ABSTRACT
Background
Thymic epithelial tumors (TETs) are rare malignancies with unique association to the autoimmune disease myasthenia gravis (MG). Heat shock proteins (HSPs) harbor great potential as cancer biomarkers and HSP inhibitors approach clinical cancer therapy.
Methods
To explore HSP pathophysiology, we assessed sera (immunoassays) and tissues (immunohistochemistry) of TETs (and thymic tissues) for HSP27, phosphorylated (p)HSP27, HSP70 and HSP90α expression in 114 TETs and 26 non-thymomatous MG patients undergoing extended thymectomy.
Results
Serum concentrations of HSP90α were significantly increased in patients with thymic carcinomas, thymomas, thymic neuroendocrine tumors and non-thymomatous MG compared to patients who underwent thymectomy revealing regular thymic morphology or controls. In thymoma patients, high serum HSP90α represented a significantly worse prognostic factor for free-from-recurrence, and complete tumor resection led to decreased levels. The expression of HSP90 in nuclei and cytoplasm of tumor cells and non-neoplastic lymphocytes varied with WHO histological subtype. HSP90 was expressed in centroblasts of thymic germinal centers in MG patients. Higher pHSP27 serum concentrations were observed in seropositive MG and those not treated with steroids.
Conclusions
HSP data suggest high potential for HSPs as TET cancer biomarkers or as candidates for targeted therapy. Caution is warranted in TET patients with associated MG overexpressing HSPs.
KEYWORDS: Cancer biomarkers, heat shock proteins, non-thymomatous myasthenia gravis, prognostic factors, thymic epithelial tumors
Background
Thymic epithelial tumors (TETs), comprising thymomas and thymic carcinomas (TCs), and thymic neuroendocrine tumors (TNETs) are the most common malignancies in the anterior mediastinum in adults.1 Although TETs are slow-growing neoplasms with long asymptomatic periods, they have the potential to metastasize. Interestingly, myasthenia gravis (MG) occurs as paraneoplastic syndrome in up to 30% of patients with thymomas, while thymomas are recorded in 10-15% of MG patients.2 Complete surgical tumor resection represents the mainstay of treatment for TETs and should be performed whenever feasible.1 Thymic pathologies are histologically categorized according to the World Health Organization (WHO) classification, which differentiates six main histopathologic types: A, AB, B1, B2, B3 thymomas and TCs.3 The Masaoka Koga staging system distinguishes TETs according to their level of invasiveness from I to IV.4 Recently, the International Association for the Study of Lung Cancer (IASLC) and International Thymic Malignancies Interest Group (ITMIG) established an evidenced-based TNM staging system (8th edition) for thymic malignancies.5
Figure 1.
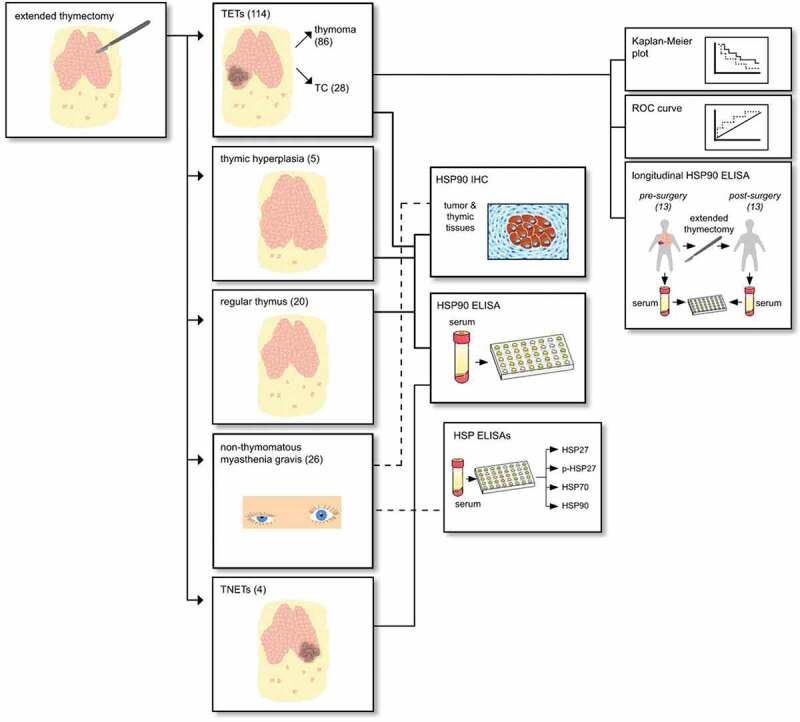
Overview description of the patient cohorts and used methodological tests in this study.
MG is a B-cell-mediated autoimmune disease characterized by muscle weakness and fatigue that are caused by pathogenic autoantibodies directed against peptides of the neuromuscular junction.6 In up to 80 percent of patients autoantibodies directed against the postsynaptic acetylcholine receptor (AChR) are detectable.6
Heat shock proteins (HSPs) are highly conserved proteins that are present in most nucleated cells. HSPs are so-called “stress proteins” that are upregulated by a variety of different stressors, such as heat shock, radiation or hypoxia.7 HSPs are grouped according to their molecular weight into main subtypes, including HSP27, HSP60, HSP70, and HSP90.8 The function of HSPs mainly depends on their localization. While intracellular HSPs function as molecular chaperones and inhibitors of apoptosis, extracellular released HSPs act in a pro-inflammatory manner through toll-like receptors.9 Dysregulated HSPs have already been associated with several autoimmune diseases, such as Rheumatoid Arthritis,10 Systemic Lupus Erythematosus11 or Multiple Sclerosis.12
Currently, HSP90 inhibitors are tested in preclinical and clinical phase I to III trials in patients with autoimmune diseases and cancers: psoriasis,13,14 breast cancer,15 gastric cancer,16 advanced epithelial ovarian and primary peritoneal carcinoma,17 hepatocellular carcinoma18 or metastatic uveal melanoma.19 So far, the exact mechanisms of anti-cancer effects remain mostly unknown. Particularly, the approach to use HSP90 inhibitors combined with other anti-neoplastic drugs has shown promising results.17,20
In 2016, we reported differentiated expression patterns in different histological types and pathological tumor stages of HSP27 and HSP70 in patients with TETs and/or MG; and the correlation of weak HSP27 and HSP70 tumor expression with worse freedom-from recurrence. Similarly, we detected increased HSP27 and HSP70 serum concentrations in TET and MG patients correlating with tumor stage and histological types. In non-thymomatous thymic glands of MG patients, we found HSP27 and HSP70 expression in dendritic cells (DCs) of germinal centers (GCs) and thymic epithelial cells with mantle zone-like distribution. There was no expression of HSP27 or HSP70 in lymphocytes of the tumor microenvironment of TETs, hyperplastic or regular thymus in patients with and without MG.21
The aim of this study was to elucidate the unknown role of HSP90 and close knowledge gaps for the role HSP27, HSP70 in patients with TETs. The primary objective was to determine HSP90α, 70, 27 and pHSP27 serum concentrations in patients with TETs (with or without MG) and non-thymomatous MG. The secondary objective was to evaluate the expression of HSP90 in tumor tissue of patients with TETs. Furthermore, we explored the diagnostic and prognostic potential of HSPs for our patient cohorts.
Material and methods
Ethics statement
Ethical approval was obtained from the Institutional Ethics Committee of the Medical University of Vienna (EC#302/2011). All participating patients and volunteers gave their written informed consent. All experiments were performed in accordance with the approved ethical guidelines.
Study population
The study cohort comprises 114 patients with TETs treated at a single European Thoracic Surgery Center (Division of Thoracic Surgery, Medical University of Vienna) between September 2000 and February 2018 (see Figure 1 for the study design). Expert pathologists (L.M. and A.I.S) performed histological subtyping according to the WHO classification system. In order to ensure consistent statistical analysis, WHO mixed type thymomas were classified with their most malignant part (e.g. B2/B3 thymomas were classified as B3 thymomas). Tumor volume, expressed in [cm3], was determined from measurements of the pathological specimen in the craniocaudal, axial and anterior – to – posterior axis.
The diagnosis of MG was established by a neurologist specialized in the diagnosis and treatment of patients with MG in every case. See supplementary material for details on MG diagnosis. In our TET patient cohort, there was one patient with thyroiditis and with Cushing syndrome.
