Abstract
Personalization of care through precision medicine and, more specifically, genetic testing is altering the management of breast cancer. Genetic testing is currently employed in germline and tumor testing, each providing distinct data to guide management. Germline testing supports more accurate risk evaluation to inform screening and risk reducing medical and surgical strategies. Tumor testing can inform cancer recurrence risk assessment and cancer treatment options. We review how genetic evaluation informs treatment and potential risks for a patient with breast cancer and the patient’s family. Hereditary cancer genetic testing of family members should include a discussion of the potential results, adverse effects, clinical management options, insurance coverage for testing, and address any concerns about privacy or discrimination with patients. Genetic professionals are available to assist with educating, testing, and managing patients with increased cancer risk.
Introduction
The following hypothetical patient presents with several clinical issues and questions common in routine practice. She is a 47-year old Ashkenazi Jewish woman who was recently diagnosed with a 0.9 cm estrogen receptor (ER)/progesterone receptor (PR)-positive, human epidermal growth factor receptor (HER2)-negative unilateral breast cancer with no lymph node involvement who presents to her internist with questions regarding how genetic testing fits into her care and what it means for her family. She has a family history of breast cancer in both her paternal aunts although no one in her family has ever been genetically tested. She is trying to make treatment decisions about surgery, radiation, and chemotherapy, and is worried about the future risk of cancer for her two daughters. She had heard about hereditary cancer genetic testing but did not think her paternal family history was relevant. Now that she has been diagnosed with cancer, she wants to learn about what genetic testing can do to inform her management decisions for herself and her family.
The general population has become more literate about cancer genetics due to extensive media and popular culture coverage. Nonetheless, misunderstandings about what genetic testing can and cannot do persist, and barriers to genetic testing still exist and include an insufficient genetic workforce, lack of knowledge about genetic services, and lack of recognition of patient risk factors (1).
Furthermore, changes in the landscape of genetic testing require that clinicians are informed to educate and counsel patients. Advances in cancer treatment and prevention in the past decades have been improved by the introduction of next generation sequencing of both the tumor for somatic mutations and the blood for germline mutations. Somatic mutations are mutations that arise after conception and are not heritable. Germline mutations are present in all cells in the body and are heritable and can be passed onto offspring. The decreasing cost of testing with the availability of next generation sequencing and the elimination of gene patents have increased the options for cancer genetic testing including some limited direct-to-consumer options for germline testing. As a result, genetic testing and tumor sequencing are accessible to more individuals, at times leaving clinicians to provide genetic education and counselling. We will provide an overview of the role of precision medicine in breast cancer management since breast cancer is a common cancer among women.
Tumor genetic testing and impact on management
The patient asks about the next steps in her management and how genetic testing of the tumor should be incorporated into her care. She was told that decisions regarding adjuvant chemotherapy should consider tumor size, grade, characteristics, and lymph node involvement.
Genetic testing in cancer is a term used loosely and should be clarified. The neoplastic process is driven by somatic mutations that lead to uncontrolled cell growth and metastases. Genetic testing can be performed on the tumor to detect somatic mutations or as gene expression or on a blood or saliva sample to sequence and assess the germline or heritable genetic factors. Gene expression profiling is currently widely used and is an integral part of recommended management for breast cancer (2). Gene expression profiling quantifies the level of gene expression, and this profile can provide information about the tumor characteristics, prognosis and risk of recurrence. Tumor sequencing detects both driver mutations that drive oncogenesis and passenger mutations that are a byproduct of increased mutations rates but do not drive oncogenesis (3). Identifying somatic mutations in the tumor may be used in the future to select treatments based upon molecular characteristics of the tumor.
In our patient, staging is first considered. For stage 1 breast tumors, the prognosis is generally good, but approximately 13% of T1N0 (no nodal involvement) and 20% of T1N1 (1–3 nodes involved) cancers will recur even after 5 years of hormonal therapy, of which 20–30% will metastasize and may then be lethal (4). Breast tumors are routinely pathologically evaluated by immunohistochemical (IHC) staining for the presence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) overexpression. This information is used to make treatment decisions about hormonal and HER2-targeted therapy (5).
