Abstract
Background:
Diabetes has been shown as a risk factor for cognitive impairments. However, it is still not clear about the time course of developing abnormal cognition in those with diabetes especially if the morbidity accelerates the cognitive deterioration process.
Objective:
To study how diabetes is related to the abnormal cognition development.
Methods:
A retrospective analysis was performed using data collected by the National Alzheimer’s Coordinating Center. Incidence, prevalence, and age at onset (AAO) of either mild cognitive impairment (MCI) or dementia were compared between participants with and without diabetes.
Results:
During a follow-up period of more than 10 years, the diabetic group had a higher incidence and prevalence of MCI or dementia than the non-diabetic group. However, the AAO of either MCI or dementia was independent of the diagnosis of diabetes.
Conclusion:
Although diabetic patients have a higher incidence and prevalence of abnormal cognition than those without diabetes, diabetes does not accelerate the cognitive deterioration process.
Keywords: Abnormal cognition, age at onset, dementia, diabetes, mild cognitive impairment
INTRODUCTION
In 1996, a positive association was reported between diabetes and dementia from the Rotterdam study [1]. In 2007, diabetes was shown to be related to an increased risk of mild cognitive impairment (MCI) [2]. In 2015, both type 1 and 2 diabetes were shown for being associated with an elevated risk of dementia [3]. On the other hand, diabetes was found to be more prevalent among these diagnosed with MCI than among those who were cognitively normal [4]. Further, even newly diagnosed diabetes was found to be associated with a 16% increase in dementia risk among seniors (>or = 66 years old) [5]. From a prospective cohort study, adults with diabetes starting in their midlife had a greater global cognitive decline than those without diabetes [6]. However, the time course of developing an abnormal cognition has been rarely studied in those with diabetes, and it is not clear if the existing morbidity of diabetes plays a role in the cognitive deterioration process. Using the National Alzheimer’s Coordinating Center’s uniform data set (NACC-UDS), elders with a normal cognition at the baseline were identified (N = 8846). The role of diabetes as a risk factor for cognitive impairment was investigated by comparing incidence, prevalence, and age at onset (AAO) of MCI or dementia between participants with and without documented diabetes who were followed for more than 10 years.
METHODS
Participants
National Alzheimer’s Coordinating Center (NACC)combines information collected from participants of all Alzheimer’s disease centers (ADC) funded by National Institute on Aging (NIA) [7]. Participants were recruited from different sources including references from a relative, friend, or clinician, ADC solicitation, non-ADC media appeal, and other community outreach efforts [8]. Demographic information, medical history, neurological examination, and neuropsychological assessment were collected during annual visits [9]. Participants included in the current study met the following two criteria: who were evaluated between 06/09/2005 and 08/14/2016 as part of the Uniform Data Set (UDS); who had a diagnosis of normal cognition (NC) at the baseline. Participants were classified into either the diabetic group or the non-diabetic group based on their medical history recorded in the UDS. In total, there were 8,846 participants at the baseline, of which 379 had diabetes and 8467 did not have diabetes. However, information on diabetes subtypes (type 1 or 2) was not collected for those with participants with diabetes at the baseline.
Cognitive diagnosis
As part of the UDS, cognitive assessment data were collected from participants on an approximately annual basis [8]. If one visit missed the annual follow-up visit window, the next assessment would be accepted by the NACC as the subsequent visit. Cognitive diagnoses were made either by a single clinician or a multi-disciplinary consensus team using neuropsychological performance, neurological examination results, and medical history details. Cognitive diagnosis classifications relevant to the current study include NC, MCI, and dementia.
NC was defined by 1) Clinical Dementia Rating (CDR) = 0 (no dementia) [10]; 2) no deficits in activities of daily living directly attributable to cognitive impairment; and 3) no evidence of objective cognitive impairment. NC was defined as performance falling less than 1.5 standard deviations within the age-adjusted normative mean on neuropsychological tests assessing language, attention, memory, and executive functioning [8].
