Abstract
Arterial stiffness is recognized mainly as an indicator of arteriosclerosis and a predictor of cardiovascular events. Cardio-ankle vascular index (CAVI), which reflects arterial stiffness from the origin of the aorta to the ankle, was developed in 2004. An important feature of this index is the independency from blood pressure at the time of measurement. A large volume of clinical evidence obtained using CAVI has been reported. CAVI is high in patients with various atherosclerotic diseases including coronary artery disease and chronic kidney disease. Most coronary risk factors increase CAVI and their improvement reduces CAVI. Many prospective studies have investigated the association between CAVI and future cardiovascular disease (CVD), and proposed CAVI of 9 as the optimal cut-off value for predicting CVD. Research also shows that CAVI reflects afterload and left ventricular diastolic dysfunction in patients with heart failure. Furthermore, relatively acute changes in CAVI are observed under various pathophysiological conditions including mental stress, septic shock and congestive heart failure, and in pharmacological studies. CAVI seems to reflect not only structural stiffness but also functional stiffness involved in acute vascular functions. In 2016, Spronck and colleagues proposed a variant index CAVI0, and claimed that CAVI0 was truly independent of blood pressure while CAVI was not. This argument was settled, and the independence of CAVI from blood pressure was reaffirmed. In this review, we summarize the recently accumulated evidence of CAVI, focusing on the proposed cut-off values for CVD events, and suggest the development of new horizons of vascular function index using CAVI.
Keywords: Cardio-ankle vascular index, Arterial stiffness, Stiffness parameter β, Cardiovascular disease, Heart failure
Introduction
Previous studies have demonstrated the significance of arterial stiffness as an indicator of arteriosclerosis and a predictor of cardiovascular (CV) events1). Several parameters have so far been utilized for clinical assessment of arterial stiffness2). Since pulse wave velocity (PWV) can be measured noninvasively and easily, this method has been used clinically for several decades as a representative arterial stiffness marker3). PWV contributed greatly to recognition of the importance of measuring arterial stiffness. However, PWV is dependent on blood pressure at the time of measurement. Therefore, use of this index is not appropriate in studies that examine the effect of hypertension or the effect of antihypertensive drugs on intrinsic arterial stiffness. To overcome this problem, Hayashi4) proposed the stiffness parameter β, an index reflecting arterial stiffness of local arterial segment, which is not influenced by blood pressure at the time of measurement. Subsequently, this theory has been applied to a new arterial stiffness index called cardio-ankle vascular index (CAVI) developed in 2004 5), and this index reflects the stiffness of the arterial tree from the origin of the aorta to the ankle. The CAVI equation was essentially derived from the stiffness parameter β, and the changes of the artery caliber in the equation during the cardiac cycle were obtained from Bramwell-Hill's equation, in which PWV is related to caliber changes6). The calculation formula for CAVI is given in eq. 1.
Ps: systolic blood pressure, Pd: diastolic blood pressure, ΔP: Ps − Pd, haPWV: heart-ankle PWV
The principle of CAVI and the calculation formula have been described in our previous review2). Independence of CAVI from blood pressure at the time of measurement was also confirmed by clinical experiments7).
CAVI has been widely used in clinical medicine for the last 15 years as an index for the evaluation of cardiovascular diseases (CVD) and risk factors. Increased CAVI is observed in persons with CVD and risk factors, and a number of studies have investigated the association between CAVI and the occurrence of CV events, as summarized in the review published in 2016 2). In the last decade, research using CAVI has continued to increase in depth and scope. Prospective studies aiming to determine the optimal cut-off values for predicting CVD have been accumulated8–18). Furthermore, the interaction between left ventricular (LV) function and CAVI has been reported, indicating that CAVI reflects not only organic stiffness but also functional stiffness. These studies may lead to the development of a new field of cardiovascular interaction using CAVI.
On the other hand, Spronck et al.19) proposed a variant index CAVI0 in 2016. They claimed that CAVI0 was an index truly independent from blood pressure while CAVI was not. Their report generated some controversies, but the issue was resolved. The independence of CAVI from blood pressure at the time of measurement was reaffirmed. We also add some comments in this review.
In this article, we review the new evidence that has been added in the last 5 years, and summarize the prospective studies aiming to determine the cut-off CAVI values for CVD events. Furthermore, we discuss the possibility of developing new horizons of vascular function index using CAVI.
Various Factors Affecting CAVI (Cross-sectional Studies)
A large number of cross-sectional studies have verified that numerous factors including arteriosclerotic diseases and coronary risk factors affect CAVI value. These studies are summarized in Table 1.
Table 1. Diseases and Coronary Risk Factors that Affect CAVI.
| Factors affecting CAVI | CAVI value | Main References |
|---|---|---|
| Age and gender | ||
| Aging | ↑ | [2, 20, 21, 22] |
| Male | ↑ | [23, 24, 25] |
| Arteriosclerotic diseases | ||
| Coronary artery disease | ↑ | [17, 20, 21, 26, 27, 28, 29, 30, 31, 32] |
| Cerebral infarction | ↑ | [33, 34] |
| Chronic kidney disease | ↑ | [35, 36, 37, 38] |
| Thickening of carotid intima-media thickness | ↑ | [20, 30, 39, 40, 41] |
| Coronary risk factors | ||
| Hypertension | ↑ | [20, 36, 40, 42, 43, 44] |
| Diabetes mellitus | ↑ | [21, 32, 40, 54] |
| Diabetic retinopathy | ↑ | [55] |
| Diabetic neuropathy | ↑ | [56] |
| Diabetic nephropathy | ↑ | [29, 32, 39] |
| Postprandial hyperglycemia and high glycemic variability | ↑ | [57, 58, 59] |
| Dyslipidemia | ↑ | [66, 67] |
| Primary hypercholesterolemia | → | [66, 68, 69] |
| Uric acid | ↑ | [73, 74, 75, 76] |
| Only in females | ↑ | [77] |
| Nonalcoholic fatty liver disease | ↑ | [113] |
| Sleep apnea syndrome | ↑ | [78, 79] |
| Smoking | ↑ | [20, 81, 82] |
| Obesity and leanness | ||
| Metabolic syndrome | ↑ or → | [32, 84, 86, 87] |
| Obesity | ↓ | [83, 84] |
| Leanness | ↑ | [85] |
| Sarcopenia | ↑ | [89, 90, 91] |
| Cognitive decline | ↑ | [92, 93] |
| Autonomic nervous system | ||
| Mental stress | ↑ | [94, 95, 97] |
| Sleep disturbance | ↑ | [96] |
| Septicshock | ↓ | [98] |
| Endocrine system | ||
| Hypothyroidism | ↑ | [114] |
| Hypogonadism | ↑ | [115] |
| Collagen diseases | ||
| Rheumatoid arthritis | ↑ | [99] |
| Systemic lupus erythematosus | ↑ | [100] |
| Psoriasis | ↑ | [116] |
| Tooth loss | ↑ | [117] |
1. Age and Gender
Several studies have indicated that CAVI increases linearly with age2, 20–22). CAVI values are on average 0.2 higher in men than in women at all ages21). Moreover, male gender is an independent determinant of higher CAVI22).
Age dependence of CAVI may be useful in the field of geriatric medicine. Nilsson23) proposed a new concept of early vascular ageing (EVA) syndrome measured by carotid-femoral PWV (cfPWV) in European countries. Based on the definition of EVA, CAVI seems to be appropriate for its evaluation, since CAVI is independent of blood pressure. Furthermore, CAVI may also be used to evaluate the effects of anti-aging supplements on vascular aging. Resveratrol, an activator of sirtuin 1 (SIRT1)24), and S-equol, a non-steroidal estrogen25), are both reported to lower CAVI. The opposite phenotype of EVA is defined as super-normal vascular aging (SUPERNOVA). The use of CAVI may contribute to the search of protective mechanisms or new therapeutic targets against the aging process.
2. Arteriosclerotic Diseases
CAVI is known to be high in patients with various atherosclerotic diseases.
