Abstract
Background
Video-assisted thoracoscopic (VATS) lobectomy has been demonstrated to offer several benefits over open surgery. The purpose of this study was to assess the feasibility and safety of an ultra-fast-track 23-hour recovery pathway for VATS lobectomy.
Methods
A prospectively maintained institutional database was queried for patients who underwent VATS lobectomy from 2006 to 2016 at the McGill University Health Centre in Montreal, Quebec, and data were supplemented with focused chart review. Patients discharged with a length of stay (LOS) of 23 hours or less were compared with those with an LOS of 2 days or more. Logistic regression was performed to identify predictors of LOS of 23 hours or less.
Results
Two hundred and five patients were included in the study. Perioperative 30-day mortality for our cohort was 0% and the major complication rate was 8.3%. The median LOS was 3 days (interquartile range [IQR] 2–4 d). Thirty-four patients were discharged within 23 hours and none of them required readmission; 171 patients were discharged on postoperative day 2 or later and 9 of them (5.3%) required readmission (p = 0.36). The proportion of patients discharged within 23 hours increased in 2016 compared with previous years (25.8% v. 12.0%, p = 0.05). Patients discharged within 23 hours had shorter chest tube duration (odds ratio [OR] 0.20, 95% confidence interval [CI] 0.09–0.46, p < 0.001), lower clinical stage disease (stages II–III v. stage I OR 0.07, 95% CI 0.01–0.52, p = 0.011), lower pathologic stage lesions (stages II–III v. stage I OR 0.26, 95% CI 0.07–0.91, p = 0.035), fewer surgical complications (OR 0.04, 95% CI 0.01–0.30, p = 0.002) and shorter operative time (surgery duration > 120 min OR 0.42, 95% CI 0.18–0.95, p = 0.04). Our exploratory prediction modelling showed that chest tube duration, clinical stage and surgeon were the most influential predictors of discharge within 23 hours.
Conclusion
The only preoperative factors that predicted shorter LOS in our cohort were clinical stage and surgeon. A significant proportion of patients can be discharged safely by adopting a VATS lobectomy 23-hour enhanced recovery pathway.
Abstract
Contexte
Il a été démontré que la lobectomie par chirurgie thoracique vidéoassistée (CTVA) offre plusieurs avantages comparativement à la chirurgie ouverte. La présente étude avait pour but d’évaluer la faisabilité et la sûreté d’un protocole de récupération ultrarapide en 23 heures pour la lobectomie par CTVA.
Méthodes
Nous avons extrait d’une base de données d’établissement maintenue de manière prospective des données sur les patients ayant subi une lobectomie par CTVA entre 2006 et 2016 au Centre universitaire de santé McGill à Montréal (Québec), complétées par un examen ciblé des dossiers. Les patients ayant reçu leur congé après une hospitalisation de 23 heures ou moins ont été comparés à ceux dont l’hospitalisation avait duré 2 jours ou plus. Nous avons ensuite mis en évidence les facteurs prédictifs d’une hospitalisation de 23 heures ou moins par une analyse de régression logistique.
Résultats
Deux cent cinq patients ont été inclus dans l’étude. La mortalité périopératoire dans les 30 jours suivant l’intervention était de 0 % dans notre cohorte, et le taux de complications majeures était de 8,3 %. La durée d’hospitalisation médiane était de 3 jours (écart interquartile [EI] 2 à 4 jours). Trente-quatre patients ont reçu leur congé dans les 23 heures suivant l’intervention, et aucun n’a dû être réhospitalisé; comparativement, 171 patients ont reçu leur congé au deuxième jour ou après, et 9 d’entre eux (5,3 %) ont dû être réhospitalisés (p = 0,36). Le pourcentage de patients ayant reçu leur congé dans les 23 heures a augmenté en 2016 par rapport aux années précédentes (25,8 % c. 12,0 %, p = 0,05). Les patients au congé dans les 23 heures conservaient leur drain thoracique moins longtemps (rapport de cotes [RC] 0,20, intervalle de confiance [IC] de 95 % 0,09 à 0,46, p < 0,001); leur stade clinique était moins élevé (stades II à III c. stade I – RC 0,07, IC de 95 % 0,01 à 0,52, p = 0,011); le stade pathologique de leurs lésions était plus faible (stades II à III c. stade I – RC 0,26, IC de 95 % 0,07 à 0,91, p = 0,035); ils avaient moins de complications chirurgicales (RC 0,04, IC de 95 % 0,01 à 0,30, p = 0,002); et la durée de leur intervention était plus courte (durée de la chirurgie > 120 minutes – RC 0,42, IC de 95 % 0,18 à 0,95, p = 0,04). Notre modèle prédictif exploratoire a montré que le délai avant le retrait du drain thoracique, le stade clinique et le chirurgien était les facteurs prédictifs les plus importants du congé dans les 23 heures.
Conclusion
Les seuls facteurs préopératoires permettant de prédire une hospitalisation plus courte dans notre cohorte étaient le stade clinique et le chirurgien. Un pourcentage important des patients peuvent recevoir leur congé sans danger si on suit un protocole de récupération optimisée en 23 heures après une lobectomie par CTVA.
Lung cancer remains the leading cause of cancer death worldwide.1 Minimally invasive lung resections, such as video-assisted thoracoscopic surgery (VATS), have supplanted open thoracotomy for the majority of early-stage lung cancers over the last decade. Many surgeons prefer VATS approaches for locally advanced lung cancers as well, which obviates the need for large intercostal incisions and rib spreading. Indeed, many studies have demonstrated the benefits of this approach over open thoracotomy: VATS lobectomy has been associated with significantly better postoperative pulmonary function and overall prognosis than conventional open thoracotomy.2–7 It has led to decreased chest tube duration, shorter hospital stays, lower average treatment costs, improvements in the likelihood of chemotherapy completion and reduced morbidity compared with the traditional open thoracotomy approach, all with equivalent oncologic outcomes.8–13
The concept of enhanced recovery after surgery (ERAS) has been studied and implemented in a multitude of surgical specialties with positive outcomes. Applying concepts of ERAS, mainly multimodal analgesia, reduction in postoperative fluid administration and favouring early oral intake, after minimally invasive procedures has increased the adoption of fast-track programs for a variety of surgical procedures. At our institution, the implementation of an enhanced recovery pathway (ERP) after esophagectomy reduced the mean length of stay (LOS) from 10 to 7 days without an increase in the rate of complications and readmissions,14 and it was associated with overall cost savings of Can$2649 per patient.15 Moreover, day-case surgery for complex laparoscopic hiatal procedures was recently shown to be feasible and safe.16 These studies indicate that significant progress has been made in postoperative care and further improvement can be made.
