ABSTRACT
Background
Whole grains and other foods containing fiber are thought to be inversely related to colorectal cancer (CRC). However, whether these associations reflect fiber or fiber source remains unclear.
Objectives
We evaluated associations of whole grain and dietary fiber intake with CRC risk in the large NIH-AARP Diet and Health Study.
Methods
We used Cox proportional hazard models to estimate HRs and 95% CIs for whole grain and dietary fiber intake and risk of CRC among 478,994 US adults, aged 50–71 y. Diet was assessed using a self-administered FFQ at baseline in 1995–1996, and 10,200 incident CRC cases occurred over 16 y and 6,464,527 person-years of follow-up. We used 24-h dietary recall data, collected on a subset of participants, to evaluate the impact of measurement error on risk estimates.
Results
After multivariable adjustment for potential confounders, including folate, we observed an inverse association for intake of whole grains (HRQ5 vs.Q1 : 0.84; 95% CI: 0.79, 0.90; P-trend < 0.001), but not dietary fiber (HRQ5 vs. Q1: 0.96; 95% CI: 0.88, 1.04; P-trend = 0.40), with CRC incidence. Intake of whole grains was inversely associated with all CRC cancer subsites, particularly rectal cancer (HRQ5 vs. Q1: 0.76; 95% CI: 0.67, 0.87; P-trend < 0.001). Fiber from grains, but not other sources, was associated with lower incidence of CRC (HRQ5 vs. Q1: 0.89; 95% CI: 0.83, 0.96; P-trend < 0.001), particularly distal colon (HRQ5 vs. Q1: 0.84; 95% CI: 0.73, 0.96; P-trend = 0.005) and rectal cancer (HRQ5 vs. Q1: 0.77; 95% CI: 0.66, 0.88; P-trend < 0.001).
Conclusions
Dietary guidance for CRC prevention should focus on intake of whole grains as a source of fiber.
Keywords: colorectal cancer, whole grains, dietary fiber, colon cancer, rectal cancer, diet, epidemiology
Introduction
With >145,000 new cases and >51,000 deaths estimated in 2019, colorectal cancer (CRC) is the third most commonly occurring cancer among both men and women in the United States (1). The most recent report published by the World Cancer Research Fund and the American Institute for Cancer Research (2) concluded that foods containing dietary fiber, especially whole grains, decrease the risk of CRC. Prior research indicates that dietary fiber may lower risk of CRC by increasing stool bulk, thus diluting potentially carcinogenic substances, decreasing transit time through the bowel, and promoting fermentation and SCFA production in the gut (3). However, studies of CRC that isolate fiber from whole foods have been inconsistent, and it is unclear whether potential fiber benefits derive from the nutrient itself or from the whole foods that naturally contain it (4–15). Moreover, observed inverse associations have varied by food source and cancer site (i.e., proximal colon, distal colon, or rectum) (9, 11, 14). The Dietary Guidelines for Americans (2015–2020) highlight dietary fiber as an under-consumed nutrient of public health concern but recommend consuming nutrient-dense foods, not supplements, that are good sources of dietary fiber, including whole grains, fruits, and vegetables (16).
The hypothesis that consumption of dietary fiber decreases risk of CRC was previously investigated in the prospective NIH-AARP Diet and Health Study more than a decade ago (15). With only 5 y of follow-up, this large cohort of nearly half a million US adults had ascertained 2974 incident CRC cases but found no association between total dietary fiber intake and CRC. They did, however, find inverse associations for intake of fiber from grains and whole grains with CRC, particularly rectal cancer, after adjustment for folate and other potential confounders (15). It is important to note that folate has been strongly and inversely associated with CRC risk in the NIH-AARP Diet and Health Study (17). Currently, the NIH-AARP Diet and Health Study has >15 y of follow-up and >10,000 incident cases of CRC. Thus, we are revisiting this important hypothesis and conducting more extensive analyses of the primary hypothesis as well as potential effect modification, subsite heterogeneity, and reverse causality.
Methods
Study population
The NIH-AARP Diet and Health Study has been described in detail elsewhere (18). In brief, beginning in 1995, 3.5 million AARP members, aged 50 to 71 y, who resided in 1 of 6 US states (California, New Jersey, Pennsylvania, Florida, Louisiana, and North Carolina) or 1 of 2 metropolitan areas (Atlanta, Georgia and Detroit, Michigan), were mailed a questionnaire on demographics, health-related behaviors, and diet. Of the 566,398 participants who satisfactorily completed the baseline questionnaire, we excluded those with proxy responders (n = 15,760); individuals with prevalent cancer except nonmelanoma skin cancer (n = 51,260); those with self-reported poor health (n = 8365) or end-stage renal disease (n = 769); those with no cancer registry report (n = 4117); and those with extreme caloric intake (n = 4270), intake of dietary fiber (n = 1733), or intake of whole grains (n = 1130) (defined as >2 IQRs above the 75th percentile or below the 25th percentile of intake). Our final analytic cohort included 285,456 men and 193,538 women (n = 478,994). The NIH-AARP Diet and Health Study was reviewed and approved by the National Cancer Institute's Special Studies Institutional Review Board.
Assessment of CRC cases
Participants were cancer-free at baseline, and person time was counted from the return date of the baseline questionnaire until the date of CRC diagnosis, date of death, date the participant moved from the study area, or the end of the follow-up (31 December, 2011), whichever came first. Incident CRC cases were ascertained through probabilistic linkage with 11 state cancer registries (8 previously mentioned and 3 additional, including Arizona, Texas, and Nevada), which was validated to capture 90% of all cancer cases (19). Linkage with the National Death Index determined vital status and matching of cohort data with the US Post Office National Change of Address database determined address changes. CRC cases were defined by the International Classification of Diseases of Oncology (third edition) (20) using codes C180–189, C199, C209, and C260 which were further broken down by site as follows: C180–C184 (proximal colon), C185–C187 (distal colon), and C199 and C209 (rectum). During the follow-up period, 34,884 participants (7.3%) moved out of the catchment area and were considered lost to follow-up. Among those who remained in the catchment area, we identified 10,200 incident CRC cases (6712 men and 3488 women).