(a). Coronary Artery Disease
Several researchers have reported that CAVI is high in patients with coronary artery disease (CAD)20, 21, 26, 27). CAVI increases according to the number of stenotic vessels26, 27). Izuhara et al.28) reported that CAVI, but not baPWV, is associated with the presence of carotid and coronary arteriosclerosis. In addition, Nakamura et al. reported that CAVI is an independent variable of coronary atherosclerosis severity, but mean intima-media thickness (IMT), maximum IMT and plaque score are not. CAVI correlates with coronary artery calcification17, 29, 30). A number of cross-sectional studies have determined the cut-off value of CAVI for the presence of CAD (Table 2). CAVI ≥ 8.0 is associated with ≥ 50% coronary artery stenosis31, 32), and CAVI ≥ 9.0 with ≥ 75% coronary artery stenosis28). In a study in Thailand, the cutoff value of CAVI for the presence of CAD is 8.0, and adding CAVI into the traditional risk score (RAMA-EGAT) improves the prediction of CAD incidence, increasing C-statistics from 0.72 to 0.85 and resulting in a net reclassification improvement (NRI) of 27.7% (p < 0.0001)31). From these findings, high CAVI is strongly associated with the presence of CAD, and the possible cut-off value is considered to be 8 or 9.
Table 2. Cross-Sectional Studies on the Association of CAVI with the Presence of Cardiovascular Disease.
| Author | Country | Subjects | Mean Age | Mean CAVI | Multivariate Analysis | What is Cut-off Value of CAVI for? | Cut-off Value | NRI |
|---|---|---|---|---|---|---|---|---|
| Nakamura et al. 2008 26) | Japan | 109 participants who underwent coronary angiography | 58.0–67.6 | Not described | CAVI was an independent variables of coronary atherosclerosis severity, but mean IMT, maximum IMT and plaque score were not. | Presence of CAD. (Significant coronary stenosis defined as ≥ 75%) | 8.81 | Not described |
| Takenaka et al. 2008 118) | Japan | 68 patients with endstage renal diseases | 60 | 7.8 | Not described. | Presence of CVD. | 7.55 | Not described |
| Park et al. 2012 32) | Korea | 158 Normoglycemic Subjects and 373 subjects with abnormal glucose metabolism | 56–58 | 7.5–7.9 | Adjusted CAVI ≥ 8.0 was independently associated with significant coronary artery stenosis (OR 3.143). | Predicting ≥ 50% coronary artery stenosis. | 8.0 | Not described |
| Yingchoncharoen et al. 2012 31) | Thailand | 1,391 patients with a moderate to high risk for CAD | 59 | Not described | There was a correlation between CAVI and the prevalence of coronary stenosis after adjusting for traditional CAD risk factors (OR 3.29). | Presence of CAD. (Significant coronary stenosis defined as ≥ 50%) | 8.0 | 0.277 (p < 0.0001) |
| Gomez-Sanchez et al. 2015 41) | Spain | 500 subjects with intermediate level of CV risk factor | 60.3 | 8.59 | IMT and PWV maintained a positive association with adjusted CAVI. | Detecting mean IMT > 0.90 mm and maxima IMT > 0.90 mm. | 8.95 (mean IMT > 0.90) (maxima IMT > 0.90) | Not described |
| Hitsumoto et al. 2019 119) | Japan | 405 patients with CV risk factors | 64 | 8.7 | CAVI was selected as independent factor for pulsatility index of common carotid artery as a subordinate factor. | High pulsatility index of common carotid artery (> 1.60) as a risk value of stroke incidence. | 9.1 | Not described |
CAVI, cardio-ankle vascular index; NRI, net reclassification improvement; IMT, intima-media thickness; CAD, coronary artery disease; CVD, cardiovascular disease; OR, odds ratio; CV, cardiovascular; PWV, pulse wave velocity
(b). Cerebral Infarction
CAVI values are high in patients with cerebral infarction33). CAVI is larger in patients with large artery atherosclerosis and small vessel occlusion than in controls33, 34). In the study of Saji et al.34), the CAVI cut-off value for indicating silent brain infarct is 9.2, and that for white matter hyperintensities is 8.9.
(c). Chronic Kidney Disease
CAVI is higher in patients with chronic kidney disease (CKD) compared to patients with normal kidney function35, 36), and is especially high in patients receiving hemodialysis35, 37). CAVI correlates with estimated glomerular filtration rate, urinary albumin creatinine ratio and cystatin C35, 36, 38).
(d). Thickening of Carotid Intima-Media Thickness
IMT is associated with CAVI20, 30, 39), and patients with carotid plaque have higher CAVI40). Spanish researchers have shown that IMT and PWV correlate positively with CAVI, and the cut-off CAVI values for detecting mean and maxima IMT > 0.90 mm are 8.95 and 8.85, respectively41).
3. Coronary Risk Factors
Overall, CAVI is high in patients with coronary risk factors. However, some conventional risk factors do not affect CAVI. In addition, the effect on CAVI differs depending on the type of treatment, even when the degree of risk factor improvement is similar. Accumulated studies reporting treatments and behavior modifications that affect CAVI are summarized in Table 3. CAVI may reveal the impact of each risk factor on arterial stiffness.
Table 3. Effects of Various Treatments and Behavior Modifications on CAVI.
| Treatments and behavior modifications | CAVI value | Main References |
|---|---|---|
| Body weight reduction | ||
| Calorie restriction (metabolic syndrome) | ↓ | Satoh N. Hypertens Res 2008 84) |
| Calorie restriction (obesity with diabetes) | ↓ | Nagayama D. Obes Res Clin Pract 2011 88) |
| Bariatric Surgery | → | Streese L. Obes Surg 2019 126) |
| Glucose control | ||
| Rapid-acting insulin analog | ↓ | Ohira M. Metabolism 2011 58) |
| ↓ | Akahori H. Diabetolo Int 2014 59) | |
| Dipeptidyl peptidase 4 inhibitors | ↓ or → | Shigiyama F. J Diabetes Investig 2016 63) |
| Sulfonylurea | ↓ or → | Nagayama D. Int J Clin Pract 2010 64) |
| Pioglitazone | ↓ | Ohira M. Diabetes Metab Syndr Obes 2014 62) |
| α-glucosidase inhibitor | ↓ | Uzui H. J Diabetes Investig 2011 61) |
| Sodium-glucose Cotransporter-2 Inhibitors | ↓ | Bekki M. Current Vascular Pharmacology 2018 65) |
| Blood pressure control | ||
| Ca blocker (L-type calcium channel blocker) | → | Sasaki H. J Atheroscler Thromb 2009 50) |
| → | Miyashita Y. J Atheroscler Thromb 2009 45) | |
| → | Shibata T. Intern Med 2015 51) | |
| → | Bokuda K. Hypertens Res 2018 52) | |
| Ca blocker (T-type calcium channel blocker) | ↓ | Sasaki H. J Atheroscler Thromb 2009 50) |
| Renin-angiotensin-aldosterone system inhibitors | ↓ | Bokuda K. Vasc Health Risk Manag 2010 46) |
| ↓ | Miyashita Y. J Atheroscler Tromb 2009 45) | |
| ↓ | Ogihara T. Exert Rev Cardiovasc Ther 2008 47) | |
| ↓ | Miyoshi T. Clin Med Res 2017 48) | |
| ↓ | Kiuchi S. Clin Pharmacol 2015 49) | |
| Mineralocorticoid receptor blocker | ↓ | Shibata T. Intern Med 2015 51) |
| Direct renin inhibitor | ↓ | Miyoshi T. Open Heart 2017 53) |
| ↓ | Bokuda K. Hypertens Res 2018 52) | |
| Lipid control | ||
| Statins | ↓ | Miyashita Y. J Atheroscler Thromb 2009 70) |
| Eicosapentaenoic acid | ↓ | Satoh N. Hypertens Res 2009 71) |
| Fibrates | ↓ | Yamaguchi T. J Atheroscler Thromb 2018 72) |
| Nitroglycerin | ↓ | Shimizu K. Vasc Health Risk Manag 2016 120) |
| ↓ | Shimizu K. J Atheroscler Thromb 2017 121) | |
| Sarpogrelate hydrochloride | ↓ | Nagayama D. Int Heart J 2014 122) |
| Anti-vascular endothelial growth factor inhibits | ↓ | Shiba T. Ophthalmologica 2016 123) |
| Chemotherapy | ↑ | Shimizu N. J Clin Med Res 2017 124) |
| Antiaging Supplements | ||
| Resveratrol | ↓ | Imamura H. Int Heart J 2017 24) |
| S-equol | ↓ | Usui T. Clin Endocrinol 2013 25) |
| Smoking cessation | ↓ | Noike H. J Atheroscler Thromb 2010 81) |
| Small amount of alcohol | ↓ | Nishiwaki M. Physiol Rep 2017 127) |
| Exercise | ↓ | Alonso-Domínguez R. BMC Cardiovasc Disord 2019 125) |
| Continuous positive airway pressure | ↓ | Kasai T. Am J Hypertens 2011 80) |
(a). Hypertension
Hypertension is well known to be a major risk factor for CVD. Numerous studies have reported high CAVI in hypertensive patients20, 36, 40, 42, 43). Logistic regression models show that 1-standard deviation increments in systolic (SBP), diastolic (DBP) and mean blood pressure (MBP) indices contribute independently to high CAVI (≥ 90th percentile)43). Moreover, most of these reports show that CAVI has a lower correlation coefficient with blood pressure than PWV5, 36, 40, 44). Since PWV depends on blood pressure at the time of measurement and CAVI does not, the lower correlation of CAVI with blood pressure is reasonable. CAVI may accurately assess whether antihypertensive agents improve arterial stiffness.
