ABSTRACT
Cancer immunosurveillance generally relies on adaptive immune programs executed by CD8+ T cells. Our findings demonstrate that CD8+ T cells fail to control early oncogenesis in a mouse model of luminal B breast cancer and suggest that natural killer (NK) cells may instead play a predominant role in this setting.
KEYWORDS: Hormonotherapy, immune checkpoint blockers, nicotinamide, PD-1, type I interferon, tumor microenvironment
It is now accepted that tumors emerge, progress and respond to treatment in the context of a complicated bidirectional interaction with the host, which involves a major immunological component.1,2 Thus, while malignant cell precursors are initially recognized and eliminated by the immune system, the accumulation of genetic and epigenetic defects in (pre)neoplastic cells enable an equilibrium phase that ultimately converts into overt immune escape and uncontrolled disease progression.1,2 A large body of evidence from multiple groups spearheaded by the Schreiber laboratory demonstrates that CD8+ cytotoxic T lymphocytes (CTLs) are critical for cancer immunosurveillance in a variety of carcinogen-driven and spontaneous mouse tumor models, including methylcholanthrene (MCA)-driven sarcomas.3 Apparently at odds with this notion, our recent findings indicate that CD8+ CTLs fail to control 7,12-dimethylbenz[a]anthracene (DMBA)-initiated, medroxyprogesterone (MPA)-accelerated mammary carcinomas emerging in immunocompetent C57BL/6 mice, whereas natural killer (NK) cells are the main effectors of early cancer immunosurveillance in this setting.4
An estimated 276,480 new cases of breast cancer (BC) and 42,170 new BC-related deaths have been quantified to affect women living in the US in 2020, a majority of which originate from hormone receptor (HR)-positive (luminal A and luminal B) disease.5 Thus, despite the progress achieved over the past decades on the early detection and clinical management of HR+ BC, many women still succumb to this devastating disease, calling for the development of therapeutic regimens with superior clinical activity. An exciting development in this sense has recently come from the realization that cyclin-dependent kinase 4 (CDK4) and CDK6 inhibitors significantly extend the overall survival of women with advanced HR+ BC receiving hormonotherapy.6 Conversely, despite substantial expectations, immunotherapy with immune checkpoint blockers (ICBs) employed as standalone agents is virtually inactive in patients with HR+ BC,7 largely reflecting the limited infiltration of these neoplasms by immune cells.8
The lack of a preclinical model recapitulating the emergence and progression of HR+ BC in fully immunocompetent hosts has considerably hampered the development of efficient immunotherapeutic regimens for the disease.9 Indeed, human HR+ BC cell lines such as MCF7 cells are intrinsically incompatible with immunological studies in vivo. Moreover, while transplantable mouse HR+ BC models such as TSA cells can be employed to generate tumors in immunocompetent syngeneic BALB/c mice, they have been considerably immunoedited in their original host, implying that they have already evaded immunosurveillance. Finally, genetically engineered mouse models of BC such as the MMTV-PyMT and MMTV-HER2 models (1) lack HR expression, de facto modeling triple negative breast cancer (TNBC) and erb-b2 receptor tyrosine kinase 2 (ERBB2, best known HER)-overexpressing BC, and (2) exhibit a relatively low mutational burden, suggesting that they are virtually invisible to the adaptive immune system.9
In view of the aforementioned considerations, we embarked in the comprehensive characterization of mammary carcinomas driven by DMBA and MPA in C57BL/6 mice as a potential model for immunological studies in the HR+ BC space. We found that these tumors are histologically and transcriptionally similar to human luminal B (highly proliferative HR+HER2−) BC, and emerge by an oncogenic process that depends on the transcriptional activity of estrogen receptor 1 (ESR1) and is coupled to the evasion of immunosurveillance.4 Consistent with this notion, oncogenesis and tumor progression (culminating in the death of tumor-bearing mice) are accelerated in highly immunodeficient Rag2−/-Il2rg−/- mice (which lack T cells, B cells and NK cells), as well as in mice lacking the key immune effector interferon gamma (IFNG).4 Moreover, palpable DMBA/MPA-driven mammary carcinomas (1) exhibit a transcriptional signature enriched in genes involved in cell cycle progression and proliferation, but deprived of genes involved in immunological functions, and (2) are scarcely infiltrated by immune effector cells as they display a CD4 compartment that is polarized toward immunosuppression by CD4+CD25+FOXP3+ regulatory T (TREG) cells. Finally, DMBA/MPA-driven mammary carcinomas resemble human HR+ BC in their limited sensitivity to ICBs targeting programmed cell death 1 (PDCD1, best known as PD-1).4