Assessment of whole grain and dietary fiber intakes
Dietary intakes were assessed with a self-administered FFQ which included questions on usual frequency of intake and portion size over the previous 12 mo. Frequency of intake was determined using 10 predefined categories from “never” to “≥6 times/day” for beverages and “never” to “≥2 times/day” for most foods as well as 3 portion size categories. Nutrients and portion sizes were estimated using the USDA's 1994–1996 Continuing Survey of Food Intake by Individuals (21), whereas food groups and serving sizes were defined by its corresponding Pyramid Servings database from 1994–1996, which allowed estimation of intake of whole grains from all sources in the FFQ. Categories of soluble and insoluble dietary fiber were assessed using the Nutrition Data System for Research software Food and Nutrient Database from the Nutrition Coordinating Center at the University of Minnesota (22). Quintiles for dietary variables including fiber, whole grain, red and processed meat, calcium, and folate were calculated using the baseline distributions for each variable in the analytic cohort. The FFQ was validated within a subset of participants using 2 nonconsecutive 24-h dietary recalls within 1 y of baseline; energy-adjusted correlations between FFQ-estimated and 24-h dietary recall–estimated fiber intakes were 0.72 and 0.66 for men and women, respectively (23).
Statistical analysis
We estimated HRs and 95% CIs for quintiles of intake of dietary fiber or whole grains and CRC using Cox proportional hazards regression models with the lowest quintile of intake of dietary fiber or whole grains as the reference category. First, we estimated age- and sex-adjusted HR estimates. Next, we adjusted risk estimates for BMI, alcohol consumption, general health status, having a first-degree relative with colon cancer, race, education, physical activity, smoking status, red and processed meat intake, calcium intake, total energy intake, and postmenopausal hormonal therapy (women only). To further explore the association between CRC and dietary fiber, we adjusted all whole grain and dietary fiber models for dietary folate intake. We also ran a model mutually adjusted for intake of dietary fiber and whole grains. Dietary variables were nutrient density–adjusted such that dietary fiber, red and processed meat, calcium, and folate are expressed in grams per 1000 kcal of total energy per day and whole grains in servings per 1000 kcal of total energy per day. We used person-years as the underlying time metric; models using age as the underlying time metric yielded similar results (data not shown). We conducted linear trend tests by assigning the median value of each quintile of intake of dietary fiber or whole grains and treating each variable as a continuous measure. We tested the proportional hazards assumption by including an interaction term for dietary fiber and person-years or whole grains and person-years in separate models. We found no evidence that the proportional hazard assumption was violated in the dietary fiber models (P = 0.99) or whole grain models (P = 0.18).
In secondary analyses, we analyzed the association between CRC and dietary fiber by source of fiber (i.e., grains, beans, fruits, and vegetables) and type of fiber (i.e., soluble and insoluble), overall and for each anatomical site (i.e., total colon, proximal colon, distal colon, and rectal cancer). To assess potential nonlinear associations of dietary fiber from different sources with CRC risk, we used restricted cubic spline analyses where the reference value for dietary fiber from a given source was set at the median intake value of the first quartile for HR estimates with 5 knots set at the 5th, 25th, 50th, 75th, and 95th percentiles of dietary fiber intake. To test for a potential nonlinear association between dietary fiber type or source and CRC risk, we compared the model with only the linear term for dietary fiber with the model containing both the linear and the cubic spline terms using a likelihood ratio test. We stratified by sex using sex-specific quintiles of intake for both whole grains and dietary fiber and also examined associations of whole grains and dietary fiber intake with CRC risk by anatomical site. We performed a lag analysis for both intake of dietary fiber and intake of whole grains with CRC risk, considering cases that occurred <5 y, 5–10 y, and >10 y after baseline. We conducted stratified models of dietary fiber by tertiles of folate and tertiles of red and processed meat. Finally, we also conducted analyses using a subset of individuals who completed two 24-h dietary recalls (n = 1975) to calibrate estimates of intakes of whole grains and dietary fiber in the larger cohort. Because dietary variables were highly correlated, we also used the residual method to adjust for these variables (24) (Supplemental Methods).
All analyses were conducted in SAS version 9.4 (SAS Institute). Tests for statistical significance were 2-sided, and P values < 0.05 that were unadjusted for multiple comparisons were considered statistically significant.
Results
Among the 478,994 participants in our analytic cohort, 10,200 incident cases of CRC occurred over 6.5 million person-years of follow-up. Mean age at baseline was 62 y and a majority of participants were non-Hispanic white and male. Median dietary fiber intake for the first and fifth quintiles ranged from 6.4 to 15.9 g . 1000 kcal−1 . d−1, and median whole grain intake ranged from 0.2 to 1.3 servings . 1000 kcal−1 . d−1. Compared with those in the lowest quintile of dietary fiber or whole grain consumption, those in the fifth quintile of dietary fiber or whole grain consumption were more likely to be older, college educated, report being in “excellent” health, be physically active, have never smoked, drink <1 alcoholic beverage per day, have received menopausal hormone therapy, be normal weight (i.e., 18.5 ≤BMI <25 kg/m2), not have a first-degree relative with colon cancer, and consume more dietary calcium and folate and fewer grams of red and processed meat and calories (Table 1).
TABLE 1.
NIH-AARP Diet and Health Study characteristics by quintiles of intakes of whole grains and dietary fiber1
| Whole grains | Dietary fiber | |||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 | |
| Whole grains,2 servings . 1000 kcal−1 . d−1 | 0.2 | 0.6 | 1.3 | 0.3 | 0.6 | 0.9 |
| Dietary fiber,2 g . 1000 kcal−1 . d−1 | 7.7 | 10.1 | 13.1 | 6.4 | 10.3 | 15.9 |
| Age, y | 61.7 ± 5.4 | 61.9 ± 5.4 | 62.5 ± 5.3 | 61.3 ± 5.5 | 62.1 ± 5.3 | 62.5 ± 5.3 |
| Men | 63.32 | 57.91 | 59.44 | 70.58 | 59.49 | 48.67 |
| Non-Hispanic white | 89.71 | 91.89 | 91.79 | 91.68 | 92.14 | 89.44 |
| College or postgraduate | 32.61 | 40.39 | 41.98 | 32.57 | 39.72 | 43.05 |
| Excellent self-reported health | 15.65 | 17.53 | 18.92 | 14.07 | 16.90 | 22.09 |
| Physical activity 3–4 times/wk | 21.79 | 28.10 | 30.24 | 20.07 | 27.98 | 31.96 |
| Never smoker | 28.84 | 36.63 | 39.24 | 25.52 | 36.97 | 42.02 |
| <1 Alcoholic drink/d | 61.82 | 72.08 | 74.69 | 57.56 | 73.81 | 74.50 |
| Current menopausal hormone therapy (women only) | 38.92 | 45.28 | 47.90 | 38.53 | 45.32 | 46.47 |
| Normal BMI (≥18.5 to <25 kg/m2) | 31.17 | 32.75 | 38.61 | 30.10 | 32.00 | 41.18 |
| No first-degree relative with colon cancer | 86.19 | 86.05 | 86.03 | 86.71 | 85.97 | 85.56 |
| Dietary calcium, mg . 1000 kcal−1 . d−1 | 380.0 ± 194.0 | 436.6 ± 176.0 | 469.4 ± 170.7 | 386.1 ± 215.2 | 429.1 ± 168.8 | 477.6 ± 162.3 |
| Dietary folate, μg . 1000 kcal−1 . d−1 | 236.6 ± 72.5 | 288.1 ± 78.0 | 332.5 ± 102.1 | 208.4 ± 59.7 | 286.3 ± 67.9 | 367.2 ± 90.5 |
| Red and processed meat, g . 1000 kcal−1 . d−1 | 41.1 ± 24.3 | 35.0 ± 20.4 | 26.7 ± 18.4 | 44.0 ± 24.4 | 36.1 ± 19.4 | 21.4 ± 16.1 |
| Energy, kcal/d | 1964.1 ± 963.3 | 1850.0 ± 753.2 | 1636.2 ± 627.7 | 2121.6 ± 975.9 | 1807.2 ± 731.9 | 1565.0 ± 603.0 |
n = 478,994. Values are means ± SDs or percentages unless otherwise indicated.
