Figure 3.
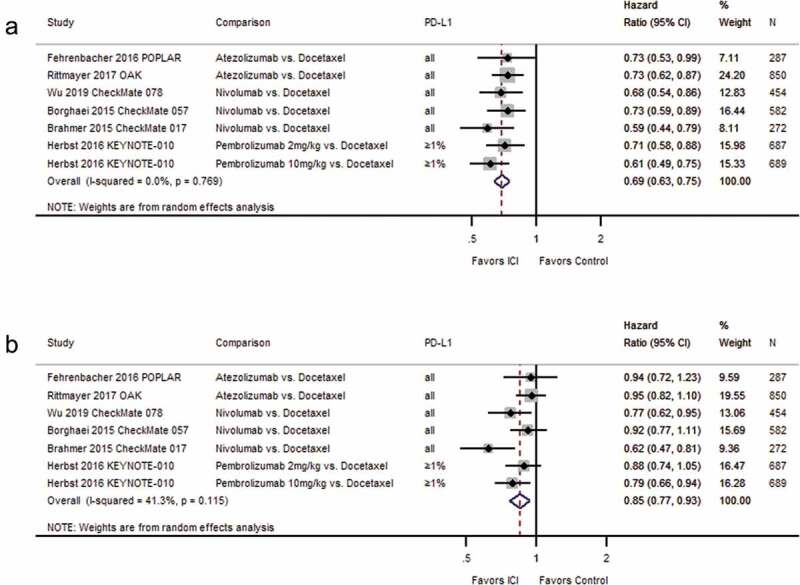
Forest plots for (a) overall survival and (b) progression-free survival in studies assessing immune checkpoint inhibitors as second-line therapy.
Wu 2019 CheckMate 078: We converted 97.7% CI (0.52–0.90) for overall survival to 95% CI. Herbst 2016 KEYNOTE-010:intention-to-treat N = 1033 including all three study arms (pembrolizumab 2 mg, N = 344, pembrolizumab 10 mg, N = 346, docetaxel 75 mg, N = 343). Percentage of randomized patients with quantifiable tumor PD-L1 expression: Borghaei 2015 CheckMate 057: 78%; Brahmer 2015 CheckMate 017: 83%; Wu 2019 CheckMate 078: 9%. Abbreviations: CI = confidence interval; D + L = DerSimonian and Laird method; ICI = immune checkpoint inhibitor; kg = kilogram; mg = milligram; N = number of patients; PD-L1 = programmed cell death ligand-1.