Characterizing the tumor’s gene expression profile allows for risk stratification for recurrence. Multigene expression assays are currently used in the care of 34.4% of women with breast cancer in the US and are used to assess the benefit of adjuvant chemotherapy for early stage breast cancer (6). For instance, a 21-gene expression assay calculates a recurrence score (RS) for patients with node negative ER/PR positive disease to assist in deciding chemotherapy use based upon risk of recurrence. The RS ranges from 0–100. Sparano et al. demonstrated that in patients with ER/PR-positive, HER2-negative, node-negative cancers and low to intermediate RS, endocrine therapy was not inferior to combination chemoendocrine therapy, thus saving many patients from side effects and burden of chemotherapy (7). The evidence reviewed by the American Society of Clinical Oncology has demonstrated clinical utility for the Oncotype DX 21-gene expression assay (2). Based upon this evidence the patient elects to have tumor expression profiling and is found to have a low RS and decides not to pursue adjuvant chemotherapy.
While not relevant to this patient because treatment recommendations are straightforward, the National Cancer Institute has created the Molecular Analysis for Therapy Choice (NCI-MATCH) trial which is exploring the effectiveness of systemic treatment based on genetic variations (8). Patients who have failed standard treatments or who have rare cancers with no standard of care are eligible. Actionable genes related to breast cancer that are included in the study are HER2 amplification, PTEN, PIK3CA, c-KIT, and mTOR. As new therapies emerge, they are being added to the trial. Growing insight into driver mutations and their pharmacologic treatment may lead to new therapeutic options. However, the yield of somatic tumor testing for breast cancer is low but still under research.
The patient asks about the limitations of tumor sampling. One limitation to genetic profiling of tumors is intra-tumor heterogeneity. Tumors are heterogeneous and constantly evolving, and distinct sub-populations of cells exist within the tumor. Clonal lines can continue to evolve as new mutations are acquired over time. There are unavoidable sampling issues that arise when obtaining a biopsy or analyzing a small part of the tumor. It is possible that the part of the tumor tested will not include all cell clones or sufficient numbers of cells with the greatest metastatic potential. These limitations of sampling may lead to undertreatment. Sampling multiple parts of the tumor is used to minimize the risk of sampling errors.
In summary, molecular analysis of the tumor can provide data to guide which patients are more likely to benefit from systemic chemotherapy, as well as targeted therapies. The patient’s tumor was evaluated and found to have a low recurrence risk score and therefore our patient decided against adjuvant chemotherapy.
Germline testing and impact on management
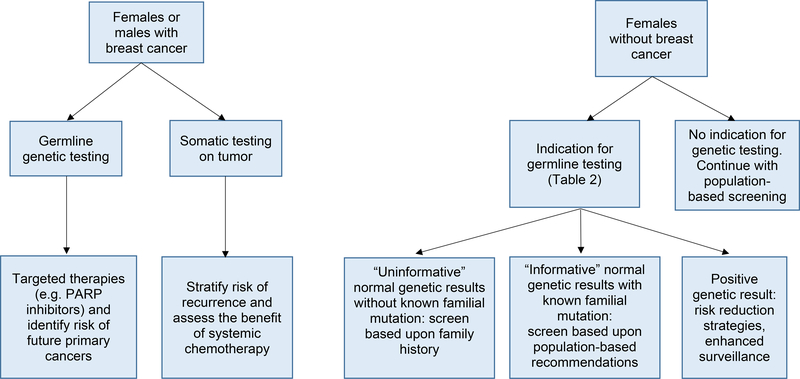
The patient has concerns about the risk of breast cancer for her daughters given her own cancer history. She is also concerned that she could be at risk for another cancer in the future. Recently, germline genetic panels have been included in breast cancer staging. Germline genetic testing is utilized for the detection of heritable mutations that increase susceptibility to cancers and can help make treatment decisions for this breast cancer while considering the risk of a future independent primary breast or other cancer (Table 1, Figure 1). Germline testing can impact management of breast cancer. For instance, the utility of germline testing in the setting of HER2-negative breast cancer has yielded therapeutic benefits. One such novel therapy is for the treatment of patients with HER2-negative breast cancer and germline BRCA1/2 mutations (2). Poly ADP ribose polymerase (PARP) inhibitors can be used to treat breast, ovarian, fallopian tube, primary peritoneal cancers, metastatic pancreatic, and prostate cancer, and are based upon the aberrant DNA repair of BRCA1/2 cancers (2,9,10).
