Abstract
BACKGROUND
Trastuzumab deruxtecan (DS-8201) is an antibody-drug conjugate composed of an anti-HER2 (human epidermal growth factor receptor 2) antibody, a cleavable tetrapeptide-based linker, and a cytotoxic topoisomerase I inhibitor. In a phase 1 dose-finding study, a majority of the patients with advanced HER2-positive breast cancer had a response to trastuzumab deruxtecan (median response duration, 20.7 months). The efficacy of trastuzumab deruxtecan in patients with HER2-positive metastatic breast cancer previously treated with trastuzumab emtansine requires confirmation.
METHODS
In this two-part, open-label, single-group, multicenter, phase 2 study, we evaluated trastuzumab deruxtecan in adults with pathologically documented HER2-positive metastatic breast cancer who had received previous treatment with trastuzumab emtansine. In the first part of the study, we evaluated three different doses of trastuzumab deruxtecan to establish a recommended dose; in the second part, we evaluated the efficacy and safety of the recommended dose. The primary end point was the objective response, according to independent central review. Key secondary end points were the disease-control rate, clinical-benefit rate, duration of response and progression-free survival, and safety.
RESULTS
Overall, 184 patients who had undergone a median of six previous treatments received the recommended dose of trastuzumab deruxtecan (5.4 mg per kilogram of body weight). In the intention-to-treat analysis, a response to therapy was reported in 112 patients (60.9%; 95% confidence interval [CI], 53.4 to 68.0). The median duration of follow-up was 11.1 months (range, 0.7 to 19.9). The median response duration was 14.8 months (95% CI, 13.8 to 16.9), and the median duration of progression-free survival was 16.4 months (95% CI, 12.7 to not reached). During the study, the most common adverse events of grade 3 or higher were a decreased neutrophil count (in 20.7% of the patients), anemia (in 8.7%), and nausea (in 7.6%). On independent adjudication, the trial drug was associated with interstitial lung disease in 13.6% of the patients (grade 1 or 2, 10.9%; grade 3 or 4, 0.5%; and grade 5, 2.2%).
CONCLUSIONS
Trastuzumab deruxtecan showed durable antitumor activity in a pretreated patient population with HER2-positive metastatic breast cancer. In addition to nausea and myelosuppression, interstitial lung disease was observed in a subgroup of patients and requires attention to pulmonary symptoms and careful monitoring. (Funded by Daiichi Sankyo and AstraZeneca; DESTINY-Breast01 ClinicalTrials.gov number, NCT03248492.)
Approximately 15 to 20% of metastatic breast cancers are characterized by overexpression or amplification of human epidermal growth factor receptor 2 (HER2).1–3 The recommended first-line therapy for HER2-positive metastatic breast cancer consists of the anti-HER2 monoclonal antibodies trastuzumab and pertuzumab given with a taxane. In the CLEOPATRA (Clinical Evaluation of Pertuzumab and Trastuzumab) trial, the combination of trastuzumab, pertuzumab, and docetaxel resulted in a median duration of progression-free survival and overall survival of 18.7 months and 56.5 months, respectively.4,5 Standard second-line therapy is the antibody-drug conjugate trastuzumab emtansine, which was associated with an objective response of 43.6% (95% confidence interval [CI], 38.6 to 48.6) and a median duration of progression-free survival of 9.6 months when the drug was administered after trastuzumab and a taxane.6 No uniformly accepted standard of care has been defined after the administration of trastuzumab emtansine, and the currently available options have limited benefit, with response rates of approximately 9 to 31% and a duration of progression-free survival of approximately 3 to 6 months for third-line therapy.7–10
Trastuzumab deruxtecan (DS-8201) is an antibody-drug conjugate that is composed of a humanized monoclonal antibody specifically targeting HER2, with the same amino acid sequence as trastuzumab, a cleavable tetrapeptide-based linker, and a potent topoisomerase I inhibitor as the cytotoxic drug (payload). Trastuzumab deruxtecan, which was designed to improve on the critical attributes of currently available antibody-drug conjugates, has a higher drug-to-antibody ratio than trastuzumab emtansine (approximately 8 vs. 3 to 4) while retaining a favorable pharmacokinetic profile.11 The proprietary tetrapeptide-based linker is stable in plasma and is selectively cleaved by cathepsins that are up-regulated in tumor cells.12–15 Unlike trastuzumab emtansine, trastuzumab deruxtecan has a released payload that easily crosses the cell membrane, which potentially allows for a potent cytotoxic effect on neighboring tumor cells regardless of target expression.16 In addition, the released payload has a short half-life, which is designed to minimize systemic exposure.15
In a phase 1 study of trastuzumab deruxtecan (called DS8201-A-J101; ClinicalTrials.gov number, NCT02564900), 111 patients with advanced HER2-positive breast cancer received trastuzumab deruxtecan at a dose of 5.4 mg or 6.4 mg per kilogram of body weight. The confirmed response rate was 59.5% (95% CI, 49.7 to 68.7), and the median response duration was 20.7 months on the basis of investigators’ assessment.17 Of the 43 patients with breast cancer who had a low expression of HER2 (a score of 1+ or 2+ [negative or borderline] on immunohistochemical analysis and no demonstrated amplification on in situ hybridization), 19 (44%) had a response to treatment.18 These results show promise in patients who have metastatic breast cancer with varying levels of HER2 expression, which supports the conduct of additional studies to evaluate the therapeutic potential of trastuzumab deruxtecan.17,19 We performed a phase 2 registration study of trastuzumab deruxtecan (DESTINY-Breast01) in patients with HER2-positive metastatic breast cancer who had previously been treated with trastuzumab emtansine.
METHODS
STUDY DESIGN
We conducted a two-part, open-label, single-group, multicenter study of trastuzumab deruxtecan in adults with pathologically documented HER2-positive, unresectable or metastatic breast cancer who had received previous treatment with trastuzumab emtansine. Positivity for HER2 was defined as a score of 3+ on immunohistochemical analysis or as positive results on in situ hybridization, as centrally confirmed on archival tissue. Eligible patients were adults (=18 years of age in all country sites except for =20 years in Japan and South Korea) and had a performance-status score of 0 or 1 on the Eastern Cooperative Oncology Group scale (ranging from 0 [no disability] to 5 [death]). Patients were excluded if they had untreated or symptomatic brain metastases or if they had a history of noninfectious interstitial lung disease or pneumonitis resulting in the use of glucocorticoids or current or suspected interstitial lung disease or pneumonitis. (Details regarding the eligibility criteria are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.)