Median intake.
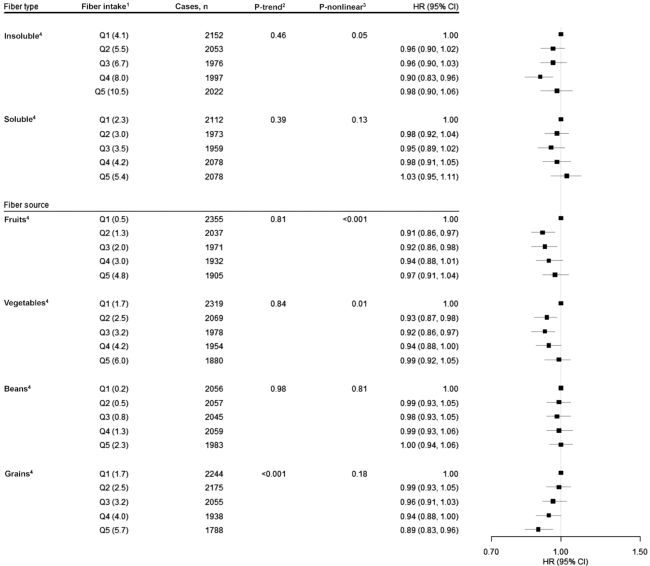
First, we estimated age- and sex-adjusted associations for whole grain and dietary fiber intakes with risk of CRC. Comparing the highest and lowest quintiles (i.e., Q5 compared with Q1), we observed statistically significant associations for both whole grains (HRQ5 vs. Q1: 0.69; 95% CI: 0.64, 0.73; P-trend < 0.001) and dietary fiber (HRQ5 vs. Q1: 0.70; 95% CI: 0.66, 0.75; P-trend < 0.0001) (Table 2). After adjustment for other potential confounders, but not folate, associations for intake of whole grains (HRQ5 vs. Q1: 0.83; 95% CI: 0.78, 0.89; P-trend < 0.001) and dietary fiber intake (HRQ5 vs. Q1: 0.92; 95% CI: 0.86, 0.99; P-trend = 0.03) with CRC were attenuated but remained statistically significant (Table 2). The association between intake of whole grains and CRC remained statistically significant after adjustment for folate (HRQ5 vs. Q1: 0.84; 95% CI: 0.79, 0.90; P-trend < 0.001) and further adjustment for dietary fiber intake (HRQ5 vs. Q1: 0.84; 95% CI: 0.78, 0.90; P-trend < 0.001) (Table 2). However, after adjustment for folate, associations for dietary fiber intake attenuated and became null (HRQ5 vs. Q1: 0.96; 95% CI: 0.88, 1.04; P-trend = 0.40). For dietary fiber type and source, associations were nonsignificant for insoluble fiber (HRQ5 vs. Q1: 0.98; 95% CI: 0.90, 1.06; P-trend = 0.46) and soluble fiber (HRQ5 vs. Q1: 1.03; 95% CI: 0.95, 1.11; P-trend = 0.39). Of potential sources, only fiber from grains was linearly and inversely associated with CRC (HRQ5 vs. Q1: 0.89; 95% CI: 0.83, 0.96; P-trend < 0.001) (Figure 1). We did, however, observe evidence of nonlinear associations for insoluble fiber (P for nonlinear association = 0.05) and for fiber from fruit (P for nonlinear association = 0.0002) and fiber from vegetables (P for nonlinear association = 0.01) with CRC risk using a restricted cubic spline approach (Supplemental Figure 1).
TABLE 2.
HRs and 95% CIs for quintiles of intakes of whole grains and dietary fiber and colorectal cancer1
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend2 | |
|---|---|---|---|---|---|---|
| Whole grains | ||||||
| Median intake, servings . 1000 kcal−1 . d−1 | 0.2 | 0.4 | 0.6 | 0.8 | 1.3 | |
| Cases/person-years | 2417/1,265,611 | 2111/1,293,497 | 2009/1,297,815 | 1882/1,304,937 | 1781/1,302,670 | |
| Age- and sex-adjusted | 1.00 | 0.86 (0.81, 0.91) | 0.81 (0.76, 0.86) | 0.74 (0.69, 0.78) | 0.69 (0.64, 0.73) | <0.0001 |
| Multivariable adjusted3 | 1.00 | 0.93 (0.87, 0.98) | 0.91 (0.86, 0.97) | 0.86 (0.81, 0.92) | 0.83 (0.78, 0.89) | <0.0001 |
| Multivariable adjusted with folate4 | 1.00 | 0.93 (0.88, 0.99) | 0.92 (0.86, 0.98) | 0.87 (0.82, 0.93) | 0.84 (0.79, 0.90) | <0.0001 |
| Multivariable adjusted with folate and fiber5 | 1.00 | 0.94 (0.88, 1.00) | 0.92 (0.87, 0.98) | 0.88 (0.82, 0.94) | 0.84 (0.78, 0.90) | <0.0001 |
| Dietary fiber | ||||||
| Median intake, g . 1000 kcal−1 . d−1 | 6.4 | 8.5 | 10.3 | 12.3 | 15.9 | |
| Cases/person-years | 2428/1,260,591 | 2107/1,289,753 | 2018/1,298,864 | 1826/1,305,506 | 1821/1,309,813 | |
| Age- and sex-adjusted | 1.00 | 0.83 (0.78, 0.88) | 0.78 (0.74, 0.83) | 0.70 (0.66, 0.75) | 0.70 (0.66, 0.75) | <0.0001 |
| Multivariable adjusted3 | 1.00 | 0.91 (0.86, 0.97) | 0.92 (0.86, 0.98) | 0.87 (0.81, 0.93) | 0.92 (0.86, 0.99) | 0.027 |
| Multivariable adjusted with folate4 | 1.00 | 0.93 (0.87, 0.99) | 0.94 (0.87, 1.00) | 0.89 (0.83, 0.96) | 0.96 (0.88, 1.04) | 0.396 |
n = 478,994.