There were 86 patients with thymomas and 28 patients with TCs. Only patients undergoing surgical tumor resection, enabling the pathologic examination of the complete tumor specimen in order to verify combined tumors, were included in this study. Three patients referred for definitive chemotherapy (ChT) after surgical tumor biopsy were excluded from outcome analysis. All other patients underwent extended thymectomy – the surgical removal of the entire thymic gland in conjunction with the en-bloc resection of mediastinal fatty tissue, potentially harboring microscopic foci of thymic tissue. Indications for surgical extended thymectomy included: histologically verified TET, radiological suspicion of TET (mediastinal mass) or thymectomy in patients with MG. All patients with a second primary malignancy were excluded from this study. The blood draw to obtain serum samples for further analyses had to be at least 4 weeks apart from the last applications of ChT or surgery. The following clinical conditions led to exclusion from the study: pneumonia, COPD exacerbation and acute cardiac insufficiency. Patients with acute infections based on medical reports were excluded from analysis.22 See Table 1 for patient demographics.
Table 1.
HSP90α serum concentrations in TETs, basic demographic data and clinicopathologic characteristics. HSP90α serum concentrations in patients with TETs according to basic demographics, tumor staging systems (Masaoka-Koga and TNM 8th edition) and the WHO histological classification system.
| n % | HSP90α [ng/ml]a | p value | |
|---|---|---|---|
| Age mean (median) SD | 55.4 (55.3)±14.9; | 0.916b | |
| <55.4 | 14.2 ± 1.4 | ||
| >55.4 | 13.4 ± 1.3 | 0.685b | |
| Female:male ratio n (%) | 58:56 (50.9):(49.1) | 0.805c | |
| Male: 13.3 ± 1.3 | |||
| Female: 14.2 ± 1.4 | 0.630b | ||
| BMI mean (median)±SD | 26.8(26.3)±5.1 | ||
| <26.8 | 14.8 ± 1.6 | ||
| >26.8 | 12.7 ± 1.2 | 0.298b | |
| TETs | |||
| Thymoma | 86 (75.4) | 12.2 ± 1.0 | |
| TC | 28(24.6) | 18.5 ± 2.3 | 0.005b |
| WHO classification | |||
| A | 12(10.5) | 11.7 ± 2.2 | |
| AB | 15(13.2) | 9.3 ± 2.1 | |
| B1 | 10(8.8) | 14.6 ± 3.2 | |
| B2 | 27(23.7) | 11.3 ± 1.6 | |
| B3 | 17(14.9) | 16.7 ± 2.7 | |
| TC | 28(24.5) | 18.5 ± 2.3 | |
| Squamous carcinoma | 24(85.6) | ||
| Basaloid carcinoma | 1(3.6) | ||
| Adenocarcinoma | 1(3.6) | ||
| Lymphoepithelioma-like carcinoma | 1(3.6) | ||
| Undifferentiated carcinoma | 1(3.6) | ||
| MNT | 5(4.4) | 7.4 ± 2.2 | 0.026b |
| Masaoka-Koga staging | |||
| I–II | 74(64.9) | 12.0 ± 1.1 | |
| III–IV | 40(35.1) | 17.0 ± 1.7 | 0.013b |
| I | 32(28.1) | 12.6 ± 2.0 | |
| II | 42(36.8) | 11.6 ± 1.3 | |
| III | 17(14.9) | 17.5 ± 2.5 | |
| IV | 23(20.2) | 16.6 ± 2.3 | 0.096b |
| TNM8th staging system | |||
| I–II | 77(67.5) | 12.3 ± 1.1 | |
| III–IV | 37(32.5) | 16.7 ± 1.8 | 0.033b |
| I | 74(64.9) | 12.2 ± 1.2 | |
| II | 3(2.6) | 15.3 ± 3.9 | |
| III | 14(12.3) | 16.9 ± 2.9 | |
| IV | 23(20.2) | 16.6 ± 2.3 | 0.190d |
*WHO combined TETs were classified according to the more malignant tumor component, e.g. B2/B3 thymomas were classified as B3 thymomas
amean ± SEM
bunpaired t test
cChi squared test (TETs (n = 114) vs. healthy volunteers (n = 58))
dOne-Way ANOVA
HSP90α, heat shock protein 90α; n, number of samples; SD, standard deviation; SEM, standard error of the mean; TC, Thymic Carcinoma; TET; Thymic Epithelial Tumor; WHO; World Health Organization.
We analyzed serum samples of 114 patients with TETs (32 [28.2%] with paraneoplastic MG). Furthermore, we included 20 patients undergoing extended thymectomy whose final pathologic report revealed regular thymic architecture (12 of those had MG and eight were without MG; none of them had a TET). Fifty-eight gender- (51.7% female; p = .805) and age-matched (54.7 ± 2.6 years; p = .916) healthy volunteers donated control serum. None of the healthy volunteers suffered from acute or chronic diseases. Further, none of them took any medication three months before the blood draw.
Table 1 lists the demographic data of 114 TET patients. Forty-five percent of patients with thymomas and 92.9% of patients with TCs underwent multimodal treatment (regimens combining surgery with radiotherapy [RT] and/or ChT, see supplementary material for details).
Longitudinal analysis
In a subgroup of 13 patients with TETs (5 TCs and 8 thymomas) pre- und and postoperative serum samples were available (at a median of 80.73 months after surgery). Inclusion criteria for repeated measures were complete surgical tumor resection of TETs (R0), no adjuvant therapy during the last month, and no sign of recurrence or a second primary malignancy.
Patient cohort with non-thymomatous MG
In addition, we analyzed 26 patients (80.8% female) with non-thymomatous MG that were age- (30.15 ± 8.39 years; p < .001) and gender-matched (80.8% female; p = .226) to healthy volunteers for the serum secretion of pHSP27, HSP27, HSP70 and HSP90α. Longitudinal analysis of matched preoperative and postoperative blood samples was performed. We included sub-analyses of response to treatment (extended thymectomy), steroid treatment, seronegative and seropositive patients. Further characterization of MG patients can be found in supplementary material (Patient cohort with non-thymomatous MG).
Patient cohort with benign thymic pathologies
Furthermore, we analyzed 20 patients (45.0% female) with a mean age of 37.0 ± 15.0 years with benign thymic pathologies (regT). This patient cohort consists of patients with thymitis (n = 5), true thymic hyperplasia (TTH) (n = 2), regular thymic morphology (n = 10), and highly atrophic thymic morphology (n = 3). Patients with regT underwent extended thymectomy because of radiological suspicion of a TET. Patients underwent surgery via video-assisted (3 patients) or robotic-assisted thoracoscopic surgery (17 patients).
Laboratory procedures
Blood samples were collected on the day before surgery. Blood was drawn from the antecubital vein into serum separator gel tubes. Serum was separated by centrifugation (15 minutes at 2845 × g). All samples were transferred to −80°C within 2 hours. The study was conducted at the research laboratories of the division of thoracic surgery (ARGE Moser, FOLAB Chirurgie) at the Medical University Vienna.
Tumor HSP90 expression assessed by immunohistochemistry
Tumor tissues of completely resected TETs from 101 patients (78 thymomas and 23 TCs) were analyzed for HSP90 protein expression by immunohistochemistry. The following thymic tissue specimens from patients without neoplasms (no TETs) were investigated for HSP90 expression: regular thymic tissue of six MG patients and four patients without MG, TTH from five patients without MG and follicular thymic hyperplasia (FTH) from five patients with MG. See supplementary material for details on immunohistochemical techniques and analysis.
Enzyme-linked immunosorbent assay (ELISA)
HSP serum concentrations were measured by commercially available ELISA kits: Human HSP27 DuoSet® (R&D Systems #DY1580, Minneapolis, MN, USA), Phospho-HSP27 (S78/S82) DuoSet®IC ELISA (R&D Systems #DYC2314-5), Total-HSP70/HSPA1A DuoSet®IC (R&D Systems #DYC1663) and HSP90α (Enzo Life Science #ADI-EKS-895, Lausen, Switzerland). All tests were performed according to the manufacturer`s instructions. Researchers performing the immunoassays and data analyses were blinded to the groups associated with each sample.
Graphical overview
The graphical overview was designed using Adobe InDesign software (version 14.0.3; Adobe Inc., San José, CA, USA).