Part 1 of the study was designed to consist of two sequential stages: pharmacokinetics and dose finding (Fig. S1 in the Supplementary Appendix). In the pharmacokinetics stage, patients were randomly assigned in a 1:1:1 ratio to receive trastuzumab deruxtecan at a dose of 5.4 mg, 6.4 mg, or 7.4 mg per kilogram administered by intravenous infusion every 3 weeks. On the basis of the pharmacokinetics analysis, two doses were identified for evaluation in the dose-finding stage, in which newly enrolled patients were randomly assigned in a 1:1 ratio. We identified the recommended dose using a predicted benefit-risk profile modeled from exposure–response, exposure–safety, and pharmacokinetic analyses as well as clinical data from this study and from the phase 1 DS8201-A-J101 study.11
In part 2 of the study, we evaluated the efficacy and safety of the recommended dose of trastuzumab deruxtecan in patients treated at the recommended dose in part 1. Part 2 consisted of two cohorts: one involved patients who had tumor progression during or after the previous administration of trastuzumab emtansine and one involved patients who had discontinued trastuzumab emtansine for reasons other than progressive disease (e.g., toxicity). Treatment continued until disease progression, the occurrence of unacceptable toxic effects, or withdrawal of consent.
STUDY OVERSIGHT
The study was funded by Daiichi Sankyo and AstraZeneca. The study was sponsored and designed by Daiichi Sankyo and was approved by the institutional review board at each participating site. After the collaboration was formed in March 2019, representatives from AstraZeneca were involved in study oversight and data interpretation. All the patients provided written informed consent. Data were analyzed and interpreted by the sponsor and the authors. All the authors reviewed the manuscript and vouch for the accuracy and completeness of the data and for the adherence of the study to the protocol, available at NEJM.org. Editorial assistance was financially supported by Daiichi Sankyo.
END POINTS
The primary end point was the overall response (complete response plus partial response) to trastuzumab deruxtecan therapy in patients who had tumor progression during or after the administration of trastuzumab emtansine and who had received the recommended dose of trastuzumab deruxtecan in both parts 1 and 2 of the study. The response was confirmed on the basis of an independent central review of imaging with the use of the modified Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.20 Secondary end points were the response duration, progression-free survival, overall survival, response rate according to investigator assessment, best percentage change in the sum of the diameters of measurable tumors, disease-control rate (response rate plus stable-disease rate), clinical-benefit rate (disease-control rate with stable disease lasting ≥6 months), safety, and pharmacokinetics. Exploratory end points included the time until response and evaluation of exposure–response relationships for efficacy and safety.
SAFETY
Adverse events were categorized with the use of the Medical Dictionary for Regulatory Activities, version 20.1, and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.03. Potential episodes of interstitial lung disease were evaluated by an external independent adjudication committee, and grading was consistent with the NCI CTCAE.
STATISTICAL ANALYSIS
We calculated that a sample of approximately 230 patients would result in approximately 150 patients being treated at the recommended part 2 dose of trastuzumab deruxtecan in both parts of the study, which would provide a 95% confidence interval within 10% of the overall response rate. Enrollment was designed to continue until at least 100 patients who had received previous treatment with pertuzumab were enrolled at the recommended dose. With 150 patients, the probability that the lower boundary of the 95% confidence interval would be more than 20% was 0.982, and the probability that the estimated response rate would be 30% or more was 0.916, according to the anticipated response rate of 35%.
We used the Clopper–Pearson method to calculate the two-sided 95% confidence intervals for the response rate. We used the Kaplan–Meier method to estimate the distribution of time-to-event end points of response duration, progression-free survival, and overall survival; corresponding two-sided 95% confidence intervals were calculated with the Brookmeyer and Crowley methods.21 The primary analysis was per formed after all the patients who had received the recommended dose of trastuzumab deruxtecan had at least 6 months of follow-up or had discontinued their participation in the study.
RESULTS
PATIENTS
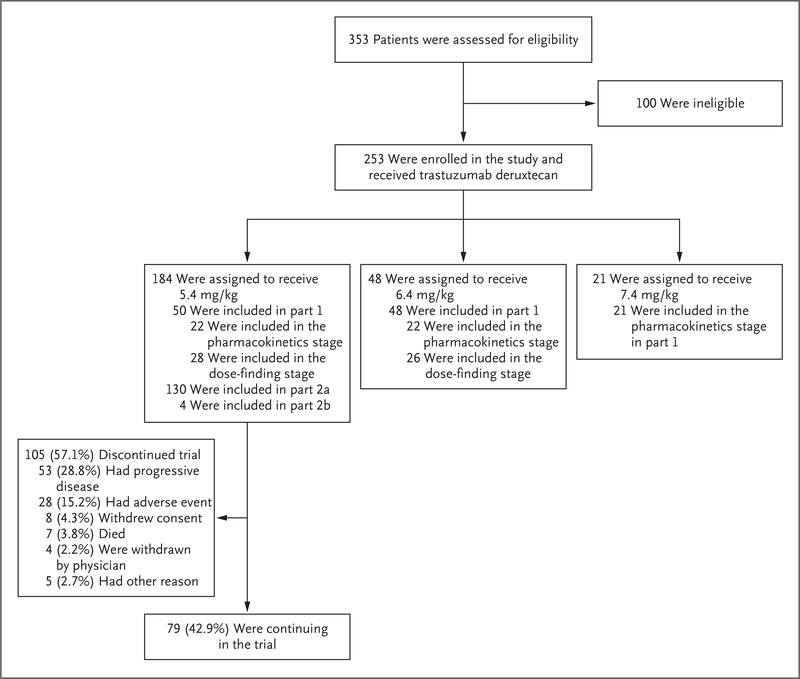
From October 2017 through September 2018, a total of 253 patients were enrolled and received at least one dose of trastuzumab deruxtecan at 72 sites in eight countries in North America, Asia, and Europe; 184 patients received the recommended dose of 5.4 mg per kilogram (Fig. 1). The cutoff date for the data analysis was August 1, 2019.