Most contemporary series on VATS lobectomy report a median LOS of between 4 and 6 days after the procedure.17,18 Although LOS is a problematic metric in comparisons of centres, particularly because of the impact of socioeconomic factors and health care system differences, the prospect of moving toward shorter hospital stays and earlier return to function after VATS lobectomy is an attractive one.19–21 Indeed, from the perspective of ERP after surgery, earlier return home and earlier return to baseline function may further support the use of surgery to treat lung cancer or pulmonary metastasis, particularly in the age of competing emerging nonsurgical ablative therapies.22–24 The implementation of an ERP for open and VATS lobectomy at our centre in 2011 resulted in a decreased LOS and a reduced complication rate with no change in readmission rate.25 Furthermore, we have recently shown that this ERP reduced overall societal costs by an average of Can$4396 per treated patient.26 With this untailored program targeting all lobectomies, VATS and open, patients are more commonly discharged before their target discharge date than patients following the traditional pathway before the implementation of the ERP. As expected, this has been most evident for patients who undergo VATS lobectomy, and a pathway focused on this patient group seemed appropriate. We noticed early in our experience that patients occasionally seemed ready for discharge even on the first postoperative day. Thus, our goal was to move toward a 23-hour care trajectory for patients undergoing VATS lobectomy and to determine if such an approach is feasible and safe for a substantial proportion of our patient population.
Methods
A video is available (https://www.youtube.com/watch?v=HE3KD5JeD5g) showing a representative case from our program: a patient who underwent uniportal right lower lobectomy for early-stage lung cancer treated according to our 23-hour recovery pathway.
Study design, setting and patient selection
Using a prospectively maintained institutional surgical database, we identified all patients who underwent a video-assisted thoracoscopic operation at the McGill University Health Centre from January 2006 to March 2016. All adult patients (aged ≥ 18 yr) who underwent a VATS anatomic lung resection (lobectomy or bilobectomy) were included in the study. Exclusion criteria were age younger than 18 years, pneumonectomy, wedge resection, segmentectomy and extended resection of adjacent organs (e.g., chest wall). Unlike lobectomy, segmentectomy is a limited pulmonary resection commonly performed in patients unable to tolerate lobectomy because of limited cardiopulmonary function. Given that patients undergoing segmentectomy frequently differ from those undergoing lobectomy by indication, we excluded them from this study to ensure that we created a homogeneous patient cohort with regard to the operative procedure. We supplemented our data capture from the institutional database with a focused chart review of all included patients. We collected demographic characteristics (sex, age and distance of home from the hospital) and baseline characteristics, including smoking status (never smoked, current smoker or exsmoker [quit for ≥ 1 yr]), pulmonary function test results (forced expiratory volume in 1 s [FEV1] and diffusing capacity of the lungs for carbon monoxide [DLCO]), medical comorbidities, American Society of Anesthesiologists (ASA) classification, indication for resection and clinical stage. We also examined perioperative features and outcomes, including operative time, timing of surgery (morning or afternoon), conversion to thoracotomy, transfer to the intensive care unit (ICU), chest tube duration and 30-day return to the emergency department (ED) and readmission. Pathology reports on the specimens were retrieved; specimens were classified as primary carcinoma, metastatic lesion or benign. The pathologic stage was confirmed for patients with primary lung carcinoma. Staging was done on the basis of the seventh edition of the American Joint Committee on Cancer (AJCC) TNM system. Data on postoperative complications and mortality were collected and scored using the Thoracic Morbidity and Mortality classification system.27,28 This study was approved by the McGill University Health Centre Research Ethics Board.
The primary outcome of the study is LOS. We classified patients in 2 groups: patients discharged within 23 hours or less of their surgery (LOS1) and patients discharged with an LOS of 2 or more days (LOS2+). Our secondary outcomes were ED visits, hospital readmissions, chest tube duration, 30-day mortality and postoperative complications (including recurrent pneumothorax, subcutaneous emphysema, prolonged air leak, pneumonia, pulmonary embolism, atrial fibrillation, bleeding and uncontrolled pain).
Patient management
During this 10-year study period several evolutions in patient care and surgical technique took place. However, in 2012 an ERP was implemented at our institution, the design and results of which were previously published.25 In brief, throughout the study period, no dedicated prehabilitation program was in place for these patients. Operative procedures consisted primarily of 2- or 3-port approaches, with a smaller number of uniportal cases being performed in the final 2 years of the study. Although the ERP that was implemented in 2012 proposed a 300 mL, 24-h cut-off for chest drainage, the 2 surgeons (L.F., J.S.) principally responsible for more than 95% of the cases in this series were tolerant to higher drainage volumes and accepted chest tube removal as long as there was no evidence of air, frank blood or chyle draining. Hence, the analysis herein does not focus on these metrics, given their changing nature over the course of the study period. Rather, the focus is on the proof of principle that a 23-hour pathway for VATS lobectomy is feasible and safe. Postoperatively, chest tubes are typically taken off suction upon the patient’s arrival to the thoracic surgery ward after discharge from the recovery room. Foley catheters are avoided for all VATS lobectomies. Pain is controlled by a combination of intercostal nerve blockade, acetaminophen, nonsteroidal anti-inflammatory agents and oral narcotics. Epidural catheters and patient-controlled intravenous analgesia are avoided as much as possible and used only in rescue situations. Within 1 week after patients were discharged home from the hospital, our nurse clinician (L.A.) followed up with them by phone to ensure that they were progressing well and did not require any assistance with their surgical recovery. Routine postoperative follow-up visits were scheduled within 1 month after surgery with the surgeon of record.
