Abstract
Background and study aims Based on pathology, locally resected T1 colorectal cancer (T1-CRC) can be classified as having low- or high-risk for irradicality and/or lymph node metastasis, the latter requiring adjuvant surgery. Reporting and application of pathological high-risk criteria is likely variable, with inherited variation regarding baseline oncological staging, treatment and surveillance.
Methods We assessed practice variation using an online survey among gastroenterologists and surgeons participating in the Dutch T1-CRC Working Group.
Results Of the 130 invited physicians, 53 % participated. Regarding high-risk T1-CRC criteria, lymphangio-invasion is used by 100 %, positive or indeterminable margins by 93 %, poor differentiation by 90 %, tumor-free margin ≤ 1 mm by 78 %, tumor budding by 57 % and submucosal invasion > 1000 µm by 47 %. Fifty-two percent of the respondents do not perform baseline staging in locally resected low-risk T1-CRC. In case of unoperated high-risk patients, we recorded 61 different surveillance strategies in 63 participants, using 19 different combinations of diagnostic tests. Endoscopy is used in all schedules. Mean follow-up time is 36 months for endoscopy, 26 months for rectal MRI and 30 months for abdominal CT (all varying 3–60 months).
Conclusion We found variable use of pathological high-risk T1-CRC criteria, creating risk for misclassification as low-risk T1-CRC. This has serious implications, as most participants will not proceed to oncological staging in low-risk patients and adjuvant surgery nor radiological surveillance is considered. On the other hand, oncological surveillance in patients with a locally resected high-risk T1-CRC who do not wish adjuvant surgery is highly variable emphasizing the need for a uniform surveillance protocol.
Introduction
Due to implementation of colorectal cancer screening programs, detection of submucosal invasive colorectal cancers (T1-CRCs) has increased 1 2 , with additional opportunities for local excisions.
Based on several pathological criteria, T1-CRCs can be divided into low risk or high risk for residual intramural cancer or lymph node metastases (LNM). However, there is variation between models using various pathological criteria contributing to a high-risk status ( Table 1 ). Availability of these significantly different models could lead to practice variation. In high-risk T1-CRC, adjuvant oncological resection should be discussed with the patient. On the other hand, in low-risk T1-CRC, baseline oncological staging is often considered unnecessary, as is additional oncological resection, due to a very low recurrence rate of 0.8 % 3 4 5 . As a consequence, (mis)classification as low-risk has important implications for the patient.
Table 1. Available pathological risk criteria and the use in various definitions.
| Lymphangio-invasion | Poor or signet cell differentiation | Grade 2–3 tumor budding | ≥ 1 mm submucosal invasion | Resection margin not free or indeterminable | Margin free, but tumor-free margin < 1 mm | |
| Dutch guideline 11 | x | x | x | uncertain | ||
| Scottish model 12 | x | x | ||||
| French model 13 | x | x | x | |||
| Japanese guideline 14 | x | x | x | x | x |
“x” confers high-risk status.
In patients with high-risk T1-CRC, there is an increasing trend towards “wait and see” with surveillance chosen by the patient on the basis of shared decision-making. This is because risk of residual cancer in the bowel wall and lymph node metastasis is 3 % to 8 % 3 6 7 8 and 12 % to 16 % 4 5 6 , respectively. Accordingly, more than 84 % of these patients have additional surgery without clear benefit while being exposed to surgical risks of morbidity (20 %–30 %), ostomy (5 %–10 %), and mortality (2.5 %) 9 10 . However, no guideline is available with advice on a specific oncological surveillance strategy as an alternative for these patients 11 .
Various definitions and applications of pathological high-risk criteria may contribute to practice variation with potential consequences for treatment, baseline oncological staging, and surveillance of patients after local excision of T1-CRC. We investigated variations in personal daily practice about T1-CRC among dedicated gastroenterologists and surgeons.