Figure 1. Enrollment and Study Design.
Of the 100 patients who were excluded from participating in the study, 94 either did not meet the criteria for inclusion or met the criteria for exclusion, 2 withdrew consent, 1 was receiving long-term glucocorticoid therapy for fibromyalgia, 1 had new intracranial metastatic lesions, 1 was lost to follow-up, and 1 missed the deadline for reconsent. Part 1 of the study consisted of two sequential stages: pharmacokinetics and dose finding. On the basis of these results, a dose of trastuzumab deruxtecan (5.4 mg per kilogram of body weight) was recommended for part 2 of the study. Part 2 consisted of an evaluation of the efficacy and safety of trastuzumab deruxtecan in patients treated at the recommended dose who had tumor progression during or after the administration of trastuzumab emtansine (part 2a) and in those who had discontinued trastuzumab emtansine for reasons other than progressive disease (e.g., toxic effects) (part 2b).
The demographic and clinical characteristics of the patients who received the recommended dose of trastuzumab deruxtecan and of all the patients are summarized in Table 1 and Table S1, respectively. Among the patients who received the recommended dose, the median age was 55 years (range, 28 to 96); 23.9% of the patients were 65 years of age or older. Of the 184 patients, 97 (52.7%) had hormone receptor–positive tumors. The median number of previous lines of therapy for metastatic disease was 6 (range, 2 to 27) and included trastuzumab emtansine (100%), trastuzumab (100%), pertuzumab (65.8%), and other anti-HER2 therapies (54.3%).
Table 1.
Demographic and Clinical Characteristics ofthe Patients at Baseline.*
| Characteristic | Patients (N = 184) |
|---|---|
| Age | |
| Median (range) — yr | 55.0 (28.0–96.0) |
| ≥65 yr — no. (%) | 44 (23.9) |
| Female sex — no. (%) | 184 (100) |
| Race — no. (%)† | |
| Asian | 70 (38.0) |
| White | 101 (54.9) |
| Other | 9 (4.9) |
| Missing data | 4 (2.2) |
| Region — no. (%) | |
| Europe | 68 (37.0) |
| Asia | 63 (34.2) |
| North America | 53 (28.8) |
| ECOG performance-status score — no. (%)‡ | |
| 0 | 102 (55.4) |
| 1 | 81 (44.0) |
| 2 | 1 (0.5) |
| Hormone-receptor status — no. (%) | |
| Positive | 97 (52.7) |
| Negative | 83 (45.1) |
| Unknown | 4 (2.2) |
| HER2 expression — no. (%)§ | |
| IHC 3+ | 154 (83.7) |
| IHC 1+ or 2+, ISH-positive | 28 (15.2) |
| Missing data | 2 (11) |
| Median sum of diameters of target lesions (range) — cm | 5.5 (1.2–24.5) |
| Median no. of previous cancer regimens (range) | 6 (2–27) |
| Previous systemic cancer therapy — no. (%) | |
| Trastuzumab | 184 (100) |
| Trastuzumab emtansin¶ | 184 (100) |
| Pertuzumab | 121 (65.8) |
| Other anti-HER2 therapy | 100 (54.3) |
| Hormone therapy | 90 (48.9) |
| Other systemic therapy | 183 (99.5) |
| Best response to trastuzumab emtansine therapy — no. (%) | |
| Complete or partial response or stable disease | 79 (42.9) |
| Progressive disease | 66 (35.9) |
| Could not be evaluated | 39 (21.2) |
Percentages may not total 100 because of rounding.
Race was reported by the patients.
Performance-status scores on the Eastern Cooperative Oncology Group (ECOG) scale range from 0 (no disability) to 5 (death).
HER2 (human epidermal growth factor receptor 2) expression was centrally confirmed by analysis of the most recent archival tissue, according to the guidelines of the American Society of Clinical Oncology-College of American Pathologists.22 According to these guidelines, HER2 positivity was defined as a HER2 immunohistochemical (IHC) analysis score of 1+ (IHC negative) or 2+ (IHC borderline) and positive results on in situ hybridization (ISH) or a score of 3+ (IHC positive). Data for patients with an IHC score indicated as 1+ or 2+ include data for patients for whom the result was equivocal or could not be evaluated. Data regarding HER2 status were missing for a patient who had an IHC 2+ result with equivocal results on ISH and for another patient who had conflicting IHC results during evaluations in 2017 and 2018.
A total of 56 patients (30.4%) received trastuzumab deruxtecan immediately after initial therapy with trastuzumab emtansine.
At the time of the data cutoff, 79 of 184 patients (42.9%) who had received the recommended dose were continuing to receive trastuzumab deruxtecan. The primary reasons for discontinuation included progressive disease according to RECIST, version 1.1 (28.8%), and adverse events (15.2%). The median treatment duration was 10.0 months (range, 0.7 to 20.5 months), and the median duration of follow-up was 11.1 months (range, 0.7 to 19.9); 128 patients (69.6%) continued to receive trastuzumab deruxtecan for more than 6 months.
DOSE JUSTIFICATION
In the pharmacokinetics stage in part 1 of the study, the dose of trastuzumab deruxtecan that was administered was 5.4 mg per kilogram in 22 patients, 6.4 mg per kilogram in 22 patients, and 7.4 mg per kilogram in 21 patients. The resulting pharmacokinetic profiles were analyzed in conjunction with those from the phase 1 study, DS8201-A-J10111; 5.4 mg and 6.4 mg per kilo gram were chosen for the dose-finding stage.
