ABSTRACT
Background
The elevated consumption of sugar-sweetened beverages (SSBs) in Mexico is an important public health concern. However, the association between SSB consumption and hyperuricemia has been scarcely studied and not well documented.
Objectives
To prospectively evaluate the association between SSB consumption and risk of hyperuricemia in Mexican adults.
Methods
A longitudinal analysis was conducted using data from the Health Workers Cohort Study. Participants were followed from 2004 to 2018, with measurements every 6 y. The analysis sample consisted of 1300 adults, aged 18 to 85 y. SSB consumption during the previous year was evaluated through a semiquantitative FFQ. Hyperuricemia was defined as a concentration of uric acid ≥7.0 mg/dL in men and ≥5.7 mg/dL in women. We evaluated the association of interest using 2 methodologies: fixed-effects logistic regression and generalized estimating equations (GEEs). Potential confounders were included in both approaches.
Results
At baseline, median intake of SSBs was 472.1 mL/wk (IQR: 198.8–1416.4 mL/wk), and 233 participants had hyperuricemia. Uric acid was higher in participants with an SSB intake ≥7 servings/wk, compared with those with an intake <1 serving/wk (P < 0.001). Participants who changed from the lowest to the highest category of servings consumption experienced 2.6 increased odds of hyperuricemia (95% CI: 1.27, 5.26). Results from the GEE model indicated the odds of hyperuricemia increased by 44% (OR=1.44; 95% CI: 1.13, 1.84) in the 2–6 servings/wk group, and by 89% (OR=1.89; 95% CI: 1.39, 2.57) in the ≥7 servings/wk categories, compared with the <1 serving/wk category. Diet soft drinks were not associated with hyperuricemia.
Conclusions
Our results suggest that the consumption of SSBs is associated with an increased risk of hyperuricemia in Mexican adults, but diet soft drink consumption is not, which supports the need to strengthen existing recommendations to reduce the intake of SSBs.
The Health Workers Cohort Study (HWCS) has been approved by the Institutional Review Board of the Mexican Social Security Institute (12CEI 09 006 14), and the National Institute of Public Health of Mexico (13CEI 17 007 36).
Keywords: SSBs, hyperuricemia, prospective cohort, GEE, fixed effects, Mexican adults
Introduction
Hyperuricemia, characterized by the elevation of plasma uric acid concentration, has been considered as the major etiologic factor of gout and inflammatory arthritis (1–3). Moreover, recent epidemiological evidence has shown a positive association between hyperuricemia and cardiometabolic disorders, such as cardiovascular disease, type 2 diabetes, and chronic kidney disease (4–8), clinical disorders that have become a global epidemic. Hyperuricemia prevalence has increased markedly worldwide over recent years (9). In Mexico, it is estimated that 20% of adults from urban areas have hyperuricemia (10, 11). It is well known that a diet with a high content of meat, seafood, and alcohol is associated with a greater risk of hyperuricemia (1, 12). More recently, the consumption of sugar-sweetened beverages (SSBs) has emerged as a factor associated with hyperuricemia (1, 10, 11, 13). The major plausible mechanism to explain this association implicates the large amounts of fructose-containing sugars present in these beverages (14, 15). There is strong evidence that fructose contributes significantly to uric acid production through hepatic degradation of ATP, leading to an accumulation of AMP, which in turn serves as a substrate for uric acid synthesis (15). High consumption of SSBs has been associated with hyperuricemia in cross-sectional studies. However, there is a dearth of robust longitudinal studies focused on examining the association between soft drink consumption and the risk of hyperuricemia (1, 10, 11, 13, 16).
Although fructose is present in many food sources, such as fruits, the largest amounts of fructose are found in SSBs, either as high-fructose corn syrup (HFCS)—a potent sweetener substitute for cane sugar—or as a mix with other simple sugars (17). Mexico has one of the highest rates of SSB consumption in the world (18). According to the National Health and Nutrition Survey (2012), the consumption of SSBs contributes 9.8% of the total caloric intake; in contrast, fructose consumption from natural sources contributes little to the total energy intake (19). Taking advantage of the large variability in SSB consumption in Mexico, we aimed to estimate the longitudinal association between SSB consumption and hyperuricemia in Mexican adults participating in a prospective cohort study with a follow-up from 2004 to 2018.
Methods
Study population
Longitudinal data were obtained from the Health Workers Cohort Study (HWCS) participants, a dynamic prospective cohort study conducted in Central Mexico. Participants are mostly health care workers from the Mexican Social Security Institute and their families, in Cuernavaca, Morelos. Baseline measurements were conducted in 2004, when all participants were asked to complete an extensive self-administered questionnaire at home and visited a research center for physical examination and a fasting blood sample collection, after providing informed consent. The 2010–2012 and 2016–2018 assessments used similar self-administered questionnaires and procedures. The goal of the HWCS is to examine the effect of genetic and lifestyle factors on the occurrence of different health outcomes of interest in the Mexican population. Further details regarding the design and methods are described in detail elsewhere (20).
Assessment of soft drink consumption and dietary information
Dietary assessment was conducted using a validated semiquantitative and self-administrated FFQ of 116 foods during the previous year (21). For each food, a commonly used portion size was described (e.g., a slice of bread). The same instrument was used to collect dietary information at each of the follow-up assessments (20). From the FFQ we considered 3 types of SSBs: 1) cola-flavored soft drinks, 2) other flavors of soft drinks, and 3) diet soft drinks (e.g., “low-calorie cola”), all with reference to a portion size of ∼355 mL (in Mexico a can, a medium-size bottle, or a glass). Participants could choose between 10 answers (never, less than 1 a month, 1–3 a month, once a week, 2–4 a week, 5–6 a week, once a day, 2–3 a day, 4–5 a day, and 6 or more a day). The consumption of energy derived from SSBs (kilocalories per portion) and the overall consumption of food was obtained from the Nutritional Habits Evaluation System and Nutriment Consumption (22). For this study, we added up the individual portions of SSBs (cola and flavored) to create a total amount of SSBs consumed. Following Schulze et al. (23), we applied a similar categorization of SSB consumption: 1) <1 serving/wk; 2) 1 serving/wk; 3) 2–6 servings/wk; and 4) ≥7 servings/wk. In the first category were included nonconsumers. Diet soft drinks were evaluated in the same categories as for SSB consumption. Total caloric intake, animal protein intake, and alcohol consumption were also estimated using the FFQ. Supplement intake was not assessed for this study.