MCI determinations were based upon Petersen criteria [11] and defined as 1) a CDR≤0.5 (reflecting mild severity of impairment); 2) relatively spared instrumental activities of daily living; 3) objective cognitive impairment in at least one domain (i.e., performance falling greater than 1.5 standard deviations outside the age-adjusted normative mean in memory, language, attention, or executive functioning) or a significant cognitive decline over time on the neuropsychological evaluation; 4) Mini-Mental State Examination (MMSE) ≥23 [12, 13]; 5 report of a cognitive change by the patient or informant or as observed by a clinician; and 6) absence of dementia.
Dementia was defined as meeting criteria for Alzheimer’s disease (AD) [14] or other dementias [15–20] defined as 1) objective cognitive impairment (i.e., performances falling greater than 1.5 standard deviations outside the age-adjusted normative mean) in at least two cognitive systems (i.e., memory, language, attention or executive functioning); and 2) cognitive impairment contributes directly to impaired activities of daily living.
Statistical analysis, tables, and figures
SPSS (version 24.0) was used for all statistical analyses. Descriptive analyses of demographic and clinical variables in diabetics versus non-diabetic participants were conducted. Means (and standard deviation) or frequencies were calculated for the demographic variables of age, education, sex, and APOEɛ4 carrier status [21]. Two-sample t-tests were used to compare age and education between the diabetic and non-diabetic groups. Chi-square tests were used to compare the sex and APOEɛ4 carrier status between the diabetic and non-diabetic groups. Both demographic information and APOE ɛ4 carrier status are comparable between the diabetic and non-diabetic groups (Table 1).
Table 1.
Participants with and without diabetes had comparable demographic and genetic information
| Diabetic group (n = 379) | Non-diabetic group (n = 8467) | |
| Age (y) | 72.13±9.46 | 72.06±10.53 |
| Education (y) | 15.48±3.23 | 15.84±2.92 |
| Sex (M/F) | 145/234 (38.26% /61.74%) | 2868/5599 (33.87% /66.13%) |
| APOE ɛ4 (+/–/unknown) | 96/231/52 (25.33% /60.95% /13.72%) | 2146/5026/1295 (25.35% / 59.36% /15.29%) |
Both age and education are shown in the format of mean±SD. APOE, Apolipoprotein epsilon; M, male; F, female; SD, standard deviation.
The incidence of MCI or dementia as well as mortality were compared between participants with and without diabetes during the whole follow-up period of more than 10 years. The prevalence of MCI or dementia was also calculated between participants with and without diabetes and plotted against the annual follow-up visits. A two-way analysis of covariance (ANCOVA) model was used to evaluate the effect of diabetes on the AAO ofMCI or dementia with controlling baseline age, education, sex, and APOE ɛ4 carrier status as possible confounding factors. Data were presented in the form of mean±standard deviation, and p < 0.05 was considered as significant in all statistical analyses. Figures were created using Microsoft Excel or Sigma plot (version 10.0).
Standard protocol approvals, registrations, and patient consents
Written informed consent was obtained from participants at each participating ADC. Research using the NACC database was approved by the University of Washington Institutional Review Board (IRB).
Data availability statement
Data and analytical methods are carefully documented for the performed study. Anonymized data will be shared by request from any qualified investigator with following the NACC regulations on data use.
RESULTS
During the follow-up period, the diabetic group had a higher incidence rate for either MCI or dementia than the non-diabetic group (Table 2). In other words, participants with diabetes are more likely to develop and be diagnosed with MCI or dementia. The risk ratios for MCI and dementia are 1.46 and 1.61, respectively, for participants with diabetes over those without diabetes (Table 2). By contrast, the diabetic group had a slightly lower mortality than the non-diabetic group. During the whole follow-up period, the mortality rate for the diabetic participants is 4.75% (18/379), which was slightly lower than that for the non-diabetic group of 6.44% (545/8467). However, the age of death for participants with pre-existing diabetes was 89.12±6.77 (N = 18), which was comparable to the same measure for those without diabetes of 89.06±8.3 (N = 545, p = 0.99).
Table 2.