Many studies have consistently reported that renin-angiotensin-aldosterone system inhibitors such as angiotensin II receptor antagonists lower CAVI45–49). Among calcium channel blockers (CCBs), amlodipine, the most commonly used L-type blocker, does not lower CAVI45, 50–52). On the other hand, CAVI is significantly reduced by the T-channel blocker efonidipine50). CAVI may indicate the differential effects of various types of CCBs on arterial stiffness. Recent studies also report that novel antihypertensive agents, mineralocorticoid receptor blocker and direct renin inhibitor, also lower CAVI51–53).
(b). Diabetes Mellitus
CAVI is higher in patients with diabetes than in those without21, 40), and CAVI is associated with HbA1c32, 54). Elevated CAVI (≥ 8.0) is independently associated with diabetes54). In patients with diabetes, CAVI is related to diabetic microvascular complications such as retinopathy, peripheral neuropathy and microalbuminuria32, 39, 55, 56), and correlates with estimated glomerular filtration rate29). Furthermore, a few studies show the association of CAVI with postprandial hyperglycemia57–59). Tsuboi et al.57) reported that 1-hour postprandial glucose levels are associated with increased CAVI in non-diabetic subjects.
The response of CAVI to glucose-lowering treatment depends on the type of agent used60). Alpha-glucosidase inhibitor61) and rapid-acting insulin analog58, 59) reduce CAVI through improvement of postprandial hyperglycemia. An insulin-sensitizer pioglitazone also decreases CAVI accompanying the adiponectin increasing effect62). Whether dipeptidyl peptidase 4 (DPP-4) inhibitors and sulfonylurea reduce CAVI remains controversial63, 64). Nagayama et al.64) reported that glimepiride, a third generation sulfonylurea, improves CAVI and markers of insulin resistance and oxidative stress, but glibenclamide, a conventional sulfonylurea, has no such effects. A recent study shows that switching DPP-4 inhibitors to the sodium-glucose cotransporter-2 inhibitor tofogliflozin ameliorates CAVI that correlates with the level of advanced glycation end products65). These findings suggest that postprandial hyperglycemia, insulin resistance and oxidative stress may influence CAVI in patients with diabetes.
(c). Dyslipidemia
CAVI is higher in patients with dyslipidemia than in controls66). Using a trend test, Nagayama et al.67) demonstrated linear relations between CAVI and all the conventional lipid parameters, and that these parameters contributed independently to high CAVI (≥ 90th percentile). Among their subjects with high low-density lipoprotein cholesterol (LDL-C), those with concurrent high triglycerides (TG) had higher CAVI, and receiver operating characteristic (ROC) analysis determined TG level of 93 mg/dl as the optimal cut-off value in predicting high CAVI (Fig. 1). On the other hand, several researchers found no elevation of CAVI in patients with primary hypercholesterolemia66, 68, 69). On the contrary, CAVI is low in patients with familial hypercholesterolemia (FHC) due to “lipidosis” in the early stage, but CAVI increases after the development of inflammation, fibrous cap or complicated lesion. Therefore, a low CAVI in FHC does not indicate a low risk of future CVD69).
Fig. 1.
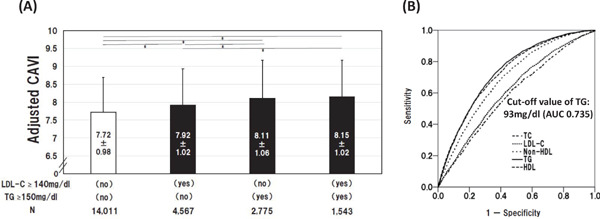
Relationship of Adjusted CAVI with Types of Dyslipidemia and Lipid Parameters
(A) Adjusted CAVI for three types of dyslipidemia were compared. CAVI was adjusted by gender, age, SBP or BMI. Data are presented as mean ± SD. *P < 0.01, one-way ANOVA followed by Bonferroni multiple comparison test. (B) Discriminatory powers of lipid parameters for high CAVI (≥ 90th percentile). Curves represent ROC analyses for discriminating the probability of high CAVI. CAVI, cardio-ankle vascular index; SBP, systolic blood pressure; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; ROC, receiver-operating-characteristics; SD, standard deviation67).
Among cholesterol-lowering agents, pitavastatin lowers CAVI, which is associated with changes in malondialdehyde-LDL70). Triglyceride-lowering agents also decrease CAVI. Eicosapentaenoic acid decreases CAVI together with a reduction in oxidized LDL71). Yamaguchi et al.72) reported that bezafibrate ameliorates CAVI accompanied by improvement of remnant-like particle cholesterol and diacron-reactive oxygen metabolites. In patients with dyslipidemia, coexisting abnormal glycolipid metabolism and oxidative stress may affect CAVI.
(d). Uric Acid
Uric acid as a risk factor for arteriosclerosis is controversial because uric acid is known to have both antioxidant73) and pro-oxidative actions in the process of production74). Several researchers documented that uric acid increases arterial stiffness measured by CAVI75, 76). Nagayama et al.76) reported that CAVI increases progressively with increasing serum uric acid tertile as shown in a multiple regression analysis, but CAVI tends to be high in male patients with lower serum uric acid. Zheng et al. 77) also reported a positive association between elevated serum uric acid and higher CAVI risk only in females.
(e). Sleep Apnea Syndrome
Sleep apnea syndrome is one of important risk factors of atherosclerosis. CAVI is also high in patients with sleep apnea syndrome78, 79). Alberto et al.79) reported that multivariable-adjusted odds ratio of increased CAVI in patients with severe nocturnal intermittent hypoxia is remarkably increased when body mass index (BMI) is 25 or higher. Furthermore, CAVI is reduced by continuous positive airway pressure therapy80).
(f). Smoking
CAVI is higher in smokers than in non-smokers81, 82). Brinkman index correlates with CAVI20). CAVI increases significantly after smoking one cigarette82), and improves after cessation of smoking81).
4. Obesity and Leanness
The obesity paradox is observed in the relationship between CAVI and BMI. In studies of relatively healthy people, CAVI is negatively related to BMI83, 84). Young females with anorexia nervosa show signs of early arteriosclerotic damage indicated by CAVI85). On the other hand, patients with visceral fat accumulation and concurrent metabolic disorders have high CAVI, as described below.
(a). Metabolic Syndrome
Several studies have reported that CAVI is high in metabolic syndrome84, 86). Kawada et al. found87) a trend of positive association between CAVI and BMI < 25, high blood pressure or hypertriglyceridemia. Park et al.32) reported that visceral and epicardial fat, but not subcutaneous fat, show a positive association with CAVI.
Body weight reduction improves CAVI and many risk factors in patients with metabolic syndrome84). Nagayama et al.88) observed that weight reduction using a calorie restriction diet decreases CAVI in obese patients with type 2 diabetes, and that change in visceral fat area is a significant independent predictor for change in CAVI. These findings suggest that subcutaneous fat may have a protective effect on blood vessels, whereas visceral fat may cause metabolic disorders, resulting in increased arterial stiffness. Further investigation is required.
(b). Sarcopenia
Kirkham et al.89) reported that CAVI is high in sarcopenic persons and CAVI is a significant predictor of skeletal mass index in women. Im et al.90) observed a positive association of muscle mass deficits with arterial stiffness in middle-aged men. Xue et al.91) showed that CAVI is associated with frailty in older patients. These findings suggest that skeletal muscle loss not only promotes vascular aging, but atherosclerosis may also promote sarcopenia.
5. Cognitive Decline
Patients with lower Mini Mental State Examination (MMSE) scores have higher CAVI92), and the annual decreases in MMSE score are significantly larger in patients with high CAVI93). CAVI ≥ 10.0 is associated with future cognitive dysfunction93). Elderly people with high CAVI may be at greater risk of cognitive decline.