Table 1:
| Gene | Associated cancers | Lifetime penetrance† for breast cancer in women | Mode of inheritance | Frequency in the population | Frequency in women with BC | Breast and gynecologic cancer treatment and prevention |
|---|---|---|---|---|---|---|
| ATM | Breast, colorectal, renal and urinary tract | 38–69% | AD | ~1% | 1% | Annual mammogram starting at the age of 40 years. |
| BRCA1/2 | Breast, ovary, fallopian tube, peritoneum, prostate, pancreas, melanoma | 41–90% | AD | 0.2–0.3%/2.5% (in Ashkenazi Jews) | 6.1% | Risk reducing salpingo-oophorectomy, prophylactic bilateral mastectomy. Oral contraceptives to reduce ovarian cancer risk. Tamoxifen and PARP inhibitors can be considered. Starting at the age of 25, clinical breast exams every 6–12 months and annual MRI and mammogram. |
| CDH1 | Hereditary diffuse gastric cancer, lobular breast cancer | 39–52% | AD | - | - | Annual mammogram starting at the age of 30 years. Risk reducing mastectomy may be considered in patients with family history. Risk reducing gastrectomy is also recommended between the ages of 18–40. If not performed, upper endoscopy and biopsies should be done every 6–12 months. |
| CHEK2 | Breast, ovary, colorectal | 28–37% | AD | 1% | ~1% | Annual mammogram starting at the age of 40. Bilateral prophylactic mastectomy may be considered based on family history. |
| NF1 | Peripheral nerve sheath, central nervous system, gastrointestinal, breast | 8.4% by age 50 | AD | 0.01% | - | Annual mammogram starting at the age of 30. |
| PALB2 | Breast, pancreas | 33–58% | AD | - | 1–3% | Annual mammogram starting at 30, Bilateral prophylactic mastectomy based on family history |
| PTEN | Breast, endometrial, colorectal, melanoma, renal and urinary tract, hereditary paragangliomas and pheochromocytomas, endocrine, brain | 25–85% | AD | 0.0005% (Cowden syndrome) | - | Annual physical exams starting at the age of 18. Annual mammogram and MRI starting at the age of 30. Bilateral prophylactic mastectomy and hysterectomy should be considered on a case-by-case basis. |
| TP53 | Cumulative lifetime risk for any cancer: ~ 100% | 54% | AD | 0.02–0.005% | 1% | Breast cancer surveillance every 6–12 months starting at the age of 20. Risk reducing mastectomy should be discussed on a case-by-case basis. These patients should avoid radiation. Comprehensive physical examinations should be done every 12 months. Every 2–5 years an endoscopy and colonoscopy should be performed starting at the age of 25. The patient should be educated about the signs and symptoms of cancer. A whole body MRI is recommended annually, if available. |
| STK11 | Gastrointestinal, breast, ovary | 8–45% | AD | - | - | Annual mammogram and MRI starting at the age of 25. Clinical breast exam every 6 months. Upper endoscopy and colonoscopy starting in the late teens and every 2–3 years. Pelvic exam and pap smear annually starting at age 18–20. Small bowel visualization with CT or MRI every 2–3 years starting at the age of 18; after baseline at the age of 8–10. |
Abbreviations: AD=autosomal dominant; BC=breast cancer; MRI=magnetic resonance imaging
Penetrance is the probability of developing the disease for individuals who have the genetic mutation.
Figure 1:
Utility of genetic testing on breast cancer management. Germline testing involves evaluating the genetic information of non-tumor origin as compared to tumor sequencing, which sequencing genetic variation and driver mutations within tumor tissue. Genetic testing and a female without breast cancer with a family history of breast cancer is ideally performed after the family member with breast cancer has had genetic testing. By doing so, it can be determined whether the cause of the breast cancer in the family is due to an identifiable genetic cause. If the genetic cause of the breast cancer in the family is known, a normal genetic test results in an unaffected family member is considered informative and significantly decreases the risk of breast cancer to population-based risk. When genetic test results on the affected family member are not available, genetic testing may start with the female without breast cancer. Normal genetic test results in an unaffected woman without genetic test results in an affected family member are considered uninformative since the genetic cause and the family is not known definitively. These normal results eliminate some but not all genetic causes of breast cancer since we do not yet know all of the genes contributing to breast cancer. Therefore, screening recommendations are based upon family history for women with uninformative genetic test results. Abbreviations: PARPi = poly ADP ribose polymerase inhibitors.
