ABSTRACT
Background
Obesity prevalence remains high in the United States, and there is an increased risk among women who do not lose their gestational weight gain during the postpartum period. Indicators of dietary carbohydrate quality including added sugar consumption, glycemic load, and glycemic index have been linked with weight gain, whereas fiber may protect against obesity. However, these dietary factors have not been examined during the postpartum period.
Objectives
The aim of this study was to determine whether dietary sugars and fiber intake were associated with changes in postpartum weight.
Methods
We examined Hispanic women from the longitudinal Southern California Mother's Milk Study (n = 99) at 1 and 6 mo postpartum. Maternal assessments included height, weight, and dietary intake based on 24-h diet recalls. We used multivariable linear regression to examine the relation between maternal diet and change in postpartum weight after adjusting for maternal age, height, and energy intake.
Results
Higher intake of added sugar was associated with postpartum weight gain (β: 0.05; 95% CI: 0.004, 0.10; P = 0.05). In addition, a half 8-ounce (8 fluid ounces = 236.6 mL) serving per day increase in soft drinks was associated with a 1.52-kg increase in weight (95% CI: 0.70, 2.34 kg; P < 0.001). A high glycemic index (β: 0.25; 95% CI: 0.07, 0.42; P = 0.006) and glycemic load (β: 0.04; 95% CI: 0.002, 0.08; P = 0.04) were associated with postpartum weight gain. Higher soluble fiber was associated with a decrease in postpartum weight (β: −0.82 kg; 95% CI: −1.35, −0.29 kg; P = 0.003) and the negative effects of added sugar, sugary beverages, and high-glycemic-index and -load diets were partially attenuated after adjusting for soluble fiber intake.
Conclusions
Increased consumption of added sugar, sugar-sweetened beverages, and high-glycemic diets were associated with greater weight gain in the first 6 mo postpartum. In addition, increased consumption of soluble fiber was associated with postpartum weight loss, which may partially offset the obesogenic effects of some dietary sugars.
Keywords: dietary sugar, added sugar, sugar-sweetened beverages, postpartum weight, Hispanics
Introduction
Over the past several decades, the prevalence of obesity in the United States has increased dramatically with large disparities by race/ethnicity (1, 2). Hispanic women are among the most affected demographics, of whom nearly 46% have obesity (2). Of note, previous work among Hispanic women has shown that prepregnancy overweight and obesity increase the risk of excess gestational weight gain (3). Although postpartum weight retention is an important predictor for women's health (4), few studies have examined how dietary factors contribute to changes in postpartum weight (5, 6). Thorough investigation of relevant modifiable risk factors during this life stage is warranted because excess postpartum weight gain increases maternal weight status for subsequent pregnancies, increasing the risk of future obesity in their offspring (7, 8).
A growing body of work has shown that dietary patterns play an important role in the development of overweight and obesity (9, 10). Carbohydrates make up the greatest proportion of human energy intake (11), and the relative amount and type of dietary carbohydrates consumed may significantly affect weight trajectories. For example, studies suggest that excess consumption of highly processed foods (12, 13), sugar-sweetened beverages (14, 15), and high-glycemic-index diets (5) may increase body weight. At the same time, dietary fiber may protect against the development of obesity by aiding in weight loss (16–18). Thus, greater consumption of dietary sugars and a reduced intake of dietary fiber have the potential to increase postpartum weight trajectories and contribute to elevated risk of obesity (19, 20).
Studies suggest that retention of excess gestational weight may increase risk of obesity and type 2 diabetes (4, 21–24). Despite this, no studies to our knowledge have examined how specific dietary factors affect postpartum weight trajectories among Hispanic women, a population already at increased risk of obesity as well as gestational diabetes and type 2 diabetes (1, 25). Therefore, the aim of this study was to determine whether dietary sugars (e.g., added sugars, total sugar, sugar-sweetened beverages, high-glycemic diets) and fiber intake were associated with postpartum weight change among high-risk Hispanic women in the first 6 mo after delivery.
Methods
Study subjects
Participants from this study were recruited from the ongoing Southern California Mother's Milk Study between 2016 and 2019. By November 2019, 217 participants were enrolled in the Mother's Milk Study and 104 had available dietary data at 1 and 6 mo postpartum. Of these 104 participants, 1 outlier was removed owing to weight loss, which was >11 SDs above the mean. Four outliers were also removed when examining dietary sugar variables (i.e., sucrose, mannitol, soft drinks, sweets) because the value for each variable was >2 SDs above the mean (Supplemental Figure 1). The Mother's Milk Study was designed to examine the impact of breast-milk factors on the development of the infant gut microbiota at 1, 6, 12, 18, and 24 mo postpartum. Participants were recruited from maternity clinics affiliated with the University of Southern California in Los Angeles County and other community clinics, with the inclusion criteria being comprised of 1) self-identification of Hispanic ethnicity; 2) ≥18 y old at the time of delivery; 3) a healthy, term, singleton birth; 4) within 1 mo postpartum; and 5) intention to breastfeed for ≥3 mo postpartum. We excluded participants if they were taking medications or had any medical conditions that could affect metabolism, nutritional status, or physical or mental health. We also excluded participants if they currently used tobacco (i.e., >1 cigarette/wk), other recreational drugs, or had a clinical diagnosis of fetal abnormalities. Before testing, we obtained informed written consent from each participant. The University of Southern California and Children's Hospital Los Angeles Institutional Review Boards approved this study, and the participants were treated according to the Helsinki Declaration of 1964.