P-trend < 0.05. All statistical tests were 2-sided.
Estimated using a Cox proportional hazards regression model (model 1) adjusted for age (years, continuous), BMI (kg/m2) (<18.5; 18.5 to <25; 25 to <30; ≥30; missing), alcohol intake (0 drinks/d; <1 drink/d; 1 to <2 drinks/d; 2 to <3 drinks/d; ≥3 drinks/d; missing), general health status (excellent; very good; good; fair; poor; unknown), first-degree relatives with colon cancer (yes; no; unknown), race/ethnicity (non-Hispanic white; non-Hispanic black; Hispanic; Asian, Pacific Islander, or American Indian/Native American; unknown), education (<12 y; 12 y or completed high school; post–high school training other than college; some college; college and postgraduate; unknown), sex, physical activity (never, rarely; <3 times/mo; 1–2, 3–4, or ≥5 times/wk; missing), smoking (never; ≤20 cigarettes/d in the past; >20 cigarettes/d in the past; ≤20 cigarettes/d currently; >20 cigarettes/d currently; missing), and intakes of red and processed meat (quintiles), dietary calcium (quintiles), and total energy (continuous).
Adjusted for all covariates in model 1 and dietary folate (quintiles).
Adjusted for all covariates in models 1 and 2 and dietary fiber (quintiles).
FIGURE 1.
Associations for type (i.e., insoluble and soluble) and source of dietary fiber (i.e., fruits, vegetables, beans, and grains) intake with risk of colorectal cancer, using quintile 1 as the reference, in the NIH-AARP Diet and Health Study (n = 478,994). 1Quintiles of dietary fiber intake (median g . 1000 kcal−1 . d−1). 2P-trend < 0.05. All statistical tests were 2-sided. 3P-nonlinear < 0.05. 4Cox proportional hazard model adjusted for age (continuous), BMI (in kg/m2) (<18.5; 18.5 to <25; 25 to <30; ≥30; missing), alcohol (0 drinks/d; <1 drink/d; 1 to <2 drinks/d; 2 to <3 drinks/d; ≥3 drinks/d; missing), general health status (excellent; very good; good; fair; poor; unknown), first-degree relatives with colon cancer (yes; no; unknown), race/ethnicity (non-Hispanic white; non-Hispanic black; Hispanic; Asian, Pacific Islander, or American Indian/Native American; unknown), education (<12 y; 12 y or completed high school; post–high school training other than college; some college; college and postgraduate; unknown), sex, physical activity (never, rarely; <3 times/mo; 1–2, 3–4, or ≥5 times/wk; missing), smoking (never; ≤20 cigarettes/d in the past; >20 cigarettes/d in the past; ≤20 cigarettes/d currently; >20 cigarettes/d currently; missing), and intakes of red and processed meat (quintiles), dietary calcium (quintiles), folate (quintiles), and total energy (continuous).
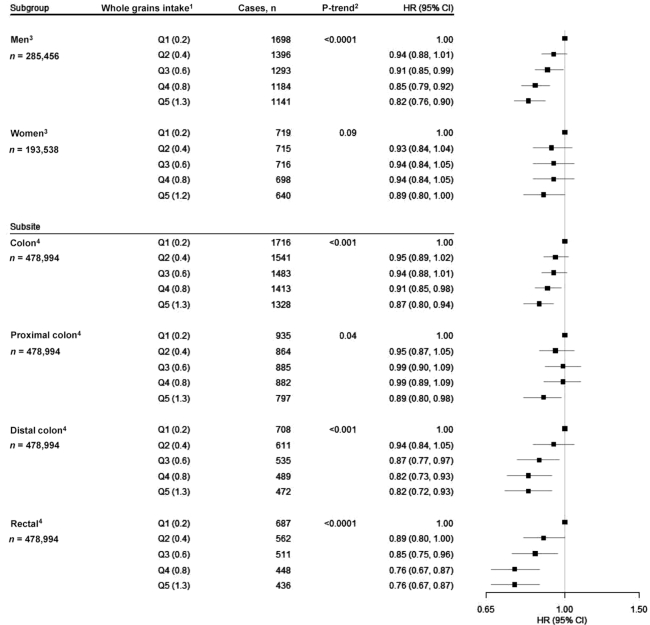
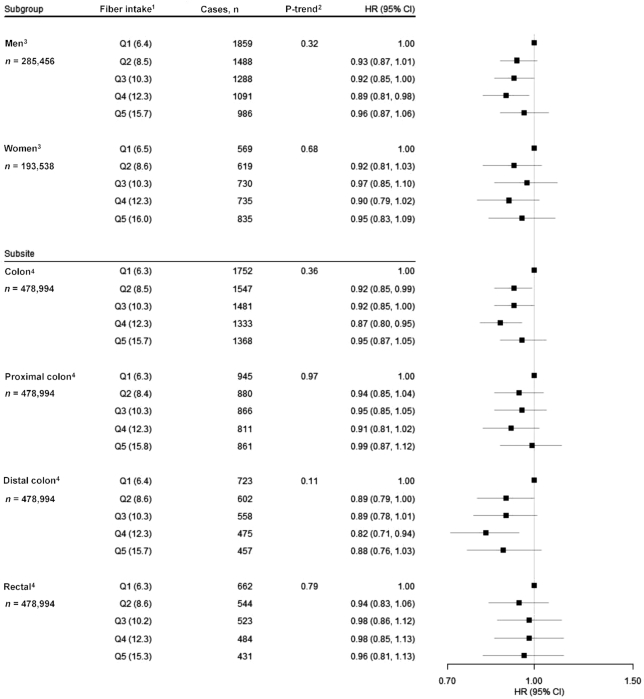
To better understand the relation between intake of whole grains and CRC, we evaluated associations by sex and according to CRC subsite and length of follow-up time. We found no statistical evidence of effect modification by sex (P-interaction = 0.13). We found statistically significant inverse associations for intake of whole grains and CRC across all subsites, with the association of intake of whole grains with rectal cancer (HRQ5 vs. Q1: 0.76; 95% CI: 0.67, 0.87; P-trend < 0.001) notably strong (Figure 2). Finally, we performed a lag analysis for intake of whole grains considering cases that occurred during the first 5 y, during years 5 through 10, and after >10 y of follow-up. HRs were generally consistent over time (Table 3). We also conducted these analyses for dietary fiber and found largely null associations across all follow-up periods (Figure 3, Table 4).