6. Autonomic Nervous System
CAVI reflects not only organic stiffness but also functional stiffness. Therefore, CAVI may be affected by the activity of the autonomic nervous system.
(a). Mental Stress and Sleep Disturbance
CAVI is significantly higher in shift workers94) and workers working long overtime hours95). There is an inverse relationship between sleep duration and CAVI in children96). Interestingly, Shimizu et al.97) reported that people had higher CAVI shortly after experiencing a huge earthquake, even though they were in a hospital situated about 300 km from the epicenter of the earthquake. These findings suggest that mental stress also increases CAVI.
(b). Septic Shock
Nagayama et al. studied patients with sepsis and found that their CAVI increased after 1-week treatment without increase in blood pressure98). This finding suggests that CAVI may reflect sepsis-induced vascular alteration which is not indicated by blood pressure change.
7. Collagen Diseases
Spinelli et al.99) reported that rheumatoid arthritis patients have significantly higher CAVI, and that CAVI is associated with anti-carbamylated proteins antibodies in a multivariate regression analysis. Carlucci et al.100) found increases in CAVI, noncalcified plaque burden and vascular inflammation quantified by 18F-fluorodeoxyglucose-PET/CT in patients with systemic lupus erythematosus. CAVI may be useful in the diagnosis and quantification of vascular inflammation in patients with collagen disease.
CAVI as a Predictor of Cardiovascular Events
Several studies have investigated the association between CAVI and future CV events (Table 4). The participants in all the studies were at high risk for CVD, such as having hypertension, diabetes, obesity, CKD, and a history of CVD. Nine studies were from Japan8–16), and the other two were from Taiwan17) and Lithuania18). In most studies, baseline CAVI was a predictor of future CV events. However, CAVI did not predict CV events in hemodialysis patients in one study9). In the metanalysis of Matsushita et al.101), the pooled hazard ratio for composite CVD events per 1 standard deviation increment in CAVI was 1.20 (95% confidence interval 1.05–1.36) in four prospective studies11, 12, 14, 18). Otsuka et al.10) reported that the incidence of CV events after 2.9 years was significantly higher in the group with no improvement in CAVI at 6 months than in the group with improvement. This seems to be an interesting report that clarifies the relationship between change in CAVI over time and the occurrence of CV events.
Table 4. Summary on Association of CAVI with Cardiovascular Outcomes in Prospective Studies.
| Author | Country | Subjects | Mean Age | Baseline CAVI | Duration of Follow-up | CV Outcomes | Incidence (%) (1,000 person-years) | Prognostic Value | Cut-off Value | NRI |
|---|---|---|---|---|---|---|---|---|---|---|
| Kubota et al. 2011 8) | Japan | 400 patients with metabolic disorders or past history of CAD | 63.2–73.9 | Not described | 27.2 months | Coronary artery disease, stroke and death | 54.0 | Hazard ratio of CVD was significantly higher in CAVI ≥ 10.0 group (HR 2.25). | 9.0 | Not described |
| Kato et al. 2012 9) | Japan | 135 hemodialysis patients | 60 | 9.7 | 63 months | Primary outcome: All-cause and CV mortalities. Secondary outcome: Fatal and non-fatal CV events. | 52.2 | Not significant. | Not described | Not described |
| Otsuka et al. 2014 10) | Japan | 211 CAD patients | 65 | 9.87–10.05 | 2.9 years | Cardiac death, non-fatal MI, unstable angina pectoris, recurrent angina pectoris requiring coronary revascularization or stroke. | 45.8 | Persistently impaired CAVI was a significant independent predictor of CV events compared with improved CAVI at 6 months (HR 3.3). | Not described | Not described |
| Laucevičius et al. 2015 18) | Lithuania | 2,106 metabolic syndrome patients | 53.83 | 7.92 | 3.8 years | MI, stroke or transient ischemic attack, and sudden cardiac death. | 11.6 | CAVI was significantly associated with the occurrence of total CV events (p = 0.045) and MI (p = 0.027). | 7.95 | Not described |
| Satoh-Asahara et al. 2015 11) | Japan | 425 obese patients | 51.5 | 7.6 | 5 years | Angina pectoris, myocardial infarction, stroke and arteriosclerosis obliterans. | 15.8 | CAVI was a significant predictor of CV events (HR 1.44 per 1 unit increase). | Not described | 0.164 (p = 0.066) |
| Sato et al. 2015 12) | Japan | 1,003 subjects with CV risk factor | 62.5 | 9.25 | 6.7 years | Myocardial infarction and stable/unstable angina pectoris. | 13.4 | CAVI was independently associated with future CV event risk (HR 1.126 per 1 unit increase). | Not described | Not described |
| Chung et al. 2015 17) | Taiwan | 626 patients with type 2 diabetes | 64 | 8.8 | 4.1 years | Death, ACS, ischemic stroke and any coronary revascularization for coronary artery disease. | 38.2 | Patients with CAVI ≥ 9.0 had greater CV events than those with CAVI < 9.0 (OR 1.23). | 9.0 | Not described |
| Gohbara et al. 2016 13) | Japan | 288 patients with ACS | 58–71 | Not described | 1.25 years | CV death, non-fatal MI, non-fatal ischemic stroke. | 52.8 | Patients with CAVI > 8.325 was an independent predictor of CV events (HR 18.0) and nonfatal ischemic stroke (HR 9.37). | 8.325 | Not described |
| Kusunose et al. 2016 14) | Japan | 114 patients with at least 2 CV risk factors | 69 | 8.5 | 4.25 years | Cardiac death, non-fatal myocardial infarction/coronary revascularization, acute pulmonary edema and stroke. | 72.2 | CAVI was not a significant predictor of CV events. CAVI was associated with a 5% per year decline in kidney function (HR: 1.52 per 1 SD increase). | 9.2 | Not described |
| Hitsumoto et al. 2018 15) | Japan | 460 patients with chronic kidney disease | 74 | 9.7 | 60.1 months | Cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke and hospital admission for heart failure. | 39.5 | A MACE was significantly higher in group CAVI > 10 than in non-group CAVI < 10 (HR 2.04). | 9.7 | Not described |
| Kirigaya et al. 2019 16) | Japan | 387 patients with ACS | 64 | 8.4–9.0 | 62 months | CV death, recurrence of ACS, heart failure requiring hospitalization, or stroke. | 31.0 | CAVI was an independent predictor of MACE (HR 1.496) and cardiovascular death (HR 2.204), but ba PWV was not. The addition of CAVI to GRACE score enhanced NRI (0.337). | 8.35 | 0.337 (p = 0.034) |
CAVI, cardio-ankle vascular index; CV, cardiovascular; CAD, coronary artery disease; CVD, cardiovascular disease; NRI, net reclassification improvement; MI, myocardial infarction; HR, hazard ratio; ACS, acute coronary syndrome; OR, odds ratio; SD, standard deviation; MACE, major adverse cardiovascular events; GRACE, global registry for acute coronary events
Many of the above studies in Asian countries determined the CAVI cut-off values for CVD events8, 13–17), and they are summarized in Fig. 2. In patients with type 2 diabetes, metabolic disorders, CKD and past history of CAD, the cut-off values for CVD events were 9.0–9.7 8, 14, 15, 17). Chung et al.17) reported that patients with CAVI ≥ 9.0 had greater risk of CV events than those with CAVI < 9.0 (odds ratio 1.23). Therefore, CAVI ≥ 9.0 seems to indicate increased cardiovascular risk. On the other hand, the cut-off values for CVD events were 8.325–8.35 in patients with acute coronary syndrome13, 16). Gohbara et al.13) reported that CAVI > 8.325 was an independent predictor of CV events (hazard ratio 18.0). Kirigaya et al.16) reported that CAVI was an independent predictor of major adverse cardiovascular events, but baPWV was not. Therefore, an optimal CAVI cut-off value of 8 may be recommended for secondary prevention of CV events.
Fig. 2.