Uric acid assessment
A venous blood sample was collected after 8 h of fasting. Determination of serum concentrations of uric acid at each of the follow-up assessments was performed with the enzymatic colorimetric method using a SYNCHRON CX system from Beckman Coulter (24). Hyperuricemia was defined as a serum concentration of uric acid ≥7.0 mg/dL in men and ≥5.7 mg/dL in women (1). The primary outcome variable for this study was hyperuricemia. No secondary outcome variables were analyzed.
Anthropometric evaluation
Weight was obtained with a calibrated electronic scale (model BC-533; Tanita). Measurements were taken with the subjects wearing minimum clothing and no shoes. Height was obtained using a conventional stadiometer (Seca 206), with subjects standing up with their shoulders in a normal position, tracing a horizontal plane perpendicular to the vertical scale, touching the vertex of the head during inhalation. Waist circumference (WC) was measured at the highest point of the iliac crest at the end of a normal exhalation, with a 0.1 cm precision, using a conventional tape (Seca) (25). The presence of abdominal obesity was defined by a WC ≥102 cm for men and ≥88 cm for women (26). BMI (in kg/m2) was classified as normal (<25), overweight (≥25 to <30), or obese (≥30). The proportion of body fat was determined by DXA, using a densitometer (model DPX-GE 73,735, series: 638405U77; GE Healthcare Lunar; software version 1.35, quick scanning mode) in fasting conditions (27). We considered proportion of body fat as an indicator of adiposity (28) along with BMI and WC. All measurements were taken by trained personnel using standardized procedures (20).
Other covariates
Demographic characteristics were collected with a self-administered questionnaire. Physical activity was evaluated through the International Physical Activity Questionnaire, translated and validated in Spanish (29). Participants reported the time they dedicated to leisure time physical activity, such as walking, running, or riding a bicycle, during a typical week of the previous year. The physical activity variable was categorized as inactive (<30 min/d) or active (≥30 min/d), based on previous studies (29, 30).
Ethics
The study was planned and conducted according to the Helsinki Declaration guidelines. The study protocol, questionnaires, procedures, and informed consent forms were approved by the Institutional Review Board of the Mexican Social Security Institute (12CEI 09 006 14), and the National Institute of Public Health of Mexico (13CEI 17 007 36). Written informed consent was obtained from all study participants.
Statistical analysis
The enrollment visit was used as the study baseline (either 2004–2006 or 2010–2012). For the descriptive analysis, central tendency and dispersion measurements of the baseline characteristics were calculated according to SSB consumption categories. Tests of equality of means were performed using the F statistic from ANOVA, and tests of equality of distributions of categorical variables were performed with the chi-square statistic. For continuous variables that showed significant skewness, the Kruskal–Wallis test was used.
A fixed-effects logistic regression model (31) was fitted to the data to estimate the within-subject association between SSB consumption categories (main predictor) and hyperuricemia (outcome). Fixed-effects models enable estimation of longitudinal associations statistically isolated from time-invariant confounders with time-invariant effects (32), allowing adjustment for these potential sources of bias. The first model was unadjusted. In the second model, we adjusted for time-varying potential confounders such as age (continuous), smoking status (ex-smoker, current smoker, and nonsmoker), physical activity (inactive <30 min/d, active ≥30 min/d), and dietary intake of meat (portions per day), seafood (portions per day), and alcohol (grams per day).
Additionally, a generalized estimating equations (GEE) model (33) was fitted using the same covariates as for the fixed-effects logistic regression but adding an indicator variable for sex. We specified a binomial family for the outcome, a logit link between the predictor and the risk of hyperuricemia, and an unstructured covariance (a specific correlation parameter for each pair of measurements). In contrast to the fixed-effects regression, estimates from the GEE models reflect both between- and within-sources of variation, and covariates that exclusively show between-subject variation (e.g., sex) can be included in the predictor. Although fixed-effects regression focuses only on within-subject variation and therefore reflects exclusively longitudinal associations, the SEs tend to be higher than approaches that incorporate both between- and within-subject variation. However, GEE coefficient estimators tend to show higher precision, but associations that reflect both between-subject and within-subject variations. The association between SSB consumption and hyperuricemia was expressed as ORs between categories of SSB consumption; odds for all pairwise comparisons for consumption increases were obtained from the model by exponentiating the proper linear combination of coefficients. We fitted both GEE and fixed-effects logistic regression models with and without adjustment covariates.
Given the potential role of the measures of adiposity as mediators between SSBs and hyperuricemia, we fitted models with and without each adiposity indicator: BMI, WC, and proportion of body fat. Models that included these indicators of adiposity show the relation from SSB consumption to hyperuricemia that does not pass through these mediators. However, in models that do not include these indicators of adiposity, their potential mediator effect is absorbed into the estimated parameters of the relation from SSB consumption to hyperuricemia.
The association between diet soft drink consumption and hyperuricemia was also evaluated, using the same 2 statistical methods as above, and adjusting for the same covariates used in the SSB analysis. All analyses were performed using Stata version 14.1 (Stata Corp LLC).