FIGURE 2.
Associations for intake of whole grains with risk of CRC, using quintile 1 as the reference, stratified by sex and examined by CRC subsite (i.e., total colon, proximal colon, distal colon, and rectal cancer), in the NIH-AARP Diet and Health Study (n = 478,994). 1Quintiles of intake of whole grains (median servings . 1000 kcal−1 . d−1). 2P-trend < 0.05. All statistical tests were 2-sided. 3Cox proportional hazard model adjusted for age (continuous), BMI (in kg/m2) (<18.5; 18.5 to <25; 25 to <30; ≥30; missing), alcohol (0 drinks/d; <1 drink/d; 1 to <2 drinks/d; 2 to <3 drinks/d; ≥3 drinks/d; missing), general health status (excellent; very good; good; fair; poor; unknown), first-degree relatives with colon cancer (yes; no; unknown), race/ethnicity (non-Hispanic white; non-Hispanic black; Hispanic; Asian, Pacific Islander, or American Indian/Native American; unknown), education (<12 y; 12 y or completed high school; post–high school training other than college; some college; college and postgraduate; unknown), physical activity (never, rarely; <3 times/mo; 1–2, 3–4, or ≥5 times/wk; missing), smoking (never; ≤20 cigarettes/d in the past; >20 cigarettes/d in the past; ≤20 cigarettes/d currently; >20 cigarettes/d currently; missing), menopausal hormone therapy in women only (never; past; current; missing), and intakes of red and processed meat (quintiles), dietary calcium (quintiles), folate (quintiles), and total energy (continuous). P-interaction = 0.42. 4Adjusted for all covariates in the previous model and sex. CRC, colorectal cancer.
TABLE 3.
HRs and 95% CIs for quintiles of intake of whole grains and CRC by lag time (<5 y, 5 to ≤10 y, >10 y)1
| Whole grains | ||||||
|---|---|---|---|---|---|---|
| CRC | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend2 |
| Cases/person-years | 2417/1,265,611 | 2111/1,293,497 | 2009/1,297,815 | 1882/1,304,936 | 1781/1,302,670 | |
| Overall3 | 1.00 | 0.93 (0.88, 0.99) | 0.92 (0.86, 0.98) | 0.87 (0.82, 0.93) | 0.84 (0.79, 0.90) | 0.001 |
| Cases/person-years | 896/459,960 | 759/461,459 | 757/461,876 | 659/462,521 | 642/462,279 | |
| <5 y3 | 1.00 | 0.91 (0.83, 1.01) | 0.93 (0.84, 1.03) | 0.82 (0.74, 0.92) | 0.81 (0.72, 0.90) | 0.018 |
| Cases/person-years | 860/410,205 | 723/418,565 | 687/419,462 | 645/421,857 | 643/420,974 | |
| ≥5 to ≤10 y3 | 1.00 | 0.90 (0.81, 1.00) | 0.89 (0.80, 0.99) | 0.85 (0.76, 0.95) | 0.86 (0.77, 0.96) | 0.055 |
| Cases/person-years | 661/395,446 | 629/413,475 | 565/416,477 | 578/420,557 | 496/419,416 | |
| >10 y3 | 1.00 | 1.00 (0.90, 1.12) | 0.93 (0.83, 1.05) | 0.98 (0.87, 1.10) | 0.86 (0.76, 0.98) | 0.118 |
n = 478,994. CRC, colorectal cancer.
P-trend < 0.05. All statistical tests were 2-sided.
Estimated using a Cox proportional hazards regression model adjusted for age (years, continuous), BMI (in kg/m2) (<18.5; 18.5 to <25; 25 to <30; ≥30; missing), alcohol intake (0 drinks/d; <1 drink/d; 1 to <2 drinks/d; 2 to <3 drinks/d; ≥3 drinks/d; missing), general health status (excellent; very good; good; fair; poor; unknown), first-degree relatives with colon cancer (yes; no; unknown), race/ethnicity (non-Hispanic white; non-Hispanic black; Hispanic; Asian, Pacific Islander, or American Indian/Native American; unknown), education (<12 y; 12 y or completed high school; post–high school training other than college; some college; college and postgraduate; unknown), sex, physical activity (never, rarely; <3 times/mo; 1–2, 3–4, or ≥5 times/wk; missing), smoking (never; ≤20 cigarettes/d in the past; >20 cigarettes/d in the past; ≤20 cigarettes/d currently; >20 cigarettes/d currently; missing), and intakes of red and processed meat (quintiles), dietary calcium (quintiles), folate (quintiles), and total energy (continuous).
FIGURE 3.
Associations for dietary fiber intake and risk of CRC, using quintile 1 as the reference, stratified by sex and examined by CRC subsite (i.e., total colon, proximal colon, distal colon, and rectal cancer), in the NIH-AARP Diet and Health Study (n = 478,994). 1Quintiles of dietary fiber intake (median g . 1000 kcal−1 . d−1). 2P-trend < 0.05. All statistical tests were 2-sided. 3Cox proportional hazard model adjusted for age (continuous), BMI (in kg/m2) (<18.5; 18.5 to <25; 25 to <30; ≥30; missing), alcohol (0 drinks/d; <1 drink/d; 1 to <2 drinks/d; 2 to <3 drinks/d; ≥3 drinks/d; missing), general health status (excellent; very good; good; fair; poor; unknown), first-degree relatives with colon cancer (yes; no; unknown), race/ethnicity (non-Hispanic white; non-Hispanic black; Hispanic; Asian, Pacific Islander, or American Indian/Native American; unknown), education (<12 y; 12 y or completed high school; post–high school training other than college; some college; college and postgraduate; unknown), physical activity (never, rarely; <3 times/mo; 1–2, 3–4, or ≥5 times/wk; missing), smoking (never; ≤20 cigarettes/d in the past; >20 cigarettes/d in the past; ≤20 cigarettes/d currently; >20 cigarettes/d currently; missing), menopausal hormone therapy in women only (never; past; current; missing), and intakes of red and processed meat (quintiles), dietary calcium (quintiles), folate (quintiles), and total energy (continuous). P-interaction = 0.49. 4Adjusted for all covariates in the previous model and sex. CRC, colorectal cancer.
TABLE 4.
