Abstract
Objectives
To investigate the mechanism of angiotensin II-induced apoptosis in cultured cardiomyocytes by determining which receptor subtype is involved, and what is the relationship between intracellular Ca2+ changes and apoptosis.
Design and methods
Neonatal rat cardiomyocytes were pretreated with either the AT1 antagonist irbesartan or the AT2 antagonist PD123319 before exposure to angiotensin II. Apoptotis was evaluated using morphological technique, staining nuclei by Feulgen and Hoechst methods followed by image analysis and by in situ terminal deoxynucleotidyl transferase nick-end (TUNEL) labelling. TUNEL-positive cardiocytes were distinguished from other cells by double staining with α-sarcomeric actin. Intracellular Ca2+ changes were assessed by indo-1 fluorescence microscopy, and the effect of Ca2+ on angiotensin II-induced apoptosis was tested using the calcium channel blocker verapamil.
Results
Exposure to angiotensin II (10 nmol/l) resulted in cell replication and a three-fold increase in programmed cell death (P < 0.05). Pretreatment with either irbesartan (an AT1receptor antagonist, 100 nmol/l) or PD123319 (an AT2 receptor antagonist, 1 μmol/l) prevented the angiotensin II-induced apoptosis, indicating the presence of both AT1 and AT2receptors on cardiomyocytes. Exposure of myocytes to angiotensin II caused an immediate and dose-dependent increase in the concentration of intracellular free Ca2+ that lasted 40–60 s. The effect was sustained in a Ca2+ free medium. Pretreatment of cells with irbesartan (100 nmol/l) and PD123319 (10 μmol/l) blocked Ca2+ elevation. Pretreatment with verapamil (10 μmol/l) prevented angiotensin II-induced apoptosis.
Conclusions
Angiotensin II-induced apoptosis in rat cardiomyocytes is mediated through activation of both AT1 and AT2 receptors. The apoptotic mechanism is not related to the immediate angiotensin II-induced Ca2+ rise from intracellular stores. However, it is accompanied by cardiomyocyte proliferation and requires Ca2+ influx through L-type channel activity.
Keywords: angiotensin II, AT1 and AT2 receptors, apoptosis, cardiomyocytes, Ca2+ concentration
Introduction
Programmed cell death (apoptosis) has been shown to occur during ageing and in various cardiac pathological processes [1–5]. Angiotensin II has been demonstrated to induce programmed cardiomyocyte death in vitro [6,7], and a direct correlation was found between angiotensin converting enzyme (ACE) activity and the apoptotic index in spontaneous hypertensive rats [8]. Nevertheless, the ability of angiotensin II to produce apoptosis is controversial. In histological studies, no signs of apoptotic cardiomyocytes were found in myocardium after infarction in vivo or in vitro [9]. In embryonic chick cardiomyocytes, angiotensin II does not induce apoptosis but rather prevents apoptosis [10]. ACE inhibition and angiotensin receptor blockade confers cardioprotection and improves survival in patients with heart failure [11–13]. It is possible, however, that part of the cardioprotection in heart failure conferred by blocking the renin–angiotensin system occurs through prevention of apoptotic cardiomyocyte loss.
The vasoactive peptide angiotensin II acts on its target tissues via membrane-bound receptors of the G-protein coupled family. These receptors have been divided into two subclasses: AT1 and AT2 [14,15]. Recently, it has been shown that stimulation of the AT2 receptor antagonizes the growth effect induced by activation of the AT1 receptor in cardiac cells [14]. However, there are contradictory data on the subtype of angiotensin II receptor which mediates apoptosis in myocyte and non-myocyte cells [16–18].
Therefore, we investigated whether blocking either AT1 or AT2 receptors can protect against apoptotic death induced by angiotensin II in neonatal rat cardiomyocyte cultures. We also studied the mechanism of the angiotensin II-induced apoptosis by measuring intracellular Ca2+ concentrations, and by blocking L-type calcium channel activity with verapamil.
Methods
Cardiac cell cultures
Sprague–Dawley rat hearts (1–2 days old) were removed under sterile conditions and washed three times in phosphate-buffered saline (PBS) to remove excess blood cells. The hearts were minced to small fragments and then agitated gently in a solution of proteolytic enzymes, RDB (Biological Institute, Ness-Ziona, Israel), which was prepared from a fig tree extract. The RDB was diluted 1 : 100 in Ca2+ and Mg2+-free PBS at 25°C for a few cycles of 10 min each, as described previously [19]. The mixture was centrifuged at 300 g for 5 min. The supernatant phase was discarded, and the cells were resuspended in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% horse serum (Biological Industries, Kibbutz Beit Haemek, Israel) and 2% chick embryo extract (Biological Industries). The suspension of the cells was diluted to 1.0 × 106 cells/ml, and 1.5 ml were placed in 35 mm plastic culture dishes on collagen/gelatin-coated cover glasses. The cultures were incubated in a humidified atmosphere of 5% CO2, 95% air at 37°C. Confluent monolayers exhibiting spontaneous contractions were developed in culture within 2 days.
Experiments with angiotensin II and angiotensin II antagonists and verapamil
Before treatment with angiotensin II, the growth medium was replaced with chemically defined medium based on DMEM supplemented with transferrin (10 μg/ml), insulin (10 μg/ml), T4 (0.1 μmol/l) and bovine serum albumin (100 μg/ml). Pretreatment with irbesartan (a selective AT1 receptor antagonist), PD123319 (a selective AT2receptor antagonist) and verapamil (an L-type calcium channel blocker), was accomplished by adding these compounds 20 min before exposure of cells to angiotensin II.
α-Sarcomeric actin assay
In order to identify cardiomyocytes, cells on coverslips were stained for immunohistochemical demonstration of α-sarcomeric actin using mouse monoclonal anti α-sarcomeric actin (C-5) and goat anti-mouse biotinylated immunoglobulin conjugated with extravidin peroxidase. The chromogen 3-amino-9-ethylcarbazole (AEC) was used as described previously [19].
Feulgen procedure
Cells on coverslips were fixed in essential fatty acid (by volume, 75 : 20 : 5 of 96% ethanol : 40% neutral formol : acetic acid) for 20 min. Fixed samples were placed in 5 N HCl for 60 min at 24°C to hydrolyse DNA and stained with the Schiff reagent as described previously [19].
In situ apoptosis assay
Apoptotic cells were identified in situ using the terminal deoxynucleotidyl transferase nick-end labelling (TUNEL-like) assay as described previously [19]. After fixation in 10% neutral-buffered formalin for 20 min at room temperature and a permeabilization in 70% ethanol for 30 min at −20°C, the assay was performed on cells on coverslips using a commercial TdT FragEL DNA fragmentation KIT. Biotinylated nucleotides were detected using a streptavidin-horseradish peroxidase conjugate. Chromogenes AEC or DAB-Black (Zymed substrate KIT; Zymed, San Francisco, California, USA) reacted with the labelled sample to generate an insoluble colored substrate at the site of DNA fragmentation.
Image analysis of Fuelgen and TUNEL-stained cells
The image analysis was performed with Scan-Array 2 Image Analyser (Galai, Israel). The analyser consisted of an Axiovert 135TV florescent microscope (Zeiss, Hallbergmoos, Germany) and a black and white Sony video camera, interfaced to an image analysis computer. Morphonuclear parameters were computed as described in detail previously [19]. In the present work, the system described the following parameters: AREA, morphometric parameter, which corresponds to the area of the nuclear profile; IOD, the integrated optical density, a densitometric parameter, related to the total DNA content and apoptotic index, percentage of apoptotic nuclei
Fluorescence DNA stains
Cells were analysed for apoptosis following visualization of the fluorescent DNA-binding dye Hoechst 33342 trihydrochloride trihydrate. The monolayers were rinsed with PBS and then incubated with 10 μg/ml H33342 for 30 min. Nuclei were visualized using fluorescent microscopy and analysed for apoptotic morphology. An average of 1000 nuclei from random fields was analysed for each slide. The apoptotic index was calculated as described by Wu et al. [20]. At least three samples were scored per group.