The incidence of mild cognitive impairment (MCI) or dementia as well as mortality were compared between participant with and without diabetes
| Diabetic group | Non-diabetic group | Risk ratio | |
| MCI | 25.27% (92/364) | 17.21% (1419/8244) | 1.47 |
| Dementia | 10.03% (38/379) | 6.15% (521/8467) | 1.63 |
| Mortality | 4.75% (18/379) | 6.44% (545/8467) | 0.74 |
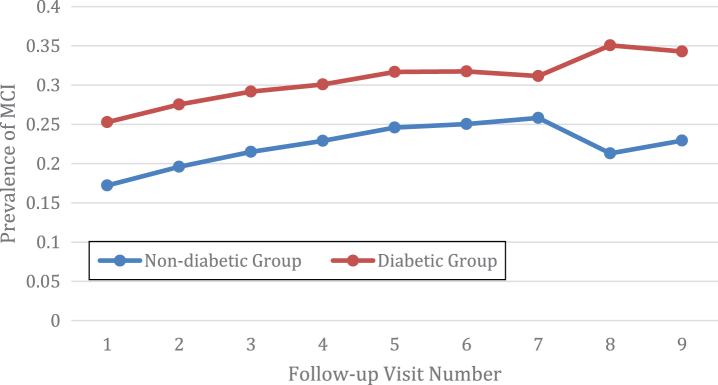
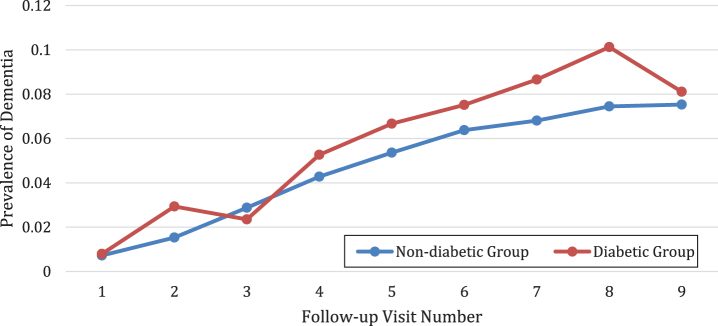
The prevalence of MCI or dementia was compared at each follow-up visit between the diabetic group and the non-diabetic group. In total, there were 10 visits including 1 baseline visit and 9 follow-up visits (Figs. 1 and 2). The average MCI prevalence was 30.66±3.09% for the diabetic group, which is significantly higher than the counterpart measure for the non-diabetic group of 22.32±2.75% (p < 0.001). For MCI, the prevalence is always higher in the diabetic group than the non-diabetic group (Fig. 1). As expected, the prevalence of dementia was higher in the diabetic group than the non-diabetic group (p = 0.01). The average prevalence of dementia was 5.82±3.19% for the diabetic group as compared to 4.77±2.56% for the non-diabetic group. The only exception is that the prevalence of dementia was slightly lower for the diabetic group (2.35%) than the non diabetic group (2.88%) at the third follow-up visit (Fig. 2).
Fig.1.
The diabetic group had a higher prevalence of mild cognitive impairment (MCI) than the non diabetic group.
Fig.2.
The diabetic group had a higher prevalence of dementia than the non diabetic group.
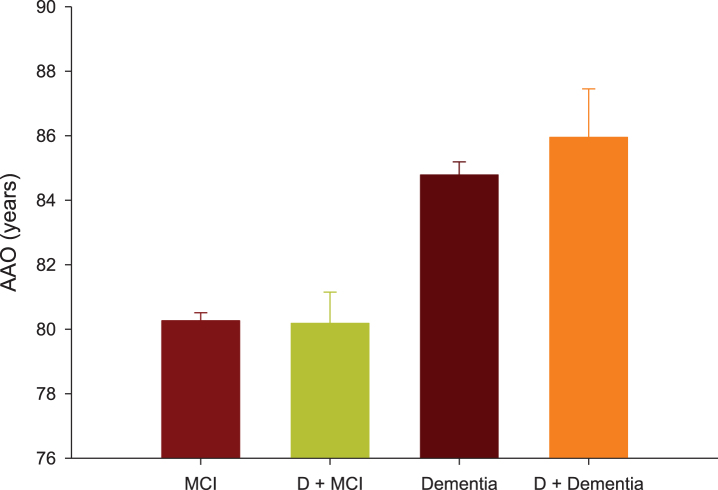
For participants with diabetes, the AAO of MCI was 80.18±0.97 years (95% CI: 78.28–82.07, N = 92), which is not significantly different from the same measure from the non-diabetic group of 80.26±0.25 years (95% CI: 79.78–82.75, N = 1410, p = 1) (Fig. 3). The AAO of dementia was 85.95±1.50 years (95% CI: 83.00–88.89, N = 38) for participants with diabetes, which is not significantly different from the same measure from those without diabetes of 84.78±0.41 years (95% CI: 83.98–85.57, N = 518, p = 1) (Fig. 3). By contrast, the AAO of dementia was significantly greater than the AAO of MCI, which is independent of the baseline diagnosis of diabetes (p < 0.001).