HRs and 95% CIs for quintiles of intake of dietary fiber and CRC by lag time (<5 y, ≥5 to ≤10 y, >10 y)1
| Dietary fiber | ||||||
|---|---|---|---|---|---|---|
| CRC | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend2 |
| Cases/person-years | 2428/1,260,591 | 2107/1,289,753 | 2018/1,298,865 | 1826/1,305,506 | 1821/1,309,813 | |
| Overall3 | 1.00 | 0.93 (0.87, 0.99) | 0.94 (0.87, 1.00) | 0.89 (0.83, 0.96) | 0.96 (0.88, 1.04) | 0.396 |
| Cases/person-years | 901/459,317 | 789/461,496 | 707/461,870 | 638/462,461 | 678/462,950 | |
| <5 y3 | 1.00 | 0.93 (0.84, 1.03) | 0.88 (0.78, 0.99) | 0.83 (0.74, 0.94) | 0.95 (0.83, 1.09) | 0.414 |
| Cases/person-years | 846/408,700 | 703/417,156 | 707/420,027 | 669/421,959 | 633/423,222 | |
| ≥5 to ≤10 y3 | 1.00 | 0.89 (0.80, 0.99) | 0.96 (0.85, 1.08) | 0.97 (0.85, 1.09) | 0.99 (0.86, 1.13) | 0.710 |
| Cases/person-years | 681/392,574 | 615/411,102 | 604/416,968 | 519/421,086 | 510/423,641 | |
| >10 y3 | 1.00 | 0.96 (0.85, 1.08) | 0.99 (0.87, 1.12) | 0.89 (0.77, 1.02) | 0.93 (0.80, 1.09) | 0.294 |
n = 478,994. CRC, colorectal cancer.
P-trend < 0.05. All statistical tests were 2-sided.
Estimated using a Cox proportional hazards regression model adjusted for age (continuous), BMI (in kg/m2) (<18.5; 18.5 to <25; 25 to <30; ≥30; missing), alcohol intake (0 drinks/d; <1 drink/d; 1 to <2 drinks/d; 2 to <3 drinks/d; ≥3 drinks/d; missing), general health status (excellent; very good; good; fair; poor; unknown), first-degree relatives with colon cancer (yes; no; unknown), race/ethnicity (non-Hispanic white; non-Hispanic black; Hispanic; Asian, Pacific Islander, or American Indian/Native American; unknown), education (<12 y; 12 y or completed high school; post–high school training other than college; some college; college and postgraduate; unknown), sex, physical activity (never, rarely; <3 times/mo; 1–2, 3–4, or ≥5 times/wk; missing), smoking (never; ≤20 cigarettes/d in the past; >20 cigarettes/d in the past; ≤20 cigarettes/d currently; >20 cigarettes/d currently; missing), and intakes of red and processed meat (quintiles), dietary calcium (quintiles), folate (quintiles), and total energy (continuous).
In the dietary fiber–CRC models, folate adjustment substantially attenuated risk estimates (i.e., >10% change). Therefore, we wanted to ascertain whether the association between fiber and CRC varied by folate intake. We found no statistical evidence of effect modification by folate intake (P-interaction = 0.63); accordingly, in models stratified by tertile of folate intake, we observed no association between dietary fiber intake and CRC among those who consumed diets low or high in folate (Supplemental Table 1). Similarly, we found no statistical evidence of effect modification by tertile of red and processed meat intake (P-interaction = 0.66) (Supplemental Table 2).
We performed calibration analyses to evaluate whether measurement error in FFQ estimates of intake of dietary fiber and whole grains biased risk estimates (Supplemental Methods). Calibrated risk estimates for intake of whole grains were stronger with smaller HRs than those in our main analysis (HR: 0.71; 95% CI: 0.61, 0.82; P value < 0.001). Calibrated dietary fiber HR estimates appeared similar to those in the main analysis (HR: 0.97; 95% CI: 0.90, 1.04; P value = 0.40) (Supplemental Table 3). To address issues of multicollinearity and potential nonlinearity of dietary variables, we adjusted for dietary covariates and their polynomials using the residual method (Supplemental Methods). Risk estimates between whole grains and CRC were attenuated but remained inversely associated (HRQ5 vs. Q1: 0.88; 95% CI: 0.83, 0.93; P-trend < 0.0001), whereas the null association between dietary fiber and CRC remained unchanged (HRQ5 vs. Q1: 0.97; 95% CI: 0.91, 1.03; P-trend = 0.57) (Supplemental Table 4). Finally, to better understand the relation between dietary fiber and CRC risk, we considered the associations with fiber source for each CRC subsite. Fiber from grains was significantly associated with CRC overall, and this association appeared to be driven by significant associations with cancers of the distal colon and rectum (HRQ5 vs. Q1: 0.84; 95% CI: 0.73, 0.96; P value = 0.005; HRQ5 vs. Q1: 0.77; 95% CI: 0.66, 0.88; P value = 0.0002, respectively) (Supplemental Table 5).
Discussion
Our study is the largest cohort analysis to date of the association of whole grain and dietary fiber intake with CRC risk. This study updated a previous analysis that demonstrated an inverse association between intake of whole grains, but not fiber, and CRC. However, the prior analysis, with <3000 cases and only 5 y of follow-up, could not fully interrogate these associations. Now, with >10,000 cases and >15 y of follow-up, we were able to conduct further analyses evaluating associations for CRC subsites and different sources and types of dietary fiber. Our study confirmed the previous findings that intake of whole grains, but not dietary fiber, was inversely associated with CRC risk. In the current analysis, compared with those in the lowest quintile of intake of whole grains, those in the highest quintile of intake of whole grains had a 16% lower risk of CRC, and we observed even stronger inverse associations for rectal cancer. We found that the association between intake of whole grains and CRC was further strengthened when we used 24-h dietary recall data to account for measurement error in the FFQ.
Similarly to our findings, prior studies have generally found an inverse association between intake of whole grains and CRC, with the magnitude of associations, comparing the highest with the lowest consumers, ranging from an 8% to a 20% risk reduction (5, 25, 26). As for dietary fiber intake, the prior literature is inconsistent, with some studies finding associations (4, 10, 12, 27) and others not (6, 14, 15, 28). It is important to consider that whole grains and other sources of fiber contain numerous other constituents, including folate. In our analyses, we found that adjustment for folate substantially attenuated observed associations between dietary fiber and CRC. Approaches to disentangle dietary fiber from folate and other constituents in whole grains and other foods have been inconsistently applied in prior studies. Our results suggest that other constituents in whole grains may be responsible for the associations. For example, after mutually adjusting our whole grain model for dietary fiber, whole grains remained inversely associated with CRC. Whole grains contain numerous micronutrients and bioactive components such as B vitamins, minerals, phenols, antioxidants, and phytoestrogens, which may protect against CRC (29, 30). In addition, whole grains, which are a good source of fiber, increase stool bulk, dilute possible carcinogens, decrease stool transit time through the bowel, and produce SCFAs, which are hypothesized to protect against CRC risk (29, 31). Indirectly, whole grains and fiber may protect against CRC by reducing weight gain (32) and type 2 diabetes (33, 34), which are both risk factors for CRC. Taken together, our results suggest the importance of considering whole foods for cancer prevention rather than individual constituents, such as fiber supplements.