Intracellular calcium measurements
Intracellular free calcium concentration was estimated from indo-1 fluorescence, using the ratio method described by Grynkiewicz et al. [21]. The cardiac cells grown on coverslips were transferred to a chamber on the stage of Zeiss inverted microscope filtered with ultraviolet epifluorescence illumination. Indo-1 was excited at 355 nm and the emitted light then split by a dichroic mirror to two photomultipliers (Hamamatsu, Japan) with input filters at 405 and 495 nm. The fluorescence ratio of 405/495 nm, which is proportional to Ca2+ concentration, was monitored. Assessment of changes in intracellular Ca2+ concentration was performed in all experiments. In addition, intracellular Ca2+ concentration was measured after exposure of cardiac cells to angiotensin II in a Ca2+-free medium.
Chemicals
Irbesartan was supplied by Bristol Myers Squibb (Princeton New Jersey, USA). Other reagents were purchased from Sigma Chemicals (St Louis, Missouri, USA).
Statistical analysis
Results are expressed as means ± SE. ANOVA and Student’s t test were used in statistical evaluation of the data. P < 0.05 was considered statistically significant.
Results
Characterization of neonatal cardiomyocytes in cell cultures after exposure to angiotensin II
Primary cultures of neonatal rat myocytes maintained in serum-free medium are depicted in Figure 1A. Myofibrils within these cells were elongated and uniformly distributed throughout the cell. Individual actin fibrils stained with α-sarcomeric actin antibody demonstrated the typical striated pattern. Each culture dish also contained a small number of dead cells, defined by a condensed granular cytoplasm and pyknotic nucleus. The cardiomyocytes in the angiotensin II (10 nmol/l)-treated cultures showed an increase in myofibril content and moderate hypertrophy. In preliminary experiments, myocytes were exposed to angiotensin II at concentrations of 1–1000 nmol/l for a period of 24 h (in serum-free medium) and only a weak dose-dependent response, was observed on apoptotic activity. Therefore, concentrations of 10–100 nmol/l of angiotensin II were employed in the present study. Morphological features of apoptosis in angiotensin II-treated myocytes include nuclear condensation and fragmentation, blebbing of plasma membrane, shrinking, retraction and condensation of cytoplasm (Fig. 1B1–4,C,E). Some apoptotic cells showed also signs of mitosis and postmitotic apoptosis (Fig. 1B1–4,E). These features were not observed in control cultures.
Fig. 1.
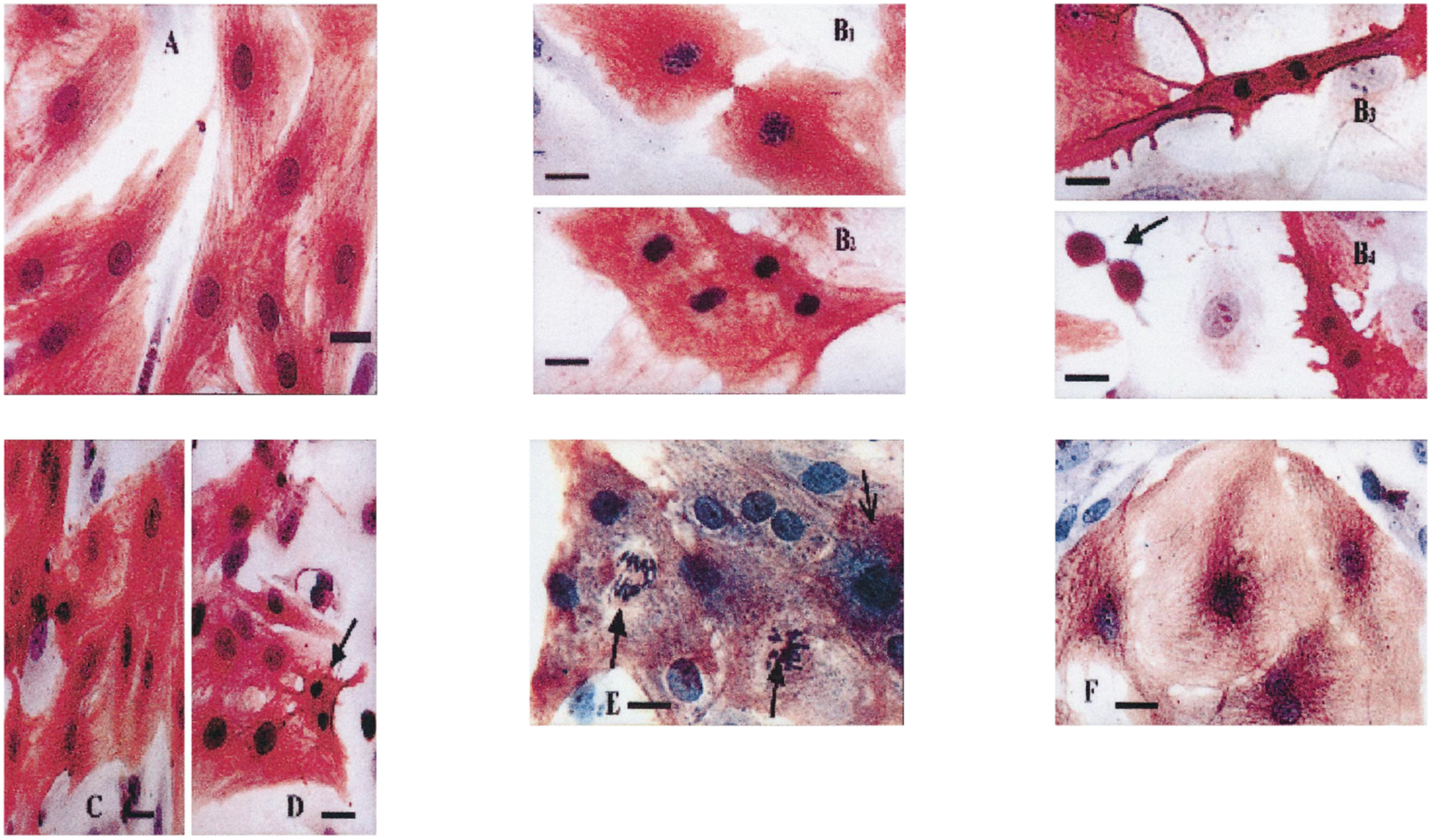
Morphology of cardiocytes following angiotensin II treatment. Cardiac cells 3 days in culture were transferred to serum-free media for 2 days before treatment with angiotensin II (10 nmol/l) for 24 h. The cells were stained with α-saromeric actin and counter-stained with haematoxylin. (A) Control cells. (B) Angiotensin II (10 nmol/l)-treated cells. Some myocardial cells entered the mitotic stage and the late telophase reappearance of myofibrils, decondesation of chromosomes and appearance of double nuclear membrane were observed (B1). Many myocytes in late telophase were characterized by further condensation of chromosomes to uniformly and tightly packed chromatin figures, shrinking, retraction, condensation of cytoplasm, loss of organized myofibrils and reappearance of nuclear membrane (B2–3). After the end of cleavage, most myocytes became typical apoptotic bodies (B4). (C) Angiotensin II (100 nmol/l)-treated cells. (D) Pretreatment with irbesartan (100 nmol/l) before exposure to angiotensin II (10 nmol/l). Arrows indicate apoptotic cardiomyocytes. (E,F) Experiments with verapamil. The cells were stained with α-saromeric actin and counter-stained with haematoxylin. (E) Angiotensin II (10 nmol/l)-treated cells. Cardiomyocytes undergoing mitosis (wide arrows) and apoptosis (narrow arrow) are demonstrated. (F) Pretreatment with verapamil (10 μmol/l) before exposure to angiotensin II (10 nmol/l). Bars = 10 μm.