Results
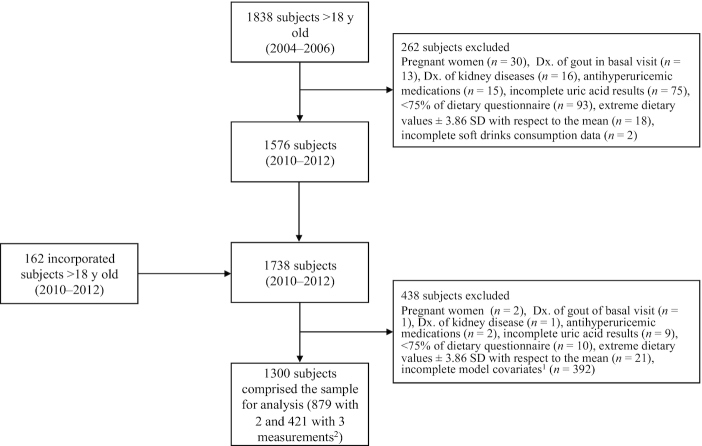
During the first assessment from 2004 to 2006, 1838 men and women between the ages of 18 and 85 y agreed to participate. From 2010 to 2012, 162 new participants aged 19 to 78 y were enrolled. During the 2016–2018 assessment, a total of 481 participants returned. Follow-up assessments were conducted approximately every 6 y. A total of 700 participants were excluded: pregnant women (n = 32), participants with a gout diagnosis (n = 14), with kidney disease (n = 17), taking medication for uric acid control (n = 17), with missing uric acid values (n = 84), who responded to <75% of the dietary questions or skipped complete sections of the FFQ (n = 103), who exceeded ±3.86 SDs in caloric intake values (n = 39) (34), and participants with incomplete SSB consumption information (n = 2) or incomplete information in covariates (n = 392). A total of 1300 participants with complete information and ≥2 measurements were included in this longitudinal analysis, and of these participants, 879 had 2 assessments and 421 had 3 (Figure 1). Average time between first and second measurement was 6.9 y, and between second and third measurement was 5.6 y.
FIGURE 1.
Flow chart of the Health Workers Cohort Study participants. 1Model covariates included sugar-sweetened beverage consumption, age, smoking status, physical activity, meat intake, seafood intake, alcohol intake, sex, and BMI. 2There were ≤3 measurements in the study; the first was taken between 2004 and 2006, the second between 2010 and 2013, and the third between 2016 and 2018. Dx: diagnosis.
At baseline, the mean age was 45.8 ± 12.9 y, and 75.2% were female. The prevalence of overweight and obesity was 60.6%. The average concentration of uric acid was 4.9 ± 1.5 mg/dL. The average energy intake from SSBs was 64.8 ± 92.0 kcal/d. Participants with the highest consumption of SSBs (≥7 servings/wk) were younger, and were more frequently smokers, compared with those with a lower SSB consumption. Uric acid concentration and the prevalence of hyperuricemia were higher in participants with higher consumption of SSBs. The total caloric intake of meat and alcohol was higher in participants who consumed ≥7 servings/wk, compared with participants who consumed <1 serving/wk (Table 1). At the first, second, and third measurement, 18.0%, 22.5%, and 30.7% of subjects had hyperuricemia, respectively.
TABLE 1.
Baseline characteristics and frequency of SSB consumption of participants from the Health Workers Cohort Study, 2004–20181
| SSB consumption (servings/wk) | |||||
|---|---|---|---|---|---|
| Variable | Total | <1/wk | 1/wk | 2–6/wk | ≥7/wk |
| n | 1300 | 339 | 456 | 340 | 165 |
| SSBs, mL/wk | 472.1 [198.8–1416.4] | 79.5 [39.8–79.5] | 397.6 [238.6–546.7] | 1416.4 [1267.3–2137.1] | 3056.5 [2683.8–4970] |
| Age, y | 45.8 ± 12.9 | 48.7 ± 12.9 | 45.0 ± 12.7 | 45.5 ± 13.3 | 42.8 ± 11.4 |
| Female | 978 (75.2) | 293 (86.4) | 366 (80.3) | 222 (65.3) | 97 (58.8) |
| Education level | |||||
| Elemental | 321 (24.7) | 86 (25.4) | 103 (22.6) | 90 (26.5) | 42 (25.5) |
| Middle school | 228 (17.5) | 35 (10.3) | 89 (19.5) | 63 (18.5) | 41 (24.8) |
| High school or higher | 751 (57.8) | 218 (64.3) | 264 (57.9) | 187 (55.0) | 82 (49.7) |
| BMI, kg/m2 | 26.4 ± 4.2 | 26.1 ± 4.3 | 26.5 ± 4.1 | 26.7 ± 4.3 | 26.4 ± 4.0 |
| Overweight2 | 555 (42.7) | 134 (39.5) | 201 (44.2) | 141 (41.5) | 79 (47.9) |
| Obese3 | 233 (17.9) | 53 (15.6) | 83 (18.2) | 70 (20.6) | 27 (16.4) |
| WC, cm | 90.5 ± 11.7 | 89.3 ± 12.4 | 90.7 ± 11.6 | 91.9 ± 11.3 | 89.8 ± 10.9 |
| Central obesity4 | 573 (44.3) | 144 (42.6) | 217 (47.6) | 158 (46.7) | 54 (33.1) |
| Body fat,5 % | 38.8 ± 8.3 | 40.3 ± 7.7 | 40.3 ± 8.0 | 39.1 ± 8.7 | 38.5 ± 9.2 |
| Physical activity, min/d | 24.5 ± 29.3 | 24.8 ± 30.6 | 23.4 ± 26.2 | 25.9 ± 31.4 | 24.4 ± 30.4 |
| Active6 | 454 (35.0) | 123 (36.3) | 154 (33.9) | 121 (35.6) | 56 (34.1) |
| Smoking status | |||||
| Active smoker | 213 (16.6) | 33 (9.9) | 58 (12.9) | 75 (22.3) | 47 (28.8) |
| Ex-smoker | 331 (25.8) | 82 (24.5) | 120 (26.7) | 83 (24.7) | 46 (28.2) |
| Uric acid, mg/dL | 4.9 ± 1.5 | 4.5 ± 1.3 | 4.8 ± 1.3 | 5.3 ± 1.7 | 5.4 ± 1.5 |
| Hyperuricemia7 | 233 (17.9) | 44 (13.0) | 72 (15.8) | 74 (21.8) | 43 (26.1) |
| Dietary variables | |||||