Statistical analysis
Descriptive sample statistical analyses were performed using proportions for categorical variables, means with standard deviations for normally distributed variables and medians with interquartile ranges (IQRs; 25th percentile and 75th percentile) for variables with a skewed distribution. Patient demographic characteristics, baseline characteristic, perioperative features and postoperative outcomes were compared for the 2 groups (LOS1 and LOS2+) using the nonparametric Wilcoxon–Mann–Whitney test for continuous variables and the Fisher exact test for categorical variables.
An exploratory predictive model was developed for the outcome LOS1 using model-building techniques for predictive modelling outlined by Vittinghoff and colleagues28 and Steyerberg.29 We determined candidate predictors that were preoperative and/or modifiable on the basis of the expert knowledge of the investigators, while also ensuring that our choices mapped onto the dimensions of risk developed by Iezzoni30 for modelling health-related outcomes, where applicable. A list of the 23 candidate predictors and how they map onto the framework is found in Table 1.
Table 1.
Categories of candidate factors considered in predictive modelling of 23-h hospital length of stay in patients undergoing video-assisted thoracosopic lobectomy
| Category | Candidate predictors |
|---|---|
| Patient characteristics | Age, sex, distance from hospital, no. of comorbidities, COPD, smoking status, smoking pack-years, DLCO, FEV1, ASA classification |
| Tumour characteristics | Pathologic stage, clinical stage, indication for resection |
| Surgical factors | Surgeon, era of surgery, surgical complications, chest tube duration, operative time* longer than 120 min, total duration of surgery† longer than 180 min, time of surgery (am v. pm), PACU duration longer than 150 min, lobe removed, additional resection |
ASA = American Society of Anesthesiologists; COPD = chronic obstructive pulmonary disease; DLCO = diffusing capacity of the lungs for carbon monoxide; FEV1 = forced expiratory volume in 1 s; PACU = postanesthesia care unit.
Time from first incision to final closure.
Total time in the operating room.
Logistic regression was used to develop the predictor model, and model assumptions were verified by graphical methods (i.e., residual × predictor plots). First, all candidate predictors were screened in a univariable analysis by computing the unadjusted odds ratios (ORs) and corresponding 95% confidence intervals (CIs). We modelled each category of candidate predictors (patient characteristics, tumour characteristics, surgical factors) (Table 1) separately. Predictors were selected using a stepwise approach on the basis of a priori clinical knowledge, magnitude of the effect and conservative p values (< 0.20). To determine which variables were included in the final logistic model, we examined measures of discrimination and calibration. Overall model fit was assessed using the Akaike information criterion (AIC), the Bayesian information criterion (BIC), the likelihood ratio R2 and the Brier score. The best prediction model that can be fit is one that maximizes adjusted R2 and minimizes AIC, BIC and Brier score. We used the C statistic to measure discrimination or how effectively the model can distinguish between events and non-events. Calibration was measured using the Hosmer–Lemeshow χ2 statistic, where a statistically significant (p < 0.05) finding indicated lack of fit.
The variables included in the final model were age, smoking pack-years, clinical stage (stage I v. stages II–III), chest tube duration and surgeon (A, D). We restricted the sample size to patients whose operation was performed by surgeon A or D (L.F., J.S.) because they operated on 97% of the study patients; the restriction also improved model statistics.
Statistical analysis was performed using Stata release 14 (StataCorp).
Results
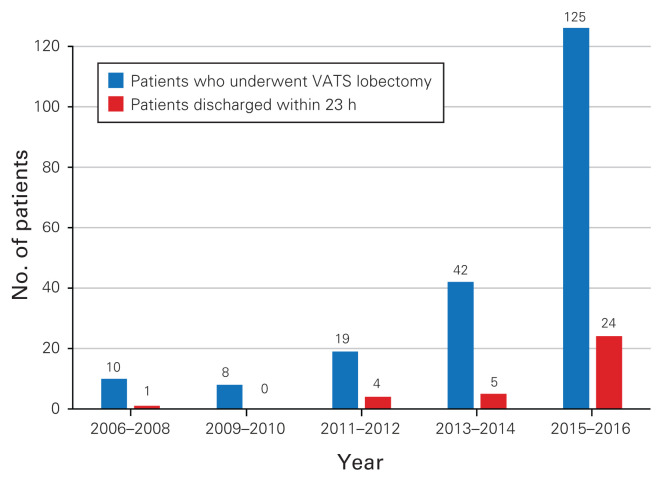
We identified 205 patients who underwent VATS lobectomy from 2006 to 2016 at our institution; only 3 of these patients underwent bilobectomies. The proportion of discharges within 23 hours (LOS1) doubled in the year 2016 compared with previous years (25.8% v. 12.05%, p = 0.05) (Fig. 1). Almost 5 times more patients were discharged within the first 23 hours after surgery in 2015–2016 than in the 2 previous years. Patient demographic characteristics, baseline characteristics, perioperative features and outcomes are summarized in Table 2. The median age of our cohort was 68 years (IQR 60–74 yr) and 41.5% of patients were men. The median LOS was 3 days (IQR 2–4 d). Patients were discharged within the first 23 hours after surgery in 16.6% of cases (n = 34, LOS1); the remainder (83.4%, n = 171) were discharged on postoperative day 2 or later (LOS2+). Patients in the 2 groups were comparable in terms of all demographic characteristics and baseline characteristics, except that patients in the LOS1 group more commonly had clinical stage I disease than patients in the LOS2+ group (95.8% v. 61.5%, p < 0.001). Although patients discharged early more frequently lived less than 100 km from our centre than patients discharged later, this difference did not reach statistical significance (64.7% LOS1 v. 53.9% LOS2+, p = 0.26).
Fig. 1.
Total number of patients who underwent video-assisted thoracoscopic lobectomy (VATS) and the number of patients who were discharged within 23 h between 2006 and 2016.
Table 2.