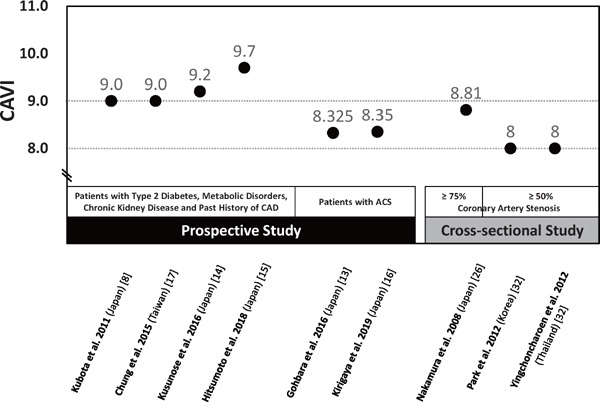
Summary of Optimal Cut-off Values of CAVI for Coronary Artery Disease Reported by Prospective and Cross-sectional Studies
CAD, coronary artery disease; ACS, acute coronary syndrome.
In several cross-sectional studies, the cut-off CAVI value for the presence of CAD defined as coronary artery stenosis ≥ 50% was 8.0, as described before31, 32). In addition, a few studies reported that coronary artery stenosis or calcification occurred as CAVI increased above 8.0 17, 21, 28–30). These findings suggest that CAVI ≥ 8.0 may be associated with subclinical or asymptomatic atherosclerosis.
The Physiological Diagnosis Criteria for Vascular Failure Committee propose cut-off CAVI values of 8.0 and 9.0 (< 8 for normal, ≥ 8 and < 9 for borderline, ≥ 9 for abnormal) (Fig. 3)102). We also agree with this definition. In Japan, two large multicenter longitudinal studies, CAVI-J and Coupling Registry, registering 3000–50000 high-risk patients are ongoing103, 104). These studies may reveal whether adding CAVI to the cardiovascular risk scoring systems improves the accuracy of CV risk prediction.
Fig. 3.

Estimated Criteria for Medial Layer Dysfunction and its Border Zone of CAVI
CAVI cut-off values of 8.0 and 9.0 (< 8 for normal, ≥ 8 and < 9 for borderline, ≥ 9 for abnormal) are proposed by the Physiological Diagnosis Criteria for Vascular Failure Committee102).
The Possible Role of CAVI in Cardio-Vascular Interaction
The proximal large vessels such as the aorta store the LV stroke volume during systole, and the elastic forces of the aortic wall forward part of this volume to the peripheral circulation during diastole, resulting in a nearly continuous peripheral blood flow. This systolic-diastolic interplay is defined as the Windkessel function. The Windkessel function influences not only the peripheral circulation but also the reduction of LV afterload. To assess the elastic properties of the aortic Windkessel, an index that theoretically indicate the “intrinsic” stiffness of the aortic wall is required. Therefore, CAVI, which is independent of blood pressure, may be a useful index reflecting this vascular function.
Schillaci et al.105) reported that subjects with inappropriately high LV masses have higher CAVI values, but not higher PWV values. A few studies show a relationship between LV ejection fraction and CAVI106). These reports provide evidence that CAVI reflects afterload, and high CAVI leads to myocardial hypertrophy. Furthermore, Sakane et al.107) reported that elevated CAVI is independently associated with LV diastolic dysfunction in patients with preserved systolic function. Other studies also found an association between high CAVI and LV diastolic dysfunction106, 108). These findings suggest that increased arterial stiffness may be a risk factor for LV diastolic heart failure, independent of blood pressure.
Since the LV function and morphology are closely related to arterial stiffness, treatments that improve CAVI may also improve LV function and prevent heart failure. Recently, Ogawa et al.109) reported that CAVI and physical function assessed by 6-minute walk distance are complementary to each other in elderly heart failure patients. Development of new cardiac rehabilitation by monitoring CAVI is anticipated.
Difference in Blood Pressure Dependence between CAVI and Variant CAVI0 and its Reason
CAVI represents blood pressure-independent arterial stiffness, as described above (eq, 1). In 2017, Spronck et al.19) proposed a variant index termed CAVI0, and the calculation formula is given in eq. 2.
P0: reference pressure (100 mmHg)
Spronck et al.19) claimed that the conventional CAVI was blood pressure-dependent based on the following reasons. First, there is slight difference between the β formula of Hayashi et al.4) and that of Kawasaki et al.110). Second, ln(Ps/Pd)/ΔP in the CAVI formula is not equal to 1/Pd. Furthermore, they pointed out that the arterial stiffness value should be corrected with a reference pressure19), and added “– ln(Pd/P0)” in the CAVI0 equation. They showed that CAVI was dependent on blood pressure based on mathematical simulation and concluded that CAVI leads to an erroneous conclusion in clinical studies19). In response to this claim, Shirai et al. reconfirmed the independency of CAVI from blood pressure at the time of measurement using measurements by the VaSera system and explained the reason for the difference between CAVI and CAVI0, both theoretically and in actual clinical studies111). In addition, it is known that PWV measured at the foot-to-foot interval differs from that measured at the top-to-top interval of the pulse wave during the cardiac cycle. The pulse transition time of PWV measured in CAVI using the VaSera system is not just the foot-to-foot period of the pulse wave at the pressure level of Pd, but almost the mid-to-mid period. Therefore, there was a concern that calculation of CAVI0 using only 1/Pd may yield unexplainable results. This concern was demonstrated in a cross-sectional study of a large population comparing CAVI with CAVI0, as shown in Fig. 4 112). CAVI was higher in the hypertensive group than in the healthy group in both men and women, whereas CAVI0 was significantly lower in women aged 30–39 years in the hypertensive group compared to the corresponding healthy control group. This unexplainable result was thought to be due to the strong dependence of CAVI0 on Pd. Incidentally, ln(Pd/P0) is actually negligible in clinical study.
Fig. 4.
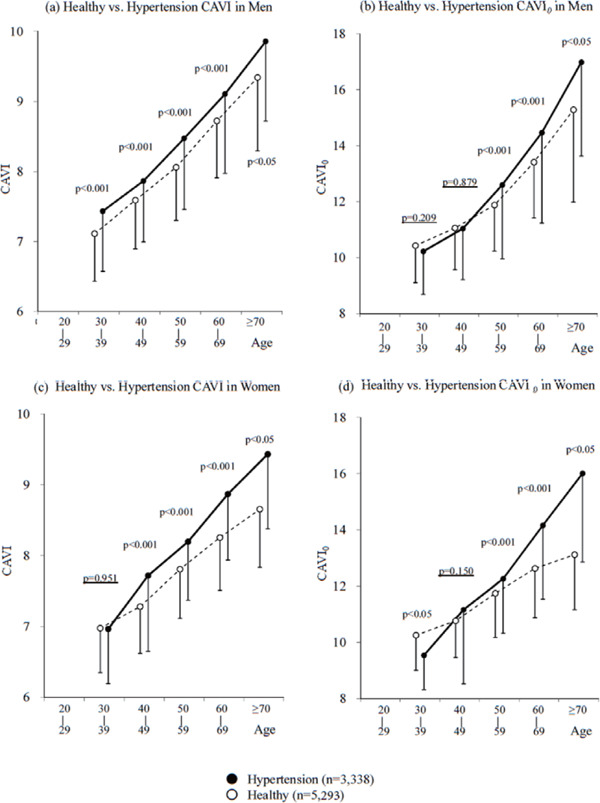
Comparison of the Significant Differences of CAVI and CAVI0 between Healthy Group and Hypertensive Group Stratified by Age in Men (a and b) and Women (c and d)112)
Conclusions
CAVI is an index reflecting arterial stiffness without being influenced by blood pressure. Since its development in 2004, a large volume of evidence has validated CAVI as a parameter for clinical evaluation of arterial stiffness. In recent years, an increasing number of studies have investigated the association between CAVI and future CV events, and CAVI of 9 has been proposed to be the optimal cut-off value for predicting CVD in Asian patients. Apart from the conventional role of being a marker of cardiovascular events, recent research has shed light on the clinical use of CAVI as an index of arterial stiffness for evaluation of a wide range of cardiovascular disorders including vasculitis in collagen diseases, septic conditions and heart failure, as well monitoring of interventions for controlling cardiovascular risk factors, as shown in Fig. 5. This research trend may open up new horizons of vascular function index using CAVI.
Fig. 5.
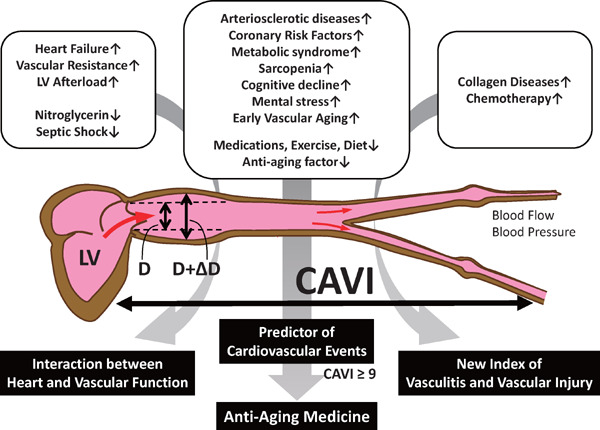
CAVI not only Predicts Cardiovascular Events, but may also be Developed as a New Index of Vascular Functions
Acknowledgement
None.
