ABSTRACT
Lurbinectedin is a DNA-binding inhibitor of transcription that potently induces immunogenic cell death (ICD). In June 2020, the Federal Drug Administration (FDA) approved lurbinectedin for the salvage treatment of small-cell lung cancer that has relapsed from platinum compound-based first-line chemotherapy. Thus, the clinical activity of lurbinectedin may originate, at least in part, from the induction of ICD.
KEYWORDS: Transcription inhibitor, anticancer immunity, immunotherapy, immune checkpoint blockade
Chemotherapeutic agents have been conceived as cytotoxic compounds that selectively kill cancer cells yet spare normal cells, thus ridding the body from malignancy. Over the past 15 years, it has become clear that there is no possible cure for cancer unless the therapeutic intervention stimulates a potent antitumor immune response. Even transient effects that lead to a significant extension of patient survival rely to a large extent on the capacity of the immune system to control tumor progression.1
The most important mechanism through which chemotherapy achieves the stimulation of anticancer immunity involves the induction of immunogenic cell death (ICD).2 Malignant cells that succumb to ICD emit a series of stress signals in the form of danger-associated molecular patterns (DAMPs) that act on pattern recognition receptors (PRRs) to attract dendritic cell (DC) precursors into their vicinity (with the DAMP ATP acting on purinergic receptors and the DAMP annexin A1 acting on the PRR formyl peptide receptor-1),3,4 transfer tumor-associated antigens via phagocytosis into such DCs (with the DAMP calreticulin on the surface of dying cells acting as an ‘eat-me’ signal on the PRR CD91), and stimulates the activation/maturation of the DC (with the DAMP high mobility group B1 acting on the PRR Toll-like receptor 4). In addition, ICD causes the recruitment of T cells into the tumor bed (secondary to the activation of the PRR Toll-like receptor 3 by ectopic nucleotides and a Type-1 interferon response) so that they can be educated by DC and finally launch an attack against residual cancer cells.2
Unfortunately, many cytotoxic agents are unable to stimulate ICD. Only a fraction of particularly efficient anticancer drugs are able to trigger the premortem stress responses that characterize ICD. Although there is a necroptotic variant of ICD,5 human cancers usually invalidate the necroptotic pathway (due to the silencing of either RIP3 or MLKL), meaning that ICD usually occurs in the context of apoptosis, requiring the activation of caspases.6 In this context of apoptotic ICD, cell death is preceded by a pathognomonic event, which is the activation of the ‘integrated stress response’ (ISR), consisting in the phosphorylation of serine 51 in eukaryotic initiation factor 2α (eIF2α) by upstream kinases.7,8 This ISR is usually activated downstream of the inhibition of DNA to RNA transcription and constitutes a central event for ICD at two levels. First, the ISR is required for the autophagy-dependent release of ATP from dying tumor cells. Second, the ISR is essential for the translocation of calreticulin from the endoplasmic reticulum lumen to the cell surface.9
We recently performed a systematic analysis of chemotherapeutic drugs, observing that drugs that inhibit transcription are particularly efficient ICD inducers. Thus, dactinomycin (also known as actinomycin D), a prototypic inhibitor of transcription that has been used for this purpose by generations of cellular and molecular biologists, turned out to be a particularly efficient ICD inducer that causes tumor growth control in preclinical models only if the immune system is intact, through a T cell-dependent mechanism that can be boosted by combination with PD-1 blockade.8 Lurbinectedin is a cytotoxicant that binds to DNA and inhibits transcription.10 Accordingly, lurbinectedin also triggers ICD and induces potent anticancer immune responses in preclinical models.11
Small-cell lung cancer is a disease with a notoriously bad prognosis. The initial treatment is usually a combination of platinum compounds (cisplatin or carboplatin) with etoposide that often leads to a partial or even apparently complete response, yet is invariably followed by a relapse of the disease that then manifests in a chemotherapy-resistant form.12 It should be noted that cisplatin and etoposide are rather poor ICD inducers, providing an explanation for this observation.6,9 Although both drugs kill a substantial portion of tumor cells, they are unable to induce an immune response that would provide long-term effects to the patients. From this perspective, the introduction of a potent ICD inducer like lurbinectedin into the clinics (Figure 1a), initially as a second-line treatment appears a logical development.13 Although the formal proof for this conjecture is still elusive, two interesting future developments await urgent exploration. First, it will be interesting to know whether lurbinectedin might be useful as a first-line treatment, thus avoiding the patient with small-cell lung cancer to endure a debilitating (and perhaps even immunosuppressive) platinum-based chemotherapy (Figure 1b). Second, it will be important to clarify whether lurbinectedin might be advantageously combined with immune checkpoint inhibitors (ICIs) targeting the PD-1/PD-L1 interaction (Figure 1c). Indeed, for other cancers, there are multiple examples in which ICD inducers have been successfully combined with ICIs, both preclinically and clinically,9 strongly suggesting that this might be an adequate strategy for the clinical management of small-cell lung cancer.