Patient baseline characteristics
| Characteristic | No. (%) of patients* | p value | ||
|---|---|---|---|---|
| LOS1 n = 34 |
LOS2+ n = 171 |
Total n = 205 |
||
| Male | 15 (44.1) | 70 (40.9) | 85 (41.5) | 0.85 |
| Age at surgery, yr, median (IQR) | 66.1 (59.4–71.5) | 68.2 (60.1–74.5) | 67.7 (60.0–74.0) | 0.21 |
| No. of pack-years, median (IQR) | 35 (0–50) | 27.5 (0–50) | 30 (0–50) | 0.71 |
| Smoking status | 0.28 | |||
| Never smoker | 8 (28.6) | 31 (22.5) | 39 (23.5) | |
| Current smoker | 4 (14.3) | 40 (28.9) | 44 (26.5) | |
| Ex-smoker | 16(57.2) | 67 (48.6) | 83 (50.0) | |
| COPD | 0.52 | |||
| No | 21 (72.4) | 83 (63.9) | 104(65.4) | |
| Yes | 8 (27.6) | 47 (36.2) | 55 (34.6) | |
| DLCO† | 0.28 | |||
| High risk (DLCO ≤ 60) | 7 (26.9) | 20 (17.7) | 27 (19.4) | |
| Low risk (DLCO > 60) | 19 (73.1) | 93 (82.3) | 112 (80.6) | |
| FEV1† | 0.44 | |||
| High risk (FEV1 ≤ 60) | 1 (3.9) | 9 (7.4) | 10 (6.8) | |
| Low risk (FEV1 > 60) | 25 (96.1) | 112 (92.6) | 137 (93.2) | |
| No. of comorbidities‡ | 0.42 | |||
| 0 | 7 (23.3) | 43 (28.5) | 50 (27.6) | |
| 1 | 16 (53.3) | 47 (31.1) | 63 (34.8) | |
| 2 | 3 (10.0) | 34 (22.5) | 37 (20.4) | |
| 3 | 3 (10.0) | 12 (7.9) | 15 (8.3) | |
| > 3 | 1 (3.4) | 15 (10.0) | 16 (8.9) | |
| Distance from hospital ≥ 100 km | 12 (35.3) | 77 (46.1) | 89 (44.3) | 0.26 |
| Indication for resection | 0.053 | |||
| Primary lung cancer | 22 (64.7) | 139 (81.3) | 161 (78.5) | |
| Metastasis | 8 (23.5) | 17 (9.9) | 25 (12.2) | |
| Benign | 3 (8.8) | 6 (3.5) | 9 (4.4) | |
| Other | 1 (2.9) | 9 (5.3) | 10 (4.9) | |
| Lobe removed | 0.20 | |||
| Right upper lobe | 12 (35.3) | 51 (30) | 63 (30.1) | |
| Right middle lobe | 6 (17.7) | 12 (7.1) | 18 (8.8) | |
| Right lower lobe | 3 (8.8) | 33 (19.4) | 36 (17.7) | |
| Left upper lobe | 11(32.2) | 55 (32.4) | 66 (32.4) | |
| Left lower lobe | 2 (5.9) | 19 (11.2) | 21 (10.3) | |
| Surgeon | 0.95 | |||
| A | 20 (58.8) | 93 (58.5) | 113 (58.6) | |
| B | 0 | 2 (1.3) | 2 (1.0) | |
| C | 0 | 4 (2.5) | 4 (2.1) | |
| D | 14 (41.2) | 60 (37.7) | 74 (38.3 | |
| ASA classification | 0.12 | |||
| 1 or 2 | 25 (73.5) | 99 (58.2) | 124 (60.8) | |
| 3 or 4 | 9 (26.5) | 71 (41.8) | 80 (39.2) | |
| Epidural | 4 (16.0) | 18 (16.1) | 22 (16.1) | 1.00 |
| Clinical stage | < 0.001 | |||
| I | 23 (95.8) | 88 (61.1) | 111 (66.1) | |
| II–III | 1 (4.2) | 56 (38.9) | 57 (33.9) | |
| Pathologic stage | 0.033 | |||
| I | 21 (87.5) | 98 (64.5) | 119 (67.6) | |
| II–III | 3 (12.5) | 54 (35.5) | 57 (32.4) | |
ASA = American Society of Anesthesiologists; COPD = chronic obstructive pulmonary disease; DLCO = diffusing capacity of the lungs for carbon monoxide; FEV1 = forced expiratory volume in 1 s; IQR= interquartile range; LOS1 = discharged within 23 hours; LOS2+ = discharged on postoperative day 2 or later.
Unless indicated otherwise.
DLCO and FEV1 cut-offs were chosen on the basis of clinically relevant cut-offs as reported in the Diagnosis and Management of Lung Cancer, American College of Chest Physicians Evidence-Based Clinical Practice Guidelines.31
Comorbidities included congestive heart failure, dementia, diabetes (with and without chronic complications), heart disease, hypertension, liver disease (mild, moderate or severe), hemiplegia or paraplegia, myocardial infarction, peptic ulcer disease, peripheral vascular disease, rheumatologic disease, HIV/AIDS, cerebrovascular disease and chronic pulmonary disease.
Postoperative outcomes
The overall 30-day mortality of our cohort was 0%. Patients in the 2 groups had similar surgical outcomes (Table 3). No patient discharged within 24 hours was readmitted to the hospital, whereas 5.3% of patients discharged on postoperative 2 or later were readmitted (p = 0.36). A single patient discharged within 24 hours returned to the emergency department, compared with 7.6% of patients discharged on postoperative day 2 or later (p = 0.47). Patients with a longer LOS had a higher rate of air leak than patients discharged within 24 hours (27.5% v. 0%, p < 0.001), and they had a longer median chest tube duration (2 d v. 1 d, p < 0.001). There was a lower proportion of complications among patients with an expedited discharge (no complications in 97% of patients in the LOS1 group v. 56.7% of patients in the LOS2+ group, p < 0.001).
Table 3.
Postoperative outcomes
| Outcome | No. (%) of patients* | p value | ||
|---|---|---|---|---|
| LOS1 n = 34 |
LOS2+ n = 171 |
Total n = 205 |
||
| Conversion to open thoracotomy | 0 | 4 (2.34) | 4 (2.0) | > 0.99 |
| Requirement for ICU stay after surgery | 0 | 5 (2.9) | 5 (2.4) | 0.59 |
| Chest tube duration, d, median (IQR) | 1 (1–1) | 2 (1–3) | 1 (1–33) | < 0.001 |
| Air leak | 0 | 47 (27.5) | 4 (22.9) | < 0.001 |
| Discharged with chest tube | 0 | 14 (8.2) | 15 (7.3) | 0.13 |
| Surgical complications† | < 0.001 | |||
| None | 33 (97.0) | 97 (56.7) | 130 (63.4) | |
| Minor (grades I and II) | 1 (2.9) | 57 (33.3) | 58 (28.3) | |
| Major (grades III and IV) | 0 | 17 (9.9) | 17 (8.3) | |
| Grade V | 0 | 0 | 0 | |
| ED visit after discharge | 1 (2.9) | 13 (7.6) | 14 (6.8) | 0.47 |
| Readmission to hospital because of complication | 0 | 9 (5.3) | 9 (4.4) | 0.36 |
ED = emergency department; ICU = intensive care unit; IQR = interquartile range; LOS1 = discharged within 23 hours; LOS2+ = discharged on postoperative day 2 or later.