In control Feulgen-stained cells, chromatin was characterized by a few small granules against a pale background or abundant granules and fibrils of deeply stained chromatin (Fig. 2A). Exposure of myocytes to angiotensin II (10 and 100 nmol/l) for 24 h led to an increase in the number of apoptotic-like nuclei (Fig. 2B,C).
Fig. 2.
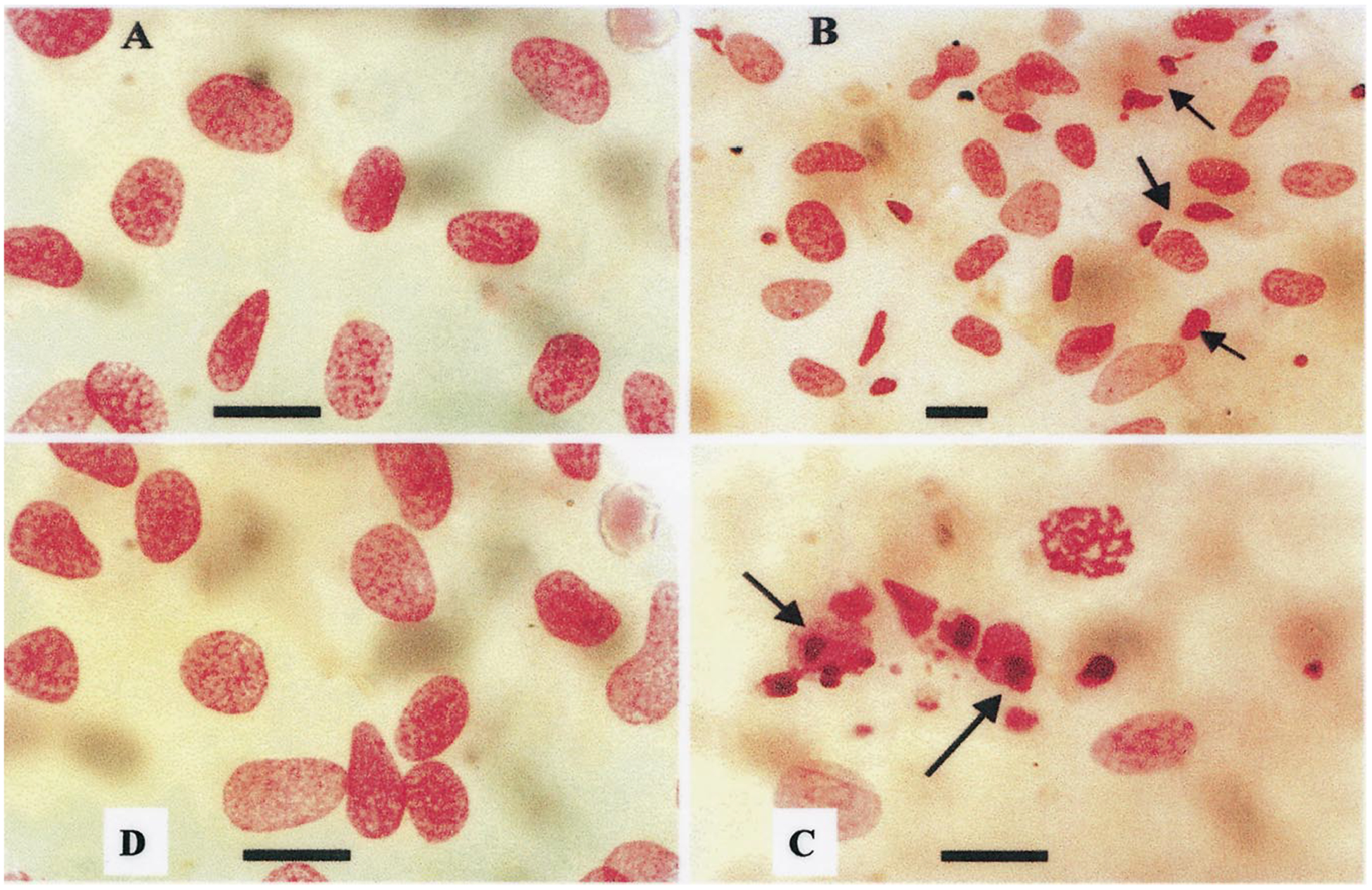
Morphology of nuclei of Feulgen-stained cultured cardiomyocytes after exposure to angiotensin II. (A) Nuclei of control cells. A few small granules against a pale background characterizes chromatin. (B) Exposure of myocytes to angiotensin II (10 nmol/l) for 24 h led to an increase in the number of apoptotic nuclei. Condensation, compacting and margination of nuclear chromatin were accompanied by disappearance of the structural framework of the nucleus and nuclear breakdown. (C) Exposure of myocytes to angiotensin II (100 nmol/l). (D) Pretreatment with PD123319 (1 μmol/l) before exposure to angiotensin II (10 nmol/l). Arrows indicate apoptotic cardiomyocytes. Bars = 10 μm.
Histograms of the distribution of integrated optical density (IOD) versus cell number are shown in Fig. 3a–d. Nuclei stained by the Feulgen technique show four distinct populations: < 2C (hypodiploid), 2C (diploid), 4C (tetraploid) and ⩾ 8C. For each of these populations, there is a constant value for IOD, despite a wide variation in nuclear size. In control cultures individual interphase nuclei, the G0/G1 population, which covered the 1.8–2.4C range of DNA content were predominant. A second peak was recorded in the range of 2.5–4.9C corresponding to cells in S-phase and tetraploid cells (G2/M). The amount of nuclei with hypodiploid DNA content (below the range of 2C) in control cultures was no more than 2–3% (Fig. 3a). The histogram of DNA content in angiotensin II-treated cells demonstrated a 4.1-fold increase in hypodiploid peak over control cells and no differences in ⩾ 2C DNA distribution (Fig. 3b,c). It is evident that the differences between angiotensin II-treated and control cells are due to the smaller nuclear area and low DNA content of hypodiploid, Feulgen-stained cells, in spite of the variability in nuclear area.
Fig. 3.