Some of the most common genes for high risk of hereditary breast cancer include BRCA1, BRCA2, TP53, and PTEN are considered to be high risk genes for the development of breast cancer. All of these genes are autosomal dominantly inherited, and first-degree relatives of individuals who carry a mutation are at 50% chance of carrying the same mutation. There are other genes that confer more modest risk such as ATM, CDH1, CHEK2, NF1, and PALB2. The treatment implications for individuals who carry mutations in these genes are summarized in Table 1. Prior to 2011, many women with breast cancer were tested for only BRCA1/2, but hereditary genetic testing panels for 5–50 genes are now readily available. With the expansion of germline testing and the efficacy of risk reducing measures, long-term survivors previously only screened for BRCA1/2 may want to return to update their genetic testing. Ultimately, selection of the number of genes to test should be guided by the family history and the amount of information sought by the patient and family and the comfort with ambiguous results if variants of uncertain significance are identified. Genetic counselors with expertise in cancer genetics can be helpful in educating and counseling patients about available genetic testing options. Simplified criteria for which patients should be offered hereditary breast cancer genetic testing are presented in table 2.
Table 2:
| Criteria that suggest obtaining genetic assessment and/or testing include: | |
|---|---|
| Personal history of breast cancer with | Diagnosis ≤ 45 years |
| Triple negative breast cancer diagnosis ≤ 60 years | |
| ≥2 primary breast cancers (bilateral or ipsilateral) | |
| Male with breast cancer | |
| Ashkenazi Jewish ancestry and breast cancer at any age | |
| Personal history of pancreatic, metastatic prostate, or ovarian cancer | |
| Individual with no personal history of breast cancer with high suspicion of hereditary cancer in 1st or 2nd degree relatives; however, testing of the affected individual should be performed when possible | |
The patient is an Ashkenazi Jewish woman with a breast cancer before age 50, so she meets NCCN criteria, and her insurance will therefore cover germline testing for a panel of hereditary cancer genes. Insurance coverage of genetic testing varies by insurance company; however, insurers will cover testing for women who meet the NCCN criteria for testing and some may have more generous criteria. Some insurers only cover cancer genetic testing once in a lifetime, and this should be considered when choosing an appropriate test panel. Medicare only covers genetic testing for individuals with a personal history of breast cancer (16).
Beyond insurance coverage, numerous other factors should be considered in the selection of testing services, such as experience of the laboratory, volume of testing, turnaround time, number of genes analyzed and types of mutations detected, options for specimen type (saliva/cheek swab or blood), accuracy of variant interpretation, transparency of variant interpretation, understandable test reports, free testing of family members to resolve variants of uncertain significance, insurance preauthorization services, and patient out-of-pocket costs.
Variants identified in genetic tests are classified into one of five categories: pathogenic; likely pathogenic; variant of unknown significance (VUS); likely benign; or benign. As the number of genes for which a patient is tested increases with larger and larger panels, the probability of identifying one or more VUS increases (17). Most VUS will eventually be reclassified as benign or likely benign over time; however, VUS have been found to increase anxiety in patients and to be a source of uncertainty for clinicians (18,19). Patients with VUSs and no personal history of cancer but with a family history of breast cancer are more likely to elect bilateral prophylactic mastectomies even though most VUS are likely to be benign (20). Pretest genetic counseling should include discussion of the risk of identifying VUSs and the associated uncertainty that increases anxiety in some individuals. Clinicians should also consider referring patients who have VUS to a genetic specialist to help with further variant interpretation, familial segregation studies, and/or to participate in research studies to assess the impact of the variant.
Interpretation of genetic variants can vary between laboratories. ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) is a central database of variants and their clinical interpretation and is maintained by the National Center for Biotechnology Information (NCBI) and is a central dynamic resource for variant interpretation. ClinVar is an open access resource that can be accessed by providers and patients to reassess additional data about VUS over time. These variants are reclassified as more data become available. Good laboratories will also notify the ordering provider as VUS are reclassified over time.
Unfortunately, it is common for women with a family history of breast cancer to be tested for germline mutations only after diagnosis and surgery for cancer, as in the case of this patient (21). The patient has germline testing, and a BRCA2 6174delT is identified. This mutation is one of the three common BRCA1/2 founder mutations found in the Ashkenazi Jewish population. The results are clear, and there is no difficulty with variant interpretation. There is an increased prevalence of mutations in certain populations due to specific founder mutations (limited genetic diversity due to a single founder from whom the mutation was inherited). In the Ashkenazi Jewish population there is a 1/40 prevalence of a carrying one of three BRCA1/2 mutations (22).