Methods
Survey development and participants
We developed a structured online survey regarding current clinical practice of patients after local excision of a T1 CRC, with the focus on four main topics: use of pathological high-risk criteria, baseline oncological staging before local excision of suspected T1-CRC, oncological staging after local excision and surveillance of unoperated high-risk T1-CRC patients. The patient’s situation was only globally addressed in the survey, for instance as a hypothetical patient, being fit for surgery, but we could not focus on individual patient details as this would render the survey impractical to fill out and difficult to analyze. This study was not intended to provide statistics on the type of local excision and the occurrence of rectal versus colonic locations. We asked the participants to answer the questions according to their own habits, which usually included counseling of the multidisciplinary oncological meeting. The survey consisted of 15 multiple choice and two open questions with examples of clinical cases (supplementary Appendix), based on guidelines and gastroenterologist’s expert opinion.
The survey was published as a Google Form between January and February 2019 and tested and improved by dedicated gastroenterologists for applicability, clarity and content. We collected data on demographics of participants and their institutes, but no data which could identify the participant or the center of employment to guarantee anonymity. Participants received an e-mail with a cover letter and a link to the online survey, with a reminder after 2 weeks. Responses were accepted up to 4 weeks from the initial e-mail. Participants comprised all gastroenterologists and surgeons who are members of the Dutch T1 CRC Working group. This is a group of Dutch specialists in the field of T1 CRC, with joint research efforts and regular meetings.
Because the survey was based on voluntary participation and information disclosure, the study protocol did not need to undergo a formal review by an Ethics Committee. Survey return was taken as consent.
Follow-up investigations were grouped as: carcinoembryonic antigen (CEA); endoscopy; perirectal imaging (rectal endo-ultrasonography and magnetic resonance imaging [MRI]); abdominal lymph node imaging (abdominal computed tomography [CT] scan); liver imaging (abdominal CT scan or liver ultrasound) and lung imaging (chest x-ray and thoracic CT scan).
Statistics
We used descriptive statistics to analyze the results using counts and proportions for categorial data and means and standard deviations for continuous variables. Missing values were not imputed. The answers to most questions regarding stage determination and surveillance were tested against the type of hospital, specialty, age group and gender using chi-square. Statistical analysis was performed with IBM SPSS version 25.
Results
The survey was sent to 130 physicians and returned by 69 (53 % participation grade). Table 2 lists baseline characteristics of the participants. Eighty-seven percent of the participants were gastroenterologists (60/69), 16 % were employed in an academic center. Ninety-six percent of physicians reported discussing every patient with a T1 CRC in a regular multidisciplinary oncological meeting. Only 21 of 69 (30 %) of the physicians reported having a local T1 CRC protocol available in their hospital.
Table 2. Demographic characteristics of the participants.
| Variable | n (%) |
| Specialist | |
|
8 (12 %) |
|
60 (87 %) |
|
1 (1 %) |
| Type of hospital | |
|
11 (16 %) |
|
6 (9 %) |
|
34 (49 %) |
|
16 (23 %) |
|
2 (3 %) |
| Sex | |
|
49 (71 %) |
|
20 (29 %) |
| Age group | |
|
21 (30 %) |
|
29 (42 %) |
|
15 (22 %) |
|
4 (6 %) |
Risk stratification
Pathological criteria used for low-risk or high-risk classification are shown in Fig. 1 . Undisputed criteria for high risk in the Netherlands are lymphovascular invasion, a resection margin that is not free or indeterminable, and poor differentiation or presence of signet cells 12 . Eighty-one percent of the respondents used all three criteria. Tumor budding is not currently standardly reported by pathology labs in the Netherlands and was used by half of the respondents. Contribution of both a tumor-free margin ≤ 1 mm and invasion depth to high-risk status are disputed and were less often used. Fifty percent of academic participants did not use a tumor-free margin ≤ 1 mm as a high-risk criterion, whereas 80 % of non-academic specialists did ( P = 0.044). The other criteria did not show significant differences between academic and non-academic specialists.
Fig. 1 .