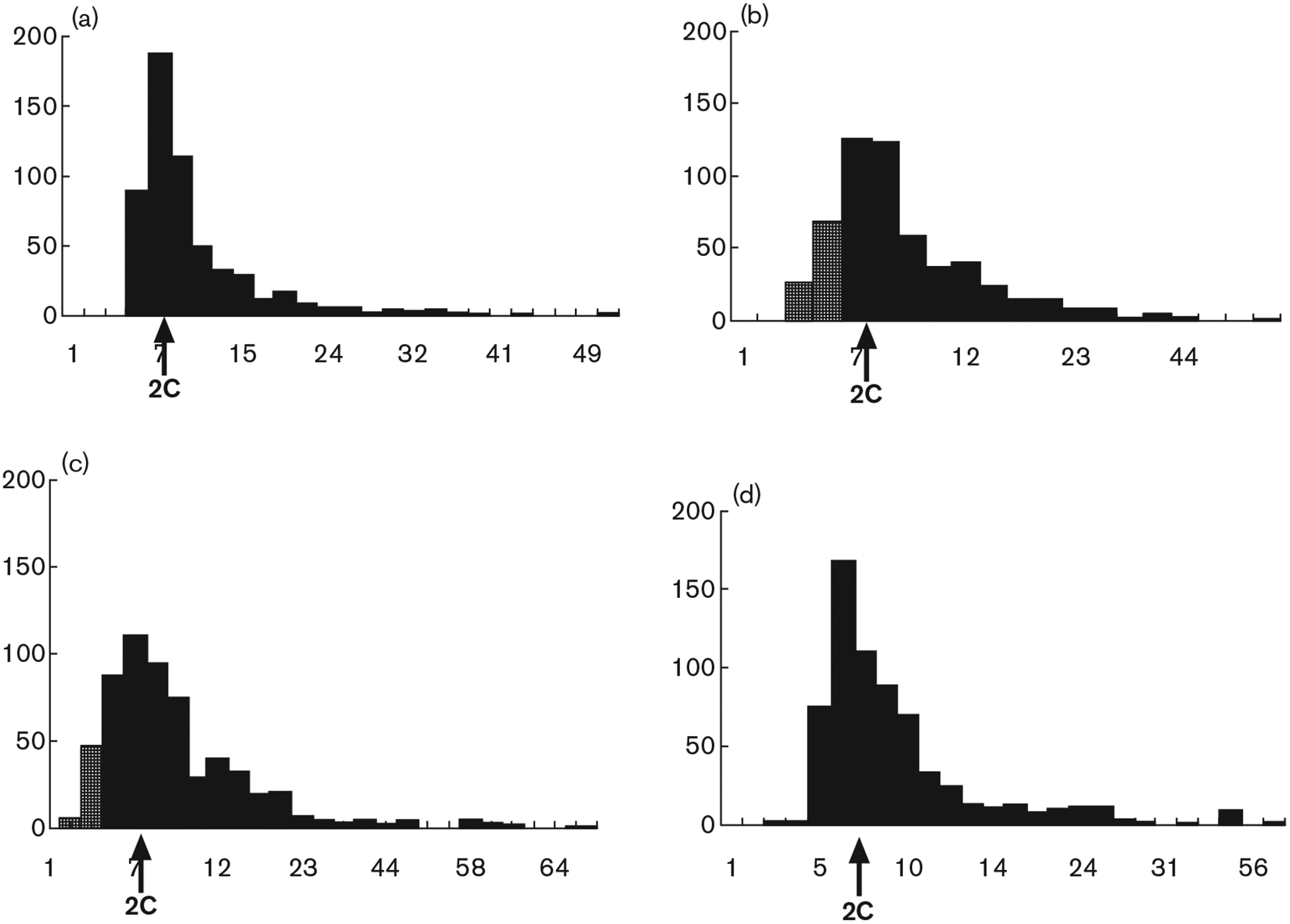
Image analysis of Feulgen-stained nuclei following exposure to angiotensin II. Histograms of DNA content are demonstrated (arbitrary units of integrated optical density, IOD). (a) Control. (b) 24 h after treatment with angiotensin II (10 nmol/l). (c) 24 h after treatment with angiotensin II (100 nmol/l). (d) Pretreatment with irbesartan (100 nmol/l) before angiotensin II (10 nmol/l) (strippled bars indicate hypoploid DNA content). 2C (arrow), normal, diploid content of DNA.
The apoptotic index (percentage of apoptotic nuclei) of Feulgen-stained cells is shown in Figure 4. Angiotensin II (10–100 nmol/l) caused a three-fold increase in apoptotic cells (from 2.4 ± 1.3% in the control cells to 6.3 ± 3.3% and 7.6 ± 2.8% in the 10 and 100 nmol/l of angiotensin II-treated cells, respectively, P < 0.05).
Fig. 4.
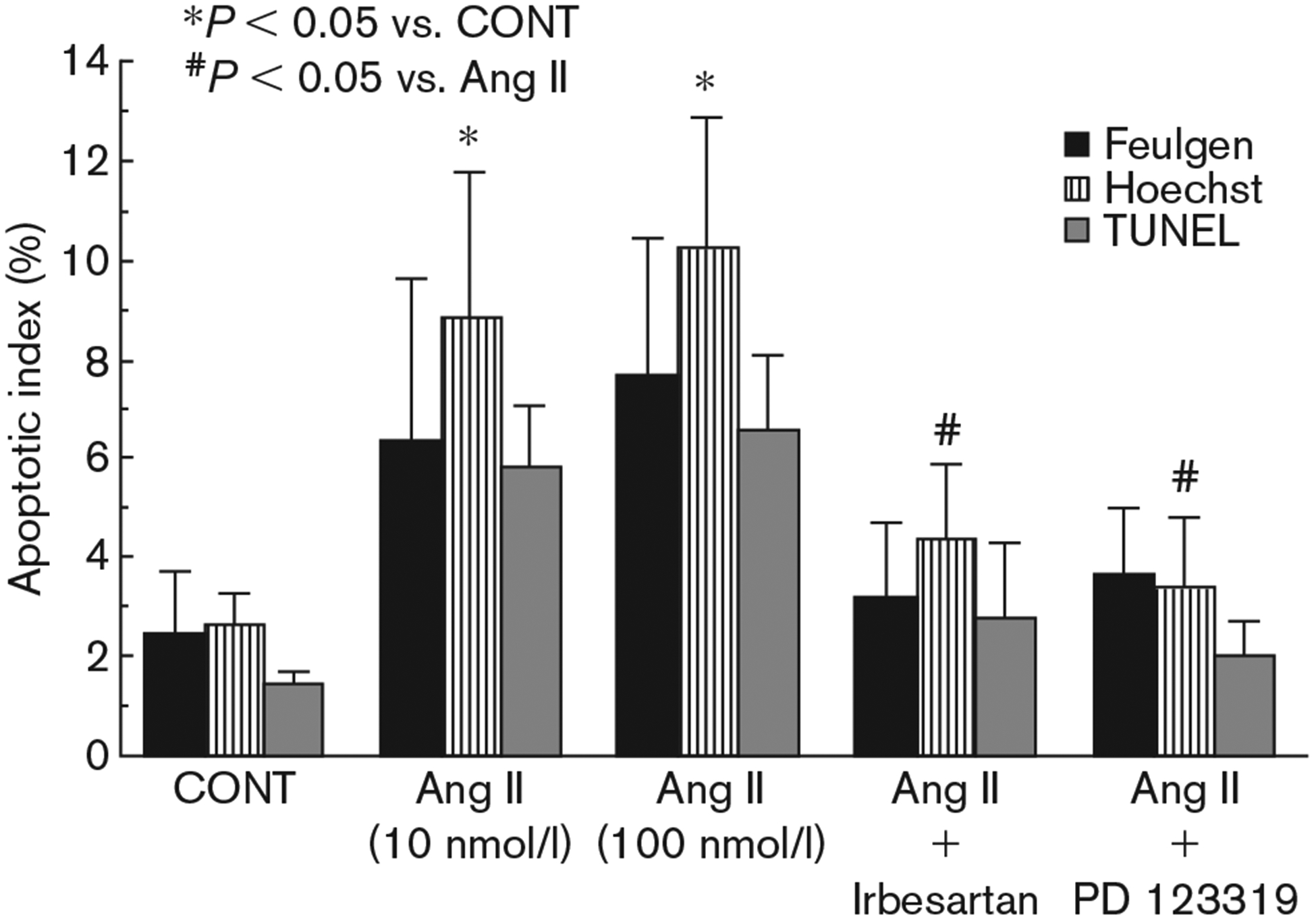
Apoptotic index of Feulgen, Hoechst and TUNEL- stained myocytes after angiotensin II treatment. Cardiocytes were treated with the indicated drugs 20 min before application of angiotensin II (10 nmol/l). Feulgen, Hoechst and TUNEL staining were performed 24 h later. CONT, control; Ang II, angiotensin II. Irbesartan, 100 nmol/l; PD123319, 1 μmol/l. Angiotensin II was employed at a concentration of 10 nmol/l except when indicated.