Unless indicated otherwise.
Graded according to the Clavien–Dindo classification system.
Univariable logistic regression showed that patients in the LOS1 group had fewer advanced carcinomas; they were less likely to have disease at a higher clinical stage (II or III) (OR 0.07, 95% CI 0.01–0.52, p = 0.011) and pathologic stage (OR 0.26, 95% CI 0.07–0.91, p = 0.035) (Table 4). These patients were also less likely to have operations exceeding 2 hours (OR 0.42, 95% CI 0.18–0.95, p = 0.04), they were less likely to have complications (OR 0.04, 95% CI 0.01–0.30, p = 0.002), and they had shorter chest tube durations (OR 0.20, 95% CI 0.09–0.46, p < 0.001) (Table 4).
Table 4.
Results of univariable logistic regression
| Predictor | OR (95% CI) | p value |
|---|---|---|
| Male | 1.14 (0.54–2.39) | 0.73 |
| Age at surgery | 0.98 (0.95–1.02) | 0.27 |
| Smoking status | ||
| Never smoker | Ref | |
| Current smoker | 0.39 (0.11–1.41) | 0.15 |
| Ex-smoker | 0.93 (0.35–2.39) | 0.87 |
| Smoking pack-years | 1.00 (0.99–1.01) | 0.19 |
| COPD | 0.67 (0.27–1.64) | 0.37 |
| Distance from hospital ≥ 100 km | 0.84 (0.56–1.26) | 0.39 |
| DLCO | ||
| High risk (DLCO ≤ 60) | Ref | |
| Low risk (DLCO > 60) | 0.58 (0.22–1.57) | 0.30 |
| FEV1 | ||
| High risk (FEV1 ≤ 60) | Ref | |
| Low risk FEV1 > 60) | 2.01 (0.24–16.59) | 0.52 |
| Clinical stage | ||
| I | Ref | |
| II–III | 0.07 (0.01–0.52) | 0.011 |
| Pathologic stage | ||
| I | Ref | |
| II–III | 0.26 (0.07–0.91) | 0.035 |
| Lobe removed | ||
| RUL | Ref | |
| RML | 2.13 (0.66–6.81) | 0.21 |
| RLL | 0.39 (0.10–1.47) | 0.16 |
| LUL | 0.85 (0.34–2.10) | 0.72 |
| LLL | 0.45 (0.09–2.19) | 0.32 |
| Indication for resection | ||
| Primary lung cancer | Ref | |
| Metastasis | 4.55 (1.47–14.51) | 0.01 |
| ASA classification | ||
| 1 or 2 | Ref | |
| 3 or 4 | 0.50 (0.22–1.14) | 0.10 |
| Time of surgery (pm v. am) | 0.90 (0.41–1.97) | 0.79 |
| Operative time longer than 120 min | 0.42 (0.18–0.95) | 0.04 |
| Total duration of surgery (longer than 180 min) | 0.35 (0.13–0.96) | 0.04 |
| PACU duration longer than 150 min | 0.43 (0.20–0.91) | 0.03 |
| Surgical complications | 0.04 (0.01–0.30) | 0.002 |
| Chest tube duration | 0.20 (0.09–0.46) | < 0.001 |
| Additional resection | 0.56 (0.12–2.54) | 0.45 |
| No. of comorbidities | 0.83 (0.61–1.14) | 0.25 |
| Surgeon | ||
| A | Ref | |
| B | 0.91 (0.04–19.72) | 0.95 |
| C | 0.51 (0.03–9.79) | 0.65 |
| D | 1.09 (0.51–2.31) | 0.82 |
| Era of surgery | ||
| Before 2015 | Ref | |
| 2015 or after | 1.62 (0.73–3.61) | 0.23 |
ASA = American Society of Anesthesiologists; CI = confidence interval; COPD = chronic obstructive pulmonary disease; DLCO = diffusing capacity of the lungs for carbon monoxide; FEV1 = forced expiratory volume in 1 s; LLL = left lower lobe; LOS1 = discharged within 23 hours; LOS2+ = discharged on postoperative day 2 or later; LUL = left upper lobe; OR = odds ratio; PACU = postanesthesia care unit; Ref = reference category; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe.
On the basis of our a priori model building strategy, we performed multivariable logistic regression to identify predictors for length of stay of 23 hours or less (Table 5). Our final model included age, pack-years, clinical stage, surgeon and chest tube duration. Year of surgery, number of comorbidities, surgical complications, operative time, LOS in the postanesthesia care unit, ASA classification, indication for resection, lobe removed, smoking status, DLCO and FEV1 did not improve the predictive accuracy of the model. The performance of the final prediction model is presented is Table 6. The final model displayed good overall fit, good discrimination power (C statistic = 0.8696) and good calibration (Hosmer–Lemeshow χ2 = 54.41, p = 0.98). The most influential predictors were chest tube duration (OR 0.27, 95% CI 0.09–0.81, p = 0.02), clinical stage (OR 0.11, 95% CI 0.01–1.05, p = 0.055) and surgeon (OR 4.98, 95% CI 1.22–20.34, p = 0.025).
Table 5.
Results of multivariable logistic regression
| Predictor | OR (95% CI) | p value |
|---|---|---|
| Age | 1.01 (0.94–1.09) | 0.81 |
| Smoking pack-years | 1.00 (0.99–1.01) | 0.15 |
| Chest tube duration | 0.27 (0.09–0.81) | 0.02 |
| Clinical stage | ||
| I Ref | 0.06 | |
| II–III | 0.11 (0.01–1.05) | |
| Surgeon (D v. A) | 4.98 (1.22–20.34) | 0.025 |
CI = confidence interval; OR = odds ratio; Ref = reference category.