Our study had many strengths, including being the largest cohort to date with >10,000 incident CRC cases and >15 y of follow-up, which allowed for more robust stratified analyses by sex, consideration of fiber source and type, and assessment by individual cancer subsites. We found some evidence of nonlinear associations for insoluble fiber, fiber from fruits, and fiber from vegetables with CRC. It is unclear what may account for these observations, which need to be replicated in other large cohorts. The extended follow-up also permitted us to estimate associations between dietary exposures and CRC cases that occurred earlier and later during follow-up. As in the main analyses, we found inverse associations for intake of whole grains and CRC and a lack of an association for dietary fiber intake and CRC for cases occurring within 5 y of baseline and those occurring >10 y after baseline, suggesting that the observed associations were unlikely to be due to reverse causality. In addition, the prospective design of our study mitigated selection and recall biases which could have affected results from previous case-control studies (35, 36). A limitation of this study is that diet was measured by FFQ in the entire cohort at baseline only, so changes in diet over time could not be assessed. However, we were able to perform calibration analyses using up to two 24-h recalls that were collected on nonconsecutive days in a subset of participants to reduce the potential impact of FFQ-related measurement error. We also explored the impact of multicollinearity between dietary exposure and adjustment variables using the residual method. These secondary analyses provided further evidence that the observed inverse associations between intake of whole grains and CRC were robust, whereas the associations between total dietary fiber intake and CRC were largely null.
In conclusion, our study is the largest prospective cohort to date to examine intake of whole grains and dietary fiber in relation to CRC risk. We found that intakes of whole grains and fiber from grains, but not total intake of dietary fiber, were inversely associated with CRC risk, particularly rectal cancer. Our findings suggest that dietary guidance for CRC prevention should focus on increasing whole grain intake as a nutrient-dense source of fiber.
Supplementary Material
Acknowledgments
Cancer incidence data were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA (for the Atlanta metropolitan area); the California Cancer Registry, California Department of Public Health's Cancer Surveillance and Research Branch, Sacramento, CA; the Michigan Cancer Surveillance Program, Community Health Administration, Lansing, MI (for the Detroit metropolitan area); the Florida Cancer Data System (Miami, FL) under contract with the Florida Department of Health, Tallahassee, FL; the Louisiana Tumor Registry, Louisiana State University Health Sciences Center School of Public Health, New Orleans, LA; the New Jersey State Cancer Registry, The Rutgers Cancer Institute of New Jersey, New Brunswick, NJ; the North Carolina Central Cancer Registry, Raleigh, NC; the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, PA; the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services, Phoenix, AZ; the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, Austin, TX; and the Nevada Central Cancer Registry, Division of Public and Behavioral Health, State of Nevada Department of Health and Human Services, Carson City, NV. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
The authors’ responsibilities were as follows—LML, EL, AGH, and RS: designed the research; LML and AGH: conducted the research; EL and LML: provided essential materials; AGH: analyzed the data; and all authors: wrote the manuscript, had primary responsibility for the final content, and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported in part by the Intramural Research Program of the National Cancer Institute at the NIH.
Supplemental Tables 1–5, Supplemental Figure 1, and Supplemental Methods are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
The data sets analyzed for this study were obtained from the National Cancer Institute. Researchers can request access to the data from the NIH-AARP Diet and Health Study online at https://dietandhealth.cancer.gov/. Analytic code can be obtained from the corresponding author upon request.
Contributor Information
Autumn G Hullings, Division of Cancer Epidemiology & Genetics, National Cancer Institute, NIH, Bethesda, MD, USA.
Rashmi Sinha, Division of Cancer Epidemiology & Genetics, National Cancer Institute, NIH, Bethesda, MD, USA.
Linda M Liao, Division of Cancer Epidemiology & Genetics, National Cancer Institute, NIH, Bethesda, MD, USA.
Neal D Freedman, Division of Cancer Epidemiology & Genetics, National Cancer Institute, NIH, Bethesda, MD, USA.
Barry I Graubard, Division of Cancer Epidemiology & Genetics, National Cancer Institute, NIH, Bethesda, MD, USA.
Erikka Loftfield, Division of Cancer Epidemiology & Genetics, National Cancer Institute, NIH, Bethesda, MD, USA.
References
- 1. Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M., Ruhl J, Tatalovich Z, Mariotto A, Lewis DR et al.et al. editors. SEER Cancer Statistics Review (CSR) 1975–2016. Bethesda, MD: National Cancer Institute; 2019. [Google Scholar]
- 2. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report: diet, nutrition, physical activity and colorectal cancer. London: World Cancer Research Fund/American Institute for Cancer Research; 2018. [Google Scholar]
- 3. Bingham SA. Mechanisms and experimental and epidemiological evidence relating dietary fibre (non-starch polysaccharides) and starch to protection against large bowel cancer. Proc Nutr Soc. 1990;49(2):153–71. [DOI] [PubMed] [Google Scholar]
- 4. Bingham S, Day NE, Luben R, Ferrari P, Slimani N, Norat T, Clavel-Chapelon F, Kesse E, Nieters A, Boeing H et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361:1496–501. [DOI] [PubMed] [Google Scholar]
- 5. Park Y, Hunter DJ, Spiegelman D, Bergkvist L, Berrino F, van den Brandt PA, Buring JE, Colditz GA, Freudenheim JL, Fuchs CS et al. Dietary fiber intake and risk of colorectal cancer: a pooled analysis of prospective cohort studies. JAMA. 2005;294(22):2849–57. [DOI] [PubMed] [Google Scholar]
- 6. Michels KB, Fuchs CS, Giovannucci E, Colditz GA, Hunter DJ, Stampfer MJ, Willett WC. Fiber intake and incidence of colorectal cancer among 76,947 women and 47,279 men. Cancer Epidemiol Biomarkers Prev. 2005;14(4):842–9. [DOI] [PubMed] [Google Scholar]