Analysis of the fluorescent morphology of Hoechst-stained cells shows that angiotensin II (10–100 nmol/l) caused a marked increase in apoptotic cell death (from 2.6 ± 0.65% in the control cells to 8.8 ± 2.9% and 10.2 ± 2.6% in the 10 and 100 nmol/l of angiotensin I-treated cells, respectively, P < 0.05) (Fig. 4).
To further examine the cells that contained fragmented nuclear DNA typical of apoptosis, we performed an in situ assay based on end-labelling of DNA strand breaks by the TUNEL-like method (Fig. 5). TUNEL positive cells were obtained following Ang II treatment (Fig. 5B) confirming our results with Fuelgen and Hoechst stainings. These TUNEL positive cells were shown to be cardiomyocytes (and not fibroblasts) as demonstrated by the double staining with both TUNEL and α-sarcomeric actin (Fig. 5C). The apoptotic index of TUNEL-stained cells is shown in Figure 4. Angiotensin II (10–100 nmol/l) caused a four-fold increase in apoptotic cell death (from 1.4 ± 0.28% in the control cells to 5.8 ± 1.24% and 6.5 ± 1.54% in the 10 and 100 nmol/l of angiotensin II-treated cells, respectively, P < 0.05).
Fig. 5.
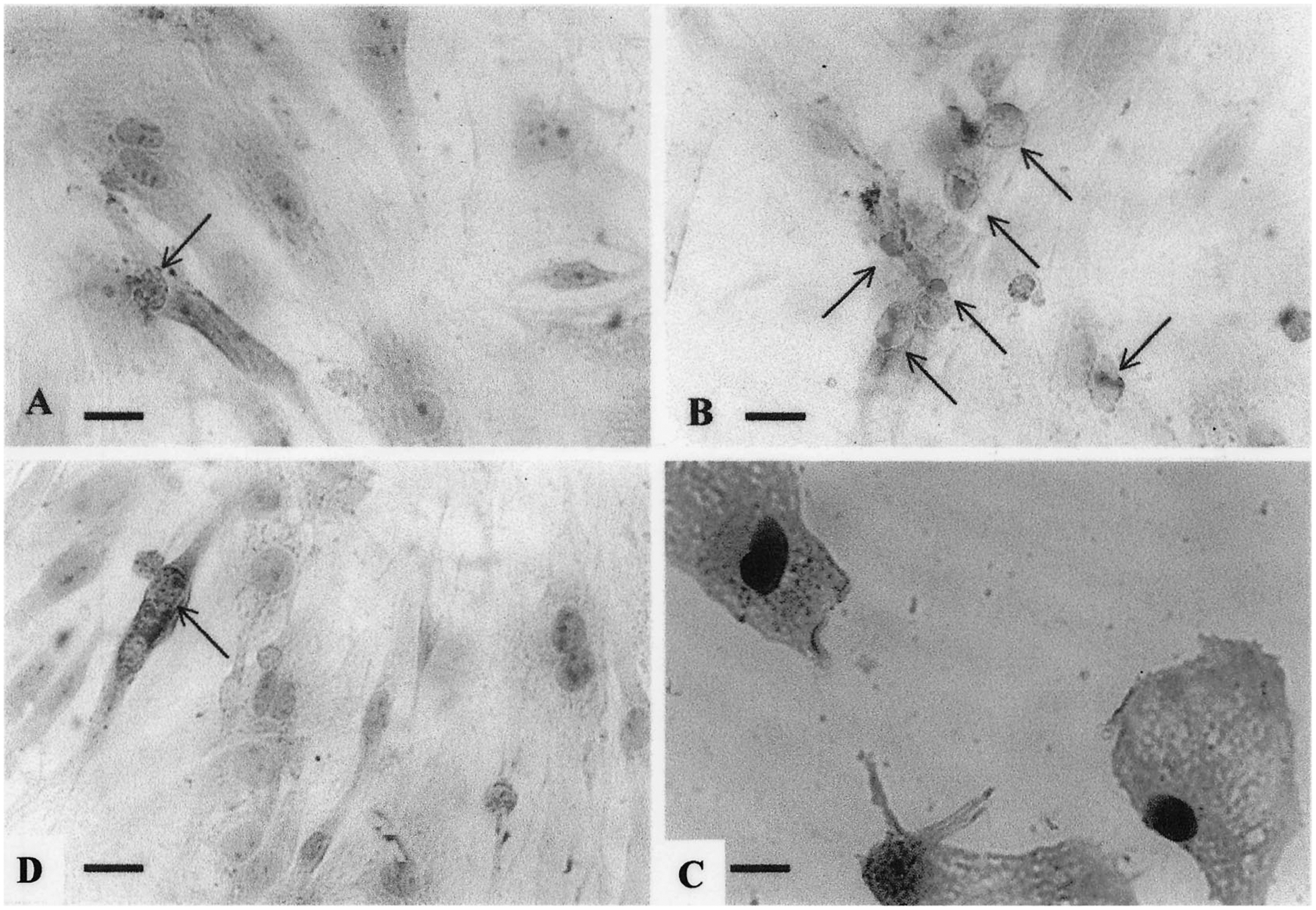
Immunohistochemical demonstration of DNA breaks (TUNEL-like) following angiotensin II treatment. Cardiac cells 3 days in cultures were transferred to serum-free media for 2 days before treatment with angiotensin II (10 nmol/l) for 24 h. (A) Control. (B) Angiotensin II (10 nmol/l)-treated cells. (C) Angiotensin II (10 nmol/l)-treated cells. Double staining: 3′-hydroxyl-free ends of DNA (DAB-black) and α-sarcomeric actin (AEC-red). (D) Pretreatment with irbesartan (100 nmol/l) before angiotensin II (10 nmol/l). Arrows indicate apoptotic cardiocytes. Bars = 1 μm.
Effects of angiotensin II antagonists on cardiomyocyte death
Exposure of cardiac cells to the AT1 selective antagonist irbesartan (100 nmol/l) or to the AT2 receptor antagonist PD123319 (1 μmol/l), alone had no effect on apoptosis (data not shown). Treatment of cardiac cells with irbesartan (100 nmol/l) completely prevented angiotensin II-mediated apoptosis (Figs 1D, 3d and 5D). Similar results were obtained with PD123319 (1 μmol/l) (Fig. 2D), indicating the presence of both receptors on the cardiomyocytes. Apoptotic indices derived from Feulgen, Hoechst and TUNEL-like assays are shown in Figure 4.
Effects of the calcium channel blocker verapamil on cardiomyocyte death
Exposure of cardiac cells to verapamil (10 μmol/l) alone had no effect on apoptosis (data not shown). Pretreatment of cardiac cells with verapamil completely prevented angiotensin II-mediated apoptosis (Fig. 1F).