Funding Sources
No funding.
Disclosure Statement
All authors declare that they have no competing interests.
References
- 1). Oliver JJ, Webb DJ: Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol, 2003; 23: 554-566 [DOI] [PubMed] [Google Scholar]
- 2). Saiki A, Sato Y, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T, Ban N, Kawana H, Nagumo A, Nagayama D, Ohira M, Endo K, Tatsuno I: The role of a novel arterial stiffness parameter, cardio-ankle vascular index (CAVI), as a surrogate marker for cardiovascular diseases. J Atheroscler Thromb, 2016; 23: 155-168 [DOI] [PubMed] [Google Scholar]
- 3). Asmar R, Benetos SA, Topuchian J, Laurent P, Pannier B, Brisac AM, Target R, Levyet BI: Assessment of arterial distensibility by automatic pulse wave velocity measurement: Validation and clinical application studies. Hypertension, 1995; 26: 485-490 [DOI] [PubMed] [Google Scholar]
- 4). Hayashi K, Yamamoto T, Takahara A, Shirai K: Clinical assessment of arterial stiffness with cardio-ankle vascular index: theory and applications. J Hypertens, 2015; 33: 1742-1757 [DOI] [PubMed] [Google Scholar]
- 5). Shirai K, Utino J, Otsuka K, Takata M: A novel blood pressure-independent arterial wall stiffness parameter: cardio-ankle vascular index (CAVI). J Atheroscler Thromb, 2006; 13: 101-107 [DOI] [PubMed] [Google Scholar]
- 6). Bramwell JC, Hill AV: Velocity of transmission of the pulse and elasticity of arteries. Lancet, 1922; 199: 891-892 [Google Scholar]
- 7). Shirai K, Song M, Suzuki J, Kurosu T, Oyama T, Nagayama D, Miyashita Y, Yamamura S, Takahashi M: Contradictory effects of b1- and a1-aderenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI): the independency of CAVI from blood pressure. J Atheroscler Thromb, 2011; 18: 49-55 [DOI] [PubMed] [Google Scholar]
- 8). Kubota Y, Maebuchim D, Takei M, Inui Y, Sudo Y, Ikegami Y, Fuse J, Sakamoto M, Momiyama Y: Cardio-Ankle Vascular Index is a predictor of cardiovascular events. Artery Res, 2011; 5: 91-96 [Google Scholar]
- 9). Kato A, Takita T, Furuhashi M, Maruyama Y, Miyajima H, Kumagai H: Brachial-ankle pulse wave velocity and the cardio-ankle vascular index as a predictor of cardiovascular outcomes in patients on regular hemodialysis. Ther Apher Dial, 2012; 16: 232-241 [DOI] [PubMed] [Google Scholar]
- 10). Otsuka T, Fukuda S, Shimada K, Suzoshikawa J: Serial assessment of arterial stiffness by cardio-ankle vascular index for prediction of future cardiovascular events in patients with coronary artery disease. Hypertens Res, 2014; 37: 1014-1020 [DOI] [PubMed] [Google Scholar]
- 11). Satoh-Asahara N, Kotani K, Yamakage H, Yamada T, Araki R, Okajima T, Adachi M, Oishi M, Shimatsu A, Japan Obesity and Metabolic Syndrome Study (JOMS) Group : Cardio-ankle vascular index predicts for the incidence of cardiovascular events in obese patients: A multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS). Atherosclerosis, 2015; 242: 461-468 [DOI] [PubMed] [Google Scholar]
- 12). Sato Y, Nagayama D, Saiki A, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T, Ban N, Kawana H, Nagumo A, Ohira M, Endo K, Kurosu T, Tomaru T, Shirai K, Tatsuno I: Cardio-ankle vascular index is independently associated with future cardiovascular events in outpatients with metabolic disorders. J Atheroscler Thromb, 2016; 23: 596-605 [DOI] [PubMed] [Google Scholar]
- 13). Gohbara M, Iwahashi N, Sano Y, Akiyama E, Maejima N, Tsukahara K, Hibi K, Kosuge M, Ebina T, Umemura S, Kimura K: Clinical impact of the cardio-ankle vascular index for predicting cardiovascular events after acute coronary syndrome. Circ J, 2016; 80: 1420-1426 [DOI] [PubMed] [Google Scholar]
- 14). Kusunose K, Sato M, Yamada H, Saijo Y, Bando M, Hirata Y, Nishio S, Hayashi S, Sata M: Prognostic implications of non-invasive vascular function tests in highrisk atherosclerosis patients. Circ J, 2016; 80: 1034-1040 [DOI] [PubMed] [Google Scholar]
- 15). Hitsumoto T: Clinical Usefulness of the cardio-ankle vascular index as a predictor of primary cardiovascular events in patients with chronic kidney disease. J Clin Med Res, 2018; 10: 883-890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Kirigaya J, Iwahashi N, Tahakashi H, Minamimoto Y, Gohbara M, Abe T, Akiyama E, Okada K, Matsuzawa Y, Maejima N, Hibi K, Kosuge M, Ebina T, Tamura K, Kimura K: Impact of cardio-ankle vascular index on longterm outcome in patients with acute coronary syndrome. J Atheroscler Thromb, 2020; 27: 657-668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Chung SL, Yang CC, Chen CC, Hsu YC, Lei MH: Coronary artery calcium score compared with cardio-ankle vascular index in the prediction of cardiovascular events in asymptomatic patients with type 2 diabetes. J Atheroscler Thromb, 2015; 22: 1255-1265 [DOI] [PubMed] [Google Scholar]
- 18). Laucevičius A, Ryliškytė L, Balsytė J, Badarienė J, Puronaitė R, Navickas R, Solovjova S: Association of cardio-ankle vascular index with cardiovascular risk factors and cardiovascular events in metabolic syndrome patients. Medicina, 2015; 51: 152-158 [DOI] [PubMed] [Google Scholar]
- 19). Spronck B, Avolio AP, Tan I, Butlin M, Reesink KD, Delhaas T: Arterial stiffness index beta and cardio-ankle vascular index inherently depend on blood pressure but can be readily corrected. J Hypertens, 2017; 35: 98-104 [DOI] [PubMed] [Google Scholar]
- 20). Takaki A, Ogawa H, Wakeyama T, Iwami T, Kimura M, Hadano Y, Matsuda S, Miyazaki Y, Matsuda T, Hiratsuka A, Matsuzaki M: Cardioankle vascular index is a new noninvasive parameter of arterial stiffness. Circ J, 2007; 71: 1710-1714 [DOI] [PubMed] [Google Scholar]
- 21). Namekata T, Suzuki K, Ishizuka N, Shirai K: Establishing baseline criteria of cardio-ankle vascular index as a new indicator of arteriosclerosis: a cross-sectional study. BMC Cardiovasc Disord, 2011; 11: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Choi SY, Oh BH, Bae Park J, Choi DJ, Rhee MY, Park S: Age-associated increase in arterial stiffness measured according to the cardio-ankle vascular index without blood pressure changes in healthy adults. J Atheroscler Thromb, 2013; 20: 911-923 [DOI] [PubMed] [Google Scholar]
- 23). Nilsson PM: Early vascular aging in hypertension. Front Cardiovasc Med, 2020; 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Imamura H, Yamaguchi T, Nagayama D, Saiki A, Shirai K, Tatsuno I: Resveratrol ameliorates arterial stiffness assessed by cardio-ankle vascular index in patients with type 2 diabetes mellitus. Int Heart J, 2017; 58: 577-583 [DOI] [PubMed] [Google Scholar]
- 25). Usui T, Tochiya M, Sasaki Y, Muranaka K, Yamakage H, Himeno A, Shimatsu A, Inaguma A, Ueno T, Uchiyama S, Satoh-Asahara N: Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin Endocrinol, 2013; 78: 365-372 [DOI] [PubMed] [Google Scholar]
- 26). Nakamura K, Tomaru T, Yamamura S, Miyashita Y, Shirai K, Noike H: Cardio-ankle vascular index is a candidate predictor of coronary atherosclerosis. Circ J, 2008; 72: 598-604 [DOI] [PubMed] [Google Scholar]