In the part 1 dose-finding stage, an additional 28 and 26 patients (who were not enrolled during the pharmacokinetic stage) received 5.4 mg and 6.4 mg per kilogram, respectively. Exposure-efficacy modeling showed a significant relationship between exposure and response rate and a trend for longer progression-free survival at higher doses. Similarly, exposure–safety modeling showed a significant relationship between exposure and key adverse events, including interstitial lung disease. On the basis of the balance of safety and efficacy, a dose of 5.4 mg per kilogram was recommended.17,23
EFFICACY
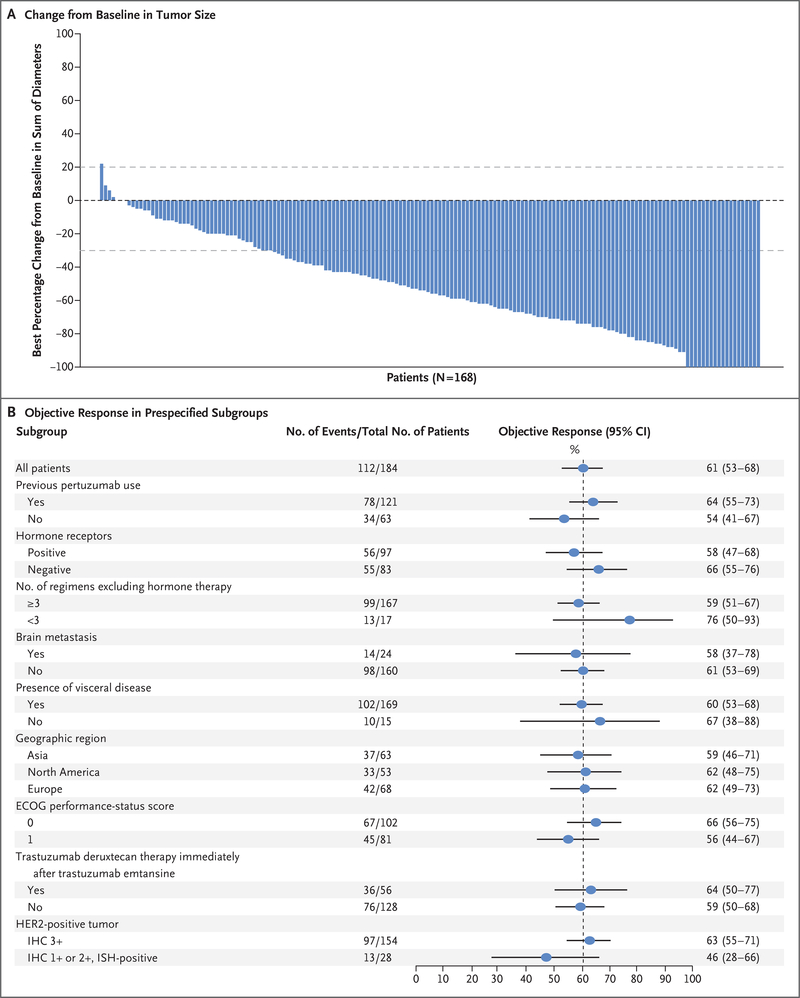
Among the 184 patients who received trastuzumab deruxtecan at the recommended dose of 5.4 mg per kilogram, the confirmed response rate on independent central review was 60.9% (95% CI, 53.4 to 68.0); of these patients, 6.0% had a complete response, and 54.9% had a partial response. Another 3 patients (1.6%) had progressive disease, and 2 (1.1%) could not be evaluated. Most of the patients for whom both baseline and postbaseline data were available had a reduction in tumor size (Fig. 2A). The disease-control rate was 97.3% (95% CI, 93.8 to 99.1), and the clinical-benefit rate was 76.1% (95% CI, 69.3 to 82.1). The median time until response was 1.6 months (95% CI, 1.4 to 2.6), an interval that corresponded to the time until the first imaging after baseline. Among the 180 patients who had tumor progression during or after the administration of trastuzumab emtansine, the confirmed response was 61.1% (95% CI, 53.6 to 68.3).
Figure 2. Response to Trastuzumab Deruxtecan, According to Tumor Size and Subgroup Analyses.
Panel A shows the best percentage change from baseline in the sum of the largest diameters of measurable tumors in 168 of 184 patients for whom data from both baseline and postbaseline assessments of target lesions by independent central review were available. The upper dashed horizontal line indicates a 20% increase in tumor size in the patients who had disease progression, and the lower dashed line indicates a 30% decrease in tumor size (partial response). Panel B shows the objective partial or complete response to trastuzumab deruxtecan therapy in all the enrolled patients who received 5.4 mg per kilogram, according to subgroup. Data for patients with an immunohistochemical (IHC) score indicated as 1+ (IHC negative) or 2+ (IHC borderline) include data for patients for whom the result was equivocal or could not be evaluated; a score of 3+ indicates positivity for HER2 (human epidermal growth factor receptor 2). HER2 positivity is also indicated by an IHC score of 1+ or 2+ and positive results on in situ hybridization (ISH). The vertical dashed line at the 61% mark indicates the median response to therapy in the overall population. ECOG denotes Eastern Cooperative Oncology Group.
Prespecified subgroup analyses showed consistent responses across demographic and prognostic subgroups, including previous receipt of pertuzumab (78 of 121 patients [64%]), hormone-receptor status (positive status, 56 of 97 patients [58%]; negative status, 55 of 83 patients [66%]), and receipt of trastuzumab deruxtecan immediately after initial trastuzumab emtansine therapy (36 of 56 patients [64%]) (Fig. 2B). The efficacy results for doses other than 5.4 mg per kilogram are provided in Table S2.