Table 6.
Performance of final prediction model for 23-h length of stay
| Performance measure | Statistic |
|---|---|
| Overall | |
| R2 | 0.2959 |
| Bier score | 0.1092 |
| AIC | 67.50 |
| BIC | 82.08 |
| Discrimination | |
| C statistic | 0.8696 |
| Calibration | |
| Hosmer–Lemeshow χ2 | 54.41* |
AIC = Akaike information criterion; BIC = Bayesian information criterion.
p = 0.98.
Discussion
This study shows that a substantial proportion of patients who undergo VATS lobectomy can be safely discharged from hospital within 23 hours after surgery. Among patients who were discharged early there were no readmissions, no major postoperative complications and, most importantly, no deaths. These findings support the implementation of a new ERP for patients at our institutions; a 23-hour trajectory for patients who undergo VATS lobectomy is now in place. It is now very well documented in the literature that VATS offers significant advantages over open thoracotomy.2–13,17–20 However, most of these studies still reported median LOS between 4 and 7 days for patients undergoing VATS lobectomy. In the fall of 2015, our centre became a regional thoracic surgical oncology referral site exclusively servicing a catchment area with a population upwards of 2 million and a geographic area covering half the area of the province of Quebec (approximately 680 000 km2). In the context of the single-payer public system in Quebec, our site must now manage the high clinical volume from this region with a fixed number of dedicated thoracic surgery beds. It is critical that we ensure that patients flow efficiently through our unit so that we can meet the demands of the population. Noting the improved recovery of our VATS patients after we implemented our ERP, we became motivated to see whether an ultra-fast-track approach was safe and whether it could in turn help us to address our clinical needs more efficiently. Our results indicate that even in the absence of a dedicated VATS lobectomy 23-hour pathway, a discharge rate of 25% within the first 23 hours after surgery was achieved in the later portion of our study period. The findings of this study have led us to modify the patient trajectory described by Madani and colleagues25 to formally address the possibility of a discharge within the first 23 hours.
An ERP for lung resections was implemented at our institution in 2006; ERPs are integrated multidisciplinary, evidence-based protocols standardizing perioperative care, including detailed standardized management plans for all phases of care. The main goal of ERPs is to return the patient to their baseline characteristics by increasing their basal level of prehabilitation, quickening recovery and decreasing postoperative deterioration.32 They have been shown to reduce postoperative complications, morbidity and the cost of patient care.14,16,24,25,26 In thoracic surgery, the daily care plan includes breathing exercises, progressive activities and physiotherapy, measures of pain control, nutrition and targeted removal dates for tubes and lines. Well-designed ERPs are grounded in evidence and multidisciplinarity; however, the evidence evolves and teams change. Hence, it is critical for thoracic surgery teams to reevaluate their ERPs over time and modify them to reflect the evolving nature of the specialty. In our case, the ERP in place up until the publication of this article does not distinguish between open and VATS lobectomies, and the preoperative patient educational material informs the patient to expect a median LOS of between 3 and 4 days. Anecdotally, patient expectations regarding timing of discharge significantly affected our ability to achieve discharge with the first 23 hours; therefore, tailored preoperative education and educational materials consistent with the current management scheme are likely to improve patient satisfaction and facilitate the achievement of target LOS metrics.
Targets for chest tube removal are standardized in our ERP to a threshold of less than 300 mL of drainage in 24 hours with no evidence of air leak or chylothorax.25 Since this ERP was implemented, several publications have reported safety with higher drainage thresholds,33,34 and these have been implemented informally in our unit. Nonetheless, chest tube removal may have been delayed in a number of cases in this series because of an output greater than 300 mL in 24 hours. In our exploratory analysis to determine predictors of discharge within 23 hours, we identified the duration of the chest tube as an influential predictor of 23-hour discharge. Indeed, none of the patients who were discharged within 23 hours were discharged with a chest tube. Beyond drainage thresholds, air leak was 1 of the more common indications for leaving the chest tube in place and therefore one of the primary reasons for later discharge, although this could not be captured in our data set because of the sample limitations of this retrospective study. Air leak is the most common postoperative complication of pulmonary resections and it is known to substantially increase LOS and health care costs.33 Patients can, however, be safely discharged with portable draining systems, allowing this common complication to be managed on an outpatient basis.36,36 Thus, introducing portable drainage systems that can be safely managed by the patient at home would constitute a substantial advantage in terms of increasing the proportion of patients discharged within 23 hours. The safety and cost effectiveness of such an approach, with chest tube removal being performed in an outpatient clinic, remains to be determined.
In the era of performance-based compensation, readmissions after thoracic surgery are a significant concern for many thoracic surgery units. Hence, there may be substantial reluctance to discharge patients early because of the risk of readmission. It is important to note that none of our patients who were discharged within 23 hours were readmitted after their discharge. We are confident in the accuracy of this finding because we have a dedicated nurse clinician who contacts all patients in the postoperative period to track their progress and takes note of all readmissions, even if they are at another hospital. This finding further demonstrates the potential benefits of such an ultra-fast-track pathway, but the pathway still requires outside validation and a larger sample size to prove its cost effectiveness in different health care systems. Rajaram and colleagues reported an 8.4% readmission rate after VATS lobectomy.37 In their study, the strongest predictor of readmission was an inpatient complication following the first surgery. In comparison, the readmission rate of our overall cohort was 4.4%.