- 27). Horinaka S, Yabe A, Yagi H, Ishimura K, Hara H, Iemura T, Matsuoka H: Comparison of atherosclerotic indicators between cardio ankle vascular index and brachial ankle pulse wave velocity. Angiology, 2009; 60: 468-476 [DOI] [PubMed] [Google Scholar]
- 28). Izuhara M, Shioji K, Kadota Y, Baba O, Takeuchi Y, Uegaito T, Mutsuo S, Matsuda M: Relationship of cardiovascular index to carotid and coronary arteriosclerosis. Circ J, 2008; 72: 1762-1767 [DOI] [PubMed] [Google Scholar]
- 29). Mineoka Y, Fukui M, Tanaka M, Tomiyasu K, Akabame S, Nakano K, Yamazaki M, Hasegawa G, Oda Y, Nakamura N: Relationship between cardio-ankle vascular index (CAVI) and coronary artery calcification (CAC) in patients with type 2 diabetes mellitus. Heart Vessels, 2012; 27: 160-165 [DOI] [PubMed] [Google Scholar]
- 30). Park JB, Park HE, Choi SY, Kim MK, Oh BH: Relation between cardio-ankle vascular index and coronary artery calcification or stenosis in asymptomatic subjects. J Atheroscler Thromb, 2013; 20: 557-567 [DOI] [PubMed] [Google Scholar]
- 31). Yingchoncharoen T, Limpijankit T, Jongjirasiri S, Laothamatas J, Yamwong S, Sritara P: Arterial stiffness contributes to coronary artery disease risk prediction beyond the traditional risk score (RAMA-EGAT score). Heart Asia, 2012; 4: 77-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Park HE, Choi SY, Kim MK, Oh BH: Cardio-ankle vascular index reflects coronary atherosclerosis in patients with abnormal glucose metabolism: assessment with 256 slice multi-detector computed tomography. J Cardiol, 2012; 60: 372-376 [DOI] [PubMed] [Google Scholar]
- 33). Suzuki J, Sakakibara R, Tomaru T, Tateno F, Kishi M, Ogawa E, Kurosu T, Shirai K: Stroke and cardio-ankle vascular stiffness index. J Stroke Cerebrovasc Dis, 2011; 22: 171-175 [DOI] [PubMed] [Google Scholar]
- 34). Saji N, Kimura K, Yagita Y, Kawarai T, Shimizu H, Kita Y: Comparison of arteriosclerotic indicators in patients with ischemic stroke: ankle-brachial index, brachialankle pulse wave velocity and cardio-ankle vascular index. Hypertens Res, 2015; 38: 323-328 [DOI] [PubMed] [Google Scholar]
- 35). Kubozono T, Miyata H, Uegama K, Nagaki A, Hamasaki S, Kusano K, Kubozono O, Tei C: Association between arterial stiffness and estimated glomerular filtration rate in the Japanese general population. J Atheroscler Thromb, 2009; 16: 840-845 [DOI] [PubMed] [Google Scholar]
- 36). Nakamura K, Iizuka T, Takahashi M, Shimizu K, Mikamo H, Nakagami T, Suzuki M, Hirano K, Sugiyama Y, Tomaru T, Miyashita Y, Shirai K, Noike H: Association between cardio-ankle vascular index and serum cystatin C levels in patients with cardiovascular risk factor. J Atheroscler Thromb, 2009; 16: 371-379 [DOI] [PubMed] [Google Scholar]
- 37). Ueyama K, Miyata M, Kubozono T, Nagaki A, Hamasaki S, Ueyama S, Tei C: Noninvasive indices of arterial stiffness in hemodialysis patients. Hypertens Res, 2009; 32: 716-720 [DOI] [PubMed] [Google Scholar]
- 38). Yamashita H, Nishino T, Obata Y, Nakazato M, Inoue K, Furusu A, Takamura N, Maeda T, Ozono Y, Kohno S: Association between cystatin C and arteriosclerosis in the absence of chronic kidney disease. J Atheroscler Thromb, 2013; 20: 548-556 [DOI] [PubMed] [Google Scholar]
- 39). Kim KJ, Lee BW, Kim HM, Shin JY, Kang ES, Cha BS, Lee EJ, Lim SK, Lee HC: Associations between cardioankle vascular index and microvascular complications in type 2 diabetes mellitus patients. J Atheroscler Thromb, 2011; 18: 328-336 [DOI] [PubMed] [Google Scholar]
- 40). Wang H, Liu J, Zhao H, Fu X, Shang G, Zhou Y, Yu X, Zhao X, Wang G, Shi H: Arterial stiffness evaluation by cardio-ankle vascular index in hypertension and diabetes mellitus subjects. J Am Soc Hypertens, 2013; 7: 426-431 [DOI] [PubMed] [Google Scholar]
- 41). Gomez-Sanchez L, Garcia-Ortiz L, Patino-Alonso MC, Recio-Rodriguez JI, Frontera G, Ramos R, Martí R, Agudo-Conde C, Rodriguez-Sanchez E, Maderuelo-Fernández JA, Gomez-Marcos MA, MARK Group : The association between the cardio-ankle vascular index and other parameters of vascular structure and function in Caucasian adults: MARK Study. J Atheroscler Thromb, 2015; 22: 901-911 [DOI] [PubMed] [Google Scholar]
- 42). Suzuki J, Kurosu T, Kon T, Tomaru T: Impact of cardiovascular risk factors on progression of arteriosclerosis in younger patients: evaluation by carotid duplex ultrasonography and cardio-ankle vascular index (CAVI). J Atheroscler Thromb, 2014; 21: 554-562 [PubMed] [Google Scholar]
- 43). Nagayama D, Watanabe Y, Saiki A, Shirai K, Tatsuno I: Difference in positive relation between cardio-ankle vascular index (CAVI) and each of four blood pressure indices in real-world Japanese population. J Hum Hypertens, 2019; 33: 210-217 [DOI] [PubMed] [Google Scholar]
- 44). Wen W, Luo R, Tang X, Tang L, Huang HX, Wen X, Hu S, Peng B: Age-related progression of arterial stiffness and its elevated positive association with blood pressure in healthy people. Atherosclerosis, 2015; 238: 147-152 [DOI] [PubMed] [Google Scholar]
- 45). Miyashita Y, Saiki A, Endo K, Ban N, Yamaguchi T, Kawana H, Nagayama D, Ohira M, Oyama T, Shirai K: Effects of olmesartan, an angiotensin II receptor blocker, and amlodipine, a calcium channel blocker, on cardio-ankle vascular index (CAVI) in type 2 diabetic patients with hypertension. J Atheroscler Thromb, 2009; 16: 621-626 [DOI] [PubMed] [Google Scholar]
- 46). Bokuda K, Ichihara A, Sakoda M, Mito A, Kinouchi K, Itoh H: Blood pressure-independent effect of candesartan on cardio-ankle vascular index in hypertensive patients with metabolic syndrome. Vasc Health Risk Manag, 2010; 6: 571-578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Ogihara T, Fujimoto A, Nakao K, Saruta T: ARB candesartan and CCB amlodipine in hypertensive patients: the CASE-J trial. Exert Rev Cardiovasc Ther, 2008; 6: 1195-1201 [DOI] [PubMed] [Google Scholar]
- 48). Miyoshi T, Suetsuna R, Tokunaga N, Kusaka M, Tsuzaki R, Koten K, Kunihisa K, Ito H: Effect of azilsartan on day-to-day variability in home blood pressure: a prospective multicenter clinical trial. J Clin Med Res, 2017; 9: 618-623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Kiuchi S, Hisatake S, Kawasaki M, Hirashima O, Kabuki T, Yamazaki J, Ikeda T: Addition of a Renin-Angiotensin-Aldosterone System Inhibitor to a Calcium Channel Blocker Ameliorates Arterial Stiffness. Clin Pharmacol, 2015; 7: 97-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Sasaki H, Saiki A, Endo K, Ban N, Yamaguchi T, Kawana H, Nagayama D, Ohhira M, Oyama T, Miyashita Y, Shirai K: Protective effects of efonidipine, a Tand L-type calcium channel blocker, on renal function and arterial stiffness in type 2 diabetic patients with hypertension and nephropathy. J Atheroscler Thromb, 2009; 16: 568-575 [DOI] [PubMed] [Google Scholar]
- 51). Shibata T, Tsutsumi J, Hasegawa J, Sato N, Murashima E, Mori C, Hongo K, Yoshimura M: Effects of add-on therapy consisting of a selective mineralocorticoid receptor blocker. Intern Med, 2015; 54: 1583-1589 [DOI] [PubMed] [Google Scholar]