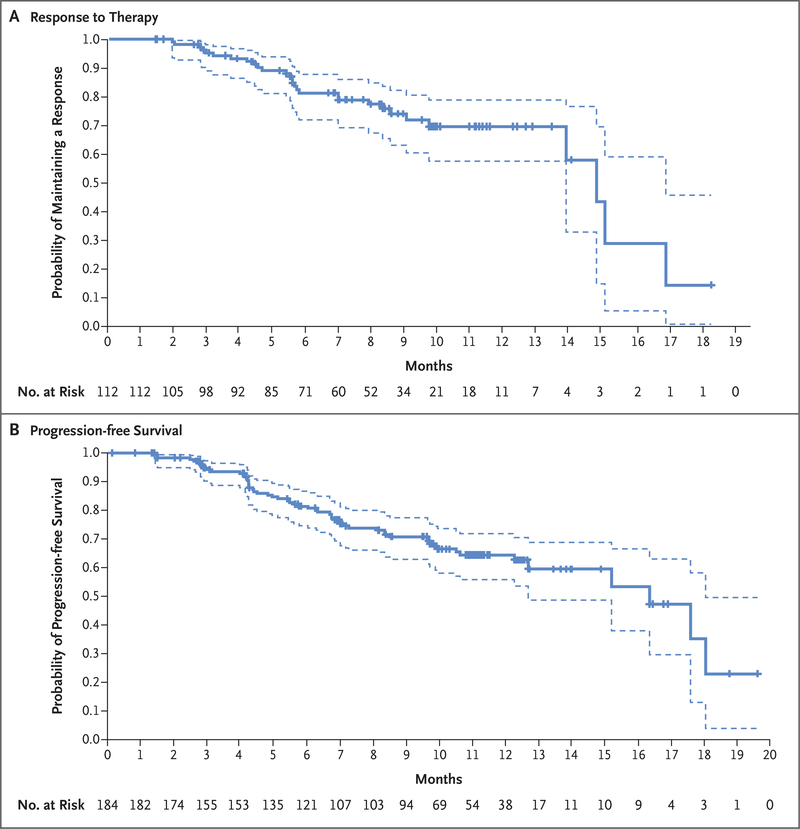
The median response duration was 14.8 months (95% CI, 13.8 to 16.9) (Fig. 3A). The median duration of progression-free survival was 16.4 months (95% CI, 12.7 to not reached) among all patients and 18.1 months (95% CI, 6.7 to 18.1) among the 24 patients who were enrolled with treated and asymptomatic brain metastases. Estimated overall survival was 93.9% (95% CI, 89.3 to 96.6) at 6 months and 86.2% (95% CI, 79.8 to 90.7) at 12 months; the median overall survival was not reached at the time of this report (Fig. S2).
Figure 3. Kaplan–Meier Analysis of Response Duration and Progression-free Survival.
Panel A shows the duration of response to trastuzumab deruxtecan therapy in the 112 patients who had a complete or partial response among the 184 patients who were enrolled in the study. Panel B shows the probability of progression-free survival in the overall population. Of the 184 patients, 48 had progressive disease and 10 had died by 20 months; data for 126 patients were censored, as indicated by tick marks in the two panels. Disease progression was assessed with the use of the modified Response Evaluation Criteria in Solid Tumors, version 1.1. In each panel, the dashed lines indicate the 95% confidence interval.
SAFETY
Of the 184 patients who received the recommended dose of trastuzumab deruxtecan, 99.5% had at least one adverse event during treatment; of these patients, 57.1% had an adverse event of grade 3 or higher (Table S3). The most common adverse events of grade 3 or higher that occurred in more than 5% of the patients were a decreased neutrophil count (in 20.7%), anemia (in 8.7%), nausea (in 7.6%), a decreased white-cell count (in 6.5%), a decreased lymphocyte count (in 6.5%), and fatigue (in 6.0%) (Table 2); 3 patients (1.6%) had febrile neutropenia. (Table S4 provides a list of all the adverse events that were reported in at least 10% of the enrolled patients.)
Table 2.
Adverse Events in the Overall Population of 184 Patients.*
| Adverse Events | Any Grade | Grade 3 | Grade 4 |
|---|---|---|---|
| number of patients (percent) | |||
| Any adverse event† | 183 (99.5) | 89 (48.4) | 7 (3.8) |
| Nausea | 143 (77.7) | 14 (7.6) | 0 |
| Fatigue | 91 (49.5) | 11 (6.0) | 0 |
| Alopecia | 89 (48.4) | 1 (0.5) | 0 |
| Vomiting | 84 (45.7) | 8 (4.3) | 0 |
| Constipation | 66 (35.9) | 1 (0.5) | 0 |
| Decreased neutrophil count‡ | 64 (34.8) | 36 (19.6) | 2 (1.1) |
| Decreased appetite | 57 (31.0) | 3 (16) | 0 |
| Anemia§ | 55 (29.9) | 15 (8.2) | 1 (0.5) |
| Diarrhea | 54 (29.3) | 5 (2.7) | 0 |
| Decreased white-cell count¶ | 39 (21.2) | 11 (6.0) | 1 (0.5) |
| Decreased platelet count|| | 39 (21.2) | 7 (3.8) | 1 (0.5) |
| Headache | 36 (19.6) | 0 | 0 |
| Cough | 35 (19.0) | 0 | 0 |
| Abdominal pain** | 31 (16.8) | 2 (1.1) | 0 |
| Decreased lymphocyte count†† | 26 (14.1) | 11 (6.0) | 1 (0.5) |
| Adverse events of special interest | |||
| Interstitial lung diseas‡‡ | 25 (13.6) | 1 (0.5) | 0 |
| Prolonged QT interval | 9 (4.9) | 2 (1.1) | 0 |
| Infusion-related reaction | 4 (2.2) | 0 | 0 |
| Decreased left ventricular ejection fraction§§ | 3 (16) | 1 (0.5)¶¶ | 0 |
Listed are adverse events that were reported in more than 15% of the patients. The safety analysis set included the 184 patients who were enrolled in part 1 or part 2 of the study and who received at least one dose of trastuzumab deruxtecan. Details regarding adverse events that were reported in more than 10% of the patients are provided in Table S5.
Adverse events that are listed in this category were reported by the investigator. They include adverse events of grade 3 or higher that were reported in at least 6% of the patients.
This category includes the preferred terms neutrophil count decreased and neutropenia.
This category includes the preferred terms hematocrit decreased, hemoglobin decreased, red-cell count decreased, and anemia.
This category includes the preferred terms white-cell count decreased and leukopenia.
This category includes the preferred terms platelet count decreased and thrombocytopenia.
This category includes the preferred terms abdominal discomfort, abdominal pain, abdominal pain lower, and abdominal pain upper.
This category includes the preferred terms lymphocyte count decreased and lymphopenia.
The presence of interstitial lung disease was determined by an independent adjudication committee, since the condition has been associated with trastuzumab deruxtecan. Four patients who had grade 5 events are included in the category of any grade.