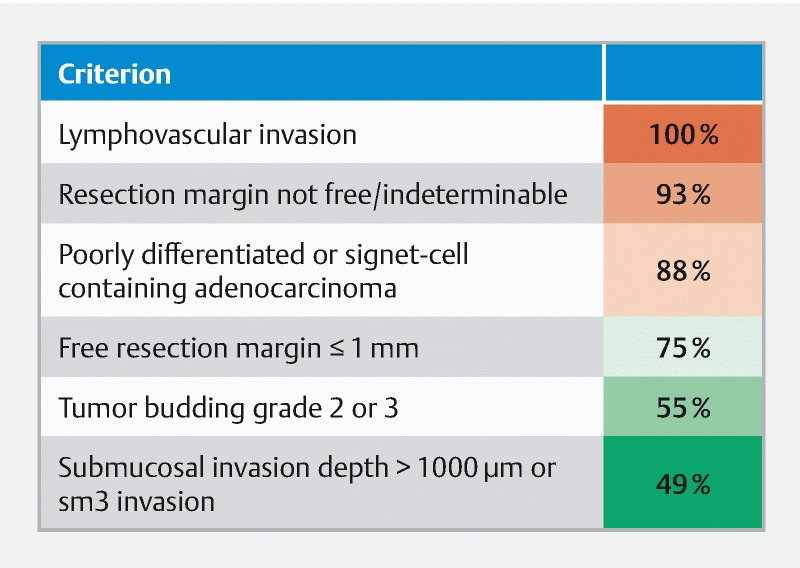
Heat map of pathology criteria used by respondents to define risk status.
Only twelve (17 %) of the participants used the exact criteria from the Dutch Guideline to determine high risk ( Table 1 ) 12 . The Scottish and French models 13 14 were used by none of the participants, the Japanese guideline 15 by five (7.2 %).
Baseline oncological staging
In the example case of a macroscopically recognized carcinoma during endoscopy, 13 (19 %) would perform oncological staging prior to local excision, whereas five (7 %) would only do so in case of rectal localization.
Of those who would not perform a priori staging (51 respondents), 53 % would stage every patient if pathology confirms a T1-CRC (low-risk and high-risk), while 47 % would do so only in case of one or more high-risk features.
When confronted with an unexpected carcinoma (“oops”-carcinoma) in a locally removed polyp, 49 % would only stage in case of high-risk features, the remainder in every case. We examined associations between type of hospital, specialty, sex, age groups, and baseline staging practices. No statistically significant associations were found, although it was remarkable that staging of suspected lesions before embarking on endoscopic resection was not performed by any of the nine academic respondents. Taken together, approximately 50 % will not perform baseline oncological staging in case of locally resected low-risk T1-CRC.
Surveillance after locally resected high-risk T1 CRC in patients who refrain from additional surgery
Details about surveillance strategies were returned by 63 participants; most participants (84 %) indicated using the same surveillance strategies after local excision of T1 CRC by submucosal dissection or full-thickness local resection techniques (such as trans-anal endoscopic microsurgery, or endoscopic full-thickness resection). On closer look, we encountered 61 different schedules, with 19 different combinations of diagnostic tests. Endoscopy is used in all schedules. Endoscopic surveillance has a median duration of 48 months (IQR 24–48 months). Radiological surveillance (including rectal endoultrasonography) has a median duration of 36 months (IQR 12–48 months), and CEA monitoring time has a median duration of 48 months (IQR 24–48 months). A heat map of the time points and follow-up investigations is shown in Fig. 2 . Significant differences between academic and non-academic respondents are summarized in Fig. 3 . In general, more follow-up investigations are performed by academic specialists, with higher use of CT-scans.
Fig. 2 .
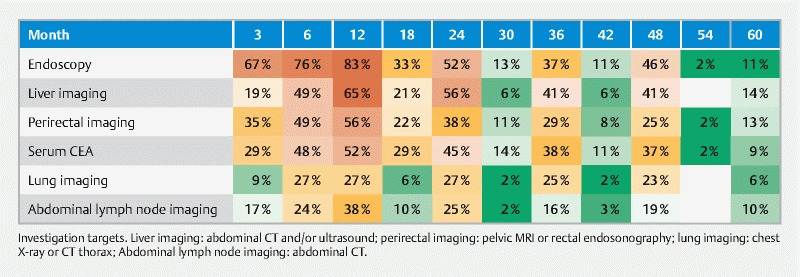
Heat map of investigation targets.