Based upon the 10.8% risk of a future independent primary breast cancer in the next 10 years and 62% by the age of 70 for patients with BRCA2 mutations, the patient decides to have a bilateral mastectomy (19). Bilateral mastectomy does not completely eliminate risk of breast cancer but significantly reduces risk (2). Additionally, due to the 16.5–27% risk of ovarian cancer in patients with BRCA2 mutations and her post-menopausal status, she also decides to have a prophylactic bilateral salpingo-oophorectomy (23). She also understands that BRCA2 mutations are inherited in an autosomal dominant fashion and that each of her children has a 50% chance to have inherited her BRCA2 mutation. Her daughters are only 19 and 25 years old, and she wonders when they should start to consider their cancer risk.
For women without breast cancer but concerned about their risk of cancer based upon their family history of cancer, testing should ideally be performed first on a person in the family with cancer (such as our patient) to determine if the cancer was due to a hereditary cause. The choice of if and when to perform genetic testing must be evaluated holistically based upon the preferences of the patient, their life stage, and how they will use the information. Genetic counselors can play an important role in educating and counseling patients about genetic testing and provide educational and emotional support through the process. Although some patients raise concerns about genetic discrimination, the Genetic Information Nondiscrimination Act (GINA) protects against discrimination for health insurance or employment but does not protect rights to life insurance or disability insurance (24).
After meeting with a cancer genetic counselor, the patient’s older daughter decides to get testing now while the younger daughter decides to wait because she would not do anything differently at age 19. The older daughter’s test shows that she inherited the BRCA2 mutation and is recommended to start annual magnetic resonance imaging (MRI) screening with contrast until the age of 30, when yearly mammograms will be recommended. She is also advised to receive a clinical breast exam every 6–12 months, and she is taught how to conduct self-breast exams. She decides to start oral contraceptives which can reduce her risk of ovarian cancer by up to 40% (25).
Summary
Following her treatment and bilateral mastectomy and prophylactic salpingo-oophorectomy, the patient feels she was empowered to make decisions to keep herself cancer free. She has some feelings of guilt about her 25-year-old daughter’s results, but feels she is a good example about how she can live well despite increased cancer risks.
As presented above, this patient’s treatment and management were affected by the ability to personalize her care and the care of her family through tumor and germline genetic testing. Genetic testing offers new opportunities to improve screening, prevention, early detection, and therapy for breast and other cancers. As the field continues to develop, improved understanding of heterogeneity across tumors with improved molecular profiling of cancer and more data on treatment and outcomes may facilitate advancements toward more specific and improved therapies that are more effective and less toxic. Additionally, as the cost decreases and interpretive ability improves, the clinical utility of population screening for germline cancer risk will be evaluated.
Box 1. Summary.
Germline genetic testing results can inform cancer care for patients with breast cancer and other cancers by identifying patients with heritable contributions that impact cancer management and future cancer risk for second primaries.
Genetic analysis of tumors provides data about cancer drivers and tumor characteristics that can inform therapy selection and clinical trial opportunities.
Germline cancer genetic testing for patients with a family history of cancer can stratify level of risk across the lifespan and define types of cancers for which there is increased risk to guide cancer surveillance strategies and risk reducing strategies.
Genetic counselors and other professionals with expertise in cancer genetics can be helpful in facilitating appropriate genetic testing and care of patients with hereditary cancer syndromes.
Acknowledgment
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Jonah Tischler received support from the Columbia University Student Affairs Office.
