulin does have anabolic effects on osteoblasts (51), and DXA aBMD at the hip and spine in subjects with and without T2DM is positively correlated with insulin levels post–oral glucose tolerance tests (52). Of note, the positive association between trabecular parameters and 5-year median HbA1c levels was present at the radius but not the tibia. The reasons for this discrepancy are unclear, but it is possible, for example, that any beneficial skeletal effect of hyperinsulinemia is diluted by the more dominant effects of weight bearing at the tibia, leading to dampening of the insulin-bone anabolic signal. It is also important to note that the µFEA using HRpQCT likely does not fully incorporate the detrimental effects of CtPo on bone strength, as HRpQCT does underestimate the extent of CtPo due to its spatial resolution (53).
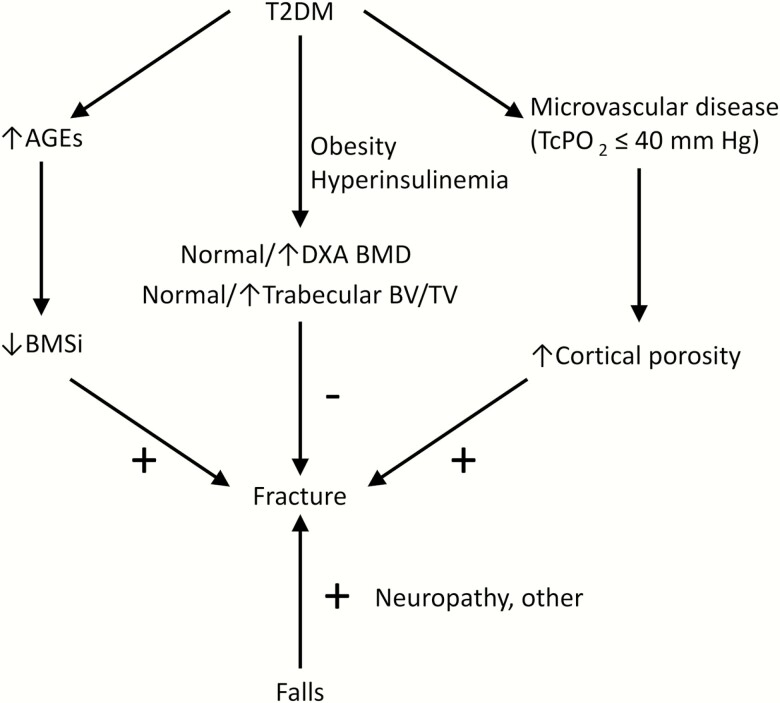
Based on our data and previous work, Fig. 4 provides a working model for the pathogenesis of skeletal abnormalities and increased fracture risk in T2DM patients. These patients have generally preserved (or even improved) trabecular bone parameters, likely related to the effects of obesity and hyperinsulinemia. However, our present findings and those of others (9, 43) indicate that the accumulation of increased AGEs contributes to impaired bone material properties, and our present findings also indicate that the conventional diabetic complication of microvascular disease contributes to increased CtPo, both of which would worsen skeletal fragility. Other factors (eg, peripheral neuropathy) may increase the risk of falls (54), further increasing fracture risk. Clearly, additional factors that remain to be defined may also contribute to reduced bone material properties and increased CtPo in T2DM patients. Moreover, our cohort of T2DM patients was relatively small for assessing fractures, so our study cannot provide direct support for the working model in Fig. 4 specifically in terms of fracture outcomes. Nonetheless, our work does identify the accumulation of AGEs and microvascular disease as potential mediators of impaired bone material properties and increased CtPo, respectively, in T2DM and serves as a framework for further human and animal studies aimed at better defining the skeletal impact of T2DM.
Figure 4.
Working model for the pathogenesis of skeletal fragility and fractures in patients with T2DM.
Acknowledgments
We thank Mr. James Peterson for help with the figures.
Financial Support: This work was supported by NIH grants AR027065 and UL1 TR002377 (Mayo Clinic CTSA).