Fig. 3 .
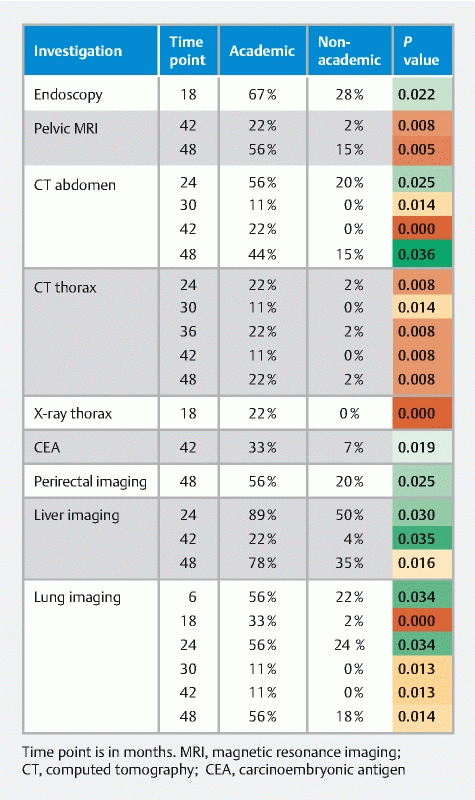
Significant differences between academic and non-academic specialists in surveillance time points and investigations
Most participants would proceed to surgery (n = 50) when local recurrence or suspicious lymphadenopathy is demonstrated during follow-up, six of them suggested also performing this in case of limited metastasis, and two respondents in case of increasing CEA levels.
Discussion
In this study, considerable practice variation among Dutch hospitals was observed in risk classification used to predict lymph node metastasis and local recurrence, on baseline oncological staging and especially on follow-up after local excision of a T1 CRC with high-risk features, but while refraining from performing surgery.
It is well known that pathological criteria that contribute to a high-risk status of a locally resected T1-CRC are subject to interobserver variation by pathologists 16 17 . Indeed, a substantial percentage of these lesions are reclassified as high risk after revision by an expert pathologist 18 . However, in the Netherlands, a decision about T1-CRC risk status is made by the treating physician, and not by the pathologist, who merely reports on presence or absence of these risk factors. Thus, it is important to understand how treating physicians deal with this information. In our survey, directed at specialists with special interest in T1-CRC, we encountered considerable practice variation.
Previous studies show conflicting results concerning the true value of submucosal invasion depth ≥ 1000 µm and a tumor-free margin < 1 mm as a high-risk pathological factor 8 19 20 21 22 23 . Indeed, this survey found that submucosal invasion depth ≥ 1000 µm is used by 49 % of physicians and a tumor-free margin < 1 mm by 75 %. Although lymphangio-invasion, poor differentiation, and a positive or indeterminable resection margin are undisputed risk factors 6 12 19 20 21 22 24 25 26 , 19 % of respondents do not use all three criteria. In the literature, high-grade tumor budding is a strong risk factor as well 19 20 21 26 , but this criterion was used by half of the respondents. This is probably explained by the fact that, in the Netherlands, reporting of tumor budding is currently not obligatory.
Taken together, incomplete use of pathological high-risk factors carries the risk of wrong low-risk classification of a locally resected T1-CRC.
Misclassification as low-risk T1-CRC has important consequences. Our study showed that half the physicians do not perform baseline oncological staging in these patients (which is theoretically defensible as risk of metastasized disease in true low-risk T1-CRC is only 0.8 % 3 4 5 ).
In addition, the option of adjuvant surgical resection will not be discussed in low-risk T1-CRC and surveillance is only performed according to the current polyp surveillance guideline using merely colonoscopy 27 .
In the second part of the survey, we addressed surveillance practices of physicians in patients after local excision of a high-risk T1-CRC without adjuvant surgery.
We found enormous practice variation among physicians, which underscores the need for a uniform surveillance protocol.