The left ventricular ejection fraction was measured on echocardiography or multigated acquisition scans every four treatment cycles.
In this patient, the left ventricular ejection fraction was more than 55% during treatment.
Adverse events led to a dose interruption in 65 patients (35.3%) and to a dose reduction in 43 patients (23.4%); 28 patients (15.2%) discontinued treatment because of an adverse event. Adverse events that led to discontinuation in at least 2 patients included pneumonitis (in 11 patients) and interstitial lung disease (in 5 patients). A total of 25 deaths were reported, including 7 that occurred during treatment as a result of either disease progression (in 3 patients) or adverse events (hemorrhagic shock, general physical health deterioration, pneumonia, and acute organ failure in 1 patient each). During survival follow-up (which was defined as 47 days after the end of treatment), 18 of the 25 deaths occurred, 2 of which were caused by events associated with interstitial lung disease that started during treatment and are among those described below; the remaining 16 deaths were considered by investigators to be unrelated to trastuzumab deruxtecan.
Eleven patients (6.0%), including 1 who could not be evaluated at baseline, tested positive for antidrug antibodies; only 3 patients had positive results for such antibodies after the baseline assessment, and all 3 cases resolved during treatment. A decrease in the left ventricular ejection fraction occurred in 3 patients (2 with grade 2 and 1 with grade 3); all the patients were asymptomatic and had recovered or were recovering after an interruption in the study treatment. No events of cardiac failure associated with the decrease in the ejection fraction were reported. No patients had an ejection fraction of less than 40% or a decrease from baseline of 20% or more, and no patients discontinued treatment because of a decrease in the ejection fraction.
Overall, 25 patients (13.6%) had interstitial lung disease related to the receipt of trastuzumab deruxtecan, as determined by an independent adjudication committee. These events were primarily NCI CTCAE grade 1 or 2 (10.9%); 1 patient (0.5%) had a grade 3 event, and no patients had a grade 4 event. Four deaths (2.2% of the patients) were attributed to interstitial lung disease by independent adjudication and were initially reported as respiratory failure, acute respiratory failure, lymphangitis, and pneumonitis in 1 patient each by the treating investigators; the primary cause of death was reported as disease progression (in 2 patients) and adverse events during survival follow-up (in 2 patients). Among the investigator-reported cases of interstitial lung disease of any grade, the median time until the onset of lung disease was 193 days (range, 42 to 535). At the time of the data cutoff, 7 patients with interstitial lung disease had recovered, 2 were recovering, 10 had ongoing interstitial lung disease, and 4 had died; status was unknown for 2 patients. Among the patients with investigator reported interstitial lung disease, the median duration from the date of onset to the date of recovery was 34 days (range, 3 to 179). Of the 20 patients who were reported to have interstitial lung disease of grade 2 or higher, 13 received glucocorticoids and 7 were hospitalized.
DISCUSSION
In this phase 2 study, trastuzumab deruxtecan (at a dose of 5.4 mg per kilogram) had durable antitumor activity in patients with HER2-positive metastatic breast cancer who had undergone extensive previous treatment, with a confirmed overall response rate of 60.9%, a median duration of progression-free survival of 16.4 months, and a median response duration of 14.8 months. Efficacy results were consistent across key subgroups, including patients who had received previous pertuzumab therapy. These results validate earlier observations from a phase 1 study (DS8201-A-J101), which showed a response of 59.5% (95% CI, 49.7 to 68.7) in a similar patient population.17
The response rate and overall efficacy observed with trastuzumab deruxtecan in this study appear to substantially exceed those of currently available HER2-directed regimens and new agents in development, although cross-trial comparisons must be interpreted with caution. In two recent phase 3 trials, the combinations of margetuximab (another anti-HER2 antibody) plus chemotherapy (SOPHIA)9 and neratinib plus chemotherapy (NALA)10 were compared with standard-of-care therapy, with response rates ranging from 16 to 32.8% in both the experimental group and the control group in the two trials. In the SOPHIA trial, the median duration of progression-free survival was 5.8 months in the experimental group and 4.9 months in the comparator group; in the NALA trial, the estimated percentage of patients with 6-month progression-free survival was 47%. Previously, investigators in the TH3RESA trial,7 which compared trastuzumab emtansine with a treatment of the physician’s choice after two previous anti-HER2 regimens, found a response rate of 31% in the trastuzumab emtansine group and 9% in the comparator group (median progression-free survival, 6.2 months vs. 3.3 months). In the EMILIA trial6 evaluating trastuzumab emtansine as a second-line therapy in patients who had previously received trastuzumab plus a taxane, the overall response was 43.6% (95% CI, 38.6 to 48.6), with a median duration of progression-free survival of 9.6 months.
Although the process is not fully understood, resistance to trastuzumab emtansine may develop through inadequate binding of the antibody to the HER2 receptor owing to down-regulation or loss of HER2 expression, heterogeneous HER2 expression (either intratumoral or from lesion to lesion), receptor mutation, or expression of a different HER2 isoform.24,25 Alterations in lysosomal trafficking and degradation also have been implicated in resistance to trastuzumab emtansine.26,27 Alternatively, cancer cells may develop resistance to the emtansine payload. In our study, trastuzumab deruxtecan retained efficacy in patients who had previously been treated with trastuzumab emtansine, a finding that may be attributable to the distinct pharmaceutical properties of trastuzumab deruxtecan, such as the potent topoisomerase I inhibitor payload instead of a microtubule inhibitor, an increased drug-to-antibody ratio (approximately 8 with trastuzumab deruxtecan vs. approximately 3.5 with trastuzumab emtansine), and the high membrane permeability of the released payload, which can result in a bystander antitumor effect.28 The reported efficacy could also be due to the use of a higher dose of trastuzumab deruxtecan (5.4 mg per kilogram) than the dose of trastuzumab emtansine (3.6 mg per kilogram). Moreover, trastuzumab deruxtecan has shown activity in patients with metastatic breast cancer with low HER2 expression (i.e., a score of <3+ on immunohistochemical analysis and negative results on in situ hybridization).18 When considered in the context of heterogeneous HER2 levels, this finding may account for activity in patients with disease that is resistant to trastuzumab emtansine.