Study visits
Participants completed a study visit at 1 and 6 mo postpartum. We collected historical health-related information regarding family health history, mental health history, maternal health history, maternal prepregnancy BMI (in kg/m2), age at delivery (y), delivery mode (natural birth or cesarean delivery), and infant sex. Maternal weight was measured using an electronic scale to the nearest 0.1 kg (Tanita, model BC-549) and standing height was measured using a stadiometer to the nearest 1 mm (Seca GmBH & Co. KG, model Seca 126). Duplicate measurements of both weight and standing height were taken and used to calculate maternal BMI at 1 and 6 mo postpartum. BMI was used to classify mothers as healthy weight, overweight, or obese based on the CDC standards (26).
Dietary intake
We performed 2 nonconsecutive 24-h diet recalls at 1 and 6 mo postpartum in order to represent mean participant dietary intake over this period. Briefly, a trained and bilingual registered dietitian assessed maternal daily intake of dietary factors separately at 1 and 6 mo postpartum. The two 24-h dietary recalls were conducted on 1 weekday and 1 weekend day using the multipass method (27) at both 1 and 6 mo postpartum. We performed the first dietary recall in person at our laboratory with the use of food models, portion booklets, and serving containers to assist in estimating serving sizes. The second recall was conducted by an unscheduled telephone call. A trained research assistant coded and analyzed all dietary recall data, which were entered into the Nutrition Data System for Research software (version 2018) from the National Coordinating Center at the University of Minnesota. For the current study, we examined added sugar (g/d), servings of sugary beverages (8 ounces/d, equivalent to 236.6 mL/d), glycemic variables (bread as a reference), and fiber variables (g/d). Servings of beverages were defined as soft drinks (sweetened soft drinks + artificially sweetened soft drinks + unsweetened soft drinks), sweet soft drinks (sweetened soft drinks), sugary beverages excluding juice (sweetened soft drinks + sweetened fruit drinks + sweetened tea + sweetened coffee + sweetened coffee substitutes + sweetened water + nondairy-based sweetened meal replacement/supplement + ready-to-drink flavored milk + sweetened flavored milk beverage powder), sugary beverages plus juice (sugary beverages excluding juice + citrus juice + fruit juice excluding citrus juice + vegetable juice), and sugary beverages excluding juice and dairy [sugary beverages excluding juice − (ready-to-drink flavored milk + sweetened flavored milk beverage powder)]. Total dietary fiber included unavailable carbohydrates (cellulose, hemicellulose, pectins, gums, and muscilages) and lignin. Values were obtained by chemical analyses or from the sum of insoluble dietary fiber and soluble dietary fiber. Soluble dietary fiber values were determined by chemical analyses or calculated from the difference between total dietary fiber and insoluble dietary fiber, which includes pectins, gums, muscilages, and some hemicellulose. Insoluble dietary fiber was defined as the portion of dietary fiber determined by the modified Van Soest neutral detergent method. This included cellulose, some hemicellulose, and lignin.
Statistical analysis
We present descriptive statistics as means ± SDs for continuous variables and as frequency (percentage) for categorical variables. Paired t tests and chi-square tests were used to assess differences in physical and dietary factors between 1 and 6 mo postpartum. Repeated-measures ANOVA and a Bonferroni correction were used to examine differences in maternal weight from prepregnancy to 1 and 6 mo postpartum. Postpartum dietary intake was estimated by averaging the dietary recalls from 1 and 6 mo (Supplemental Table 1). Based on the literature, specific dietary factors were examined for their potential impact on body weight including added sugar variables (i.e., added sugar, soft drinks, sugar-sweetened beverages excluding juice, sugar-sweetened beverages plus juice), glycemic variables (i.e., glycemic index, glycemic load), and fiber variables (i.e., total, soluble, and insoluble). For these analyses, multiple linear regression models were used to examine the associations between dietary factors and change in maternal weight from 1 to 6 mo postpartum. These models adjusted for maternal age, maternal height, and mean energy intake as a priori covariates. Further adjustment for prepregnancy weight or change in breastfeeding was also examined (Supplemental Table 2). We conducted additional analyses that adjusted for soluble fiber intake when examining the associations of added sugar, sugary beverages, and glycemic index and load with change in postpartum weight. We also examined interaction terms to determine whether the effects of added sugar, sugary beverages, or high-glycemic-index/load diets were modified by soluble fiber intake. Whereas beverage intake was assessed in 8-ounce servings per day (equivalent to 236.6 mL/d), effect estimates (β) are reported for a half serving per day increase in beverage consumption, which better reflects the observed variability in our data. All analyses were performed in RStudio version 1.1.456 (RStudio, Inc.). Statistical significance was set at P value <0.05 and 95% CIs are reported.