Postoperative pain is a modifiable factor that has been addressed by various approaches over the years. Initially, our VATS patients had epidural catheters inserted preoperatively; however, now these catheters are seldom used and they are reserved for the rare patient who requires a conversion to thoracotomy, in whom it is placed postoperatively. At our centre, all patients are now treated with regional analgesia using multilevel intercostal nerve blocks with 20 mL of 0.5% ropivacaine mixed with 1% epinephrine and 10 mg of dexamethasone, injected under thoracoscopic guidance immediately after entering the chest. Long-acting liposomal formulations are not currently available in Canada; given their demonstrated effectiveness for minimally invasive and open thoracic procedures, it is likely that such products could further improve our 23-hour trajectory rate.38
We wished to explore whether there were preoperative predictors to determine the patients for whom a VATS lobectomy 23-hour trajectory would be most suitable. The final model selected, on the basis of the best overall fit, discrimination and calibration statistics, included clinical stage, age, pack-years and surgeon as preoperative predictors of discharge within 23 hours. We hypothesize that patients with higher-stage lesions may require more complex and longer surgery and may have higher rates of perioperative surgical complications. In our series, patients discharged on postoperative day 2 or later did indeed have longer operative times, which may reflect the higher clinical and pathologic stages of the lesions of patients in this cohort. Interestingly, the odds of patients undergoing pulmonary metastatectomy was 4.55 higher in the cohort discharged within 23 hours. Although the indication for lobectomy did not improve model prediction, it stands to reason that patients undergoing metastatectomy may have superior lung function and a less extensive smoking history than patients with primary lung cancer. Moreover, many patients who undergo surgery at our centre are referred from remote areas. We expected patients who lived farther than 100 km from our centre to have longer hospital stays. However, when we included distance from hospital in our model it did not improve prediction accuracy; this may be have been due to insufficient power. A widely used guideline suggests having 10 events for 1 candidate predictor. In our study sample, we had 34 events, which would allow around 3 or 4 predictors to be included in the model. Our model had good calibration and discrimination even with 5 predictors; however, the small number of events did limit the inclusion of other pertinent factors. Our approach going forward will be to include all patients undergoing VATS lobectomy into our 23-hour trajectory, with the expectation that patients with more advanced stage disease will probably require longer hospital stays.
Limitations
The limitations of this study are those inherent to a retrospective cohort study. However, most of the data in our database are prospectively collected, thus reducing the introduction of recall or documentation bias. The fact that this was a single-centre study involving primarily 2 surgeons at different phases of their career restricts the generalizability of the findings; the results should be externally validated before our pathway is implemented in other centres. Given this limitation, it is difficult to determine surgeon-specific differences in practice that may allow for earlier discharge. However, this is a subject of active study via positive deviance seminars.39 Our study is also limited by the lack of data on chest tube drainage volumes, which constitute strict benchmarks for many thoracic surgeons. Finally, we did not quantify the degree of air leak. This is a potentially important metric, particularly in the era of digital thoracic drainage systems or when consideration is being given to early chest tube removal and discharge home with chest tubes in place.
Conclusion
Our study suggests that an ultra-fast-track VATS lobectomy pathway is feasible, and our rate of 23-hour trajectories more than doubled in the last year of the study. We showed that it is a safe approach with low complication rates, very few returns to the emergency department, no readmissions and no deaths, despite the absence of a formal protocol. We did not identify any factors that clearly precluded a patient from being enrolled in a VATS lobectomy 23-hour trajectory. In the future, we would like to trial the use of long acting liposomal formulations for intercostal nerve blockade with the hope that this may further diminish postoperative pain. In addition, portable chest tube collection devices that can easily be managed on an outpatient basis could further reduce the need for hospitalization beyond the 23-hour window. Hence, a VATS lobectomy 23-hour pathway is feasible for as many as 25% of patients. Prospective multisurgeon and multicentre studies will be required to further demonstrate the safety, efficacy and potential pitfalls of this type of ultra-fast-track pathway.
Footnotes
Presented at the Canadian Association of Thoracic Surgeons Annual Meeting, Canadian Surgery Forum, Sept. 16, 2017, Victoria, B.C., and at the European Society of Thoracic Surgeons Annual Meeting, May 28–31, 2017, Innsbruck, Austria.
Competing interests: None declared.
Contributors: T.-C. Dumitra, J.-C. Molina, D. Mulder, L. Ferri and J. Spicer designed the study. T.-C. Dumitra, J.-C. Molina, J. Mouhanna, A. Siblini, D. Mulder and J. Spicer acquired the data, which T.-C. Dumitra, J.-C. Molina, J. Mouhanna, I. Nicolau, S. Renaud, D. Mulder and J. Spicer analyzed. T.-C. Dumitra, J.-C. Molina, J. Mouhanna, I. Nicolau and J. Spicer wrote the article, which all authors critically reviewed. All authors gave final approval of the article to be published.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Kaseda S, Aoki T, Hangai N, et al. Better pulmonary function and prognosis with video-assisted thoracic surgery than with thoracotomy. Ann Thorac Surg. 2000;70:1644–6. doi: 10.1016/s0003-4975(00)01909-3. [DOI] [PubMed] [Google Scholar]
- 3.Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg. 2013;16:244–9. doi: 10.1093/icvts/ivs472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begum S, Hansen HJ, Papagiannopoulos K. VATS anatomic lung resections—the European experience. J Thorac Dis. 2014;6(Suppl 2):S203–10. doi: 10.3978/j.issn.2072-1439.2014.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen HJ, Petersen RH. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach - the Copenhagen experience. Ann Cardiothorac Surg. 2012;1:70–6. doi: 10.3978/j.issn.2225-319X.2012.04.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86:2008–16. doi: 10.1016/j.athoracsur.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Deng B, Cassivi SD, de Andrade M, et al. Clinical outcomes and changes in lung function after segmentectomy versus lobectomy for lung cancer cases. J Thorac Cardiovasc Surg. 2014;148:1186–1192.e3. doi: 10.1016/j.jtcvs.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicastri DG, Wisnivesky JP, Litle VR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg. 2008;135:642–7. doi: 10.1016/j.jtcvs.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non–small-cell lung cancer. J Clin Oncol. 2009;27:2553–62. doi: 10.1200/JCO.2008.18.2733. [DOI] [PubMed] [Google Scholar]