Figure 1.
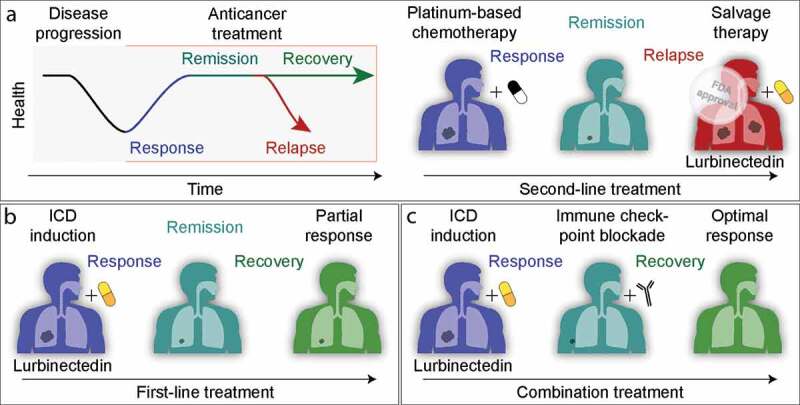
Clinical development of lurbinectedin for the treatment of small-cell lung carcinoma. a. Current FDA-approved use of lurbinectedin, as a salvage therapy after failure of platinum compound-based chemotherapy. b. Hypothetical use of lurbinectedin as a first-line treatment. c. Hypothetical combination of lurbinectedin used in first or second line with PD-1/PD-L1-blocking antibodies.
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association “Ruban Rose”; Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085), Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology (ANR-18-IDEX-0001); the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Conflict of interest statement
GK and OK worked on a research contract with PharmaMar, the company that identified lurbinectedin.
References
- 1.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G.. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–3. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Martinez AB, Chan TA, Coukos G, Demaria S, Deutsch E, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8:e000337. doi: 10.1136/jitc-2019-000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 4.Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science. 2015;350:972–978. doi: 10.1126/science.aad0779. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Ma Y, Chen G, Zhou H, Yamazaki T, Klein C, Pietrocola F, Vacchelli E, Souquere S, Sauvat A, et al. Contribution of RIP3 and MLKL to immunogenic cell death signaling in cancer chemotherapy. Oncoimmunology. 2016;5:e1149673. doi: 10.1080/2162402X.2016.1149673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bezu L, Sauvat A, Humeau J, Gomes-da-Silva LC, Iribarren K, Forveille S, Garcia P, Zhao L, Liu P, Zitvogel L, et al. eIF2alpha phosphorylation is pathognomonic for immunogenic cell death. Cell Death Differ. 2018;25:1375–1393. doi: 10.1038/s41418-017-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humeau J, Sauvat A, Cerrato G, Xie W, Loos F, Iannantuoni F, Bezu L, Lévesque S, Paillet J, Pol J, et al. Inhibition of transcription by dactinomycin reveals a new characteristic of immunogenic cell stress. EMBO Mol Med. 2020;12:e11622. doi: 10.15252/emmm.201911622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galluzzi L, Humeau J, Buque A, Zitvogel L, Kroemer G. Immunostimulation by chemotherapy in the era of immune checkpoint blockers. Nat Rev Clin Oncol. 2020;17:49-64. doi: 10.1038/s41571-019-0272-7 [DOI] [PubMed] [Google Scholar]
- 10.Santamaria Nunez G, Robles CMG, Giraudon C, Martinez-Leal JF, Compe E, Coin F, Aviles P, Galmarini CM, Egly J-M. Lurbinectedin specifically triggers the degradation of phosphorylated RNA polymerase ii and the formation of DNA breaks in cancer cells. Mol Cancer Ther. 2016;15:2399–2412. doi: 10.1158/1535-7163.MCT-16-0172. [DOI] [PubMed] [Google Scholar]
- 11.Xie W, Forveille S, Iribarren K, Sauvat A, Senovilla L, Wang Y, Humeau J, Perez-Lanzon M, Zhou H, Martínez-Leal JF. Lurbinectedin synergizes with immune checkpoint blockade to generate anticancer immunity. Oncoimmunology. 2019;8:e1656502. doi: 10.1080/2162402X.2019.1656502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saltos A, Antonia S. Breaking the impasse: advances in treatment of small cell lung cancer. Clin Chest Med. 2020;41:269–280. doi: 10.1016/j.ccm.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Trigo J, Subbiah V, Besse B, Moreno V, López R, Sala MA, Peters S, Ponce S, Fernández C, Alfaro V, et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020;21:645–654. doi: 10.1016/S1470-2045(20)30068-1. [DOI] [PubMed] [Google Scholar]