Fig.3.
Age at onset (AAO) of mild cognitive impairment (MCI) or dementia were compared between participants with and without pre-existing diabetes (D).
DISCUSSION
Our current study has verified diabetes as a risk factor of cognitive impairments measured by increased incidence and prevalence of MCI or dementia during a follow-up of more than 10 years. In other words, people with diabetes are more likely to develop and be diagnosed with cognitive impairments. The risk ratios for both MCI and dementia in people with diabetes are comparable to what have been reported in earlier studies (Table 2) [3, 22]. Unexpectedly, a diagnosis of diabetes at the baseline is associated with a slightly lower mortality than those with diabetes. The people with diabetes might see their healthcare providers more often than the comparison group. However, the underlying reason need be further investigated.
The AAO for MCI is around 80 years old, which is 5–6 years younger than the AAO for dementia on average. However, the AAO of either MCI or AD is independent of the pre-existing diabetes. Then the question remains: how does diabetes as existing morbidity increase the risk of abnormal cognition development? Vascular disease and severe hypoglycemia are believed to post the greatest risk for dementia in a people with newly diagnosed diabetes. Besides hypoglycemia, hyperglycemia was reported to be associated with poor baseline cognitive performance or accelerated cognitive decline [23, 24]. However, we did not observe an accelerated cognitive decline in participants with diabetes compared to their non-diabetic counterparts. It is worthy to note that participants comprising the NACC dataset represent a convenience sample, including clinical-referrals and community-based volunteers who are predominantly Caucasians and well-educated. Therefore, the participants with diabetes have a relatively decent care for their known morbidity.
The use of the NACC UDS represents a number of strengths including a large sample size, a comprehensive and standardized neuropsychological protocol, and standardized diagnostic criteria for differentiating cognitive impairments (MCI or dementia) from normal cognition (NC). The diagnostic criteria for NC, MCI, and dementia are standard across all participating ADCs. The longitudinal follow-up represents an important methodological strength, allowing us to better dissect the role of a pre-existing diagnosis of diabetes in increasing the risk of cognitive impairments. Despite these strengths, there are some limitations for the current study. Presence of diabetes is based on a self-reported medical history and treatment information, which might bring some information bias. Although both duration and subtype of diabetes have important effects on the declining rate of cognitive functions [6, 25], this information is not available in the NACC-UDS.
To summarize, diabetes is a risk factor for developing cognitive impairments. Although it does not accelerate the cognitive deterioration process, diabetes increases the risk of developing abnormal cognition by increasing incidence and prevalence of either MCI or dementia. Therefore, it is important to monitor cognitive performance in elders with diabetes, perform regular evaluations and implement timely interventions to prevent or delay the onset of cognitive impairments including dementia.
CONFLICT OF INTEREST
The authors report no disclosures relevant to the manuscript.