| Caloric intake from SSBs, kcal/d | 64.8 ± 92.0 | 3.7 ± 3.0 | 25.0 ± 9.2 | 91.2 ± 25.6 | 246.1 ± 137.0 |
| Energy percentage for SSBs | 3.1 ± 4.1 | 0.2 ± 0.2 | 1.4 ± 0.7 | 4.7 ± 2.0 | 10.7 ± 5.9 |
| Caloric intake from diet soft drinks, kcal/d | 0.5 ± 2.1 | 0.7 ± 2.5 | 0.3 ± 2.0 | 0.4 ± 1.5 | 0.6 ± 2.0 |
| Total caloric intake, kcal/d | 2145 ± 877 | 2031 ± 863 | 2111 ± 923 | 2141 ± 752 | 2480 ± 939 |
| Carbohydrates, % of total energy | 61.0 ± 8.6 | 62.1 ± 9.7 | 60.9 ± 7.8 | 60.1 ± 8.4 | 61.3 ± 8.4 |
| Fat, % of total energy | 20.0 ± 5.3 | 19.3 ± 5.7 | 20.7 ± 5.7 | 20.0 ± 4.5 | 19.5 ± 4.3 |
| Total meat intake,8 portions/d | 1.2 ± 0.9 | 1.0 ± 0.8 | 1.1 ± 0.8 | 1.3 ± 0.7 | 1.6 ± 1.3 |
| Total seafood intake,9 portions/d | 0.2 ± 0.3 | 0.3 ± 0.5 | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.2 ± 0.3 |
| Alcohol intake, g/d | 1.0 [0.2–3.9] | 0.6 [0.0–1.8] | 1.0 [0.2–3.2] | 1.6 [0.2–6.3] | 1.6 [0.2–4.9] |
Values are mean ± SD or median [IQR] for continuous type variables, and n (%) for categorical variables. SSB, sugar-sweetened beverage; WC, waist circumference.
Defined as BMI ≤25 to <30 kg/m2.
Defined as BMI ≥30 kg/m2.
Defined as WC ≥102 cm in men and ≥88 cm in women.
Percentage of body fat obtained by DXA.
Leisure time physical activity ≥30 min/d.
Hyperuricemia: plasma uric acid ≥5.7 mg/dL for women and ≥7.0 mg/dL for men.
Total meat included main or mixed dish of beef/pork/lamb/processed meat (sausage, bacon, ham, cecina, chorizo), poultry, chicken liver, beef liver, fried pork chunks.
Seafood intake included tuna, sardines, and other seafood.
There was a total of 309 subjects with variation of their hyperuricemia status across their measurements. These subjects changed SSB consumption categories as follows: 112 changes between <1/wk and 1/wk (in either direction); 33 changes between <1/wk and 2–6/wk; 17 changes between <1/wk and ≥7/wk; 89 changes between 1/wk and 2–6/wk; 22 changes between 1/wk and ≥7/wk; and 53 changes between 2–6/wk and ≥7/wk. The number of changes does not match the number of subjects because some subjects contributed two or three changes between different consumption categories and others did not change between consumption categories.
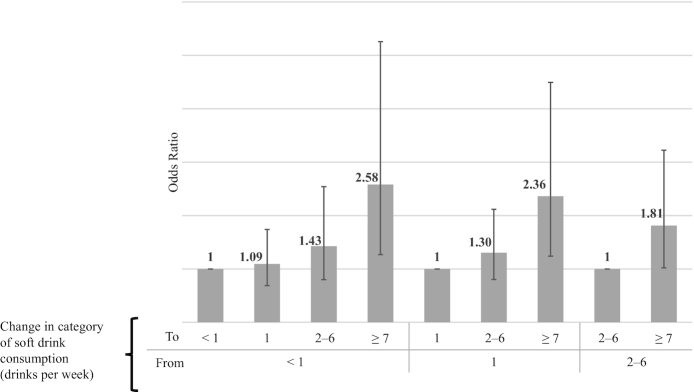
Results from the fixed-effects logistic regression showed increased odds of hyperuricemia when changing from any of the lower SSB consumption categories to the highest soft drinks consumption category. For example, the odds of hyperuricemia increased 2.6-fold (OR = 2.58; 95% CI: 1.27, 2.56) when changing from the <1 serving/wk category to the ≥7 servings/wk category (Figure 2, Table 2—Model 2), or reciprocally, a 61% reduction (OR = 1/2.58 = 0.39; 95% CI: 0.19, 0.79) in the odds of hyperuricemia when changing from the highest to the lowest SSB consumption categories. There were no statistically significant differences between the remaining categories of SSB consumption. In contrast, changes between diet soft drink consumption categories were not associated with hyperuricemia [e.g., OR = 1.55 (95% CI: 0.32, 7.41) when changing from the <1 serving/wk category to the ≥7 servings/wk category; Table 3]. The GEE model showed results in the same direction as the fixed-effects analysis for SSBs. Compared with the <1 serving/wk category, the odds of hyperuricemia were 44% (OR = 1.44; 95% CI: 1.13, 1.84) higher for the 2–6 servings/wk category and 89% higher for the ≥7 servings/wk category (OR = 1.89; 95% CI: 1.39, 2.57) (Table 2—Model 5). However, diet soft drinks were not associated with hyperuricemia in the GEE model. The OR for the diet soft drink consumption category ≥7 serving/d was 1.14 (95% CI: 0.67, 1.93), compared with the <1 serving/wk category (Table 3).
FIGURE 2.
Odds of hyperuricemia and change of category of sugar-sweetened beverage consumption over a period from 6 to 12 y. Obtained from a fixed-effects logistic regression including changes in age, smoking status, physical activity, intake of protein from animal origin, intake of shellfish, and intake of alcohol as covariates. Analysis sample comprised a total of 309 subjects (146 with 2 measurements and 163 with 3 measurements) that showed variation in hyperuricemia status across repeated measurements. Errors bars represent 95% CIs.
TABLE 2.