Statistical analyses
Data were computed with SPSS software (version 25.0; IBM SPSS Inc., Chicago, IL, USA). Gaussian distribution of data was tested by graphical means (histograms). Normally distributed data were reported as mean ± standard error of the mean. To compare means of two or more than two independent groups with Gaussian distributions, unpaired student’s t tests and one-way ANOVA were employed, respectively. Non-normal distributions were evaluated using Kruskal-Wallis rank and Mann-Whitney-U test. Post hoc comparisons were computed with the Tukey’s-B and Bonferroni correction. Paired student`s t test was employed to analyze two dependent datasets. Chi-square test was employed to analyze dichotomous variables (e.g. gender).
The probability of making a type I error was set at an α value of 0.05. Null hypotheses were rejected if the p-value was less than α. Testing for two-tailed p-values was employed. Pearson correlation r was used to analyze linear relationships between two numerical measurements, whereas Spearman rho was used for ranked variables. The product limit method of Kaplan and Meier was employed to estimate survival. The log-rank was used to compare survival distributions of two samples. The following outcome measures as defined by ITMIG (67) and the European Society of Thoracic Surgeons (ESTS) Thymic Working group were tested: overall survival (OS), cause-specific survival (CSS) and freedom-from recurrence (FFR; see Supplementary Material for definitions).23,24 Cox regression was performed to assess the prognostic impact of HSP90 on outcome and to calculate Hazard Ratios (HR) with corresponding 95%-confidence intervals (CI). Univariable and multivariable analyses were calculated for the following parameters: gender (male vs. female), age (continuous), MG (positive vs. negative), histology (TC vs. thymoma), resection status (R1 + 2 vs. R0), tumor stage (III–IV vs. I+ II), tumor volume [cm3] (continuous) and HSP90α (high vs. low). In order to determine optimal cutoff values for HSP90α, we plotted receiver operating characteristic curves and calculated the Youden Index. GraphPad Prism6 (GraphPad Software Inc., California, USA) was used for graphical display of all box plots, Kaplan-Meier curves and correlations. Boxplots were designed as follows: Box: 1st and 3rd quartile, Bar: median, Whiskers: minimum to maximum, +: mean. All outliers are shown.
Results
HSP90α serum concentrations in patients with TETs
Higher systemic HSP90α levels in patients with TETs compared to benign thymic pathologies or healthy volunteers
Patients with TETs had significantly higher HSP90α serum concentrations compared to healthy volunteers (HSP90α[ng/ml] TETs 13.8 ± 1.0 vs. healthy volunteers 6.2 ± 0.4; p < .001; Figure 2a) and regTs (HSP90α[ng/ml] TETs vs. regT 6.2 ± 0.9; p < .001; Figure 2a), respectively. Moreover, HSP90α serum concentrations were significantly increased in patients with TCs (HSP90α[ng/ml] TC 18.5 ± 2.3 vs. regT; p < .001; Figure 2b) and thymomas (HSP90α[ng/ml] thymoma 12.2 ± 1.0 vs. regT; p < .001; Figure 2b) compared to regTs. Furthermore, HSP90α serum levels were significantly increased in patients with TCs (HSP90α[ng/ml] TC vs. healthy volunteers; p < .001; Figure 2b) and thymomas (HSP90α[ng/ml] thymoma vs. healthy volunteers; p < .001; Figure 2b) compared to healthy volunteers. There was no significant difference in HSP90α serum concentrations between regTs and healthy volunteers (p = .935, Figure 2b). HSP90α serum concentrations in patients with TNETs (n = 4) were significantly higher compared to those of healthy volunteers (HSP90α[ng/ml]: TNETs 11.8 ± 1.6 vs. healthy volunteers; p = .001; Figure 2c), respectively.
Figure 2.
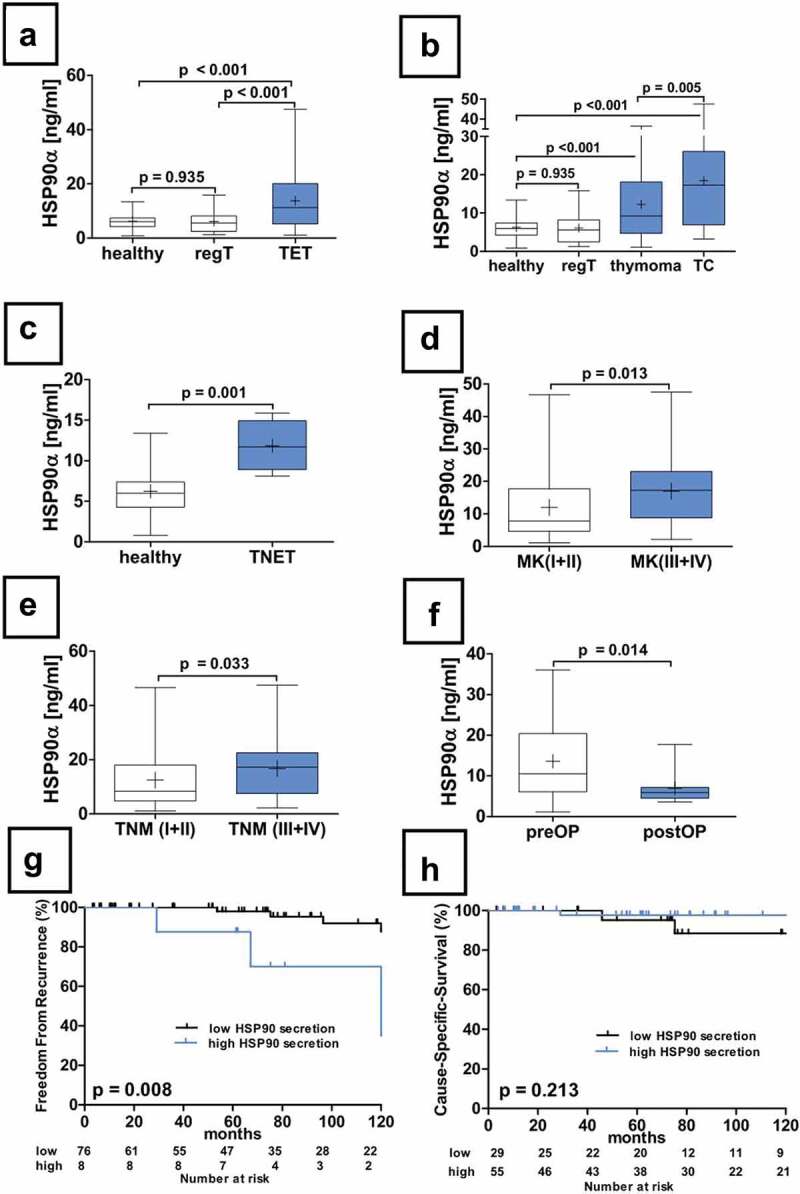
Serum concentrations of HSP90α in patients with TETs. The differences in serum concentrations of healthy volunteers, patients with regular thymus as well as TETs are depicted (a). HSP90α serum concentrations in healthy volunteers or patients with regular thymus in comparison to patients with thymomas and TCs are shown (b). An analysis HSP90α serum concentrations of the particularly rare cases of TNETs (n = 4) is shown (c). Further, the analysis of HSP90α serum levels in patients according to Masaoka-Koga stages (d), and the 8th TNM edition (e) are displayed. The longitudinal analysis of HSP90α serum concentrations before and after extended thymectomy for TETs is depicted (f). The FFR survival analysis in thymomas separated according to high or low HSP90 serum level status is depicted (g). The CSS analysis in thymomas separated according to high or low HSP90α serum level status is shown (h).
Increased HSP90α serum concentrations in advanced Masaoka-Koga and TNM stages
Patients with TETs in Masaoka-Koga stages III–IV (n = 40) had significantly higher HSP90α serum concentrations compared to stage I–II tumors (n = 74) (HSP90α[ng/ml]: III–IV 17.0 ± 1.7 vs. I–II 12.0 ± 1.1; p = .013; Figure 2d), respectively. See also Table 1.
In addition, we retrospectively staged all TETs (n = 114) according to the TNM 8th edition. Stages I–II displayed significantly lower HSP90α levels compared to stages III–IV (HSP90α[ng/ml]: I–II 12.3 ± 1.1 vs. III–IV 16.7 ± 1.8; p = .033; Figure 2e). One-way ANOVA did not show statistically significant differences of HSP90α serum concentrations between stages I, II, III and IV (p = .190; Table 1).