Results
The current study was comprised of 99 participants from the Mother's Milk Study. At the 1-mo postpartum visit, 100% of women breastfed and 80.8% breastfed at the 6-mo visit (n = 80) based on self-reports. Table 1 reports participants’ general characteristics at 1 and 6 mo postpartum and Table 2 reports dietary characteristics. Before pregnancy, 29.3% of women were healthy weight, 38.4% were overweight, and 32.3% were obese. Mean maternal body weight significantly increased over time (P < 0.0001), including from prepregnancy to 1 mo postpartum (+2.1 ± 6.9 kg; P = 0.01), prepregnancy to 6 mo postpartum (+3.5 ± 7.2 kg; P < 0.0001), and 1 to 6 mo postpartum (+1.4 ± 4.2 kg; P = 0.005).
TABLE 1.
Characteristics of Hispanic women from the Southern California Mother's Milk Study at 1 and 6 mo postpartum1
| 1 Mo | 6 Mo | |
|---|---|---|
| Age, y | 29.4 ± 6.5 | 30.0 ± 6.5* |
| Height, m | 1.6 ± 0.1 | — |
| Weight, kg | 73.1 ± 13.1 | 74.5 ± 14.6 |
| BMI, kg/m2 | 29.8 ± 4.8 | 30.3 ± 5.5 |
| Healthy weight, n (%) | 17 (17.2) | 15 (15.2) |
| Overweight, n (%) | 40 (40.4) | 37 (37.4) |
| Obese, n (%) | 42 (42.4) | 47 (47.5) |
n = 99. Values are means ± SDs unless otherwise indicated. Paired t tests or chi-square tests were used to compare 1- and 6-mo values. *P <0.0001. Otherwise, the percentage of women that were healthy weight [BMI (in kg/m2) ≤24.9], overweight (BMI < 30 and >24.9), or obese (BMI > 30) is shown at 1 and 6 mo postpartum.
TABLE 2.
Mean dietary intake from recalls performed at 1 and 6 mo postpartum1
| Nutrient | Mean ± SD | IQR |
|---|---|---|
| Energy, protein, and fat | ||
| Energy intake, kcal | 1666 ± 375 | 1442–1887 |
| Protein, g/d | 76.2 ± 17.9 | 61.5–88.6 |
| Percentage protein, %kcal/d | 18.9 ± 3.3 | 16.8–21.0 |
| Fat, g/d | 58.2 ± 16.8 | 48.0–67.3 |
| Percentage fat, %kcal/d | 30.4 ± 4.9 | 26.9−34.8 |
| Total carbohydrates | ||
| Carbohydrates, g/d | 214.7 ± 55.3 | 169.9–253.5 |
| Percentage carbohydrates, %kcal/d | 50.4 ± 6.0 | 46.4−53.9 |
| Total sugar, g/d | 87.7 ± 31.9 | 65.2−110.9 |
| Percentage total sugar, %kcal/d | 20.8 ± 5.5 | 17.5−24.3 |
| Added sugar, g/d | 46.9 ± 22.8 | 28.4−61.6 |
| Percentage added sugar, %kcal/d | 10.9 ± 4.4 | 7.8−14.2 |
| Beverage intake | ||
| Water, mL/d | 2753.4 ± 617.5 | 2352.0−3103.0 |
| All soft drinks, 8 oz/d | 0.5 ± 0.5 | 0.0−0.8 |
| Sweet soft drinks, 8 oz/d | 0.4 ± 0.5 | 0.0−0.8 |
| Sugary beverages excluding juice, 8 oz/d | 0.9 ± 0.7 | 0.3−1.4 |
| Sugary beverages excluding juice and dairy, 8 oz/d | 0.8 ± 0.7 | 0.3−1.4 |
| Sugary beverages including juice, 8 oz/d | 2.1 ± 1.8 | 0.8−2.8 |
| Citrus juice, 8 oz/d | 0.7 ± 1.2 | 0.0−1.0 |
| Fruit juice excluding citrus juice, 8 oz/d | 0.5 ± 0.8 | 0.0−0.8 |
| Glycemic index and glycemic load | ||
| Glycemic index (bread as a reference) | 82.8 ± 4.8 | 80.3−86.2 |
| Glycemic load (bread as a reference) | 163.9 ± 47.1 | 126.5−198.6 |
| Fiber | ||
| Total fiber, g/d | 17.8 ± 5.3 | 14.4−22.1 |
| Soluble fiber, g/d | 5.4 ± 1.8 | 4.1−6.3 |
| Insoluble fiber, g/d | 12.1 ± 3.9 | 9.5−14.3 |
Values are means ± SDs with the IQRs for the 99 Hispanic women included in this study. Dietary intake was determined from 24-h dietary recalls performed at 1 and 6 mo postpartum. Each dietary factor was derived from the mean of the 1- and 6-mo recalls. Dietary factors are shown as kilocalories, grams, 8-oz (equivalent to 236.6 mL) servings, or milliliters per day.