Fixed-effects logistic regression and generalized estimating equations (GEE) model for the relation between SSB consumption and probability of hyperuricemia1
| Fixed-effects logistic regression2 | GEE3 | |||||
|---|---|---|---|---|---|---|
| Subjects | 309 | 1300 | ||||
| Observations | 781 | 3021 | ||||
| OR (95% CI) | OR (95% CI) | |||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
| SSB, 355-mL portions | ||||||
| <1/wk | 1 ref.4 | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. |
| 1/wk | 0.83 (0.54, 1.28) | 1.09 (0.69, 1.74) | 1.03 (0.64, 1.66) | 1.11 (0.89, 1.38) | 1.09 (0.87, 1.36) | 1.01 (0.80, 1.26) |
| 2–6/wk | 0.98 (0.58, 1.66) | 1.43 (0.80, 2.54) | 1.32 (0.73, 2.39) | 1.50 (1.18, 1.91) | 1.44 (1.13, 1.84) | 1.34 (1.04, 1.72) |
| ≥7/wk | 1.54 (0.81, 2.96) | 2.58 (1.27, 5.26) | 2.60 (1.25, 5.42) | 2.00 (1.48, 2.69) | 1.89 (1.39, 2.57) | 1.77 (1.29, 2.43) |
SSB, sugar-sweetened beverage.
Fixed-effects logistic regression is based on within-subject variations; subjects with no variation in the outcome variable across time are not included. Model 1: without adjustment covariates. Model 2: adjusted for age, smoking status, physical activity, and dietary intake of meat, seafood, and alcohol. Model 3: includes adjustment covariates in Model 2 and BMI (kg/m2).
Generalized estimating equations model. Specified with unstructured covariance, binomial distribution for the outcome and logit link. Model 4: without adjustment covariates. Model 5: with adjustment covariates in Model 2 and sex. Model 6: with adjustment covariates in Model 3 and sex.
ref: Reference category.
TABLE 3.
Fixed-effects logistic regression and generalized estimating equations (GEE) model for the relation between diet soft drinks consumption and probability of hyperuricemia1
| Fixed-effects logistic regression2 | GEE3 | |||||
|---|---|---|---|---|---|---|
| Subjects | 309 | 1300 | ||||
| Observations | 781 | 3021 | ||||
| OR (95% CI) | OR (95% CI) | |||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
| SSB, 355-mL portions | ||||||
| <1/wk | 1 ref.4 | 1 ref. | 1 ref. | 1 ref. | 1 ref. | 1 ref. |
| 1/wk | 0.68 (0.28, 1.66) | 0.74 (0.29, 1.89) | 0.80 (0.30 2.16) | 1.00 (0.63, 1.52) | 1.02 (0.65, 1.60) | 0.91 (0.57, 1.44) |
| 2–6/wk | 1.01 (0.49, 2.07) | 1.01 (0.48, 2.14) | 1.16 (0.53, 2.54) | 1.01 (0.72, 1.60) | 1.07 (0.72, 1.61) | 1.07 (0.71, 1.60) |
| ≥7/wk | 1.89 (0.42, 8.55) | 1.55 (0.32, 7.41) | 1.42 (0.27, 7.43) | 1.14 (0.68, 1.92) | 1.14 (0.67, 1.93) | 1.01 (0.58, 1.73) |
SSB, sugar-sweetened beverage.
Fixed-effects logistic regression is based on within-subject variations; subjects with no variation in the outcome variable across time are not included. Model 1: without adjustment covariates. Model 2: adjusted for age, smoking status, physical activity, and dietary intake of meat, seafood, and alcohol. Model 3: includes adjustment covariates in Model 2 and BMI (kg/m2).
Generalized estimating equations model. Specified with unstructured covariance, binomial distribution for the outcome and logit link. Model 4: without adjustment covariates. Model 5: with adjustment covariates in Model 2 and sex. Model 6: with adjustment covariates in Model 3 and sex.
ref: Reference category.
Results from adjusting covariates in the fixed-effects logistic regression indicated the presence of suppression in some of the coefficients. When adjustment covariates were not included, the difference in the odds of hyperuricemia between the highest and the lowest SSB consumption categories was no longer significant (Table 2—Model 1). However, coefficients of SSB consumption categories from the GEE model with and without adjustment covariates were similar and statistical significance was maintained (Table 2—Models 4 to 6).
The association between SSB consumption and hyperuricemia was similar to the other models when BMI was added as a covariate (Table 2—Models 3 and 6). Additionally, BMI was related to an increased odds of hyperuricemia in both the fixed-effects logistic regression (OR = 1.24; 95% CI: 1.13, 1.37) and the GEE model (OR = 1.12; 95% CI: 1.09, 1.14). However, diet soft drink consumption remained nonsignificantly associated with hyperuricemia when BMI was added as a covariate (Table 3—Models 3 and 6). Results from models with either WC or proportion of body fat added as covariates showed results in the same direction as those with BMI included as a covariate (see Supplemental Tables 1 and 2).
Discussion
The results of this prospective study indicate that increases in SSB consumption were associated with a greater risk of hyperuricemia. This risk of hyperuricemia was 89% higher in participants who consumed ≥7 servings/wk, compared with those consuming <1 SSBs/wk. This finding was robust to model specification; a fixed-effects model rendered a 2.6 OR of hyperuricemia comparing groups with the highest and the lowest consumption. This association was independent of potential confounding variables and showed similar results when the indicators of adiposity were added as covariates in separated models. This suggests that our association holds even when the path from SSB consumption to hyperuricemia that passes through each of these adiposity indicators is statistically isolated. Our findings support the hypothesis that consumption of SSBs is positively associated with higher concentrations of uric acid. Conversely, we did not observe an association between consumption of diet soft drinks and risk of hyperuricemia, which can be related to the type of added sugar used in these beverages (35, 36). Unlike diet soft drinks that use artificial sweeteners, SSBs are sweetened with sucrose and HFCS, which contain fructose (14, 35). Fructose enhances the production of uric acid through hepatic degradation of ATP, leading to an accumulation of AMP, a substrate for the production of uric acid (15).