Effects of angiotensin II and angiotensin II antagonists on intracellular Ca2+ concentrations
Exposure of cardiomyocytes to angiotensin II (1–200 nmol/l) caused an increase in the concentration of intracellular free Ca2+ in a concentration-dependent manner. The drug produced a rapid rise followed by a sustained increase in Ca2+ that lasted 40–60 s (Fig. 6a). Treatment of cells in a Ca2+-free medium did not abolish the intracellular Ca2+ rise induced by angiotensin II, indicating that Ca2+ elevation following response to angiotensin II arises from intracellular stores (not shown). Pretreatment of cells with the AT1 antagonist irbesartan (100 nmol/l) completely blocked the angiotensin II-induced increase in intracellular calcium (Fig. 6b). Pretreatment of cardiomyocytes with the AT2 antagonist PD123319 at a concentration of 1 μmol/l only mildly attenuated the intracellular Ca2+ rise but 10 μmol/l of the antagonist completely blocked elevation in intracellular free calcium induced by angiotensin II (Fig. 6c).
Fig. 6.
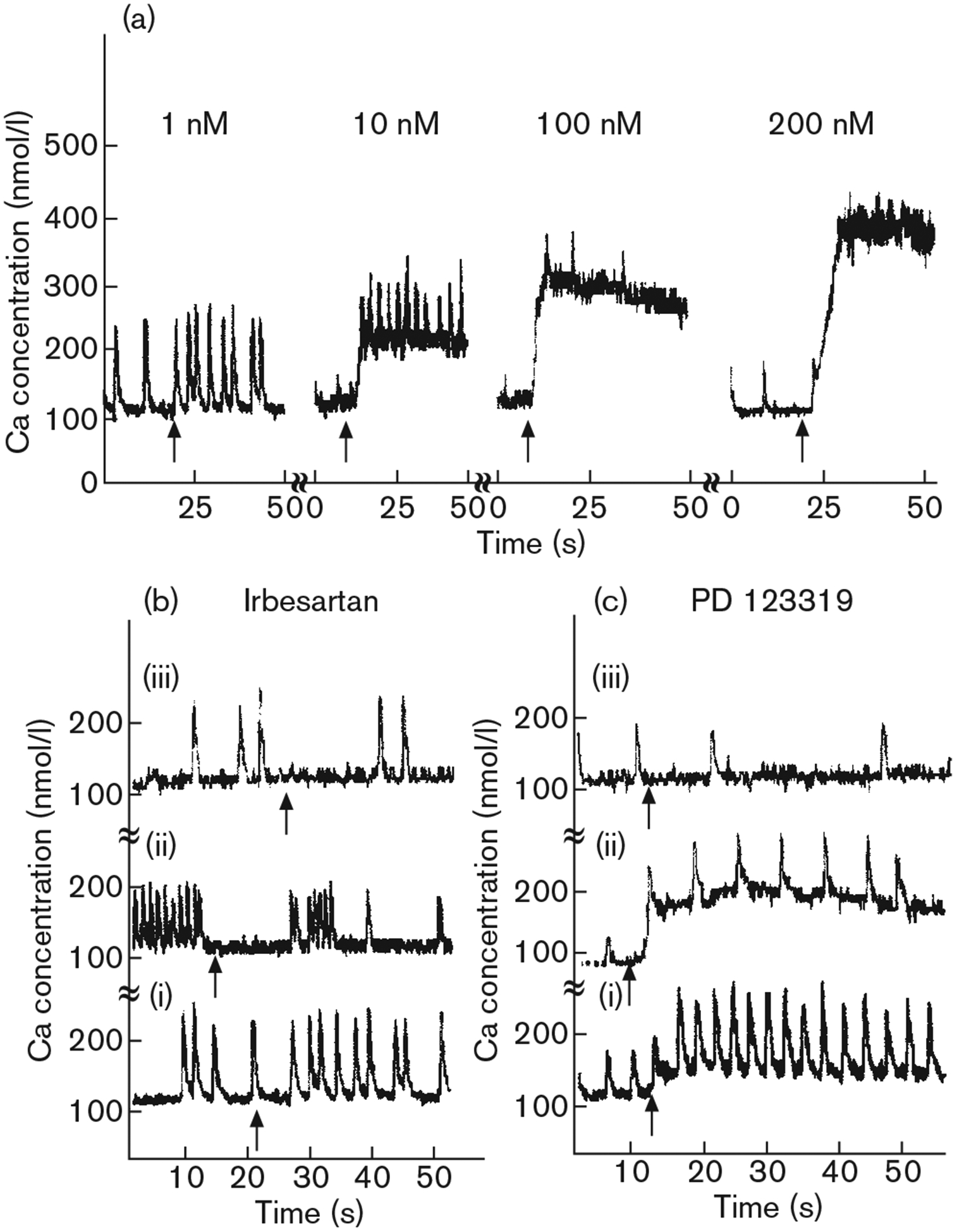
Effect of angiotensin II on intracellular free calcium in cultured rat cardiomyocytes. (a) Effects of various concentrations of angiotensin II on intracellular calcium transience. Typical tracings are shown. (b) Effect of the selective angiotensin II AT1 receptor antagonist irbesartan on the change in intracellular free calcium induced by angiotensin II (arrows): (i) irbesartan, 100 nmol/l, angiotensin II, 10 nmol/l; (ii) irbesartan, 100 nmol/l, angiotensin II, 100 nmol/l; (iii) irbesartan 1 μmol/l, angiotensin II, 100 nmol/l. (c) Effect of the selective angiotensin II AT2 receptor antagonist PD123319 on the change in intracellular free calcium induced by angiotensin II (arrows): (i) PD123319, 1 μmol/l, angiotensin II, 10 nmol/l; (ii) PD123319, 1 μmol/l, angiotensin II, 100 nmol/l; (iii) PD123319, 10 μmol/l, angiotensin II, 100 nmol/l. Cultures were incubated with antagonists for 5 min at room temperature prior to the addition of angiotensin II. Similar results were observed in five independent experiments.
Discussion
In the present study, we demonstrated that angiotensin II induces apoptosis in neonatal rat cardiomyocytes which had been grown in culture for 5–6 days. Recently, using the terminal deoxytransferase dUTP nick-end labelling (TUNEL-like) analysis, it has been shown that angiotensin II induces apoptosis in cardiac myocytes [6–8,18] and many other cells [16,17,22,23]. The scoring of apoptotic cells based on the TUNEL-like assays was found to be very ambiguous because it does not discriminate between apoptotic, necrotic and autolytic cells [9,24]. Our results were confirmed by various methods of analysis, including careful morphological and immunocytochemical investigation of dying cardiomyocytes.
At present, the function of the AT2 receptors in cardiomyocytes is still not well defined. Stimulation of AT2 receptors has been demonstrated to inhibit AT1-receptor-dependent growth [25,26], and to induce apoptosis in cultured neonatal rat cardiomyocytes [27]
To establish whether angiotensin II-induced myocytes apoptosis is mediated by activation of the AT1 or AT2 receptor subtypes, we used irbesartan and PD123319, as receptor antagonists. Pretreatment with the selective AT1 antagonist irbesartan, before exposure, conferred a full protection against the apoptotic effect of angiotensin II, indicating that angiotensin II induces apoptosis through activation of the AT1 receptor. Similar results have been previously described with another non-peptide AT1 antagonist, losartan [6,7], but this is the first documentation that irbesartan can also prevent angiotensin II-induced apoptosis.