Higher systemic HSP90α levels in patients with TCs compared to thymomas
Serum concentrations of HSP90α were significantly lower in thymoma compared to TC patients ([ng/ml]: 12.2 ± 1.0 vs. 18.5 ± 2.3, p = .005). In contrast, serum concentrations of other HSPs showed no statistically significant differences between thymoma and TC: pHSP27[pg/ml]: 172.5 ± 55.0 vs. 462.9 ± 184.2 (p = .068), HSP27[pg/ml]: 1215.6 ± 260.0 vs. 1512.3 ± 467.0 (p = .552), and HSP70[pg/ml]: 2391.2 ± 351.1 vs. 3716.6 ± 600.0 (p = .051), respectively. One-way ANOVA computed a statistically significant difference for HSP90α secretion among WHO types micronodular tumor (MNT), A, AB, B1, B2, B3 and TC (p = .026; Table 1). Unpaired comparisons of the thymoma subtypes revealed a difference for AB vs. B3 (p = .043), but no other differences: A vs. AB (p = .442), A vs. B1 (p = .448), A vs. B2 (p = .895), A vs. B3 (p = .194), AB vs. B1 (p = .155), AB vs. B2 (p = .452), B1 vs. B2 (p = .324), B1 vs. B3 (p = .616), B2 vs. B3 (p = .077), MNT vs. A (p = .268), MNT vs. AB (p = .623), MNT vs. B1 (p = .143), MNT vs. B2 (p = .320), MNT vs. B3 (p = .090). TCs displayed higher serum HSP90α compared to B2 (p = .015), and AB thymoma (p = .013), but not to other thymoma subtypes (MNT (p = .058), B3 (p = .642), B1 (p = .373) or A (p = .088)).
Nonetheless, forming groups with different WHO subtypes revealed statistically significant differences: MNT+A+AB+B1 vs. B2+ B3+ TC group (HSP90α[ng/ml]: 11.0 ± 1.3 vs. 15.4 ± 1.3; p = .029) and MNT+A+ AB+B1+ B2 vs. B3+ TC (HSP90α[ng/ml]: 11.1 ± 1.0 vs. 17.8 ± 1.8; p = .001), respectively. The comparison of the groups with thymoma patients only (TCs omitted): A+ AB+B1 vs. B2+ B3 (HSP90α[ng/ml]: 11.5 ± 1.4 vs. 13.4 ± 1.5; p = .358) and similarly MNT+A+ AB+B1 vs. B2+ B3 (HSP90α[ng/ml]: 11.0 ± 1.3 vs.13.4 ± 1.5; p = .225) did not show statistically significant differences, respectively.
Decreased serum concentrations of HSP90α after surgical tumor resection
Longitudinal analysis of pre- and postoperative serum samples revealed significantly lower HSP90α serum concentrations after surgical TET resection (HSP90α[ng/ml]: preoperative 13.6 ± 2.8 vs. postoperative 6.9 ± 1.1; p = .014; Figure 2f).
Equal HSP90α serum concentrations in TET patients with and without MG
There was no significant difference in HSP90α serum concentrations in TET patients with or without paraneoplastic MG (HSP90α[ng/ml]: TET(MG+) 11.7 ± 1.5 vs. TET(MG−) 14.6 ± 1.2; p = .186; Figure 3a), TET(MG+) and non-thymomatous MG (HSP90α[ng/ml]: TET(MG+) 11.7 ± 1.5 vs. MG 10.3 ± 0.8; p = .525; Figure 3a). However, we were able to reveal statistically significant differences between non-thymomatous MG and TET(MG−) patients (HSP90α[ng/ml]: MG 10.3 ± 0.8 vs. TET(MG−) 14.6 ± 1.2; p = .007; Figure 3a).
Figure 3.
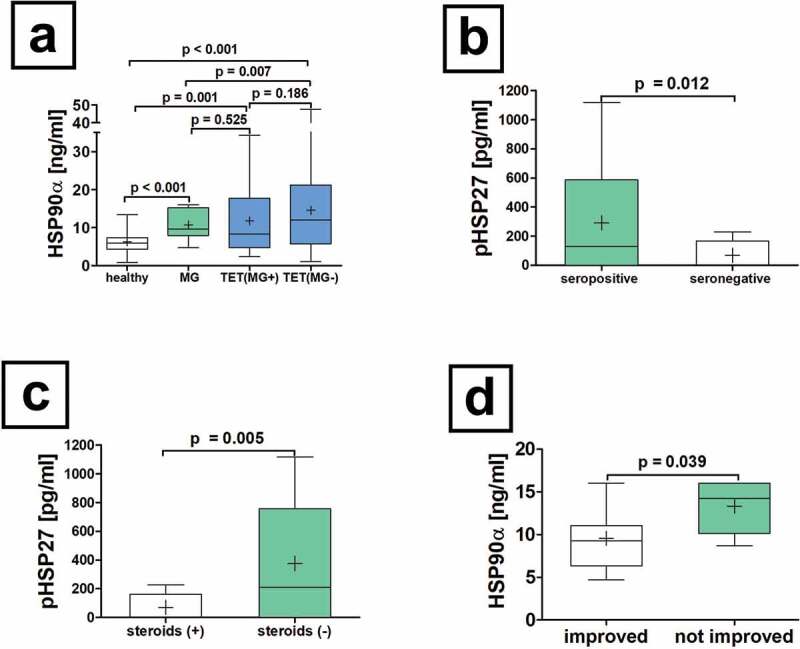
Serum concentrations of HSP90α in patients with non-thymomatous MG. The serum differences of HSP90α in patients with non-thymomatous MG, TET(MG+), TET (MG−) and healthy volunteers are shown (a). Further analysis of non-thymomatous MG patients according to the detection of antibodies to acetylcholine receptor antibodies (seropositive vs. seronegative is depicted (b). Moreover, the analysis of pHSP27 serum concentrations in patients with non-thymomatous MG receiving treatment with steroids is shown (c). The HSP90α serum concentration in patients improved after thymectomy and those who did not respond properly is depicted (d).
Only very weak correlations of HSP90α serum concentrations with clinical and pathological parameters
There were weak statistical correlations of HSP90α serum concentrations with Masaoka-Koga (I+ II vs. III+IV: r = 0.249, p = .008) and TNM8th staging (I+ II vs. III+IV: r = 0.209, p = .026). There were no statistically significant correlations with age, gender, multimodality treatment, paraneoplastic MG, and tumor volume, respectively.
Survival analysis
Worse FFR in thymoma patients with high HSP90α serum concentrations
High HSP90α serum concentrations in patients with thymomas (n = 84); cutoff: 24.9 ng/ml) were associated with significantly worse FFR (p = .008, Figure 2g). The diagnostic accuracy to predict tumor recurrences can be described with a sensitivity of 37.5% (3 out of 8), specificity of 93.4% (71 out of 76), PPV of 37.5% (3 out of 8), and NPV of 93.4% (71 out of 76). One-, five- and ten-year FFR for patients with thymomas separated into groups with high and low HSP90α levels were as following: 87.5% vs. 98.6%, 87.5% vs. 94.8% and 70% vs. 83.5%, respectively. There was no statistically significant difference in OS (cutoff: 5.5 ng/ml; p = .390) and CSS for thymomas (cutoff: 5.5 ng/ml; p = .213, Figure 2h), respectively.
The analysis of HSP90α serum concentrations in patients with TETs (thymomas and TCs together) revealed no statistically significant differences in OS (cutoff: 18.3 ng/ml; p = .077), CSS (cutoff: 6.4 ng/ml; p = .116) and FFR (cutoff: 20.8 ng/ml; p = .058), respectively.
Univariable and multivariable analyses
HSP90α serum concentrations in thymoma patients are an independent prognostic factor at multivariable analysis
At univariable cox-regression analysis for thymoma patients, tumor Masaoka stage (III+IV vs. I+ II) (HR = 8.045, p = .011, CI-95%:1.620–39.949) and HSP90α serum concentrations (high vs. low) (HR = 5.612, p = .019, CI-95%:1.332–23.654) were independent prognostic factors for worse FFR. Other tested parameters, including gender, age, MG, and tumor volume displayed no statistically significant prognostic factors. Analogously, multivariable analysis of thymoma patients revealed Masaoka tumor stage III+IV (HR = 7.173, p = .016, CI-95%:1.436–35.831) and high HSP90α serum concentrations (HR = 4.548, p = .040, CI-95%:1.070–19.325) as prognostic factors for worse FFR.