In our study, gastrointestinal and hematologic toxic effects were the most common adverse events. Other HER2-targeted therapies, such as trastuzumab and pertuzumab, have been associated with a risk of cardiomyopathy, particularly left ventricular dysfunction.29–32 In contrast, clinically significant cardiotoxicity was not observed here or in the DS8201-A-J101 study.17,33
Trastuzumab deruxtecan was associated with a substantial risk of interstitial lung disease (13.6%), which led to death in some patients. Other HER2-directed therapies, including trastuzumab and trastuzumab emtansine, and topoisomerase I inhibitors have been associated with pulmonary toxicity.30,34,35 In accordance with the study protocol, investigators managed interstitial lung disease with dose reductions or discontinuations, the administration of glucocorticoids, and supportive care. (Details regarding recommendations for management are provided in the Supplementary Appendix.) Education and close monitoring for signs and symptoms of interstitial lung disease (including fever, cough, or dyspnea) is recommended for early detection. If interstitial lung disease is suspected, evaluations should include high-resolution computed tomography, consultation with a pulmonologist, and testing of pulmonary function and oxygen saturation, and other tests could be considered, as needed (Table S6). Although data on treatment for interstitial lung disease associated with trastuzumab deruxtecan are limited, interruption of the drug regimen and prompt intervention with glucocorticoids when appropriate may help reduce the severity of this complication.17,36 However, more research is needed to determine who is at greatest risk for interstitial lung disease and the most effective method for mitigating this toxic effect.
DESTINY-Breast01 was a single-group study with a median follow-up of only 11 months. Yet, it provides validation of the earlier phase 1 results,and randomized trials are under way (NCT03523585 and NCT03529110) to confirm these findings as well as a direct comparison with trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Trastuzumab deruxtecan is also being evaluated for the treatment of metastatic breast cancer in patients with low HER2 expression (NCT03734029) and has shown early activity with a response rate of 44.2% in a patient population for which currently available HER2-directed therapies are ineffective.18
In this global, phase 2 study, trastuzumab deruxtecan had a high level of clinical activity in patients with HER2-positive metastatic breast cancer who had undergone extensive previous therapies. Such therapy was associated with a substantial risk of interstitial lung disease, a toxic effect that requires attention to pulmonary symptoms and careful monitoring.
Supplementary Material
Acknowledgments
Supported by Daiichi Sankyo and AstraZeneca.
Footnotes
A full list of investigators in the DESTINY-Breast01 trial is provided in the Supplementary Appendix, available at NEJM.org.
The authors’ full names, academic degrees, and affiliations are listed in the Appendix.
APPENDIX
The authors’ full names and academic degrees are as follows: Shanu Modi, M.D., Cristina Saura, M.D., Ph.D., Toshinari Yamashita, M.D., Yeon Hee Park, M.D., Sung-Bae Kim, M.D., Ph.D., Kenji Tamura, M.D., Ph.D., Fabrice Andre, M.D., Ph.D., Hiroji Iwata, M.D., Ph.D., Yoshinori Ito, M.D., Junji Tsurutani, M.D., Ph.D., Joohyuk Sohn, M.D., Ph.D., Neelima Denduluri, M.D., Christophe Perrin, M.D., Kenjiro Aogi, M.D., Ph.D., Eriko Tokunaga, M.D., Seock-Ah Im, M.D., Ph.D., Keun Seok Lee, M.D., Ph.D., Sara A. Hurvitz, M.D., Javier Cortes, M.D., Ph.D., Caleb Lee, M.D., Ph.D., Shuquan Chen, Ph.D., Lin Zhang, M.D., Ph.D., Javad Shahidi, M.D., Antoine Yver, M.D., and Ian Krop, M.D., Ph.D.
Contributor Information
S. Modi, Memorial Sloan Kettering Cancer Center, New York
C. Saura, Vall d’Hebron University Hospital, Vall d’Hebron Institute of Oncology, Barcelona
T. Yamashita, Kanagawa Cancer Center, Yokohama, Japan
Y.H. Park, Samsung Medical Center, South Korea
S.-B. Kim, Asan Medical Center, University of Ulsan College of Medicine, South Korea
K. Tamura, National Cancer Center Hospital, Japan
F. Andre, Institut Gustave Roussy, Université Paris-Sud, Villejuif, France
H. Iwata, Tokyo, Aichi Cancer Center Hospital, Nagoya, Japan
Y. Ito, Cancer Institute Hospital of the Japanese Foundation for Cancer Research, Japan
J. Tsurutani, Advanced Cancer Translational Research Institute, Showa University, Japan Kindai University Faculty of Medicine, Osaka, Japan.
J. Sohn, Yonsei Cancer Center, Yonsei University Health System, South Korea
N. Denduluri, US Oncology Network, Virginia Cancer Specialists, Arlington
C. Perrin, Centre Eugène Marquis, Rennes, France
K. Aogi, Shikoku Cancer Center, Matsuyama, Japan
E. Tokunaga, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan
S.-A. Im, Seoul National University Hospital, Cancer Research Institute, Seoul National University College of Medicine, Seoul, South Korea
K.S. Lee, National Cancer Center, Gyeonggi, South Korea
S.A. Hurvitz, University of California, Los Angeles–Jonsson Comprehensive Cancer Center, Los Angeles
J. Cortes, Vall d’Hebron University Hospital, Vall d’Hebron Institute of Oncology, Barcelona IOB Institute of Oncology, Quiron Group, Barcelona and Madrid.