Recently, it has been shown, in rat new-born cardiomyocytes, that angiotensin II-induced apoptosis was prevented by pretreatment with AT1 antagonist, but not by the AT2 antagonist PD123319 [6]. Similar findings were demonstrated by Kajstura et al. in adult (3 months) rat cardiomyocytes [7]. Nevertheless, in-vitro experiments suggested that AT2 type receptors were involved in apoptosis of non-myocyte cells [23,28]. Horiuchi et al. have shown that activation of AT2 receptors in cardiomyocytes inhibits cellular growth and exerts a proapoptotic effect by antagonizing the effects of the AT1 receptors [16]. Moreover, it has been demonstrated recently that adult spontaneous hypertensive rats (SHR) exhibit increased susceptibility to angiotensin II-induced apoptosis and that blockade of both the AT1 and the AT2 receptor blunted the apoptotic response to angiotensin II [29]. We have found that activation of both AT1 and AT2 receptors is necessary for angiotensin II to mediate apoptosis. Both receptor subtypes have a role in cardiovascular development and remodelling. The AT1 receptor stimulates cell proliferation, which is associated with sustained mitogen-activated protein (MAP)-kinase activation. The AT2 receptor inhibits growth and induces apoptosis in cultured cells and in vivo through activation of tyrosine phosphotase(s), such as MAP-kinase-phosphatase, resulting in Bcl-2 dephosphorylation (an inhibitor of apoptosis) and upregulation of Bax (an inducer of apoptosis). The mitogenic response induced by angiotensin II is associated with sustained MAP-kinase activation [28,30]. Despite the strong evidence that the AT1 and AT2 receptors play different roles in the regulation of apoptosis in other cell types, in which AT1 receptor activation inhibits apoptosis and AT2 receptor activation exerts antigrowth and proapoptotic effects, it has been shown that in cardiomyocytes the AT1 receptors behave differently and are involved in the promotion of apoptosis [6,7,31].
The role of angiotensin II in inducing apoptosis was also demonstrated in vivo. In SHR, chronic blockade of AT1 receptors was found to prevent Bax oncoprotein overexpression and to normalize apoptosis in the left ventricle. The effect was shown to be independent of the haemodynamic effects of AT1 blockade [8]. In a model of dogs with moderate heart failure (ejection fraction 30–40%), it was also demonstrated that early long-term monotherapy with an ACE inhibitor significantly attenuates cardiomyocyte apoptosis [32].
The apoptotic effects of angiotensin II in a normal healthy heart are unknown. However, in pathological conditions, such as heart failure, a reduction in the extent of cardiomyocyte apoptosis may be one mechanism by which ACE inhibitors and AT1 antagonists preserve global left ventricular function. It has also been suggested that apoptosis might be a mechanism involved in the reduction of cardiomyocyte mass that accompanies the transition from stable compensation to heart failure in hypertensive heart disease [33].
The effect of angiotensin II on intracellular Ca2+ concentration was immediate and lasted 40–60 s. Because treatment of cells in a calcium-free medium failed to prevent the intracellular Ca2+ elevation induced by angiotensin II, it can be concluded that Ca2+ arise from intracellular stores (e.g. the sarcoplasmic reticulum). The calcium rise induced by angiotensin II was concentration-dependent and had an effect on the whole population of cultured myocytes. Shao et al. [34], demonstrated that exposure of cardiomyocytes to angiotensin II (0.01–10 μmol/l) resulted in an immediate and sustained increase in intracellular Ca2+ in a concentration-dependent manner. This increase was blocked by antagonists of both AT1 and AT2 receptors. In contrast, in the experiments performed by Touyz et al., where only a low PD123319 concentration was employed, the AT2 antagonist had no effect on Ca2+ transience [35]. Our results are in accordance and explain both observations.
The calcium rise induced by angiotensin II was concentration-dependent, while angiotensin II-induced apoptosis was not concentration-dependent. Pretreatment with the AT1 antagonist irbesartan prevented both apoptosis and Ca2+ rise, whereas, pretreatment with the AT2 antagonist PD123319 at a concentration of 1 μmol/l prevented angiotensin II-induced apoptosis but had little effect on the angiotensin II-induced rise in intracellular Ca2+. Thus, it seems that angiotensin II-induced apoptosis is not related to the immediate intracellular Ca2+ rise induced by angiotensin II.
We have demonstrated that the apoptotic process induced by angiotensin II was associated with cardiomyocyte replication, was not dose-dependent and occurred only in a small group of cardiomyocytes. Therefore, we can assume that the number of receptors is not the limiting factor for angiotensin II-induced apoptosis. Only cells that undergo mitosis are susceptible to apoptosis. This observation is supported by our finding that verapamil completely prevented cardiomyocyte proliferation and angiotensin II-induced apoptosis. Calcium channel blockers have been shown to inhibit cell replication [36,37]; thus, both cardiomyocyte proliferation and post-mitotic apoptosis are prevented with the use of verapamil.
Our morphological results can explain why blockade of independent AT1 and AT2 receptor subtypes inhibits the proapoptotic effects of angiotensin II. Activation of the AT1 receptor is needed for cell replication and AT2 receptor activation induces mitotic arrest and apoptotic expressions only on replicated cells. Therefore, stimulation of both receptor types is required for the apoptotic effects of angiotensin II, and blockade of either AT1 or AT2 receptor subtypes inhibits the proapoptotic effects of angiotensin II.
Recognition of the factors responsible for the initiation or prevention of programmed cell death may eventually lead to therapeutic interventions.
Acknowledgements
We thank A. Isaac and T. Zinman for their valuable technical assistance and S. Victor for typing the manuscript.
Sponsorship: This study was supported in part by Sanofi-Bristol-Myers-Squibb Foundation, and by the Health Sciences Research Center at Bar-Ilan University.