As far as we know, prospective studies evaluating the association between SSB consumption and hyperuricemia are scarce (14). A previous study by Bomback et al. (13), conducted over a 3-y follow-up period, did not show any significant association between SSBs or diet soft drinks and risk of hyperuricemia. The ORs for SSBs and diet soft drinks they reported were 1.17 (95% CI: 0.95, 1.43) and 0.97 (95% CI: 0.83, 1.14), respectively. Yet, the direction of the association estimated was similar to ours for the SSBs and hyperuricemia. It is possible that variation in the ages of our participants and those of the Bomback et al. study (13), which were older than ours, affects the level of beverage consumption resulting in differences in the findings. It has been shown that SSB consumption decreases at older ages (1, 10, 11). Although there is a lack of epidemiological studies exploring the effect of SSB consumption on uric acid concentrations, we identified 2 US studies that evaluated the consumption of SSBs and gout. The first was carried out in men from the Health Professionals Study and the second examined women who participated in the Nurses’ Health Study. Both studies reported that the risk of gout increased significantly as consumption of SSBs increased (RR = 1.85; 95% CI: 1.08, 3.16; P-trend = 0.002; and RR = 2.39; 95% CI: 1.34, 4.26; P-trend < 0.001, respectively). In contrast, diet soft drinks were not associated with hyperuricemia (37, 38). Thus, our findings suggest a missing link between the consumption of SSBs and gout, by indicating the potential association between SSB intake and hyperuricemia.
Our results are of particular importance to the Mexican population, because hyperuricemia is a risk factor for other metabolic disorders that are contributing to a significant public health crisis due to the high rates of metabolic syndrome, high blood pressure, kidney disease, type 2 diabetes, and cardiovascular diseases in the country (3–8). The national prevalences of metabolic syndrome, hypertension, and diabetes are 40%, 25.5%, and 9.4%, respectively (39, 40). Previous analyses of the burden of disease generated by SSB consumption have focused on obesity, diabetes, cardiovascular disease, and obesity-related cancers (41–43). However, if the association between hyperuricemia and the consumption of SSBs is confirmed, gout and other related diseases could be added to the increasing health and economic toll caused by the excessive consumption of SSBs.
To our knowledge, no previous study has analyzed the risk of developing hyperuricemia using a fixed-effects approach. Fixed-effects models isolate within-person changes in exposure and outcome, to provide a risk estimate that is free of observed and unobserved time-invariant confounders, which are assumed to be related to the outcome in the same manner at any given time point (e.g., sex or race). Even though the time interval between assessments is long (∼6 y between each visit), we were able to detect an increased odds of hyperuricemia when changing from any of the lower SSB consumption categories to the highest consumption category based exclusively on within-subject variation. The GEE approach (31–33), which incorporates both within- and between-subject variation, showed results in the same direction as the fixed-effects logistic regression but with lower SEs. The fact that we obtained consistent results when using these 2 approaches strengthens our findings regarding the association between SSB consumption and hyperuricemia.
Our study has several strengths and some limitations. Diet was evaluated by a self-reported semiquantitative FFQ. However, the questionnaire has been validated in the Mexican population (22), and any misclassification would probably predispose the results to nullity. One limitation of our study regards SSB intake measurement: subjects were asked to report the consumption frequency of crude SSB portions (i.e., a can, a medium-size bottle, or a glass). It would be helpful to include more accurate measurements of consumption in future work and test its validity. We did not consider statins and metformin as drugs affecting the serum uric acid concentrations but excluded subjects who reported taking allopurinol and diuretics. Despite the importance of hyperuricemia as a risk factor for various metabolic disorders, there is no consensus on the cutoff point for hyperuricemia (11). Many studies (1, 10, 13) define hyperuricemia with concentrations ≥5.7 mg/dL in women. This could be the best cutoff point to prevent specific chronic diseases associated with hyperuricemia. An additional limitation is the relatively small sample size of the third assessment (n = 421), because not all participants were followed up. Furthermore, the HWCS is not representative of the Mexican population as a whole but could represent adults residing in urban areas in the center of the country (20). Most of the study participants were health workers, who are probably healthier, and have a higher socioeconomic status and educational level than the general population. However, if these conditions do not modify the effect of SSBs on hyperuricemia, our results could be applicable to the general population. To our knowledge, our longitudinal study is the first to confirm the association between the consumption of SSBs and hyperuricemia in a sample of Mexican adults. The present study did not analyze the association between sources of natural fructose and the outcome of interest given limitations in the FFQ used to isolate natural fructose from other sources in some food items. Future studies are necessary to study this issue. The longitudinal nature of our data allows us to infer with more certainty the relation between SSB consumption and hyperuricemia. Randomized intervention studies could help to confirm this association.
In conclusion, our study adds new evidence regarding the potential role of SSB consumption in the development of hyperuricemia, which leads to the possibility of additional metabolic effects of SSBs. In contrast, diet soft drinks were not associated with hyperuricemia. Although our findings need to be confirmed, they provide a clear indication of another negative metabolic impact of SSBs and reinforce the international call to reduce their consumption.
Supplementary Material
Acknowledgments
We thank Ramon A Durazo-Arvizu (Loyola University Chicago) and Delfino Vargas-Chanes (Universidad Nacional Autónoma de México) for comments on the statistical analyses.
The authors’ responsibilities were as follows—JS and JM-L: designed the study; JM-L, LL-M, NM, LT-I, RH-L, BR-P, MF, YNF, TB-G, RV-C, ADQ-S, and JS: reviewed, edited, and drafted the manuscript; JM-L, LT-I, TB-G, ADQ-S, and JS: reviewed the methodology and analyzed data: JM-L: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
The authors report no conflicts of interest.