Dietary sugar, glycemic index, and soluble fiber were associated with postpartum weight gain
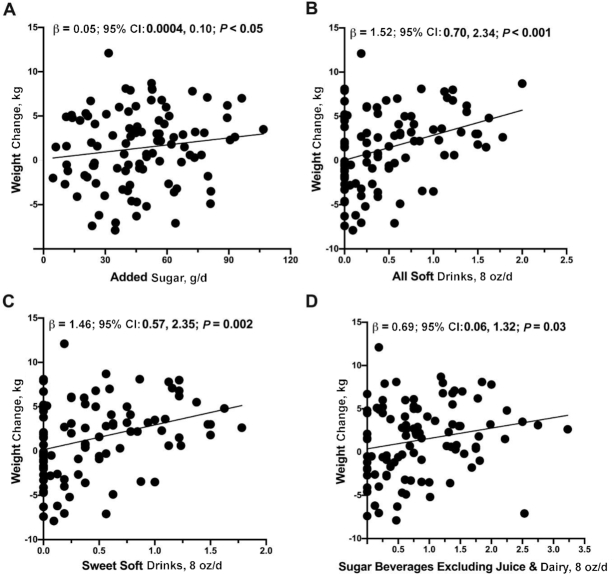
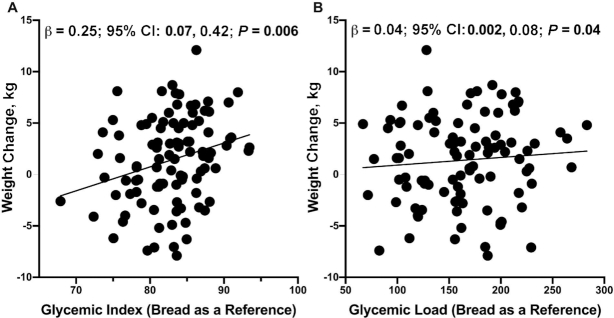
The most recent Dietary Guidelines for Americans (2015–2020) recommend that Americans should consume no more than 10% of their daily calories from added sugars (28). In this study, 59.6% of participants consumed >10% of their daily calories from added sugars, which was equal to ∼46.9 g added sugar per day. Sugary beverages were the largest source of added sugar among postpartum Hispanic women including soda (e.g., Coca-Cola, Pepsi, Fanta), sweetened juices (e.g., Tampico, Hawaiian Punch), sweetened teas (e.g., Arizona, Snapple), and sports drinks (e.g., Gatorade). Higher dietary sugar intake was associated with a higher maternal weight gain from 1 to 6 mo postpartum—this finding was observed when dietary sugars were analyzed as added sugar, all soft drinks, sweet soft drinks, sugar-sweetened beverages excluding juice, and sugar-sweetened beverages excluding juice and dairy (Table 3, Figure 1). As an example, a half 8-ounce serving per day increase in soft drink consumption was associated with a 1.52-kg increase (95% CI: 0.70, 2.34 kg; P < 0.001) in postpartum maternal weight. In addition, a half 8-ounce serving per day increase in sugary beverages excluding juice and dairy was associated with a 0.69-kg increase (95% CI: 0.06, 1.32 kg; P = 0.03) in postpartum weight. Lastly, 1-unit increases in glycemic index (bread as a reference) and glycemic load (bread as a reference) were associated with a 0.25-kg (95% CI: 0.07, 0.42 kg; P = 0.006) and a 0.04-kg (95% CI: 0.002, 0.08 kg; P = 0.04) increase in postpartum weight, respectively (Table 3, Figure 2).
TABLE 3.
Dietary factors associated with weight from 1 to 6 mo postpartum1
| β | 95% CI | P value | |
|---|---|---|---|
| Total carbohydrates | |||
| Carbohydrates, g/d | 0.01 | −0.03, 0.04 | 0.69 |
| Total sugar, g/d | −0.01 | −0.05, 0.03 | 0.58 |
| Added sugar, g/d | 0.05 | 0.0004, 0.10 | 0.05 |
| Beverage intake | |||
| Water, mL/d | −0.00004 | −0.001, 0.001 | 0.96 |
| All soft drinks, 8 oz/d | 1.52 | 0.70, 2.34 | <0.001 |
| Sweet soft drinks, 8 oz/d | 1.46 | 0.57, 2.35 | 0.002 |
| Sugary beverages excluding juice, 8 oz/d | 0.70 | 0.07, 1.33 | 0.03 |
| Sugary beverages excluding juice and dairy, 8 oz/d | 0.69 | 0.06, 1.32 | 0.03 |
| Sugary beverages including juice, 8 oz/d | 0.03 | −0.22, 0.28 | 0.82 |
| Citrus juice, 8 oz/d | 0.03 | −0.32, 0.39 | 0.85 |
| Fruit juice excluding citrus juice, 8 oz/d | −0.47 | −1.02, 0.07 | 0.09 |
| Glycemic index and glycemic load | |||
| Glycemic index (bread as a reference) | 0.25 | 0.07, 0.42 | 0.006 |
| Glycemic load (bread as a reference) | 0.04 | 0.002, 0.08 | 0.04 |
| Fiber | |||
| Total fiber, g/d | −0.15 | −0.33, 0.03 | 0.09 |
| Soluble fiber, g/d | −0.82 | −1.35, −0.29 | 0.003 |
| Insoluble fiber, g/d | −0.12 | −0.35, 0.12 | 0.33 |
Associations between the dietary factors and weight change from 1 to 6 mo postpartum among the 99 women included in this study. Models adjusted for baseline maternal age, height, and energy intake (kcal). Dietary factors are shown for grams, 8-oz (equivalent to 236.6 mL) servings, or milliliters per day.