References
- 1.Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, Nitahara JA, et al. Necrotic and apoptotic myocyte cell death in the aging heart of 344 Fischer rats Am J Physiol 1996; 271:H1215–H1228. [DOI] [PubMed] [Google Scholar]
- 2.Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, et al. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol 1996; 28:2005–2016. [DOI] [PubMed] [Google Scholar]
- 3.Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, et al. Apoptosis in myocytes in end-stage heart failure. N Engl J Med 1996; 335:1182–1189. [DOI] [PubMed] [Google Scholar]
- 4.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, et al. Apoptosis in the failing human heart. N Engl J Med 1997; 336: 1131–1141. [DOI] [PubMed] [Google Scholar]
- 5.Teiger E, Than VD, Richard L, Wisnewsky C, Tea BS, Gaboury L, et al. Apoptosis, in pressure overload-induced heart hypertrophy in the rat. J Clin Invest 1996; 97:2891–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cigola E, Kajstura J, Li B, Meggs LG, Anversa P. Angiotensin II activates programmed myocyte cell death on vitro. Exp Cell Res 1997; 231: 363–371. [DOI] [PubMed] [Google Scholar]
- 7.Kajstura J, Cigola E, Malhotra A, Li P, Cheng W, Meggs LG, Anversa P. Angiotensin II induces apoptosis of adult ventricular myocytes in vitro J Mol Cell Cardiol 1997; 29:859–870. [DOI] [PubMed] [Google Scholar]
- 8.Diez J, Panizo A, Hemandez M, Vega F, Sola I, Fortuno MA, Pardo J. Cardiomyocyte apoptosis and cardiac angiotensin-converting enzyme in spontaneously hypertensive rats. Hypertension 1997; 30:1029–1034. [DOI] [PubMed] [Google Scholar]
- 9.Shneyvays V, Safran N, Halili-Rutman I, Shainberg A. Insights into adenosine A1 and A3 receptors function: cardiotoxicity and cardioprotection. Drug Dev Res 2000; 50:324–337. [Google Scholar]
- 10.Kong JY, Rabkin SW. Angiotensin II does not induce apoptosis but rather prevents apoptosis in cardiomyocytes. Peptides 2000; 21:1237–1247. [DOI] [PubMed] [Google Scholar]
- 11.Konstam MA, Kronenberg MW, Rousseau MF, Udelson JE, Melin J, Stewart D, et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dilatation in patients with asymptomatic systolic dysfunction. SOLVD (Studies of Left Ventricular Dysfunction) Investigators. Circulation 1993; 88:2277–2283. [DOI] [PubMed] [Google Scholar]
- 12.Pitt B. Use of converting enzyme inhibitors in patients with asymptomatic left ventricular dysfunction J Am Coll Cardiol 1993; 22:158A–161A. [DOI] [PubMed] [Google Scholar]
- 13.Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial – the Losartan Heart Failure Survival Study ELITE II. Lancet 2000; 355:1582–1587. [DOI] [PubMed] [Google Scholar]
- 14.Griendling KK, Lassegue B, Murphy TJ, Alexander RW. Angiotensin II receptor pharmacology. Adv Pharmacol 1994; 28:269–306. [DOI] [PubMed] [Google Scholar]
- 15.Mukoyama M, Nakajima M, Horiuchi M, Sasamura H, Pratt RE, Dzau VJ. Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors B. J Biol Chem 1993; 268: 24539–24542. [PubMed] [Google Scholar]
- 16.Horiuchi M, Akishita M, Dzau VJ. Molecular and cellular mechanism of angiotensin II-mediated apoptosis. Endocr Res 1998; 24:307–14. [DOI] [PubMed] [Google Scholar]
- 17.Tea BS, Der Sarkissian S, Touyz RM, Hamet P, deBlois D. Proapoptotic and growth-inhibitory role of angiotensin II type 2 receptor in vascular smooth muscle cells of spontaneously hypertensive rats in vivo. Hypertension 2000; 35:1069–1073. [DOI] [PubMed] [Google Scholar]
- 18.Leri A, Liu Y, Li B, Fiordaliso F, Malhotra A, Latini R, et al. Up-regulation of AT(1) and AT(2) receptors in postinfarcted hypertrophied myocytes and stretch-mediated apoptotic cell death. Am J Pathol 2000; 156: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shneyvays V, Nawrath H, Jacobson A, Shainberg A. Induction of apoptosis in cardiac myocytes by an A3 adenosine receptor agonist. Exp Cell Res 1998; 243:383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C-F, Bishopric NH, Pratt RE. Atrial natriuretic peptide induces apoptosis in neonatal rat cardiac myocytes. J Biol Chem 1997; 272:14860–14866. [DOI] [PubMed] [Google Scholar]
- 21.Grynkiewicz G, Poenie M, Tsien TY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985; 260:3440–3450. [PubMed] [Google Scholar]
- 22.Wang R, Zagariya A, Ibarra-Sunga O, Gidea C, Ang E, Deshmukh S, et al. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am J Physiol 1999; 276:L885–L889. [DOI] [PubMed] [Google Scholar]
- 23.Yamada T, Horiuchi M, Dzau VJ. Angiotensin II type 2 receptor mediates programmed cell death. Proc Natl Acad Sci USA 1996; 93:156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanoh M, Takemura G, Misao J, Hayakawa Y, Aoyama T, Nishigaki K, et al. Significance of myocytes with positive DNA in situ nick end-labeling (TUNEL) in hearts with dilated cardiomyopathy: not apoptosis but DNA repair. Circulation 1999; 99:2757–2764. [DOI] [PubMed] [Google Scholar]
- 25.Booz GW, Baker KM. Role of type 1 and type 2 angiotensin receptors in angiotensin II-induced cardiomyocyte hypertrophy. Hypertension. 1996; 28:635–640. [DOI] [PubMed] [Google Scholar]
- 26.van Kesteren CA, van Heugten HA, Lamers JM, Saxena PR, Schalekamp MA, Danser AH. Angiotensin II-mediated growth and antigrowth effects in cultured neonatal rat cardiac myocytes and fibroblasts. J Mol Cell Cardiol 1997; 29:2147–2157. [DOI] [PubMed] [Google Scholar]
- 27.Hariuchi M, Akishita M, Dzau VJ. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension 1999; 33:613–621. [DOI] [PubMed] [Google Scholar]
- 28.Lehtonen JY, Horiuchi M, Daviet L, Akishita M, Dzau VJ Activation of the de novo biosynthesis of sphingolipids mediates angiotensin II type 2 receptor-induced apoptosis. J Biol Chem 1999; 274:16901–16906. [DOI] [PubMed] [Google Scholar]
- 29.Ravassa S, Fortuno MA, Gonzalez A, Lopez B, Zalba G, Fortuno A, Diez J. Mechanisms of increased susceptibility to angiotensin II-induced apoptosis in ventricular cardiomyocytes of spontaneous hypertensive rats. Hypertension 2000; 36:1065–1071. [DOI] [PubMed] [Google Scholar]
- 30.Wen Y, Nadler JL, Gonzales N, Scott S, Clauser E, Natarajan R. Mechanisms of ANG II-induced mitogenic responses: role of 12-lipoxygenase and biphasic MAP kinase. Am J Physiol 1996; 271: C1212–C1220. [DOI] [PubMed] [Google Scholar]
- 31.Hasegawa K, Iwai-Kanai E, Sasayama S. Neurohormonal regulation of myocardial cell apoptosis during the development of heart failure. J Cell Physiol 2001; 186:11–18. [DOI] [PubMed] [Google Scholar]
- 32.Goussev A, Sharov VG, Shimoyama H, Tanimura M, Lesch M, Goldstein S, Sabbah HM. Effects of ACE inhibition on cardiomyocyte apoptosis in dogs with heart failure. Am J Physiol 1998; 275:H626–H631. [DOI] [PubMed] [Google Scholar]
- 33.Diez J, Fortuno M, Ravassa S. Apoptosis in hypertensive heart disease. Curr Opin Cardiol 1998; 13:317–325. [DOI] [PubMed] [Google Scholar]
- 34.Shao Q, Saward L, Zahradka P, Dhalla NS. Ca2+ mobilization in adult rat cardiomyocytes by angiotensin type 1 and 2 receptors. Biochem Pharmacol 1998; 55:1413–1418. [DOI] [PubMed] [Google Scholar]
- 35.Touyz RM, Sventek P, Lariviere R, Thibault G, Fareh J, Reudelhuber T, Schiffrin EL. Cytosolic calcium changes induced by angiotensin II in neonatal rat atrial and ventricular cardiomyocytes are mediated via angiotensin subtype 1 receptors. Hypertension 1996; 27:1090–1096. [DOI] [PubMed] [Google Scholar]
- 36.Zieleniewski W. Verapamil inhibits proliferation but not steroidogenesis of regenerating rat adrenal cortex. Life Sci 1990; 46:1851–1855. [DOI] [PubMed] [Google Scholar]
- 37.Blitstein-Willinger E, Diamantstein T. Inhibition by isoptin (a calcium antagonist) of the mitogenic stimulation of lymphocytes prior to the S-phase. Immunology 1978; 34:303–307. [PMC free article] [PubMed] [Google Scholar]