- 10.Kuritzky AM, Aswad BI, Jones RN, et al. Lobectomy by video-assisted thoracic surgery vs muscle-sparing thoracotomy for stage I lung cancer: a critical evaluation of short- and long-term outcomes. J Am Coll Surg. 2015;220:1044–53. doi: 10.1016/j.jamcollsurg.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 11.Chen FF, Zhang D, Wang YL, et al. Video-assisted thoracoscopic surgery lobectomy versus open lobectomy in patients with clinical stage I non-small cell lung cancer: a meta-analysis. Eur J Surg Oncol. 2013;39:957–63. doi: 10.1016/j.ejso.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17:836–44. doi: 10.1016/S1470-2045(16)00173-X. [DOI] [PubMed] [Google Scholar]
- 13.Jiang G, Yang F, Li X, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for administration of adjuvant chemotherapy after lobectomy for non-small cell lung cancer. World J Surg Oncol. 2011;9:170. doi: 10.1186/1477-7819-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, Ferri LE, Mulder DS, et al. An enhanced recovery pathway decreases duration of stay after esophagectomy. Surgery. 2012;152:606–16. doi: 10.1016/j.surg.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Lee L, Li C, Robert N, et al. Economic impact of an enhanced recovery pathway for oesophagectomy. Br J Surg. 2013;100:1326–34. doi: 10.1002/bjs.9224. [DOI] [PubMed] [Google Scholar]
- 16.Molina JC, Misariu AM, Nicolau I, et al. Same day discharge for benign laparoscopic hiatal surgery: a feasibility analysis. Surg Endosc. 2018;32:937–44. doi: 10.1007/s00464-017-5769-5. [DOI] [PubMed] [Google Scholar]
- 17.Yang CJ, Kumar A, Klapper JA, et al. A national analysis of long-term survival following thoracoscopic versus open lobectomy for stage I non-small-cell lung cancer. Ann Surg. 2019;269:163–71. doi: 10.1097/SLA.0000000000002342. [DOI] [PubMed] [Google Scholar]
- 18.Eustache J, Ferri LE, Feldman LS, et al. Enhanced recovery after pulmonary surgery. J Thorac Dis. 2018;10(Suppl 32):S3755–60. doi: 10.21037/jtd.2018.09.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 2009;138:11–8. doi: 10.1016/j.jtcvs.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 20.Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg. 2012;93:1027–32. doi: 10.1016/j.athoracsur.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Lujan JA, Parrilla P, Robles R, et al. Laparoscopic cholecystectomy vs open cholecystectomy in the treatment of acute cholecystitis: a prospective study. Arch Surg. 1998;133:173–5. doi: 10.1001/archsurg.133.2.173. [DOI] [PubMed] [Google Scholar]
- 22.Ezer N, Veluswamy RR, Mhango G, et al. Outcomes after stereotactic body radiotherapy versus limited resection in older patients with early-stage lung cancer. J Thorac Oncol. 2015;10:1201–6. doi: 10.1097/JTO.0000000000000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–7. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiore JF, Bejjani J, Conrad K, et al. Systematic review of the influence of enhanced recovery pathways in elective lung resection. J Thorac Cardiovasc Surg. 2016;151:708–715.e6. doi: 10.1016/j.jtcvs.2015.09.112. [DOI] [PubMed] [Google Scholar]
- 25.Madani A, Fiore JF, Wang Y, et al. An enhanced recovery pathway reduces duration of stay and complications after open pulmonary lobectomy. Surgery. 2015;158:899–908. doi: 10.1016/j.surg.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 26.Paci P, Madani A, Lee L, et al. Economic impact of an enhanced recovery pathway for lung resection. Ann Thorac Surg. 2017;104:950–7. doi: 10.1016/j.athoracsur.2017.05.085. [DOI] [PubMed] [Google Scholar]
- 27.Ivanovic J, Seely AJE, Anstee C, et al. Measuring surgical quality: comparison of postoperative adverse events with the American College of Surgeons NSQIP and the Thoracic Morbidity and Mortality classification system. J Am Coll Surg. 2014;218:1024–31. doi: 10.1016/j.jamcollsurg.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 28.Vittinghoff E, Glidden D, Shiboski S, McCulloch C. Regression methods in biostatistics. 2nd ed. New York (NY): Springer; 2012. [Google Scholar]
- 29.Steyerberg EW. Clinical prediction models: a practical approach to development, validation, and updating. New York (NY): Springer; 2009. [Google Scholar]
- 30.Iezzoni LI, editor. Risk adjustment for measuring health care outcomes. Ann Arbour (MI): Foundation of the American College of Healthcare Executives; 1994. [Google Scholar]
- 31.Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e166S–e190S. doi: 10.1378/chest.12-2395. [DOI] [PubMed] [Google Scholar]
- 32.Lee L, Tran T, Mayo NE, et al. What does it really mean to “recover” from an operation? Surgery. 2014;155:211–6. doi: 10.1016/j.surg.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Yap KH, Soon JL, Ong BH, et al. The safe volume threshold for chest drain removal following pulmonary resection. Interact Cardiovasc Thorac Surg. 2017;25:822–6. doi: 10.1093/icvts/ivx161. [DOI] [PubMed] [Google Scholar]
- 34.Cerfolio RJ. Chest tube management after pulmonary resection. Chest Surg Clin N Am. 2002;12:507–27. doi: 10.1016/s1052-3359(02)00015-7. [DOI] [PubMed] [Google Scholar]
- 35.Varela G, Jiménez MF, Novoa N. Portable chest drainage systems and outpatient chest tube management. Thorac Surg Clin. 2010;20:421–6. doi: 10.1016/j.thorsurg.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Royer AM, Smith JS, Miller A, et al. Safety of outpatient chest tube management of air leaks after pulmonary resection. Am Surg. 2015;81:760–3. [PubMed] [Google Scholar]
- 37.Rajaram R, Ju MH, Bilimoria KY, et al. National evaluation of hospital readmission after pulmonary resection. J Thorac Cardiovasc Surg. 2015;150:1508–1514.e2. doi: 10.1016/j.jtcvs.2015.05.047. [DOI] [PubMed] [Google Scholar]
- 38.Rice DC, Cata JP, Mena GE, et al. Posterior intercostal nerve block with liposomal bupivacaine: an alternative to thoracic epidural analgesia. Ann Thorac Surg. 2015;99:1953–60. doi: 10.1016/j.athoracsur.2015.02.074. [DOI] [PubMed] [Google Scholar]
- 39.Ivanovic J, Antsee C, Ramsay T, et al. Using surgeon-specific outcome reports and positive deviance for continuous quality improvement. Ann Thorac Surg. 2015;100:1188–94. doi: 10.1016/j.athoracsur.2015.04.012. [DOI] [PubMed] [Google Scholar]