We recognize, however, that such a protocol is difficult to conceive. Literature on surveillance of unoperated high-risk T1-CRC is scanty and retrospective. Asian studies describe use of CEA and thoraco-abdominal CT every 6 months for 3 years, thereafter every 12 months for 2 years, combined with annual colonoscopy 4 28 . A less invasive strategy with CEA, CT of the chest, abdomen, and pelvis, and a colonoscopy every year for 5 years was used by Kouyama et al 25 . However, the benefit of these surveillance strategies has not been established. In particular, baseline radiological oncological staging of lymph nodes in colorectal cancer is hampered by moderate sensitivity and specificity of approximately 75 % 29 30 31 32 33 34 35 36 , and there exists no literature on the benefits of sequential imaging over time.
Our study has some limitations.
Only physicians participating in the T1-CRC working group were invited, which may create response bias towards experts in the field of T1-CRC. Accordingly, the reader should be aware of the fact that application of pathological high-risk criteria may be more incomplete in less involved physicians.
It should be noted that only some of the questions addressed the rectum and colon separately. Although this had no influence on our findings regarding application of pathological high-risk criteria and oncological staging, surveillance strategies differed between these tumor locations.
Each respondent provided his or her own opinion. Although reflecting the usual outcome of the multidisciplinary oncology meetings of the local hospital was encouraged, it is possible that the actual advice from a multidisciplinary meeting would have been different from the answers provided by the respondents. Nevertheless, the answers do reflect an overview of the respondents’ personal habits.
We limited patient details in our questionnaires to the most important to improve generalization, for instance “fit for surgery,” while realizing that patient-specific additional factors will play a role in daily practice.
Conclusion
In conclusion, we encountered considerable practice variation with regard to use of pathological high-risk T1-CRC criteria, with potential misclassification as low-risk T1-CRC. This has serious consequences, as a lot of these patients will not have baseline oncological staging and counseling about adjuvant surgery, or radiological surveillance. For the growing group of patients who do not wish to have adjuvant surgery in case of locally resected high-risk T1-CRC, a standardized oncological surveillance protocol is urgently needed.
Acknowledgements
This work was funded by the Boks Scholten Foundation and the Hans Diels Foundation.
Footnotes
Competing interests The authors declare that they have no conflict of interest.
Supplementary material :
References
- 1.Mengual-Ballester M, Pellicer-Franco E, Valero-Navarro G et al. Increased survival and decreased recurrence in colorectal cancer patients diagnosed in a screening programme. Cancer Epidemiol. 2016;43:70–75. doi: 10.1016/j.canep.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Levin T R, Corley D A, Jensen C D et al. Effects of Organized Colorectal Cancer Screening on Cancer Incidence and Mortality in a Large Community-Based Population. Gastroenterology. 2018;155:1383–1391 e1385. doi: 10.1053/j.gastro.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backes Y, de Vos Tot Nederveen Cappel W H, van Bergeijk J et al. Risk for Incomplete Resection after Macroscopic Radical Endoscopic Resection of T1 Colorectal Cancer: A Multicenter Cohort Study. Am J Gastroenterol. 2017;112:785–796. doi: 10.1038/ajg.2017.58. [DOI] [PubMed] [Google Scholar]
- 4.Ikematsu H, Yoda Y, Matsuda T et al. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology. 2013;144:551–559; quiz e514. doi: 10.1053/j.gastro.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Yoda Y, Ikematsu H, Matsuda T et al. A large-scale multicenter study of long-term outcomes after endoscopic resection for submucosal invasive colorectal cancer. Endoscopy. 2013;45:718–724. doi: 10.1055/s-0033-1344234. [DOI] [PubMed] [Google Scholar]