FIGURE 1.
Added sugar and servings of sugary beverages were associated with increased maternal weight from 1 to 6 mo postpartum. Servings of (A) added sugar (g/d), (B) all soft drinks (8-oz servings/d), (C) sweet soft drinks (8-oz servings/d), and (D) sugary beverages excluding juice and dairy (8-oz servings/d) were positively associated with maternal weight change in 99 women. Dietary factors are shown for grams or 8-oz (equivalent to 236.6 mL) servings per day. Unadjusted values for change in maternal weight from 1 to 6 mo compared with servings of soft drinks are shown. Multiple linear regression was performed to obtain the effect sizes (β) and 95% CIs after adjustment for mothers’ baseline age, height, and total energy intake (kcal). Plots show beverage servings in 8 oz/d and effect estimates (β) are reported for a half serving per day difference in beverage consumption.
FIGURE 2.
Glycemic index (bread as a reference) and glycemic load (bread as a reference) were associated with increased maternal weight from 1 to 6 mo postpartum. Mean daily intake of (A) glycemic index (bread as a reference) and (B) glycemic load (bread as a reference) were positively associated with change in postpartum weight in 99 women. Unadjusted values for change in maternal weight from 1 to 6 mo compared with each dietary factor are shown. Multiple linear regression was performed to obtain the effect sizes (β) and 95% CIs after adjustment for mothers’ baseline age, height, and total energy intake (kcal).
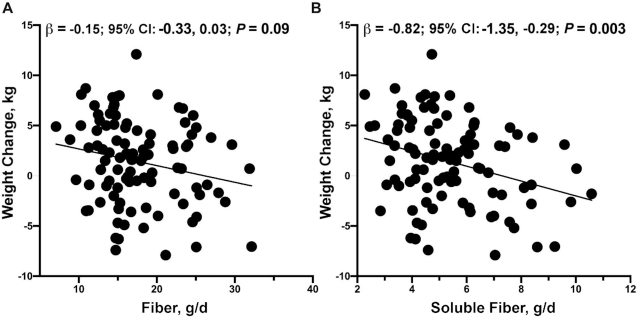
Although dietary fiber may protect against weight gain (29), only 33.3% of women in this study consumed ≥6 g soluble fiber per day, which is in line with the suggested 6–8 g/d (30). The most common sources of soluble fiber included granola bars, fortified instant oatmeal, pinto beans, vegetables, and fruit. Although consumption of dietary fiber was relatively low in this sample, we found that a 1-g/d higher dietary fiber consumption was associated with a 0.15-kg lower postpartum weight gain; however, this finding did not reach statistical significance (95% CI: −0.33, 0.03 kg; P = 0.09). We did, however, find that a 1-g/d increment of soluble fiber was associated with a 0.82-kg lower (95% CI: −1.35, −0.29 kg; P = 0.003) postpartum weight gain (Table 3, Figure 3).
FIGURE 3.
Fiber was associated with decreased maternal weight from 1 to 6 mo postpartum. Mean daily intakes of (A) fiber and (B) soluble fiber (g/d) were negatively associated with change in postpartum weight in 99 women. Unadjusted values for change in maternal weight from 1 to 6 mo compared with dietary fiber and soluble fiber intakes are shown. Multiple linear regression was performed to obtain the effect sizes (β) and 95% CIs after adjustment for mothers’ baseline age, height, and total energy intake (kcal).