HSP90 tissue expression
HSP90 expression in tumor tissue of TETs
The expression of HSP90 in nuclei and cytoplasm of tumor cells and non-neoplastic lymphocytes varied with WHO histological subtype. In Table 2 results are detailed for TET patients with and without MG.
Table 2.
Tumor expression of HSP90 in TET patients with or without MG. Immunohistochemical evaluation of tumor HSP90 expression in relation to WHO histological subtype classification is shown separately for TETs with and without MG. HSP90 expression was evident in nuclear and cytoplasmic compartments of tumor cells and non-neoplastic lymphatic cells.
| HSP90 |
||||
|---|---|---|---|---|
| MG (+) | tumor cells | lymphocytes | ||
| WHO | n | nucleus (+) | cytoplasm (+) | |
| MNT | 1 | 0% | 100% | 0% |
| A | 2 | 100% | 50% | 0% |
| AB | 2 | 100% | 0% | 0% |
| B1 | 2 | 100% | 0% | 0% |
| B2 | 15 | 87% | 20% | 47% |
| B3 | 5 | 80% | 100% | 80% |
| TC | 2 | 50% | 100% | 0% |
| MG (-) | ||||
| MNT | 4 | 50% | 100% | 0% |
| A | 8 | 100% | 63% | 0% |
| AB | 12 | 92% | 8% | 8% |
| B1 | 7 | 86% | 14% | 0% |
| B2 | 11 | 82% | 0% | 45% |
| B3 | 9 | 67% | 100% | 56% |
| TC | 21 | 67% | 95% | 0% |
HSP90, heat shock protein 90; MG, Myasthenia Gravis; MNT, Micronodular Thymoma; n, number of samples; TC, Thymic Carcinoma; TET, Thymic Epithelial Tumor; WHO, World Health Organization.
HSP90 expression in FTH in MG patients, TTH and regular thymus
We detected HSP90 expression in centroblasts, but not centrocytes, of GCs in 100% of MG patients with FTH (n = 5). The HSP90 expression in lymphoid follicles of myasthenic patients is depicted in Figure 4h. Hassall`s Corpuscles showed no HSP90 expression. We did not detect thymic HSP90 expression in four patients with regular thymic morphology or five patients with TTH.
Figure 4.

HSP90 expression in TETs. Immunohistochemical expression of HSP90 in WHO type A (a), AB (b), B1 (c), B2 (d), B3 (e), TC- Squamous Cell Carcinoma (f) and MNT (g) is shown (20X and 40X magnification). The representative sections (ac, eg) are from patients without MG, while section (d) is from a patient with paraneoplastic MG. Immunohistochemical staining of HSP90 in patients with non-thymomatous MG: a germinal center is depicted (H).
Elevated HSP serum concentrations in non-thymomatous MG
Increased HSP90α serum concentrations in patients with non-thymomatous MG compared to healthy volunteers
The analysis of baseline serum concentrations of HSP90α in patients with non-thymomatous MG before extended thymectomy revealed elevated HSP90α concentrations compared to healthy volunteers (HSP90α[ng/ml]: 10.3 ± 0.8 vs. 6.2 ± 0.4; p < .001, Figure 3a). Investigating baseline serum concentrations of HSP27, p27 and 70 in patients with non-thymomatous MG before extended thymectomy compared to volunteers showed no statistically significant differences for HSP27, pHSP27, and HSP70.
Higher HSP90α serum concentrations in TET patients compared to non-thymomatous MG patients with FTH
Fourteen gender- (78.6% female; p = .226) and age-matched (27.9 ± 7.9 years; p < .001) non-thymomatous MG patients diagnosed with a histologically verified FTH were compared to patients with TETs. HSP90α serum concentrations showed significantly elevated serum levels in patients with thymic neoplasms when compared to those with FTH (HSP90α[ng/ml]: TETs 13.8 ± 1.0 vs. FTH 9.7 ± 1.3; p = .017).
Non-thymomatous MG: no differences in serum HSPs in FTH compared to regular thymic morphology
Furthermore, we compared non-thymomatous MG patients with FTH (n = 14) to those with regular thymic morphology (n = 12). Serum concentrations of HSP27, pHSP27, HSP70, and HSP90α showed no statistically significant differences.
Increased preoperative pHSP27 in seropositive and non-steroid treated non-thymomatous MG patients
Phosphorylated HSP27 serum concentrations were significantly increased in patients with AChR positive non-thymomatous MG compared to seronegative patients (pHSP27[pg/ml]: seropositive 329.5 ± 98.9 vs. seronegative 90.5 ± 42.2; p = .012, Figure 3b). Interestingly, serum AChR antibody titer correlated significantly with pHSP27 in patients with non-thymomatous MG (r = 0.450, p = .031). In patients with non-thymomatous MG, pHSP27 serum concentrations were significantly increased in those not treated with steroids compared to those without (p = .005, Figure 3c). Neither the onset, early- vs late, nor the anatomical involvement of muscular weakness (ocular or generalized) affected HSP p27, 27, 70 and 90α serum concentrations before thymectomy.
Increased HSP90α serum concentrations in non-thymomatous MG patients without improvement following thymectomy
Patients with non-thymomatous MG who did not benefit from thymectomy with improving symptoms had significantly increased preoperative HSP90α serum concentrations (HSP90α[ng/ml]: not improved 13.3 ± 1.2 vs. improved 9.6 ± 0.9; p = .039, Figure 3d).
Discussion
Biomarkers, like HSPs (p27, 27, 70 and 90), enzymes, oncogenes and RNA, are factors generated by tumor cells, immune cells or other cells that are involved in tumor progression or infiltration processes.25 HSP27, HSP70 and HSP90, are overexpressed in several tumor entities26−29 and their overexpression correlates with tumor growth, resistance to ChT, metastases and poor survival.30,31
HSP90 is overexpressed in breast-,28 colon-29 and prostate cancer.32 High HSP90 tumor expression reflects lower malignant potential in colon cancer. Its tumor expression was associated with earlier tumor stages and the absence of lymph nodes and distant metastases. Also, patients with high HSP90 tumor expression in colon cancer had better OS.29 This is in contrast to our findings in TET patients, as we found no association with OS or CSS, while worse FFR was evident in thymoma patients with increased HSP90α serum concentrations. In prostate and breast cancer, HSP90 tumor expression is elevated in higher tumor stages,28,32 in contrast to our findings that HSP90 tumor expression was indifferent of TET tumor staging systems. We detected increased HSP90 serum concentrations in TETs of higher Masaoka-Koga or TNM stages (I–II vs. II–IV).
Experimental HSP90 inhibition caused a significant reduction in the vitality, apoptosis ability, and cell cycle arrest in cells of TC cell line TC1889 and thymoma cell line T1682. HSP90 inhibition triggers the degradation of multiple proto-oncogenic molecules, like insulin-like growth factor 1 receptor (IGF-1 R) and CDK4, and the inactivation of PI3 K/Akt and RAF/Erk signaling pathways, respectively. The IGF-1 R is commonly overexpressed in TETs and in-vitro the IGF/IGF-1 R–signaling axis contributes to the establishment of the antiapoptotic phenotype of thymic cancer cell lines. The authors concluded that HSP90 inhibition represents a therapeutic strategy in TETs that may merit evaluation in clinical trials.33 In their functional study on HSP90 inhibition, the contribution of HSP90 in thymic epithelial cells in contrast to non-neoplastic lymphocytes of the tumor microenvironment (found particularly in B type thymomas) or regular thymic epithelial cells was not addressed. Our immunohistochemical result of HSP90 expression in TETs closes this gap by pinpointing HSP90 expression to malignant thymic epithelial cells as well as the non-neoplastic lymphocytic compartment of TETs. HSP90 protein expression was not detected by immunohistochemistry in regular thymic epithelium or TTH. The effects on MG disease activity may be of interest in clinical trials of HSP90 inhibition. While we did not detect HSP90 protein expression in regular thymus or TTH, we found immunohistochemical HSP90 expression in GCs of MG patients.