- 6.Belderbos T D, van Erning F N, de Hingh I H et al. Long-term Recurrence-free Survival After Standard Endoscopic Resection Versus Surgical Resection of Submucosal Invasive Colorectal Cancer: A Population-based Study. Clin Gastroenterol Hepatol. 2017;15:403–411 e401. doi: 10.1016/j.cgh.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Benizri E I, Bereder J M, Rahili A et al. Additional colectomy after colonoscopic polypectomy for T1 colon cancer: a fine balance between oncologic benefit and operative risk. Int J Colorectal Dis. 2012;27:1473–1478. doi: 10.1007/s00384-012-1464-0. [DOI] [PubMed] [Google Scholar]
- 8.Shin J W, Han K S, Hyun J H et al. Risk of recurrence after endoscopic resection of early colorectal cancer with positive margins. Endoscopy. 2018;50:241–247. doi: 10.1055/s-0043-120441. [DOI] [PubMed] [Google Scholar]
- 9.Richards C H, Leitch F E, Horgan P G et al. A systematic review of POSSUM and its related models as predictors of post-operative mortality and morbidity in patients undergoing surgery for colorectal cancer. J Gastrointest Surg. 2010;14:1511–1520. doi: 10.1007/s11605-010-1333-5. [DOI] [PubMed] [Google Scholar]
- 10.Vermeer N CA, Backes Y, Snijders H S et al. National cohort study on postoperative risks after surgery for submucosal invasive colorectal cancer. BJS Open. 2019;3:210–217. doi: 10.1002/bjs5.50125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutch Working Group for Gastrointestinal Tumours Dutch Colorectal Cancer Guideline 2014 http://www.oncoline.nl/colorectaalcarcinoom(Accessed on 7 October 2019)
- 12.Boulkedid R, Abdoul H, Loustau M et al. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One. 2011;6:e20476. doi: 10.1371/journal.pone.0020476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards C H, Ventham N T, Mansouri D et al. An evidence-based treatment algorithm for colorectal polyp cancers: results from the Scottish Screen-detected Polyp Cancer Study (SSPoCS) Gut. 2018;67:299–306. doi: 10.1136/gutjnl-2016-312201. [DOI] [PubMed] [Google Scholar]
- 14.Lopez A, Bouvier A M, Jooste V et al. Outcomes following polypectomy for malignant colorectal polyps are similar to those following surgery in the general population. Gut. 2019;68:111–117. doi: 10.1136/gutjnl-2016-312093. [DOI] [PubMed] [Google Scholar]
- 15.Hashiguchi Y, Muro K, Saito Y et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2019 doi: 10.1007/s10147-019-01485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peck M, Moffat D, Latham B et al. Review of diagnostic error in anatomical pathology and the role and value of second opinions in error prevention. J Clin Pathol. 2018;71:995–1000. doi: 10.1136/jclinpath-2018-205226. [DOI] [PubMed] [Google Scholar]
- 17.Davenport A, Morris J, Pritchard S A et al. Interobserver variability amongst gastrointestinal pathologists in assessing prognostic parameters of malignant colorectal polyps: a cause for concern. Tech Coloproctol. 2016;20:647–652. doi: 10.1007/s10151-016-1513-8. [DOI] [PubMed] [Google Scholar]
- 18.Rampioni Vinciguerra GL, Antonelli G, Citron F et al. Pathologist second opinion significantly alters clinical management of pT1 endoscopically resected colorectal cancer. Virchows Arch. 2019 doi: 10.1007/s00428-019-02603-y. [DOI] [PubMed] [Google Scholar]
- 19.Bosch S L, Teerenstra S, de Wilt J H et al. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013;45:827–834. doi: 10.1055/s-0033-1344238. [DOI] [PubMed] [Google Scholar]
- 20.Yasue C, Chino A, Takamatsu M et al. Pathological risk factors and predictive endoscopic factors for lymph node metastasis of T1 colorectal cancer: a single-center study of 846 lesions. J Gastroenterol. 2019 doi: 10.1007/s00535-019-01564-y. [DOI] [PubMed] [Google Scholar]