Because dietary fiber may protect against obesity, we next examined whether soluble fiber intake attenuated the positive associations of added sugar and sugary beverages with maternal weight. We examined this by adjusting for soluble fiber intake when determining the associations of change in postpartum weight with added sugar, sugary beverages, and a high-glycemic-index diet. Overall, we found that the positive associations between maternal weight and added sugar (β: 0.03; 95% CI: −0.03, 0.08; P = 0.32), sugar beverages excluding juice (β: 0.55; 95% CI: −0.07, 1.17; P = 0.08), sugar beverages excluding juice and dairy (β: 0.55; 95% CI: −0.06, 1.17; P = 0.08), and glycemic index (bread as a reference) (β: 0.15; 95% CI: −0.04, 0.35; P = 0.12) were no longer significant after adjusting for soluble fiber intake. However, the positive associations between maternal weight and soft drinks (β: 1.21; 95% CI: 0.35, 2.07; P = 0.006), sweet soft drinks (β: 1.11; 95% CI: 0.18, 2.04; P = 0.02), and glycemic load (bread as a reference) (β: 0.04; 95% CI: 0.001, 0.07; P = 0.04) were only partially attenuated after adjusting for soluble fiber intake, and the effects remained statistically significant. Despite this, the associations between dietary sugar and postpartum weight gain did not differ by the amount of soluble fiber intake (Pinteraction all > 0.44). Further adjustment for prepregnancy weight or change in breastfeeding status did not significantly change results (Supplemental Table 2). Lastly, no other macronutrients (e.g., protein, fat) were significantly associated with change in maternal weight (data not shown).
Discussion
Results from this study show that dietary sugars in the form of added sugars and sugar-sweetened beverages were positively associated with greater postpartum weight gain among Hispanic women. For example, those who consumed added sugar at the 75th percentile (61.6 g) compared with the 25th percentile (28.4 g) would be predicted to gain 2.08 kg as opposed to 0.45 kg over 6 mo. In addition, high-glycemic-index and high-glycemic-load diets were associated with greater weight gain in this population. These results are consistent with previous studies that suggest that low-glycemic-index diets foster weight loss (31, 32) and that sugar-sweetened beverages contribute to the obesity epidemic (33). Similarly to previous studies (6, 34, 35), we also found that dietary soluble fiber was associated with postpartum weight loss and appeared to partially, although not completely, attenuate the associations between weight gain and added sugar intake (e.g., added sugar, sugary beverages). These results provide the first evidence that dietary added sugars and soluble fiber may affect postpartum weight trajectories among Hispanic women, a group with high rates of overweight and obesity (1, 2).
In this study, general carbohydrate intake was not found to be associated with weight trajectories; however, specific carbohydrates (e.g., added sugar, sugary beverages) did have significant associations with greater postpartum weight gain. This adds to a body of literature (5, 12–14, 31, 32) suggesting that carbohydrate quality and particularly added sugar and sugar-sweetened beverages may have obesogenic effects. We also found that dietary soluble fiber was associated with postpartum weight loss and partially attenuated some of the positive associations of added sugars and sugary beverages with postpartum weight gain. These observed associations may be due to reductions in the glycemic response to carbohydrate ingestion (36), increased satiety via delayed gastric emptying and hormonal signaling (37), and/or improved regulation of fasting blood glucose and insulin concentrations (33). In addition, previous studies among Hispanic women and children have found that dietary fiber was inversely associated with weight gain and visceral adipose tissue, respectively (18, 35). Thus, results from the current study are in line with previous findings and suggest that weight loss among postpartum Hispanic women may be influenced by dietary soluble fiber.
To date, only 1 study to our knowledge has examined the impact of carbohydrate consumption on postpartum weight trajectories, and this was among largely white Irish women. This study found that a low-glycemic-index intervention resulted in greater weight loss from prepregnancy to 3 mo postpartum (5). To our knowledge, our study is the first to examine the associations of dietary carbohydrate and fiber intake with changes in postpartum weight among Hispanic women. However, the current study is limited in that results may not be generalizable to other racial/ethnic groups. For example, an intervention study among overweight and obese adolescents found that reductions in sugar-sweetened beverage intake resulted in a reduced BMI among Hispanics but not non-Hispanic whites (38). This study is also limited by the use of only 2 dietary assessments that were performed at both the 1- and 6-mo postpartum visits. Despite this, the 24-h diet recalls provided a detailed assessment of macronutrient and sugary beverage consumption that allowed us to examine the relevant maternal dietary factors of interest. It is important to note that our moderate sample size may have influenced our ability to detect significant associations of additional carbohydrate and fiber types with postpartum weight. Although a larger sample size may have revealed other novel associations, 8 dietary characteristics were found to be associated with postpartum weight in this understudied population. To address these potential limitations, future studies should include additional dietary assessment among a larger and racially/ethnically diverse population of postpartum women. Finally, future studies should also examine the impact of these dietary variables during the pregnancy period because it is estimated that 1 in 5 pregnant women consume ≥1 sugar-sweetened beverage per day (39) and high-glycemic-load, low-fiber diets have been linked with pregnancy-induced hypertension, which may be attributable to reduced satiety and/or specific food constituents (40).