ACKNOWLEDGMENTS
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
REFERENCES
- [1]. Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MM (1996) Association of diabetes mellitus and dementia: The Rotterdam Study. Diabetologia 39, 1392–1397. [DOI] [PubMed] [Google Scholar]
- [2]. Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R (2007) Relation of diabetes to mild cognitive impairment. Arch Neurol 64, 570–575. [DOI] [PubMed] [Google Scholar]
- [3]. Smolina K, Wotton CJ, Goldacre MJ (2015) Risk of dementia in patients hospitalised with type 1 and type 2 diabetes in England, 1998–2011: A retrospective national record linkage cohort study. Diabetologia 58, 942–950. [DOI] [PubMed] [Google Scholar]
- [4]. Sano M, Zhu CW, Grossman H, Schimming C (2017) Longitudinal cognitive profiles in diabetes: Results from the National Alzheimer’s Coordinating Center’s Uniform Data. J Am Geriatr Soc 65, 2198–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Haroon NN, Austin PC, Shah BR, Wu J, Gill SS, Booth GL (2015) Risk of dementia in seniors with newly diagnosed diabetes: A population-based study. Diabetes Care 38, 1868–1875. [DOI] [PubMed] [Google Scholar]
- [6]. Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D, Griswold M, Gottesman RF, Wagenknecht LE, Windham BG, Selvin E (2014) Diabetes in midlife and cognitive change over 20 years: A cohort study. Ann Intern Med 161, 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA (2006) The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 20, 210–216. [DOI] [PubMed] [Google Scholar]
- [8]. Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC (2009) The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The neuropsychologic test battery. Alzheimer Dis Assoc Disord 23, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA (2007) The National Alzheimer’s Coordinating Center (NACC) database: The Uniform Data Set. Alzheimer Dis Assoc Disord 21, 249–258. [DOI] [PubMed] [Google Scholar]
- [10]. Morris JC (1993) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43, 2412–2414. [DOI] [PubMed] [Google Scholar]
- [11]. Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256, 183–194. [DOI] [PubMed] [Google Scholar]
- [12]. Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [13]. Mitchell AJ (2009) A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res 43, 411–431. [DOI] [PubMed] [Google Scholar]
- [14]. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944. [DOI] [PubMed] [Google Scholar]
- [15]. McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH (1996) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology 47, 1113–1124. [DOI] [PubMed] [Google Scholar]
- [16]. Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, Moody DM, O’Brien MD, Yamaguchi T, Grafman J, Drayer BP, Bennett DA, Fisher M, Ogata J, Kokmen E, Bermejo F, Wolf PA, Gorelick PB, Bick KL, Pajeau AK, Bell MA, DeCarli C, Culebras A, Korczyn AD, Bogousslavsky J, Hartmann A, Scheinberg P (1993) Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 250–260. [DOI] [PubMed] [Google Scholar]
- [17]. Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF (1998) Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 51, 1546–1554. [DOI] [PubMed] [Google Scholar]
- [18]. Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP international workshop. Neurology 47, 1–9. [DOI] [PubMed] [Google Scholar]
- [19]. Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, Quinn N, Sethi KD, Shults C, Wenning GK (2003) Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 18, 467–486. [DOI] [PubMed] [Google Scholar]
- [20]. Mesulam MM (2001) Primary progressive aphasia. Ann Neurol 49, 425–432. [PubMed] [Google Scholar]
- [21]. Irie F, Fitzpatrick AL, Lopez OL, Kuller LH, Peila R, Newman AB, Launer LJ (2008) Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: The Cardiovascular Health Study Cognition Study. Arch Neurol 65, 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Sulkava R, Kivipelto M (2010) Diabetes, Alzheimer disease, and vascular dementia: A population-based neuropathologic study. Neurology 75, 1195–1202. [DOI] [PubMed] [Google Scholar]
- [23]. Weinstein G, Maillard P, Himali JJ, Beiser AS, Au R, Wolf PA, Seshadri S, DeCarli C (2015) Glucose indices are associated with cognitive and structural brain measures in young adults. Neurology 84, 2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Feinkohl I, Aung PP, Keller M, Robertson CM, Morling JR, McLachlan S, Deary IJ, Frier BM, Strachan MW, Price JF (2014) Severe hypoglycemia and cognitive decline in older people with type 2 diabetes: The Edinburgh type 2 diabetes study. Diabetes Care 37, 507–515. [DOI] [PubMed] [Google Scholar]
- [25]. Li W, Huang E, Gao S (2017) Type 1 diabetes mellitus and cognitive impairments: A systematic review. J Alzheimers Dis 57, 29–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and analytical methods are carefully documented for the performed study. Anonymized data will be shared by request from any qualified investigator with following the NACC regulations on data use.