Additional Information
Disclosure Summary: Dr. Dyck receives grant support from Inonis Inc., Alnylam Inc., and Prothena, Inc.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Zimmet PZ. Diabetes and its drivers: the largest epidemic in human history? Clin Diabetes Endocrinol. 2017;3:1. doi: 10.1186/s40842-016-0039-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL; IOF Bone and Diabetes Working Group . Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13(4):208–219. [DOI] [PubMed] [Google Scholar]
- 3. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes-a meta-analysis. Osteoporosis Int. 2007;18(4):427–444. [DOI] [PubMed] [Google Scholar]
- 4. Schwartz AV, Vittinghoff E, Bauer DC, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305(21):2184–2192. doi: 10.1001/jama.2011.715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koromani F, Oei L, Shevroja E, et al. Vertebral fractures in individuals with type 2 diabetes: more than skeletal complications alone. Diabetes Care. 2020;43(1):137–144. doi: 10.2337/dc19-0925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dawson-Hughes B, Tosteson AN, Melton LJ 3rd, et al. ; National Osteoporosis Foundation Guide Committee . Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19(4):449–458. [DOI] [PubMed] [Google Scholar]
- 7. Giangregorio LM, Leslie WD, Lix LM, et al. FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res. 2012;27(2):301–308. [DOI] [PubMed] [Google Scholar]
- 8. Farr JN, Drake MT, Amin S, MeltonLJ, 3rd, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014;29(4):787–795. doi: 10.1002/jbmr.2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furst JR, Bandeira LC, Fan WW, et al. Advanced glycation endproducts and bone material strength in type 2 diabetes. J Clin Endocrinol Metab. 2016;101(6):2502–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nilsson AG, Sundh D, Johansson L, et al. Type 2 diabetes mellitus is associated with better bone microarchitecture but lower bone material strength and poorer physical function in elderly women: a population-based study. J Bone Miner Res. 2017;32(5):1062–1071. [DOI] [PubMed] [Google Scholar]
- 11. Dawson-Hughes B, Bouxsein M, Shea K. Bone material strength in normoglycemic and hyperglycemic black and white older adults. Osteoporos Int. 2019;30(12):2429–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patsch JM, Burghardt AJ, Yap SP, et al. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013;28(2):313–324. doi: 10.1002/jbmr.1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heilmeier U, Cheng K, Pasco C, et al. Cortical bone laminar analysis reveals increased midcortical and periosteal porosity in type 2 diabetic postmenopausal women with history of fragility fractures compared to fracture-free diabetics. Osteoporos Int. 2016;27(9):2791–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bala Y, Zebaze R, Ghasem-Zadeh A, et al. Cortical porosity identifies women with osteopenia at increased risk for forearm fractures. J Bone Miner Res. 2014;29(6):1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bala Y, Chapurlat R, Cheung AM, et al. Risedronate slows or partly reverses cortical and trabecular microarchitectural deterioration in postmenopausal women. J Bone Miner Res. 2014;29(2):380–388. doi: 10.1002/jbmr.2101 [DOI] [PubMed] [Google Scholar]
- 16. Burghardt AJ, Kazakia GJ, Sode M, de Papp AE, Link TM, Majumdar S. A longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. J Bone Miner Res. 2010;25(12):2558–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borah B, Dufresne T, Nurre J, et al. Risedronate reduces intracortical porosity in women with osteoporosis. J Bone Miner Res. 2010;25(1):41–47. [DOI] [PubMed] [Google Scholar]
- 18. Burghardt AJ, Issever AS, Schwartz AV, et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95(11):5045–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu EW, Putman MS, Derrico N, Abrishamanian-Garcia G, Finkelstein JS, Bouxsein ML. Defects in cortical microarchitecture among African-American women with type 2 diabetes. Osteoporos Int. 2015;26(2):673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paccou J, Ward KA, Jameson KA, Dennison EM, Cooper C, Edwards MH. Bone microarchitecture in men and women with diabetes: the importance of cortical porosity. Calcif Tissue Int. 2016;98(5):465–473. [DOI] [PubMed] [Google Scholar]
- 21. Shanbhogue VV, Hansen S, Frost M, et al. Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur J Endocrinol. 2016;174(2):115–124. [DOI] [PubMed] [Google Scholar]
- 22. Shu A, Yin MT, Stein E, et al. Bone structure and turnover in type 2 diabetes mellitus. Osteoporosis Int. 2012;23(2):635–641. doi: 10.1007/s00198-011-1595-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khosla S, Atkinson EJ, Riggs BL, Melton LJ 3rd. Relationship between body composition and bone mass in women. J Bone Miner Res. 1996;11(6):857–863. [DOI] [PubMed] [Google Scholar]
- 25. Melton LJ 3rd, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48(6):625–630. [PubMed] [Google Scholar]
- 26. Melton LJ 3rd, Riggs BL, Achenbach SJ, et al. Does reduced skeletal loading account for age-related bone loss? J Bone Miner Res. 2006;21(12):1847–1855. [DOI] [PubMed] [Google Scholar]