In summary, our results indicate that increased consumption of added sugar, sugar-sweetened beverages, and a higher-glycemic-index and -load diet have the potential to increase risk of obesity by contributing to postpartum weight gain among Hispanic women. In addition, results from this study suggest that dietary soluble fiber may aid in postpartum weight loss and may partially protect against weight gain from dietary added sugars and high-glycemic-index and -load diets. Notably, our results indicate that a half-serving per day (or half of a standard 8-ounce serving size) increase of soft drinks would be associated with a 1.52-kg increase in postpartum weight, whereas a 1-g increase in soluble fiber intake was associated with a 0.82-kg decrease in postpartum weight between 1 and 6 mo postpartum. These findings suggest that specific dietary factors should be considered when developing preventive strategies for alleviating the burden of obesity during the pregnancy and postpartum periods, which are sensitive periods for women's health. For example, interventions targeting reduction of sugar-sweetened beverages (e.g., soda, sweetened juice) and increased consumption of soluble fiber (e.g., fortified instant oatmeal, pinto beans, vegetables, fruit) may be successful in preventing postpartum weight gain.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—TLA and MIG: designed the research and had primary responsibility for the final content; TLA, SMM, and LEW: performed the statistical analyses; TLA, LEW, SMM, MCB, WBP, PKB, RBJ, and JFP: wrote the paper; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by National Institute of Diabetes and Digestive and Kidney Diseases grant NIH R01 DK110793 (to MIG), the Gerber Foundation (to MIG), and National Institute of Environmental Health Sciences grant NIH R00 ES027853 (to TLA).
The Gerber Foundation provided research funding but was not involved in the design, implementation, analysis, or interpretation of the data.
Supplemental Tables 1 and 2 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Contributor Information
Tanya L Alderete, Department of Integrative Physiology, University of Colorado Boulder, Boulder, CO, USA.
Laura E Wild, Department of Integrative Physiology, University of Colorado Boulder, Boulder, CO, USA.
Savannah M Mierau, Department of Integrative Physiology, University of Colorado Boulder, Boulder, CO, USA.
Maximilian J Bailey, Department of Integrative Physiology, University of Colorado Boulder, Boulder, CO, USA.
William B Patterson, Department of Integrative Physiology, University of Colorado Boulder, Boulder, CO, USA.
Paige K Berger, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, University of Southern California, Los Angeles, CA, USA.
Roshonda B Jones, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, University of Southern California, Los Angeles, CA, USA.
Jasmine F Plows, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, University of Southern California, Los Angeles, CA, USA.
Michael I Goran, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, University of Southern California, Los Angeles, CA, USA.
References
- 1. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017(288):1–8. [PubMed] [Google Scholar]
- 2. Ogden CL, Fakhouri TH, Carroll MD, Hales CM, Fryar CD, Li X, Freedman DS. Prevalence of obesity among adults, by household income and education—United States, 2011–2014. MMWR Morb Mortal Wkly Rep. 2017;66:1369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walker LO, Hoke MM, Brown A. Risk factors for excessive or inadequate gestational weight gain among Hispanic women in a U.S.‐Mexico border state. J Obstet Gynecol Neonatal Nurs. 2009;38:418–29. [DOI] [PubMed] [Google Scholar]
- 4. Endres LK, Straub H, McKinney C, Plunkett B, Minkovitz CS, Schetter CD, Ramey S, Wang C, Hobel C, Raju T et al. Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstet Gynecol. 2015;125:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horan MK, McGowan CA, Gibney ER, Donnelly JM, McAuliffe FM. Maternal diet and weight at 3 months postpartum following a pregnancy intervention with a low glycaemic index diet: results from the ROLO randomised control trial. Nutrients. 2014;6:2946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis JN, Shearrer GE, Tao W, Hurston SR, Gunderson EP. Dietary variables associated with substantial postpartum weight retention at 1-year among women with GDM pregnancy. BMC Obes. 2017;4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VWV, Eriksson JG, Broekman BFP. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Catalano PM. Obesity and pregnancy—the propagation of a viscous cycle?. J Clin Endocrinol Metab. 2003;88:3505–6. [DOI] [PubMed] [Google Scholar]
- 9. Guo X, Warden BA, Paeratakul S, Bray GA. Healthy Eating Index and obesity. Eur J Clin Nutr. 2004;58:1580–6. [DOI] [PubMed] [Google Scholar]
- 10. Nicklas TA, Baranowski T, Cullen KW, Berenson G. Eating patterns, dietary quality and obesity. J Am Coll Nutr. 2001;20:599–608. [DOI] [PubMed] [Google Scholar]
- 11. Institute of Medicine. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: The National Academies Press; 2005. [Google Scholar]
- 12. Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, Chung ST, Costa E, Courville A, Darcey V et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30:67–77..e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poti JM, Braga B, Qin B. Ultra-processed food intake and obesity: what really matters for health – processing or nutrient content?. Curr Obes Rep. 2017;6:420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–34. [DOI] [PubMed] [Google Scholar]