A point-of-care diagnostic assay employing HS-27 (fluorescently labeled HSP90 inhibitor) was developed to detect surface HSP90 in tissue biopsies of breast cancer patients. The HSP90 expression was not detected in non-tumor tissue like adipose and fibroglandular tissues. The testing with HS-27 for patients with suspicion for TETs may be feasible and warrants testing. As reiterated above, the immunohistochemical expression of HSP90 is not only detectable in malignant cells of all WHO tumor subtypes of TETs but also to varying degrees in lymphocytes of the tumor microenvironment and centroblasts of lymphoid follicles of MG patients. Therefore, the testing of whole tissue biopsies for HSP90 by HS-27 may not be feasible in MG patients, as adjacent thymic tissue may deliver a false positive diagnostic test. At least in MG patients, histology will remain the gold standard.34
Concerning the measurement of HSPs with currently available techniques several critical points must be considered. The quantitative differences in HSP90 serum concentrations between our and other studies may pertain to differences in MG treatment and/or ELISA methods (HSP90[ng/ml] healthy volunteers Chen et al.35 vs. our study: 60 vs. 13.4, MG patients: 170 vs. 47.5) (reference values were derived from bar graphs).35 In our study, patients with MG with TETs and non-thymomatous MG received pyridostigmine, immunosuppressants and steroids. A study on selected lung cancer patients with known increased HSP27 serum concentrations evaluated five commercially available assays for HSP27 measurement concerning their capabilities to distinguish between non-small cell lung cancer patients and healthy controls. Not all commercially available assays were equally useful for the detection of increased HSP27 serum levels, as the determined areas under the curve of the receiver operating characteristic curves ranged from 0.523 to 0.834.36,37 We could not identify a clinical methods study comparing different HSP90α ELISA methods. In a Chinese study enrolling 300 cancer patients, plasma HSP90α was reported as a novel pan‐cancer diagnostic biomarker including colon, lung, breast and liver cancer. Secretion of HSP90α was dependent upon its up-stream regulator ADAM10 (affecting HSP90 content in exosomes) and plasma HSP90α detection by ELISA was particularly effective in patients with high ADAM10 expression. Western blotting and proteomics may identify ELISA false negative results for HSP90α, particularly in patients with low ADAM10 expression and those with hyperphosphorylated HSP90α.38 Differences between HSP90 serum and plasma concentrations may be due to the role of HSP90 in the coagulation system. Therefore, HSP90 serum concentrations may be lower because many HSP90 molecules may have been processed by the coagulation system.39−41
Involvement of HSP90 in the pathogenesis of several autoimmune diseases, such as Systemic Lupus Erythematosus (HSP90),11 Sjogren’s syndrome (HSP90α),42 and Alzheimer’s disease (HSP90)43 were reported. One hypothesis on the role of HSPs in autoimmune diseases states that extracellular HSPs act as “danger signals” for the immune system and stimulate a pro-inflammatory immune reaction, which is often related to the severity of autoimmune diseases. Another hypothesis concerns the molecular mimicry effect of host HSPs and bacterial HSPs (5060% homology at amino acid level) that is important for autoantibody formation and therefore related to pathogenesis.44 Evidence that HSPs are relevant for the pathogenesis of MG have accumulated in the peer-reviewed literature starting in 1996, reporting of a humoral immune response with antibodies directed against human HSP60 in 30.5% of patients with MG compared to 8.5% in healthy subjects.45 In addition, elevated levels of serum HSP70-antibodies could be found in patients with generalized and ocular MG.46 Potentially, cross-reactive epitopes were shared between human HSP60 and AChRs and chlamydial HSP60 and AChRs.47 Of possible clinical benefit could be the administration of microbial HSP70 as a non-disease specific immunotherapeutic approach promoting an immunosuppressive phenotype on DCs, myeloid-derived suppressor cells and monocytes.48 Our finding of HSP27 and 70 expressing DCs and thymic epithelial cells forming marginal zone-like distributions in the GCs of the thymus may add to the intrathymic pathogenesis of MG.21 Briefly, nicotinic AChRα epitopes are presented to autoreactive CD4+ thymic immigrants by thymic antigen-presenting (possibly HSP expressing) DCs. This may lead to their activation helping in the stimulation of AChR-reactive B cells forming GCs that produce anti- AChR antibodies – one of the hallmarks of MG development.49
Patients with ocular and generalized MG had significantly increased serum concentrations of anti-HSP70 antibodies compared to healthy volunteers and patients with multiple sclerosis.46 HSP70 is selectively located in the centroblasts and/or B-immunoblasts of the GCs and the paracortex in reactive lymph nodes.50 In our study, HSP90 could be detected in the centroblasts of GCs (FTH) in patients with MG. In a previous study we reported strong HSP27 and HSP70 expression in medullary thymic epithelial cells and cortical thymic epithelial cells in regular and hyperplastic thymus. In thymus of patients with MG, we reported on HSP27 and HSP70 expression in DCs of GCs and thymic epithelial cells with mantle zone-like distribution. In our study, serum HSP90α was elevated in patients with TETs compared to FTH, analogously to HSP70 in a previous study that showed no difference for HSP27.21 There was no expression of HSP27 or HSP70 in lymphocytes of TETs, hyperplastic or regular thymus in patients with and without MG. Both, HSP In contrast,27 and 70, were expressed in cells of the tumor microenvironment of TETs: endothelial cells, fibroblasts, adipocytes and macrophages.21 These data indicate that HSPs may influence the immune response of patients in inflammatory, tumorous, and autoimmune disorders. However, a better understanding of the biological significance of the overexpression of HSPs, particularly in germinal centers of MG thymus, requires further research.
In conclusion, our group described that HSP90 is expressed in TET and MG patients. Increased serum concentrations were detected in patients with TETs and correlated with advanced tumor stages. In thymoma patients high HSP90α is a prognostic factor for worse freedom-from recurrence. Our longitudinal data are promising that HSP90α serum concentrations may be utilized as a cancer biomarker. HSP90α may play also a diagnostic role and may have an impact on therapeutic procedures. Our HSP data corroborate potential for HSPs as TET cancer biomarkers or drug targets for HSP inhibitors. The studied toxicity and adverse effects of HSP90 inhibition may result from disturbing the constitutive expression of HSP90 in the rest of the body.51 Caution is warranted in TET patients with associated MG overexpressing HSPs. TETs comprise a variety of histological subtypes, different staging systems and an association with autoimmune diseases. Therefore, orphan disease research on patients with TETs requires international efforts such as those of the ESTS and the ITMIG with concurrent evaluation of the autoimmune diseases.10,24 Prospective evaluation of HSP90α as a cancer biomarker is warranted.
Limitations of the study
This project carries the characteristic weaknesses of orphan disease research.52 One particular limitation is that only patients, which were candidates for surgical resection, were available for this study. Patients not amenable to surgery because of non-resectability (organ infiltration deemed unresectable or metastasized patients) or patients with resectable disease but comorbities not allowing surgery and/or anesthesia were not accessible for this research effort. So, there is an obvious selection bias toward surgical patients. Patients that underwent ChT and/or RT without were not included. In order to increase the amount of available tumor tissue samples this study was performed in a prospective and retrospective manner. The number of tested TNETs is very small. A recent multi-institutional outcome study on TNETs (not experimental research on tumor tissue or serum) reported on 205 patients.53
Supplementary Material
Acknowledgments
We want to thank the research laboratories ARGE Moser and ARGE Ankersmit (FOLAB Chirurgie – Department of Surgery, Medical University Vienna) for funding of the study.
Abbreviations
- AChR
Acetylcholine receptor
- ChT
Chemotherapy
- CSS
Cause specific survival
- ELISA
Enzyme-linked immunosorbent assay
- ESTS
European Society of Thoracic Surgeons
- FFR
Freedom from recurrence
- FTH
Follicular thymic hyperplasia
- HSP
Heat shock protein
- IHC
Immunohistochemistry
- IASLC
International Association for the Study of Lung Cancer
- ITMIG
International Thymic Malignancies Interest Group
- MG
Myasthenia gravis
- MGFA
Myasthenia Gravis Foundation of America
- MNT
Micronodular thymoma
- NPV
Negative Predictive Value
- OS
Overall survival
- pHSP27
Phosphorylated heat shock protein 27
- PPV
Positive Predictive Value
- regT
Regular thymus (benign thymic pathology)
- RChT
Radio- and chemotherapy
- RT
Radiotherapy
- TC
Thymic carcinoma
- TET
Thymic epithelial tumor
- TNET
Thymic neuroendocrine tumor
- TNM
Tumor Nodes and Metastasis
- TTH
True thymic hyperplasia
- WHO
World Health Organization
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Author contributions statement
Study design and supervision: BM, JT, CB. Contributed to data collection: JT, CB, CV, SJ, AS, LM, WK, MD, HJA, and BM. Experimental test: JT, CB, AS and PB. Analyzed the data: JT, CB, CV, ML, HJA, and BM. Wrote the paper: JT, CB HJA, and BM.