C. Lee, Daiichi Sankyo, Basking Ridge, NJ
S. Chen, Daiichi Sankyo, Basking Ridge, NJ
L. Zhang, Daiichi Sankyo, Basking Ridge, NJ
J. Shahidi, Daiichi Sankyo, Basking Ridge, NJ
A. Yver, Daiichi Sankyo, Basking Ridge, NJ
I. Krop, Dana–Farber Cancer Institute, Boston
REFERENCES
- 1.Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol 2009;27:5700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res 2009;7:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/ neu oncogene. Science 1987;235:177–82. [DOI] [PubMed] [Google Scholar]
- 4.Swain SM, Baselga J, Kim S-B, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swain SM, Miles D, Kim S-B, et al. End-of-study analysis from the phase III, randomized, double-blind, placebo (Pla)-controlled CLEOPATRA study of first-line (1L) pertuzumab (P), trastuzumab (H), and docetaxel (D) in patients (pts) with HER2-positive metastatic breast cancer (MBC). J Clin Oncol 2019;15:Suppl:102 abstract. [Google Scholar]
- 6.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krop IE, Kim S-B, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15: 689–99. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. Breast cancer (version 2). 2019. (https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf).
- 9.Rugo HS, Im S-A, Write GLS, et al. SOPHIA primary analysis: a phase 3 study of margetuximab + chemotherapy versus trastuzumab + chemotherapy in patients with HER2+ metastatic breast cancer after prior anti-HER2 therapies. J Clin Oncol 2019;37:Suppl:1000 abstract. [Google Scholar]
- 10.Saura C, Oliveira M, Feng Y-H, et al. Neratinib + capecitabine versus lapatinib + capecitabine in patients with HER2+ metastatic breast cancer previously treated with = 2 HER2-directed regimens: findings from the multinational, randomized, phase III NALA trial. J Clin Oncol 2019; 37:Suppl:1002 abstract. [Google Scholar]
- 11.Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol 2017;18:1512–22. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal N, Sloane BF. Cathepsin B: multiple roles in cancer. Proteomics Clin Appl 2014;8:427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruan J, Zheng H, Fu W, Zhao P, Su N, Luo R. Increased expression of cathepsin L: a novel independent prognostic marker of worse outcome in hepatocellular carcinoma patients. PLoS One 2014;9(11) e112136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakada T, Sugihara K, Jikoh T, Abe Y, Agatsuma T. The latest research and development into the antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem Pharm Bull (Tokyo) 2019;67:173–85. [DOI] [PubMed] [Google Scholar]
- 15.Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 2016;22:5097–108. [DOI] [PubMed] [Google Scholar]
- 16.Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 2016;107:1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamura K, Tsurutani J, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol 2019;20: 816–26. [DOI] [PubMed] [Google Scholar]
- 18.Modi S, Tsurutani J, Tamura K, et al. Trastuzumab deruxtecan (DS-8201a) in subjects with HER2-low expressing breast cancer:updated results of a large phase 1 study. Presented at the 2018 San Antonio Breast Cancer Symposium, San Antonio, TX, December 4–8, 2018. [Google Scholar]
- 19.Modi S, Tsurutani J, Takahashi S, et al. Safety and efficacy results from a phase 1 study of trastuzumab deruxtecan (DS-8201a) in subjects with HER2 expressing metastatic breast cancers. Presented at the 2017 San Antonio Breast Cancer Symposium, San Antonio, TX, December 5–9, 2017. [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 21.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics 1982;38:29–41. [Google Scholar]
- 22.Lin L, Sirohi D, Coleman JF, Gulbahce HE. American Society of Clinical Oncology/College of American Pathologists 2018 focused update of breast cancer HER2 FISH testing guidelines: results from a national reference laboratory. Am J Clin Pathol 2019;152:479–85. [DOI] [PubMed] [Google Scholar]
- 23.Tamura K, Modi S, Tsurutani J, et al. Dose justification for DS-8201a, a HER2-targeted antibody-drug conjugate, for HER2-positive breast cancer:observed clinical data and exposure-response analyses. Presented at the 2018 San Antonio Breast Cancer Symposium, San Antonio, TX, December 4–8, 2018. [Google Scholar]
- 24.Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res 2014;16:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loganzo F, Tan X, Sung M, et al. Tumor cells chronically treated with a trastuzumab-maytansinoid antibody-drug conjugate develop varied resistance mechanisms but respond to alternate treatments. Mol Cancer Ther 2015;14:952–63. [DOI] [PubMed] [Google Scholar]
- 26.Ríos-Luci C, García-Alonso S, Díaz-Rodríguez E, et al. Resistance to the antibody-drug conjugate T-DM1 is based in a reduction in lysosomal proteolytic activity. Cancer Res 2017;77:4639–51. [DOI] [PubMed] [Google Scholar]
- 27.Sung M, Tan X, Lu B, et al. Caveolaemediated endocytosis as a novel mechanism of resistance to trastuzumab emtansine (T-DM1). Mol Cancer Ther 2018;17: 243–53. [DOI] [PubMed] [Google Scholar]
- 28.Marcoux J, Champion T, Colas O, et al. Native mass spectrometry and ion mobility characterization of trastuzumab emtansine, a lysine-linked antibody drug conjugate. Protein Sci 2015;24:1210–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herceptin:summary of product characteristics. Welwyn Garden City, United Kingdom: Roche Registration, 2010. [Google Scholar]
- 30.Herceptin for injection fiu: full prescribing information. South San Francisco, CA: Genentech, 2017. [Google Scholar]
- 31.Perjeta: summary of product characteristics. Welwyn Garden City, United Kingdom: Roche Registration; 2013. [Google Scholar]
- 32.Perjeta for injection fiu:full prescribing information. South San Francisco, CA: Genentech, 2017. [Google Scholar]
- 33.Shitara K, Iwata H, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol 2019;20:827–36. [DOI] [PubMed] [Google Scholar]
- 34.Kadcyla for injection fiu:full prescribing information. South San Francisco, CA: Genentech, 2016. [Google Scholar]
- 35.Dimopoulou I, Bamias A, Lyberopoulos P, Dimopoulos MA. Pulmonary toxicity from novel antineoplastic agents. Ann Oncol 2006;17:372–9. [DOI] [PubMed] [Google Scholar]
- 36.Powell CA, Camidge DR, Gemma A, et al. P6–17-06: Characterization, monitoring and management of interstitial lung disease in patients with metastatic breast cancer: analysis of data available from multiple studies of DS-8201a, a HER2-targeted antibody drug conjugate with topoisomerase I inhibitor payload. Presented at the 2018 San Antonio Breast Cancer Symposium, San Antonio, TX, December 4–8, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.