Intriguingly, we found that DMBA/MPA-driven oncogenesis is not altered by the Rag2−/- genotype (which imposes a defect in T cells and B cells, but largely spares NK cells), nor by the antibody-mediated co-depletion of CD4+ and CD8+ T cells. Conversely, the depletion of cells expressing killer cell lectin like receptor K1 (KLRK1, best known as NKG2D), which encompass NK cells and a fraction of T cells, phenocopied the detrimental effects of the Rag2−/-Il2rg−/- genotype on disease emergence and progression.4 Moreover, while MCA-driven sarcomas emerging in immunocompetent, syngeneic mice can be transplanted (at least in a fraction of cases) into immunocompetent recipients, reflecting the ability of T cells from the original host to limit the antigenicity of evolving tumors,3 the same did not hold true for DMBA/MPA-driven carcinomas.4 That said, the therapeutic efficacy of anthracycline-based chemotherapy and/or the experimental caloric restriction mimetics (CRMs) hydroxycitrate and nicotinamide (both of which potently induce autophagy) against established DMBA/MPA-driven carcinomas can be abrogated by the antibody-mediated co-depletion of CD4+ and/or CD8+ T cells.4,10 Thus, adaptive immune responses specific for DMBA/MPA-driven tumors can be (re-)instated by various agents and ultimately support therapeutic efficacy, delineating a role for CD8+ CTLs in tumor control elicited by treatment.
In summary, our observations indicate that the early emergence of mammary carcinomas driven by MPA and DMBA in C57BL/6 mice involves the evasion of natural immunosurveillance by NK cells, not CD8+ CTLs. The precise mechanisms underlying this observation remain to be elucidated. NK cell activation is indeed controlled by a balance of activating and inhibitory signals,11 and the relative contribution of such signals to the immunosurveillance of DMBA/MPA-driven tumors remains to be systematically dissected. Despite this and other open questions, our data raise the intriguing possibility that the immune compartment of established DMBA/MPA-driven mammary tumors may be susceptible to re-activation by NK cell-targeting maneuvers, including recombinant interleukin 15 (IL-15) and ICBs targeting killer cell lectin like receptor C1 (KLRC1, best known as NKG2A) (Figure 1). As this possibility awaits experimental verification, signs of evasion from NK-cell immunosurveillance including increased expression levels of NKG2A have already been documented in patients with HR+ BC.12 Altogether, these findings point to the NKG2A-targeting ICB monalizumab, which has already been tested for safety and demonstrated promising clinical activity in patients with squamous cell carcinoma of the head and neck,13 as to a potential immunotherapeutic agent for the management of HR+ BC. To the best of our knowledge, no clinical trials have yet been initiated to investigate the therapeutic profile of monalizumab in this patient subset.
Figure 1.
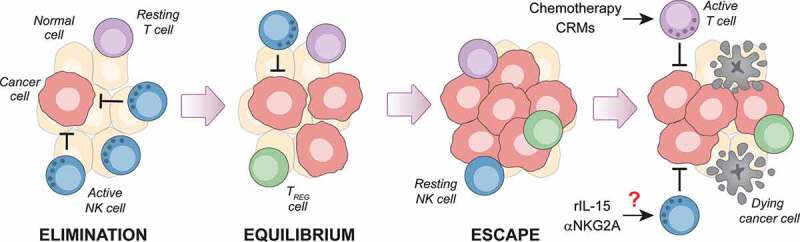
Failing NK cell immunosurveillance may reveal novel immunotherapeutic approaches for luminal B breast cancer. Endogenous mammary carcinomas driven in immunocompetent C57BL/6 female mice by 7,12-dimethylbenz[a]anthracene (DMBA) and medroxyprogesterone acetate (MPA) – a model of luminal B breast cancer in women – are under early immunosurveillance by natural killer (NK) cells, not CD8+ cytotoxic T cells. This observation raises the interesting and hitherto untested possibility that agents targeting NK cells, including recombinant interleukin 15 (rIL-15) and antibodies blocking killer cell lectin like receptor C1 (KLRC1, best known as NKG2A), may mediate therapeutic activity not only against DMBA/MPA-driven tumors in mice, but also against luminal B breast cancers in women. That said, the adaptive immune system of mice bearing DMBA/MPA-driven tumors has not lost the ability to mount tumor-targeting responses upon administration of immunostimulatory chemotherapeutics or caloric restriction mimetics (CRMs). TREG, regulatory T.