References
- 1.Detterbeck FC. Evaluation and treatment of stage I and II thymoma. J Thorac Oncol. 2010;5(10 Suppl 4):S318–12. doi: 10.1097/JTO.0b013e3181f20dab. [DOI] [PubMed] [Google Scholar]
- 2.Marx A, Pfister F, Schalke B, Saruhan-Direskeneli G, Melms A, Strobel P. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun Rev. 2013;12(9):875–884. doi: 10.1016/j.autrev.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Marx A, Strobel P, Badve SS, Chalabreysse L, Chan JKC, Chen G, de Leval L, Detterbeck F, Girard N, Huang J, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol. 2014;9(5):596–611. doi: 10.1097/JTO.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 4.Koga K, Matsuno Y, Noguchi M, Mukai K, Asamura H, Goya T, Shimosato Y. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int. 1994;44(5):359–367. doi: 10.1111/j.1440-1827.1994.tb02936.x. [DOI] [PubMed] [Google Scholar]
- 5.Bhora FY, Chen DJ, Detterbeck FC, Asamura H, Falkson C, Filosso PL, Giaccone G, Huang J, Kim J, Kondo K, et al. The ITMIG/IASLC thymic epithelial tumors staging project: a proposed lymph node map for thymic epithelial tumors in the forthcoming 8th edition of the TNM classification of malignant tumors. J Thorac Oncol. 2014;9(9 Suppl 2):S88–96. doi: 10.1097/JTO.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 6.Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14(10):1023–1036. doi: 10.1016/S1474-4422(15)00145-3. [DOI] [PubMed] [Google Scholar]
- 7.Schlesinger MJ. Heat shock proteins. J Biol Chem. 1990;265:12111–12114. [PubMed] [Google Scholar]
- 8.Khalil AA, Kabapy NF, Deraz SF, Smith C. Heat shock proteins in oncology: diagnostic biomarkers or therapeutic targets? Biochim Biophys Acta. 2011;1816(2):89–104. doi: 10.1016/j.bbcan.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of toll-like receptors. Curr Top Microbiol Immunol. 2002;270:169–184. doi: 10.1007/978-3-642-59430-4_11. [DOI] [PubMed] [Google Scholar]
- 10.Huang M-N, Yu H, Moudgil KD. The involvement of heat-shock proteins in the pathogenesis of autoimmune arthritis: a critical appraisal. Semin Arthritis Rheum. 2010;40(2):164–175. doi: 10.1016/j.semarthrit.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukla HD, Pitha PM. Role of hsp90 in systemic lupus erythematosus and its clinical relevance. Autoimmune Dis. 2012;2012:728605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turturici G, Tinnirello R, Sconzo G, Asea A, Savettieri G, Ragonese P, Geraci F. Positive or negative involvement of heat shock proteins in multiple sclerosis pathogenesis: an overview. J Neuropathol Exp Neurol. 2014;73(12):1092–1106. doi: 10.1097/NEN.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 13.Bao R, Lai C-J, Qu H, Wang D, Yin L, Zifcak B, Atoyan R, Wang J, Samson M, Forrester J, et al. CUDC-305, a novel synthetic HSP90 inhibitor with unique pharmacologic properties for cancer therapy. Clin Cancer Res. 2009;15(12):4046–4057. doi: 10.1158/1078-0432.CCR-09-0152. [DOI] [PubMed] [Google Scholar]
- 14.Mishra S, Singh S, Misra K. Restraining pathogenicity in candida albicans by taxifolin as an inhibitor of Ras1-pka pathway. Mycopathologia. 2017;182(11–12):953–965. doi: 10.1007/s11046-017-0170-4. [DOI] [PubMed] [Google Scholar]
- 15.Jensen MR, Schoepfer J, Radimerski T, Massey A, Guy CT, Brueggen J, Quadt C, Buckler A, Cozens R, Drysdale MJ, et al. NVP-AUY922: a small molecule HSP90 inhibitor with potent antitumor activity in preclinical breast cancer models. Breast Cancer Res. 2008;10(2):R33. doi: 10.1186/bcr1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H, Saini N, Parris AB, Zhao M, Yang X. Ganetespib induces G2/M cell cycle arrest and apoptosis in gastric cancer cells through targeting of receptor tyrosine kinase signaling. Int J Oncol. 2017;51(3):967–974. doi: 10.3892/ijo.2017.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendrickson AEW, Oberg AL, Glaser G, Camoriano JK, Peethambaram PP, Colon-Otero G, Erlichman C, Ivy SP, Kaufmann SH, Karnitz LM, et al. A phase II study of gemcitabine in combination with tanespimycin in advanced epithelial ovarian and primary peritoneal carcinoma. Gynecol Oncol. 2012;124(2):210–215. doi: 10.1016/j.ygyno.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Augello G, Emma MR, Cusimano A, Azzolina A, Mongiovi S, Puleio R, Cassata G, Gulino A, Belmonte B, Gramignoli R, et al. Targeting HSP90 with the small molecule inhibitor AUY922 (luminespib) as a treatment strategy against hepatocellular carcinoma. Int J Cancer. 2019;144(10):2613–2624. doi: 10.1002/ijc.31963. [DOI] [PubMed] [Google Scholar]
- 19.Shah S, Luke JJ, Jacene HA, Chen T, Giobbie-Hurder A, Ibrahim N, Buchbinder EL, McDermott DF, Flaherty KT, Sullivan RJ, et al. Results from phase II trial of HSP90 inhibitor, STA-9090 (ganetespib), in metastatic uveal melanoma. Melanoma Res. 2018;28(6):605–610. doi: 10.1097/CMR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 20.McConnell JR, McAlpine SR. Heat shock proteins 27, 40, and 70 as combinational and dual therapeutic cancer targets. Bioorg Med Chem Lett. 2013;23(7):1923–1928. doi: 10.1016/j.bmcl.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janik S, Schiefer AI, Bekos C, Hacker P, Haider T, Moser J, Klepetko W, Müllauer L, Ankersmit HJ, Moser B, et al. HSP27 and 70 expression in thymic epithelial tumors and benign thymic alterations: diagnostic, prognostic and physiologic implications. Sci Rep. 2016;6(1):24267. doi: 10.1038/srep24267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janik S, Bekos C, Hacker P, Raunegger T, Ghanim B, Einwallner E, Beer L, Klepetko W, Müllauer L, Ankersmit HJ, et al. Elevated CRP levels predict poor outcome and tumor recurrence in patients with thymic epithelial tumors: A pro- and retrospective analysis. Oncotarget. 2017;8(29):47090–47102. doi: 10.18632/oncotarget.17478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J, Detterbeck FC, Wang Z, Loehrer PJ. SR. Standard Outcome Measures for Thymic Malignancies. J Thorac Oncol. 2010;5:2017–2023. [DOI] [PubMed] [Google Scholar]
- 24.Ruffini E, Detterbeck F, van Raemdonck D, Rocco G, Thomas P, Weder W, Brunelli A, Evangelista A, Venuta F, Khaled A, et al. Tumours of the thymus: a cohort study of prognostic factors from the European society of thoracic surgeons database. Eur J Cardiothorac Surg. 2014;46(3):361–368. doi: 10.1093/ejcts/ezt649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu S, Wang J, He Y, Meng N, Yan G-R. Peptides/proteins encoded by non-coding RNA: a novel resource bank for drug targets and biomarkers. Front Pharmacol. 2018;9:1295. doi: 10.3389/fphar.2018.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yallowitz A, Ghaleb A, Garcia L, Alexandrova EM, Marchenko N. Heat shock factor 1 confers resistance to lapatinib in ERBB2-positive breast cancer cells. Cell Death Dis. 2018;9(6):621. Available from https://www.ncbi.nlm.nih.gov/pubmed/29799521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tu Y, Tian Y, Wu Y, Cui S. Clinical significance of heat shock proteins in gastric cancer following hyperthermia stress: indications for hyperthermic intraperitoneal chemoperfusion therapy. Oncol Lett. 2018;15(6):9385–9391. https://www.ncbi.nlm.nih.gov/pubmed/29946371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gautam J, Bae YK, Kim J-A. Up-regulation of cathepsin S expression by HSP90 and 5-HT7 receptor-dependent serotonin signaling correlates with triple negativity of human breast cancer. Breast Cancer Res Treat. 2017;161(1):29–40. doi: 10.1007/s10549-016-4027-1. [DOI] [PubMed] [Google Scholar]