- 27. Khosla S, Riggs BL, Atkinson EJ, et al. Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res. 2006;21(1):124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nicks KM, Amin S, Atkinson EJ, Riggs BL, Melton LJ 3rd, Khosla S. Relationship of age to bone microstructure independent of areal bone mineral density. J Bone Miner Res. 2012;27(3):637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farr JN, Charkoudian N, Barnes JN, et al. Relationship of sympathetic activity to bone microstructure, turnover, and plasma osteopontin levels in women. J Clin Endocrinol Metab. 2012;97(11):4219–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bridges D, Randall C, Hansma PK. A new device for performing reference point indentation without a reference probe. Rev Sci Instrum. 2012;83(4):044301. doi: 10.1063/1.3693085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meerwaldt R, Graaff R, Oomen PHN, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47(7):1324–1330. [DOI] [PubMed] [Google Scholar]
- 32. Williams GA, Scott IU, Haller JA, Maguire AM, Marcus D, McDonald HR. Single-field fundus photography for diabetic retinopathy screening: a report by the American Academy of Opthalmology. Opthalmology. 2004;111(5):1005–1062. [DOI] [PubMed] [Google Scholar]
- 33. Dyck PJ, Hansen JD, Winkler JA, Witt LV, Davies JL. Thermal disk quantitative sensation testing of cooling discrimination. Companion to Peripheral Neuropathy, Illustrated Cases and New Developments. Saunders: Elsevier; 2010:331–333: Chapter 77. [Google Scholar]
- 34. Dyck PJ, Winkler JA, Andrews KL, Kavros SJ, Vella A, Davies JL. Testing of touch-pressure sensation: introduction of the touch-pressure sensogram. Companion to Peripheral Neuropathy, Illustrated Cases and New Developments. Saunders: Elsevier; 2010:327–330: Chapter 76. [Google Scholar]
- 35. Dyck PJ, Argyros B, Eegt R, et al. Multicenter trial of the proficiency of smart quantitative sensation tests. Muscle & Nerve. 2014;49(5):645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dyck PJ, O’Brien PC, Kosanke JL, Gillen DA, Karnes JL. A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology. 1993;43(8): 1508–1512. [DOI] [PubMed] [Google Scholar]
- 37. Desai CS, Blumenthal RS, Greenland P. Screening low-risk individuals for coronary artery disease. Curr Atheroscler Rep. 2014;16(4):402. doi: 10.1007/s11883-014-0402-8 [DOI] [PubMed] [Google Scholar]
- 38. Padberg FT, Back TL, Thompson PN, Hobson RW 2nd. Transcutaneous oxygen (TcPO2) estimates probability of healing in the ischemic extremity. J Surg Res. 1996;60(2):365–369. [DOI] [PubMed] [Google Scholar]
- 39. Bacharach JM, Rooke TW, Osmundson PJ, Gloviczki P. Predictive value of transcutaneous oxygen pressure and amputation success by use of supine and elevation measurements. J Vasc Surg. 1992;15(3):558–563. [PubMed] [Google Scholar]
- 40. Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5(1):194–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Selvin E, Halushka MK, Rawlings AM, et al. sRAGE and risk of diabetes, cardiovascular disease, and death. Diabetes. 2013;62(6):2116–2121. doi: 10.2337/db12-1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Karim L, Moulton J, Van Vliet M, et al. Bone microarchitecture, biomechanical properties, and advanced glycation end-products in the proximal femur of adults with type 2 diabetes. Bone. 2018;114:32–39. doi: 10.1016/j.bone.2018.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rubin MR, Paschalis EP, Poundarik A, et al. Advanced glycation endproducts and bone material properties in type 1 diabetic mice. PLoS One. 2016;11(5):e0154700–e0154700. doi: 10.1371/journal.pone.0154700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dinh T, Veves A. Microcirculation of the diabetic foot. Curr Pharm Des. 2005;11(18):2301–2309. [DOI] [PubMed] [Google Scholar]
- 45. Gaylarde PM, Fonseca VA, Llewellyn G, Sarkany I, Thomas PK, Dandona P. Transcutaneous oxygen tension in legs and feet of diabetic patients. Diabetes. 1988;37(6):714–716. [DOI] [PubMed] [Google Scholar]
- 46. Rossi M, Carpi A. Skin microcirculation in peripheral arterial obliterative disease. Biomed Pharmacother. 2004;58(8):427–431. doi: 10.1016/j.biopha.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 47. Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bahrambeigi S, Yousefi B, Rahimi M, Shafiei-Irannejad V. Metformin; an old antidiabetic drug with new potentials in bone disorders. Biomed Pharmacother. 2019;109:1593–1601. doi: 10.1016/j.biopha.2018.11.032 [DOI] [PubMed] [Google Scholar]
- 49. Starr JF, Bandeira LC, Agarwal S, et al. Robust trabecular microstructure in type 2 diabetes revealed by individual trabecula segmentation analysis of HR-pQCT images. J Bone Miner Res. 2018;33(9):1665–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thrailkill KM, Lumpkin CK Jr, Bunn RC, Kemp SF, Fowlkes JL. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab. 2005;289(5):E735–E745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lecka-Czernik B. Safety of anti-diabetic therapies on bone. Clin Rev Bone Miner Metab. 2013;11(1):49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stolk RP, Van Daele PL, Pols HA, et al. Hyperinsulinemia and bone mineral density in an elderly population: The Rotterdam Study. Bone. 1996;18(6):545–549. [DOI] [PubMed] [Google Scholar]
- 53. Tjong W, Nirody J, Burghardt AJ, Carballido-Gamio J, Kazakia GJ. Structural analysis of cortical porosity applied to HR-pQCT data. Med Phys. 2014;41(1):013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Timar B, Timar R, Gaiță L, Oancea C, Levai C, Lungeanu D. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. Plos One. 2016;11(4):e0154654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.