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085 and GDW20181100051), Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology; the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM). The LG lab is supported by a Breakthrough Level 2 grant from the US Department of Defense (DoD), Breast Cancer Research Program (BRCP) (#BC180476P1), by the 2019 Laura Ziskin Prize in Translational Research (#ZP-6177, PI: Formenti) from the Stand Up to Cancer (SU2C), by a Mantle Cell Lymphoma Research Initiative (MCL-RI, PI: Chen-Kiang) grant from the Leukemia and Lymphoma Society (LLS), by a startup grant from the Dept. of Radiation Oncology at Weill Cornell Medicine (New York, US), by a Rapid Response Grant from the Functional Genomics Initiative (New York, US), by industrial collaborations with Lytix (Oslo, Norway) and Phosplatin (New York, US), and by donations from Phosplatin (New York, US), the Luke Heller TECPR2 Foundation (Boston, US) and Sotio a.s. (Prague, Czech Republic).
Disclosures
AB, NB and GP have no conflicts of interest to disclose. GK has been holding research contracts with Bayer Healthcare, Genentech, Glaxo Smyth Kline, Institut Mérieux, Lytix Pharma, PharmaMar, Sotio and Vasculox, he is on the Board of Directors of the Bristol Myers Squibb Foundation France, and he is a scientific co-founder of everImmune, Samsara therapeutics and Therafast Bio. LG received consulting fees from OmniSEQ, Astra Zeneca, Inzen and the Luke Heller TECPR2 Foundation, and he is member of the Scientific Advisory Committee of Boehringer Ingelheim, The Longevity Labs and OmniSEQ.
References
- 1.Chen DS, Mellman I.. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–3. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 2.Galluzzi L, Chan TA, Kroemer G, Wolchok JD, Lopez-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med. 2018;10:eaat7807. doi: 10.1126/scitranslmed.aat7807. [DOI] [PubMed] [Google Scholar]
- 3.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 4.Buque A, Bloy N, Perez-Lanzón M, Iribarren K, Humeau J, Pol JG, Levesque S, Mondragon L, Yamazaki T, Sato A, et al. Immunosurveillance of hormone receptor-positive breast cancer - Prophylactic and therapeutic effects of nicotinamide. Nat Commun. 2020;in press. doi: 10.1038/s41467-020-17644-0. [DOI] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martín M, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382:514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 7.Rugo HS, Delord JP, Im SA, Ott PA, Piha-Paul SA, Bedard PL, Sachdev J, Tourneau CL, van Brummelen EMJ, Varga A, et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res. 2018;24:2804–2811. doi: 10.1158/1078-0432.CCR-17-3452. [DOI] [PubMed] [Google Scholar]
- 8.Kroemer G, Senovilla L, Galluzzi L, Andre F, Zitvogel L. Natural and therapy-induced immunosurveillance in breast cancer. Nat Med. 2015;21:1128–1138. doi: 10.1038/nm.3944. [DOI] [PubMed] [Google Scholar]
- 9.Buque A, Galluzzi L. Modeling Tumor Immunology and Immunotherapy in Mice. Trend Cancer. 2018;4:599–601. doi: 10.1016/j.trecan.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Pietrocola F, Pol J, Vacchelli E, Rao S, Enot DP, Baracco EE, Levesque S, Castoldi F, Jacquelot N, Yamazaki T, et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell. 2016;30:147–160. doi: 10.1016/j.ccell.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galluzzi L, Yamazaki T, Kroemer G. Linking cellular stress responses to systemic homeostasis. Nat Rev Mol Cell Biol. 2018;19:731–745. doi: 10.1038/s41580-018-0068-0. [DOI] [PubMed] [Google Scholar]
- 12.Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Gonçalves A, André P, Romagné F, Thibault G, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121:3609–3622. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andre P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, Bléry M, Bonnafous C, Gauthier L, Morel A, et al. Anti-NKG2A mab is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175:1731–43 e13. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