- 29.Drecoll E, Nitsche U, Bauer K, Berezowska S, Slotta-Huspenina J, Rosenberg R, Langer R. Expression analysis of heat shock protein 90 (HSP90) and Her2 in colon carcinoma. Int J Colorectal Dis. 2014;29(6):663–671. doi: 10.1007/s00384-014-1857-3. [DOI] [PubMed] [Google Scholar]
- 30.Lianos GD, Alexiou GA, Mangano A, Mangano A, Rausei S, Boni L, Dionigi G, Roukos DH. The role of heat shock proteins in cancer. Cancer Lett. 2015;360(2):114–118. doi: 10.1016/j.canlet.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Mumin NH, Drobnitzky N, Patel A, Lourenco LM, Cahill FF, Jiang Y, Kong A, Ryan AJ. Overcoming acquired resistance to HSP90 inhibition by targeting JAK-STAT signalling in triple-negative breast cancer. BMC Cancer. 2019;19(1):102. doi: 10.1186/s12885-019-5295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardillo MR, Ippoliti F. IL-6, IL-10 and HSP-90 expression in tissue microarrays from human prostate cancer assessed by computer-assisted image analysis. Anticancer Res. 2006;26:3409–3416. [PubMed] [Google Scholar]
- 33.Breinig M, Mayer P, Harjung A, Goeppert B, Malz M, Penzel R, Neumann O, Hartmann A, Dienemann H, Giaccone G, et al. Heat shock protein 90-sheltered overexpression of insulin-like growth factor 1 receptor contributes to malignancy of thymic epithelial tumors. Clin Cancer Res. 2011;17(8):2237–2249. doi: 10.1158/1078-0432.CCR-10-1689. [DOI] [PubMed] [Google Scholar]
- 34.Crouch B, Murphy H, Belonwu S, Martinez A, Gallagher J, Hall A, Soo MS, Lee M, Hughes P, Haystead T, et al. Leveraging ectopic Hsp90 expression to assay the presence of tumor cells and aggressive tumor phenotypes in breast specimens. Sci Rep. 2017;7(1):17487. doi: 10.1038/s41598-017-17832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen R, Chen S, Liao J, Chen X, Xu X. The mechanism of acetylcholine receptor in binding MuSK in myasthenia gravis and the role of HSP90 molecular chaperone. Am J Transl Res. 2016;8:1763–1768. [PMC free article] [PubMed] [Google Scholar]
- 36.Ankersmit HJ, Lambers C, Zimmermann M, Hacker S, Moser B. Serendipity and technical considerations for the measurement of serum heat shock protein HSP27 in patients with COPD and lung cancer. Cell Stress Chaperones. 2015;20(5):727–728. doi: 10.1007/s12192-015-0619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann M, Mueller T, Dieplinger B, Bekos C, Beer L, Hofbauer H, Dome B, Ankersmit HJ. Circulating heat shock protein 27 as a biomarker for the differentiation of patients with lung cancer and healthy controls–a clinical comparison of different enzyme linked immunosorbent assays. Clin Lab. 2014;60(6):999–1006. doi: 10.7754/Clin.Lab.2013.130526. [DOI] [PubMed] [Google Scholar]
- 38.Liu W, Li J, Zhang P, Hou Q, Feng S, Liu L, Cui D, Shi H, Fu Y, Luo Y, et al. A novel pan-cancer biomarker plasma heat shock protein 90alpha and its diagnosis determinants in clinic. Cancer Sci. 2019;110(9):2941–2959. doi: 10.1111/cas.14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392(6678):821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 40.Bender AT, Silverstein AM, Demady DR, Kanelakis KC, Noguchi S, Pratt WB, Osawa Y. Neuronal nitric-oxide synthase is regulated by the hsp90-based chaperone system in vivo. J Biol Chem. 1999;274(3):1472–1478. doi: 10.1074/jbc.274.3.1472. [DOI] [PubMed] [Google Scholar]
- 41.Brouet A, Sonveaux P, Dessy C, Balligand JL, Feron O. Hsp90 ensures the transition from the early Ca2+-dependent to the late phosphorylation-dependent activation of the endothelial nitric-oxide synthase in vascular endothelial growth factor-exposed endothelial cells. J Biol Chem. 2001;276(35):32663–32669. doi: 10.1074/jbc.M101371200. [DOI] [PubMed] [Google Scholar]
- 42.Bardsen K, Nilsen MM, Kvaloy JT, Norheim KB, Jonsson G, Omdal R. Heat shock proteins and chronic fatigue in primary Sjogren’s syndrome. Innate Immun. 2016;22(3):162–167. doi: 10.1177/1753425916633236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuehlke AD, Moses MA, Neckers L. Heat shock protein 90: its inhibition and function. Philos Trans R Soc Lond B Biol Sci. 2018;373:1738. doi: 10.1098/rstb.2016.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghasemi Y, Dabbagh F, Rasoul-Amini S, Borhani Haghighi A, Morowvat MH. The possible role of HSPs on Behcet’s disease: a bioinformatic approach. Comput Biol Med. 2012;42(11):1079–1085. doi: 10.1016/j.compbiomed.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Astarloa R, Martinez Castrillo JC. Humoral response to the human heat shock 60 kDa protein in myasthenia gravis. J Neurol Sci. 1996;135(2):182–183. doi: 10.1016/0022-510X(95)00191-4. [DOI] [PubMed] [Google Scholar]
- 46.Helgeland G, Petzold A, Hoff JM, Gilhus NE, Plant GT, Romi FR. Anti-heat shock protein 70 antibody levels are increased in myasthenia gravis and Guillain-barre syndrome. J Neuroimmunol. 2010;225(1–2):180–183. doi: 10.1016/j.jneuroim.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 47.Cappello F, Marino Gammazza A, Zummo L, Conway de Macario E, Macario AJL. Hsp60 and AChR cross-reactivity in myasthenia gravis: an update. J Neurol Sci. 2010;292:117–118. [DOI] [PubMed] [Google Scholar]
- 48.Borges TJ, Wieten L, van Herwijnen, Broere F, Martijn JC, Broere F, van der Zee R, Bonorino C. The anti-inflammatory mechanisms of Hsp70. Front Immunol. 2012;3:95. doi: 10.3389/fimmu.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levinson AI. Modeling the intrathymic pathogenesis of myasthenia gravis. J Neurol Sci. 2013;333(1–2):60–67. doi: 10.1016/j.jns.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 50.Leopardi O, Naughten W, Giannulis I, Mirra M, Frigo B. HSP70 is selectively over-expressed in the blast cells of the germinal centres and paracortex in reactive lymph nodes. Histopathology. 2001;39(6):566–571. doi: 10.1046/j.1365-2559.2001.01293.x. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Chen M, Zhou J, Zhang X. HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (Review). Int J Oncol. 2014;45(1):18–30. doi: 10.3892/ijo.2014.2399. [DOI] [PubMed] [Google Scholar]
- 52.Sun P, Garrison LP. Retrospective outcomes studies for orphan diseases: challenges and opportunities. Curr Med Res Opin. 2012;28(4):665–667. doi: 10.1185/03007995.2012.673480. [DOI] [PubMed] [Google Scholar]
- 53.Filosso PL, Yao X, Ahmad U, Zhan Y, Huang J, Ruffini E, Travis W, Lucchi M, Rimner A, Antonicelli A, et al. Outcome of primary neuroendocrine tumors of the thymus: a joint analysis of the international thymic malignancy interest group and the european society of thoracic surgeons databases. J Thorac Cardiovasc Surg. 2015;149(1):103–9.e2. doi: 10.1016/j.jtcvs.2014.08.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