References
- 1.Delikurt T, Williamson GR, Anastasiadou V, Skirton H. A systematic review of factors that act as barriers to patient referral to genetic services. Eur J Hum Genet. 2014;23(6):739–745. doi: 10.1038/ejhg.2014.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN Clinical Practice Guideline in Oncology Breast Cancer (Version 1.2019). [cited 2019 Apr 5] Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- 3.McFarland CD, Yaglom JA, Wojtkowiak JW, et al. The Damaging Effect of Passenger Mutations on Cancer Progression. Cancer Res. 2017;77(18):4763–4772. doi: 10.1158/0008-5472.CAN-15-3283-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan H, Gray R, Braybrooke J, Davies C, Taylor C, Mcgale P, et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. New England Journal of Medicine. 2017;377(19):1836–46. doi: 10.1056/NEJMoa1701830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amin MB, American Joint Committee on Cancer, American Cancer Society, eds. AJCC Cancer Staging Manual. Eight edition / editor-in-chief, Amin Mahul B.; editors, Edge Stephen B.[and 16 others]; Gress Donna M., CTR-Technical editor; Meyer Laura R., CAPM-Managing editor. Chicago IL: American Joint Committee on Cancer, Springer; 2017. [Google Scholar]
- 6.Bhutiani N, Egger ME, Ajkay N, Scoggins CR, Martin RC, Mcmasters KM. Multigene Signature Panels and Breast Cancer Therapy: Patterns of Use and Impact on Clinical Decision Making. Journal of the American College of Surgeons. 2018;226(4). Doi: 10.1016/j.jamcollsurg.2017.12.043 [DOI] [PubMed] [Google Scholar]
- 7.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. New England Journal of Medicine. 2018;379(2):111–121. doi: 10.1056/NEJMoa1804710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NCI-MATCH Trial (Molecular Analysis for Therapy Choice) [Internet]. National Cancer Institute. [cited 2018Aug15]. Available from: https://www.cancer.gov/about-cancer/treatment/clinical-trials/nci-supported/nci-match
- 9.Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med. 2015;373(18):1697–1708. doi: 10.1056/NEJMoa1506859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib Monotherapy in Patients With Advanced Cancer and a Germline BRCA1/2 Mutation. JCO. 2015;33(3):244–250. doi: 10.1200/JCO.2014.56.2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okur V, Chung WK. The impact of hereditary cancer gene panels on clinical care and lessons learned. Cold Spring Harbor Molecular Case Studies. 2017;3(6):a002154. doi 10.1101/mcs.a002154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 2.2018). [cited 2018Aug15] Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- 13.Hereditary Cancer [Internet]. Causes of Cancer – Genetic Mutations | BRCA & HBOC Mutations. [cited 2018 Aug 15]. Available from: http://www.facingourrisk.org/understanding-brca-and-hboc/information/cancertreatment/breast-treatment/basics/treatment-options-hereditary-breast.php
- 14.National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast and Ovarian (Version 3.2019). [cited 2019 Jul 21] Available from: https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf
- 15.Moyer VA, on behalf of the U.S. Preventive Services Task Force. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer in Women: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2014;160:271–281. doi: 10.7326/M13-2747 [DOI] [PubMed] [Google Scholar]
- 16.Local coverage determination: MolDx BRCA1 and BRCA2 genetic testing. Noridian Medicare. https://med.noridianmedicare.com/documents/10546/6990981/MolDX+BRCA1+and+BRCA2+Genetic+Testing+LCD/220c55c8-8bc8-4319-80a1-66c4a1d51dd8. Accessed Jun 29, 2019
- 17.Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of Multigene Panel Testing on Surgical Decision Making in Breast Cancer Patients. Journal of the American College of Surgeons. 2018;226(4):560–5. doi: 10.1016/j.jamcollsurg.2017.12.037 [DOI] [PubMed] [Google Scholar]
- 18.Brédart A, Kop JL, Depauw A, et al. Short-term psychological impact of the BRCA1/2 test result in women with breast cancer according to their perceived probability of genetic predisposition to cancer. Br J Cancer. 2013;108(5):1012–1020. doi: 10.1038/bjc.2012.599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eccles BK, Copson E, Maishman T, Abraham JE, Eccles DM. Understanding of BRCA VUS genetic results by breast cancer specialists. BMC Cancer. 2015;15:936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsh JL, Hoskin TL, Day CN, et al. Clinical Decision-Making in Patients with Variant of Uncertain Significance in BRCA1 or BRCA2 Genes. Ann Surg Oncol. 2017;24(10):3067–3072. doi: 10.1245/s10434-017-5959-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurian AW, Ward KC, Hamilton AS, et al. Uptake, Results, and Outcomes of Germline Multiple-Gene Sequencing After Diagnosis of Breast Cancer. JAMA Oncol 2018;4(8):1066–1072. doi: 10.1001/jamaoncol.2018.0644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Struewing JP, Hartge P, Wacholder S, et al. The Risk of Cancer Associated with Specific Mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408. doi: 10.1056/NEJM199705153362001 [DOI] [PubMed] [Google Scholar]
- 23.Petrucelli N BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer [Internet]. GeneReviews. U.S. National Library of Medicine; 2016. [cited 2018Aug15]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1247/ [Google Scholar]
- 24.What is genetic discrimination? - Genetics Home Reference - NIH [Internet]. U.S. National Library of Medicine. National Institutes of Health; [cited 2018Aug15]. Available from: https://ghr.nlm.nih.gov/primer/testing/discrimination [Google Scholar]
- 25.Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers. JAMA Oncology. 2018;4(4):516–21. doi: 10.1001/jamaoncol.2017.4942 [DOI] [PMC free article] [PubMed] [Google Scholar]