Notes
The Health Workers Cohort Study was supported by the following grants from the Consejo Nacional de Ciencia y Tecnología (CONACYT): 7876, 87783, 262233, 26267M, SALUD-2010-01-139796, SALUD-2011-01-161930, and CB-2013-01-221628. YNF was supported by NIH/NCI K07CA197179. This work was supported by Bloomberg Philanthropies, which had no role in the design, analysis, interpretation, or writing of this article.
Data described in the manuscript, code book, and analytic code will be made available upon request.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: GEE, generalized estimation equation; HFCS, high-fructose corn syrup; HWCS, Health Workers Cohort Study; SSB, sugar-sweetened beverage; WC, waist circumference.
Contributor Information
Joacim Meneses-León, Research Center in Policy, Population, and Health, School of Medicine, National Autonomous University of Mexico, Mexico City, Mexico.
Leith León-Maldonado, CONACYT, Center for Population Health Research, National Institute for Public Health, Cuernavaca, Morelos, Mexico.
Nayeli Macías, Center for Nutrition and Health Research, National Institute for Public Health, Cuernavaca, Morelos, Mexico.
Leticia Torres-Ibarra, Research Center in Policy, Population, and Health, School of Medicine, National Autonomous University of Mexico, Mexico City, Mexico; Center for Population Health Research, National Institute for Public Health, Cuernavaca, Morelos, Mexico.
Rubí Hernández-López, Research Center in Policy, Population, and Health, School of Medicine, National Autonomous University of Mexico, Mexico City, Mexico.
Berenice Rivera-Paredez, Research Center in Policy, Population, and Health, School of Medicine, National Autonomous University of Mexico, Mexico City, Mexico.
Mario Flores, Center for Nutrition and Health Research, National Institute for Public Health, Cuernavaca, Morelos, Mexico.
Yvonne N Flores, Unidad de Investigación Epidemiológica y en Servicios de Salud, Delegación Morelos, Instituto Mexicano del Seguro Social, Cuernavaca, Morelos, Mexico; UCLA Department of Health Policy and Management, Center for Cancer Prevention and Control Research, Fielding School of Public Health and Jonsson Comprehensive Cancer Center, Los Angeles, CA, USA.
Tonatiuh Barrientos-Gutiérrez, Center for Population Health Research, National Institute for Public Health, Cuernavaca, Morelos, Mexico.
Amado D Quezada-Sánchez, Center for Evaluation and Survey Research, National Institute for Public Health, Cuernavaca, Morelos, Mexico.
Rafael Velázquez-Cruz, Genomics of Bone Metabolism Laboratory, National Institute of Genomic Medicine, Mexico City, Mexico.
Jorge Salmerón, Research Center in Policy, Population, and Health, School of Medicine, National Autonomous University of Mexico, Mexico City, Mexico.
References
- 1. Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008;59(1):109–16. [DOI] [PubMed] [Google Scholar]
- 2. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–41. [DOI] [PubMed] [Google Scholar]
- 3. Roubenoff R, Klag MJ, Mead LA, Liang KY, Seidler AJ, Hochberg MC. Incidence and risk factors for gout in white men. JAMA. 1991;266(21):3004–7. [PubMed] [Google Scholar]
- 4. Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290(3):F625–31. [DOI] [PubMed] [Google Scholar]
- 5. Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robles-Cervantes JA, Ramos-Zavala MG, González-Ortíz M, Martínez-Abundis E, Valencia-Sandoval C, Torre-Chávez A, Espinel-Bermúdez C, Santiago-Hernández NJ, Hernández-González SO. Relationship between serum concentration of uric acid and insulin secretion among adults with type 2 diabetes mellitus. Int J Endocrinol. 2011;2011:107904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahta T, Nuccio E, McFann K, Madero M, Sarnak MJ, Jalal D. Association of uric acid with vascular stiffness in the Framingham Heart Study. Am J Hypertens. 2015;28(7):877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vargas-Santos AB, Neogi T. Management of gout and hyperuricemia in CKD. Am J Kidney Dis. 2017;70(3):422–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rho YH, Zhu Y, Choi HK. The epidemiology of uric acid and fructose. Semin Nephrol. 2011;31(5):410–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meneses-León J, Denova-Gutiérrez E, Castañón-Robles S, Granados-García V, Talavera JO, Rivera-Paredez B, Huitrón-Bravo GG, Cervantes-Rodríguez M, Quiterio-Trenado M, Rudolph SE et al. Sweetened beverages consumption and the risk of hyperuricemia in Mexican adults: a cross-sectional study. BMC Public Health. 2014;14:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. López-Molina R, Parra-Cabrera S, López-Ridaura R, González-Villalpando ME, Ferrannini E, González-Villalpando C. Sweetened beverages intake, hyperuricemia and metabolic syndrome: the Mexico City Diabetes Study. Salud Publica Mex. 2013;55(6):557–63. [DOI] [PubMed] [Google Scholar]
- 12. Villegas R, Xiang YB, Elasy T, Xu WH, Cai H, Cai Q, Linton MF, Fazio S, Zheng W, Shu XO. Purine-rich foods, protein intake and prevalence of hyperuricemia: the Shanghai Men's Health Study. Nutr Metab Cardiovasc Dis. 2012;22(5):409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bomback AS, Derebail VK, Shoham DA, Anderson CA, Steffen LM, Rosamond WD, Kshirsagar AV. Sugar-sweetened soda consumption, hyperuricemia and kidney disease. Kidney Int. 2010;77(7):609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jamnik J, Rehman S, Blanco Mejia S, de Souza RJ, Khan TA, Leiter LA, Wolever TM, Kendall CW, Jenkins DJ, Sievenpiper JL. Fructose intake and risk of gout and hyperuricemia: a systemic review and meta-analysis of prospective cohort studies. BMJ Open. 2016;6(10):e013191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58(5 Suppl):754S–65S. [DOI] [PubMed] [Google Scholar]