- 21.Tamaru Y, Oka S, Tanaka S et al. Long-term outcomes after treatment for T1 colorectal carcinoma: a multicenter retrospective cohort study of Hiroshima GI Endoscopy Research Group. J Gastroenterol. 2017;52:1169–1179. doi: 10.1007/s00535-017-1318-1. [DOI] [PubMed] [Google Scholar]
- 22.Meining A, von Delius S, Eames T M et al. Risk factors for unfavorable outcomes after endoscopic removal of submucosal invasive colorectal tumors. Clin Gastroenterol Hepatol. 2011;9:590–594. doi: 10.1016/j.cgh.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Kouyama Y, Kudo S E, Miyachi H et al. Practical problems of measuring depth of submucosal invasion in T1 colorectal carcinomas. Int J Colorectal Dis. 2016;31:137–146. doi: 10.1007/s00384-015-2403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Z Q, Ma S, Zhou Q B et al. Prognostic value of lymph node metastasis in patients with T1-stage colorectal cancer from multiple centers in China. World J Gastroenterol. 2017;23:8582–8590. doi: 10.3748/wjg.v23.i48.8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kouyama Y, Kudo S E, Miyachi H et al. Risk factors of recurrence in T1 colorectal cancers treated by endoscopic resection alone or surgical resection with lymph node dissection. Int J Colorectal Dis. 2018;33:1029–1038. doi: 10.1007/s00384-018-3081-z. [DOI] [PubMed] [Google Scholar]
- 26.Di Gregorio C, Bonetti L R, de Gaetani C et al. Clinical outcome of low- and high-risk malignant colorectal polyps: results of a population-based study and meta-analysis of the available literature. Intern Emerg Med. 2014;9:151–160. doi: 10.1007/s11739-012-0772-2. [DOI] [PubMed] [Google Scholar]
- 27.NVMDL (Dutch Association of Gastroenterology) Dutch Guideline Colonoscopy surveillance 2013 http://www.mdl.nl/kwaliteitszaken/richtlijnen(Accessed on 7 October 2019)
- 28.Yamashita K, Oka S, Tanaka S et al. Long-term prognosis after treatment for T1 carcinoma of laterally spreading tumors: a multicenter retrospective study. Int J Colorectal Dis. 2019;34:481–490. doi: 10.1007/s00384-018-3203-7. [DOI] [PubMed] [Google Scholar]
- 29.Bipat S, Glas A S, Slors F J et al. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773–783. doi: 10.1148/radiol.2323031368. [DOI] [PubMed] [Google Scholar]
- 30.Li X T, Sun Y S, Tang L et al. Evaluating local lymph node metastasis with magnetic resonance imaging, endoluminal ultrasound and computed tomography in rectal cancer: a meta-analysis. Colorectal Dis. 2015;17:O129–135. doi: 10.1111/codi.12909. [DOI] [PubMed] [Google Scholar]
- 31.Bogach J, Tsai S, Zbuk K et al. Quality of preoperative pelvic computed tomography (CT) and magnetic resonance imaging (MRI) for rectal cancer in a region in Ontario: A retrospective population-based study. J Surg Oncol. 2018;117:1038–1042. doi: 10.1002/jso.25000. [DOI] [PubMed] [Google Scholar]
- 32.de Vries F E, da Costa D W, van der Mooren K et al. The value of pre-operative computed tomography scanning for the assessment of lymph node status in patients with colon cancer. Eur J Surg Oncol. 2014;40:1777–1781. doi: 10.1016/j.ejso.2014.08.483. [DOI] [PubMed] [Google Scholar]
- 33.Leufkens A M, van den Bosch M A, van Leeuwen M S et al. Diagnostic accuracy of computed tomography for colon cancer staging: a systematic review. Scand J Gastroenterol. 2011;46:887–894. doi: 10.3109/00365521.2011.574732. [DOI] [PubMed] [Google Scholar]
- 34.Dighe S, Purkayastha S, Swift I et al. Diagnostic precision of CT in local staging of colon cancers: a meta-analysis. Clin Radiol. 2010;65:708–719. doi: 10.1016/j.crad.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Esparrach G, Ayuso-Colella J R, Sendino O et al. EUS and magnetic resonance imaging in the staging of rectal cancer: a prospective and comparative study. Gastrointest Endosc. 2011;74:347–354. doi: 10.1016/j.gie.2011.03.1257. [DOI] [PubMed] [Google Scholar]
- 36.Al-Sukhni E, Milot L, Fruitman M et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:2212–2223. doi: 10.1245/s10434-011-2210-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