- 7. Peters U, Sinha R, Chatterjee N, Subar AF, Ziegler RG, Kulldorff M, Bresalier R, Weissfeld JL, Flood A, Schatzkin A et al. Dietary fibre and colorectal adenoma in a colorectal cancer early detection programme. Lancet. 2003;361:1491–5. [DOI] [PubMed] [Google Scholar]
- 8. Burkitt DP. Epidemiology of cancer of the colon and rectum. Cancer. 1971;28(1):3–13. [DOI] [PubMed] [Google Scholar]
- 9. Bakken T, Braaten T, Olsen A, Kyrø C, Lund E, Skeie G. Consumption of whole-grain bread and risk of colorectal cancer among Norwegian women (the NOWAC Study). Nutrients. 2016;8(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hansen L, Skeie G, Landberg R, Lund E, Palmqvist R, Johansson I, Dragsted LO, Egeberg R, Johnsen NF, Christensen J et al. Intake of dietary fiber, especially from cereal foods, is associated with lower incidence of colon cancer in the HELGA cohort. Int J Cancer. 2012;131(2):469–78. [DOI] [PubMed] [Google Scholar]
- 11. Kunzmann AT, Coleman HG, Huang WY, Kitahara CM, Cantwell MM, Berndt SI. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr. 2015;102(4):881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murphy N, Norat T, Ferrari P, Jenab M, Bueno-de-Mesquita B, Skeie G, Dahm CC, Overvad K, Olsen A, Tjønneland A et al. Dietary fibre intake and risks of cancers of the colon and rectum in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS One. 2012;7(6):e39361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park Y, Subar AF, Hollenbeck A, Schatzkin A. Dietary fiber intake and mortality in the NIH-AARP Diet and Health Study. Arch Intern Med. 2011;171(12):1061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vulcan A, Brändstedt J, Manjer J, Jirström K, Ohlsson B, Ericson U. Fibre intake and incident colorectal cancer depending on fibre source, sex, tumour location and Tumour, Node, Metastasis stage. Br J Nutr. 2015;114(6):959–69. [DOI] [PubMed] [Google Scholar]
- 15. Schatzkin A, Mouw T, Park Y, Subar AF, Kipnis V, Hollenbeck A, Leitzmann MF, Thompson FE. Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and Health Study. Am J Clin Nutr. 2007;85:1353–60. [DOI] [PubMed] [Google Scholar]
- 16. US Department of Health and Human Services (US DHHS) and USDA. 2015–2020 Dietary Guidelines for Americans. Washington (DC): US DHHS and USDA; 2015. [Google Scholar]
- 17. Gibson TM, Weinstein SJ, Pfieffer RM, Hollenbeck AR, Subar AF, Schatzkin A, Mayne ST, Stolzenberg-Solomon R. Pre- and postfortification intake of folate and risk of colorectal cancer in a large prospective cohort study in the United States. Am J Clin Nutr. 2011;94(4):1053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, Hurwitz PE, Coyle L, Schussler N, Michaud DS et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: The National Institutes of Health–American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–25. [DOI] [PubMed] [Google Scholar]
- 19. Michaud DS, Midtune D, Hermansen S, Leitzmann MF, Harlan LC, Kipnis V, Schatzkin A. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and Health Study. J Regist Manag. 2005;32(2):70–5. [Google Scholar]
- 20. WHO. International Classification of Disease for Oncology (ICD-O). 3rd ed Geneva: World Health Organization; 2013. [Google Scholar]
- 21. Tippett KS, Cypel YS editors. Design and operation: the Continuing Survey of Food Intakes by Individuals and the Diet and Health Knowledge Survey, 1994–96. Nationwide Food Surveys Report No. 96–1 Washington (DC): USDA, Agriculture Research Service; 1997. [Google Scholar]
- 22. Dennis B, Ernst N, Hjortland M, Tillotson J, Grambsch V. The NHLBI nutrition data system. J Am Diet Assoc. 1980;77(6):641–7. [PubMed] [Google Scholar]
- 23. Thompson FE, Kipnis V, Midthune D, Freedman LS, Carroll RJ, Subar AF, Brown CC, Butcher MS, Mouw T, Leitzmann M et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr. 2008;11(2):183–95. [DOI] [PubMed] [Google Scholar]
- 24. Brown CC, Kipnis V, Freedman LS, Hartman AM, Schatzkin A, Wacholder S. Energy adjustment methods for nutritional epidemiology: the effect of categorization. Am J Epidemiol. 1994;139(3):323–38. [DOI] [PubMed] [Google Scholar]
- 25. Jacobs DR, Marquart L, Slavin J, Kushi LH. Whole-grain intake and cancer: an expanded review and meta-analysis. Nutr Cancer. 1998;30(2):85–96. [DOI] [PubMed] [Google Scholar]
- 26. Egeberg R, Olsen A, Loft S, Christensen J, Johnsen NF, Overvad K, Tjønneland A. Intake of wholegrain products and risk of colorectal cancers in the Diet, Cancer and Health cohort study. Br J Cancer. 2010;103(5):730–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He X, Wu K, Zhang X, Nishihara R, Cao Y, Fuchs CS, Giovannucci EL, Ogino S, Chan AT, Song M et al. Dietary intake of fiber, whole grains and risk of colorectal cancer: an updated analysis according to food sources, tumor location and molecular subtypes in two large US cohorts. Int J Cancer. 2019;145(11):3040–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Slavin JL, Martini MC, Jacobs DR Jr, Marquart L. Plausible mechanisms for the protectiveness of whole grains. Am J Clin Nutr. 1999;70(3 Suppl):459S–63S. [DOI] [PubMed] [Google Scholar]
- 30. Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre?. Nutr Res Rev. 2010;23(1):65–134. [DOI] [PubMed] [Google Scholar]
- 31. Lipkin M, Reddy B, Newmark H, Lamprechyt SA. Dietary factors in human colorectal cancer. Annu Rev Nutr. 1999;19:545–86. [DOI] [PubMed] [Google Scholar]
- 32. Bazzano LA, Song Y, Bubes V, Good C, Manson JE, Liu S. Dietary intake of whole and refined grain breakfast cereals and weight gain in men. Obes Res. 2005;13(11):1952–60. [DOI] [PubMed] [Google Scholar]
- 33. de Munter JSL, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med. 2007;4(8):e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffman K, Boeing H. Fiber and magnesium intake and incidence of type 2 diabetes. Arch Intern Med. 2007;167:956–65. [DOI] [PubMed] [Google Scholar]
- 35. Song Y, Liu M, Yang FG, Cui LH, Lu XY, Chen C. Dietary fibre and the risk of colorectal cancer: a case-control study. Asian Pac J Cancer Prev. 2015;16(9):3747–52. [DOI] [PubMed] [Google Scholar]
- 36. Howe GR, Benito E, Castelleto R, Cornée J, Estève J, Gallagher R, Iscovich JM, Deng-ao J, Kaaks R, Kune GA et al. Dietary intake of fiber and decreased risk of cancers of the colon and rectum: evidence from the combined analysis of 13 case-control studies. J Natl Cancer Inst. 1992;84(24):1887–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.