- 16. Ebrahimpour-Koujan S, Saneei P, Larijani B, Esmaillzadeh A. Consumption of sugar sweetened beverages and dietary fructose in relation to risk of gout and hyperuricemia: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2018;2:1–10. [DOI] [PubMed] [Google Scholar]
- 17. Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537–43. [DOI] [PubMed] [Google Scholar]
- 18. Pan American Health Organization. Taxes on sugar-sweetened beverages as a public health strategy: the experience of Mexico, [Internet]. PAHO; 2015. Available from: http://iris.paho.org/xmlui/handle/123456789/18391 [Accessed September 19, 2019]. [Google Scholar]
- 19. Aburto TC, Pedraza LS, Sánchez-Pimienta TG, Batis C, Rivera JA. Discretionary foods have a high contribution and fruit, vegetables, and legumes have a low contribution to the total energy intake of the Mexican population. J Nutr. 2016;146(9):1881S–7S. [DOI] [PubMed] [Google Scholar]
- 20. Denova-Gutiérrez E, Flores YN, Gallegos-Carrillo K, Ramírez-Palacios P, Rivera-Paredez B, Muñoz-Aguirre P, Valázquez-Cruz R, Torres-Ibarra L, Meneses-León J, Méndez-Hernández P et al. Health workers cohort study: methods and study design. Salud Publica Mex. 2016;58(6):708–16. [DOI] [PubMed] [Google Scholar]
- 21. Hernández-Avila M, Romieu I, Parra S, Hernández-Avila J, Madrigal H, Willett W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex. 1998;10:133–40. [DOI] [PubMed] [Google Scholar]
- 22. Hernández-Ávila M, Resoles M, Parra S. Sistema de evaluación de hábitos nutricionales y consumo de nutrimentos (SNUT). Cuernavaca, Mexico: INSP; 2000. [Google Scholar]
- 23. Schulze MB, Manson JE, Ludwig DS, Colditz GA. Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-age women. JAMA. 2004;292(8):927–34. [DOI] [PubMed] [Google Scholar]
- 24. Tietz NW. Specimen collection and processing; sources of biological variation. In: Young DS, Bernes EW, Jr, editors. Textbook of Clinical Chemistry. 2nd ed.Philadelphia, PA: WB Saunders; 1994. p. 34–58. [Google Scholar]
- 25. Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign (IL): Human Kinetics Press; 1988. [Google Scholar]
- 26. Expert Panel of Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel of Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–97. [DOI] [PubMed] [Google Scholar]
- 27. Clark P, Denova-Gutierrez E, Ambrosi R, Szulc R, Rivas-Ruiz R, Salmerón J. Reference values of total lean mass, appendicular lean mass, and fat mass measured with dual-energy X ray absorptiometry in a healthy Mexican population. Calcif Tissue Int. 2016;99(5):462–71. [DOI] [PubMed] [Google Scholar]
- 28. Deurenberg P, Yap M, Staveren WA. Body mass index and percent body fat: a meta-analysis among different ethnic groups. Int J Obes. 1998;22(12):1164–71. [DOI] [PubMed] [Google Scholar]
- 29. Martínez-González MA, López-Fontana C, Varo JJ, Sánchez-Villegas A, Martinez JA. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005;8(7):920–7. [DOI] [PubMed] [Google Scholar]
- 30. Méndez-Hernández P, Flores Y, Siani C, Lamure M, Dosamantes-Carrasco LD, Halley-Castillo E, Huitrón G, Talavera JO, Gallegos-Carrillo K, Salmerón J. Physical activity and risk of metabolic syndrome in an urban Mexican cohort. BMC Public Health. 2009;9:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McFadden DL. Conditional logit analysis of qualitative choice behavior. In: Zerembka P, editor. Frontiers in Econometrics. New York: Academic Press; 1974. p. 105–142. [Google Scholar]
- 32. Allison PD. Fixed effects logistic models. In: Liao TF, editor. Fixed Effects Regression Models. SAGE; 2009. p. 28–48. [Google Scholar]
- 33. Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 34. Rosner B. Percentage points for generalized ESD many-outlier procedure. Technometrics. 1983;25:165–72. [Google Scholar]
- 35. Gardner C, Wylie-Rosett J, Gidding SS, Steffen LM, Johnson RK, Reader D, Lichtenstein AH, American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism, Council on Arteriosclerosis . Nonnutritive sweeteners: current use and health perspectives: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2012;35(8):1798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fitch C, Keim KS; Academy of Nutrition and Dietetics . Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112(5):739–58. [DOI] [PubMed] [Google Scholar]
- 37. Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336(7639):309–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Choi HK, Willett W, Curhan G. Fructose-rich beverages and risk of gout in women. JAMA. 2010;304(20):2270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shaman Levy T, Cuevas Nasu L, Dommarco R, Hérnandez Avila M. Encuesta Nacional de Salud y Nutrición de Medio Camino 2016. Informe final de resultados. Cuernavaca, Mexico: Instituto Nacional de Salud Pública; 2016. [Google Scholar]
- 40. Flores YN, Auslander A, Crespi CM, Rodríguez M, Zhang ZF, Durazo F, Salmerón J. Longitudinal association of obesity, metabolic syndrome and diabetes with risk of elevated aminotransferase levels in a cohort of Mexican health workers. J Dig Dis. 2016;17(5):304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Long MW, Gortmaker SL, Ward ZJ, Resch SC, Moodie ML, Sacks G, Swinburn BA, Carter RC, Wang YC. Cost effectiveness of a sugar-sweetened beverage excise tax in the U.S. Am J Prev Med. 2015;49(1):112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Basto-Abreu A, Braverman-Bronstein A, Camacho-García-Formentí D, Zepeda-Tello R, Popkin BM, Rivera-Dommarco J, Hernández-Ávila M, Barrientos-Gutiérrez T. Expected changes in obesity after reformulation to reduce added sugar in beverages: a modeling study. PLoS Med. 2018;15(10):e1002664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sánchez-Romero LM, Penko J, Coxson PG, Fernández A, Mason A, Moran AE, Ávila-Burgos L, Odden M, Barquera S, Bibbins-Domingo K. Projected impact of Mexico's sugar sweetened beverages tax policy on diabetes and cardiovascular disease: a modeling study. PLoS Med. 2016;13(11):e1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.