- 15. Malik VS, Hu FB. Sugar-sweetened beverages and cardiometabolic health: an update of the evidence. Nutrients. 2019;11:1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sylvetsky AC, Edelstein SL, Walford G, Boyko EJ, Horton ES, Ibebuogu UN, Knowler WC, Montez MG, Temprosa M, Hoskin M et al. A high-carbohydrate, high-fiber, low-fat diet results in weight loss among adults at high risk of type 2 diabetes. J Nutr. 2017;147:2060–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zou J, Chassaing B, Singh V, Pellizzon M, Ricci M, Fythe MD, Kumar MV, Gewirtz AT. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe. 2018;23:41–53.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davis JN, Alexander KE, Ventura EE, Toledo-Corral CM, Goran MI. Inverse relation between dietary fiber intake and visceral adiposity in overweight Latino youth. Am J Clin Nutr. 2009;90:1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Öhlin A, Rössner S. Trends in eating patterns, physical activity and socio-demographic factors in relation to postpartum body weight development. Br J Nutr. 1994;71:457–70. [DOI] [PubMed] [Google Scholar]
- 20. Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstet Gynecol. 2002;100:245–52. [DOI] [PubMed] [Google Scholar]
- 21. Berggren EK, Groh-Wargo S, Presley L, Hauguel-de Mouzon S, Catalano PM. Maternal fat, but not lean, mass is increased among overweight/obese women with excess gestational weight gain. Am J Obstet Gynecol. 2016;214:745.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin CL, Tate DF, Schaffner A, Brannen A, Hatley KE, Diamond M, Munoz-Christian K, Pomeroy J, Sanchez T, Mercado A et al. Acculturation influences postpartum eating, activity, and weight retention in low-income Hispanic women. J Womens Health (Larchmt). 2017;26:1333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ehrlich SF, Hedderson MM, Quesenberry CP Jr, Feng J, Brown SD, Crites Y, Ferrara A. Post‐partum weight loss and glucose metabolism in women with gestational diabetes: the DEBI Study. Diabetic Med. 2014;31:862–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin North Am. 2009;36:317–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: CDC, US Department of Health and Human Services; 2017. [Google Scholar]
- 26. CDC. Defining adult overweight and obesity. Atlanta, GA: CDC, US Department of Health and Human Services; 2019. [Google Scholar]
- 27. Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77:1171–8. [DOI] [PubMed] [Google Scholar]
- 28. USDA, US Department of Health and Human Services. Dietary Guidelines for Americans 2015–2020 [Internet]. 8th ed Washington, DC: US Government Printing Office; 2015; [cited October 4, 2019] Available from: https://health.gov/our-work/food-and-nutrition/2015-2020-dietary-guidelines/. [Google Scholar]
- 29. Wang ZQ, Zuberi AR, Zhang XH, Macgowan J, Qin J, Ye X, Son L, Wu Q, Lian K, Cefalu WT. Effects of dietary fibers on weight gain, carbohydrate metabolism, and gastric ghrelin gene expression in mice fed a high-fat diet. Metabolism. 2007;56:1635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. University of California San Francisco (UCSF). Increasing fiber intake. [Internet]. San Francisco, CA: UCSF; 2019. [cited October 4, 2019]. Available from: https://www.ucsfhealth.org/education/increasing-fiber-intake p. 1–2. [Google Scholar]
- 31. Spieth LE, Harnish JD, Lenders CM, Raezer LB, Pereira MA, Hangen SJ, Ludwig DS. A low-glycemic index diet in the treatment of pediatric obesity. Arch Pediatr Adolesc Med. 2000;154:947–51. [DOI] [PubMed] [Google Scholar]
- 32. Pittas AG, Das SK, Hajduk CL, Golden J, Saltzman E, Stark PC, Greenberg AS, Roberts SB. A low-glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE trial. Diabetes Care. 2005;28:2939–41. [DOI] [PubMed] [Google Scholar]
- 33. Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thompson SV, Hannon BA, An R, Holscher HD. Effects of isolated soluble fiber supplementation on body weight, glycemia, and insulinemia in adults with overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2017;106:1514–28. [DOI] [PubMed] [Google Scholar]
- 35. Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr. 2003;78:920–7. [DOI] [PubMed] [Google Scholar]
- 36. Ullrich IH, Albrink MJ. The effect of dietary fiber and other factors on insulin response: role in obesity. J Environ Pathol Toxicol Oncol. 1985;5:137–55. [PubMed] [Google Scholar]
- 37. Warrilow A, Mellor D, McKune A, Pumpa K. Dietary fat, fibre, satiation, and satiety—a systematic review of acute studies. Eur J Clin Nutr. 2019;73:333–44. [DOI] [PubMed] [Google Scholar]
- 38. Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, Ludwig DS. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med. 2012;367:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lundeen EA, Park S, Baidal JAW, Sharma AJ, Blanck HM. Sugar-sweetened beverage intake among pregnant and non-pregnant women of reproductive age. Matern Child Health J. 2020;24:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanjarimoghaddam F, Bahadori F, Bakhshimoghaddam F, Alizadeh M. Association between quality and quantity of dietary carbohydrate and pregnancy-induced hypertension: a case-control study. Clin Nutr ESPEN. 2019;33:158–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.