- 52). Bokuda K, Morimoto S, Seki Y, Yatabe M, Watanabe D, Yatabe J, Ando T, Shimizu S, Itoh H, Ichihara A: Greater reductions in plasma aldosterone with aliskiren in hypertensive patients with higher soluble (Pro)renin receptor level. Hypertens Res, 2018; 41: 435-443 [DOI] [PubMed] [Google Scholar]
- 53). Miyoshi T, Murakami T, Sakuragi S, Doi M, Nanba S, Mima A, Tominaga Y, Oka T, Kajikawa Y, Nakamura K, Ito H: Comparable effect of aliskiren or a diuretic added on an angiotensin II receptor blocker on augmentation index in hypertension: a multicentre, prospective, randomised study. Open Heart, 2017; 4: e000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54). Shimizu Y, Nakazato M, Sekita T, Kadota K, Yamasaki H, Takamura N, Aoyagi K, Maeda T: Association of arterial stiffness and diabetes with triglycerides-to-HDL cholesterol ratio for Japanese men: the Nagasaki Islands Study. Atherosclerosis, 2013; 228: 491-495 [DOI] [PubMed] [Google Scholar]
- 55). Lamacchia O, Sorrentino MR, Picca G, Paradiso M, Maiellaro P, De Cosmo S: Cardio-ankle vascular index is associated with diabetic retinopathy in younger than 70 years patients with type 2 diabetes mellitus. Diabetes Res Clin Pract, 2019; 155: 107793. [DOI] [PubMed] [Google Scholar]
- 56). Kim ES, Moon SD, Kim HS, Lim DJ, Cho JH, Kwon HS, Ahn CW, Yoon KH, Kang MI, Cha BY, Son HY: Diabetic peripheral neuropathy is associated with increased arterial stiffness without changes in carotid intima-media thickness in type 2 diabetes. Diabetes Care, 2011; 34: 1403-1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57). Tsuboi A, Ito C, Fujikawa R, Yamamoto H, Kihara Y: Association between the postprandial glucose levels and arterial stiffness measured according to the cardio-ankle vascular index in non-diabetic subjects. Intern Med, 2015; 54: 1961-1969 [DOI] [PubMed] [Google Scholar]
- 58). Ohira M, Endo K, Oyama T, Yamaguchi T, Ban N, Kawana H, Nagayama D, Nagumo A, Ohira M, Oyama T, Murano T, Miyashita Y, Yamamura S, Suzuki Y, Shirai K, Tatsuno I: Improvement of postprandial hyperglycemia and arterial stiffness upon switching from premixed human insulin 30/70 to biphasic insulin aspart 30/70. Metabolism, 2011; 60: 78-85 [DOI] [PubMed] [Google Scholar]
- 59). Akahori H: Clinical evaluation of thrice-daily lispro 50/50 versus twice-daily aspart 70/30 on blood glucose fluctuation and postprandial hyperglycemia in patients with type 2 diabetes mellitus. Diabetology International, 2015; 6: 275-283 [Google Scholar]
- 60). Ibata J, Sasaki H, Hanabusa T, Wakasaki H, Furuta H, Nishi M, Akamizu T, Nanjo K: Increased arterial stiffness is closely associated with hyperglycemia and improved by glycemic control in diabetic patients. J Diabetes Investig, 2013; 29; 4: 82-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61). Uzui H, Nakano A, Mitsuke Y, Geshi T, Sakata J, Sarazawa K, Morishita T, Satou T, Ishida K, Lee JD: Acarbose treatments improve arterial stiffness in patients with type 2 diabetes mellitus. J Diabetes Investig, 2011; 2: 148-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62). Ohira M, Yamaguchi T, Saiki A, Ban N, Kawana H, Nagumo A, Murano T, Shirai K, Tatsuno I: Pioglitazone improves the cardio-ankle vascular index in patients with type 2 diabetes mellitus treated with metformin. Diabetes Metab Syndr Obes, 2014; 7: 313-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63). Shigiyama F, Kumashiro N, Miyagi M, Iga R, Kobayashi Y, Kanda E, Uchino H, Hirose T: Linagliptin improves endothelial function in patients with type 2 diabetes: A randomized study of linagliptin effectiveness on endothelial function. J Diabetes Investig, 2017; 8: 330-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Nagayama D, Saiki A, Endo K, Yamaguchi T, Ban N, Kawana H, Ohira M, Oyama T, Miyashita Y, Shirai K: Improvement of cardio-vascular vascular index by glimepiride in type 2 diabetic patients. Int J Clin Pract, 2010; 64: 1796-1801 [DOI] [PubMed] [Google Scholar]
- 65). Bekki M, Tahara N, Tahara A, Igata S, Honda A, Sugiyama Y, Nakamura T, Sun J, Kumashiro Y, Matsui T, Fukumoto Y, Yamagish SI: Switching dipeptidyl peptidase-4 inhibitors to tofogliflozin, a selective inhibitor of sodium-glucose cotransporter 2 improves arterial stiffness evaluated by cardio-ankle vascular index in patients with type 2 diabetes: a pilot study. Current Vascular Pharmacology, 2018; 16, 1-10 [DOI] [PubMed] [Google Scholar]
- 66). Dobsak P, Soska V, Sochor O, Jarkovsky J, Novakova M, Homolka M, Soucek M, Palanova P, Lopez-Jimenez F, Shirai K: Increased cardio-ankle vascular index in hyperlipidemic patients without diabetes or hypertension. J Atheroscler Thromb, 2015; 22: 272-283 [DOI] [PubMed] [Google Scholar]
- 67). Nagayama D, Watanabe Y, Saiki A, Shirai K, Tatsuno I: Lipid parameters are independently associated with cardio-ankle vascular index (CAVI) in healthy Japanese subjects. J Atheroscler Thromb, 2018; 25: 621-633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68). Soska V, Dobsak P, Dusek L, Shirai K, Jarkovsky J, Novakova M, Brhel P, Stastna J, Fajkusova L, Freiberger T, Yambe T: Cardio-ankle vascular index in heterozygous familial hypercholesterolemia. J Atheroscler Thromb, 2012; 19: 453-461 [DOI] [PubMed] [Google Scholar]
- 69). Suzuki M, Takahashi M, Iizuka T, Terada H, Noike H, Shirai K: Frequency of coronary artery stenosis in patients with asymptomatic familial hypercholesterolemia and its association with carotid intimal thickness and cardio-ankle vascular index. Research Reports in Clinical Cardiology, 2016; 7; 83-90 [Google Scholar]
- 70). Miyashita Y, Endo K, Saiki A, Ban N, Yamaguchi T, Kawana H, Nagayama D, Ohira M, Oyama T, Shirai K: Effects of pitavastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, on cardio-ankle vascular index in type 2 diabetic patients. J Atheroscler Thromb, 2009; 16: 539-545 [DOI] [PubMed] [Google Scholar]
- 71). Satoh N, Shimatsu A, Kotani K, Himeno A, Majima T, Yamada K, Suganami T, Ogawa Y: Highly purified eicosapentaenoic acid reduces cardio-ankle vascular index in association with decreased serum amyloid A-LDL in metabolic syndrome. Hypertens Res, 2009; 32: 1004-1008 [DOI] [PubMed] [Google Scholar]
- 72). Yamaguchi T, Shirai K, Nagayama D, Nakamura S, Oka R, Tanaka S, Watanabe Y, Imamura H, Sato Y, Kawana H, Ohira M, Saiki A, Shimizu N, Tatsuno I: Bezafibrate ameliorates arterial stiffness assessed by cardio-ankle vascular index in hypertriglyceridemic patients with type 2 diabetes mellitus. J Atheroscler Thromb, 2019; 26: 659-669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73). Kang DH, Ha SK: Uric acid puzzle: dual role as antioxidant and pro-oxidant. Electrolyte Blood Press, 2014; 12: 1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74). Niskanen LK, Laaksonen DE, Nyyssonen K, Alfthan G, Lakka HM, Lakka TA, Salonen JT: Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med, 2004; 164: 1546-1551 [DOI] [PubMed] [Google Scholar]
- 75). Liu H, Liu J, Zhao H, Zhou Y, Li L, Wang H: Relationship between serum uric acid and vascular function and structure markers and gender difference in a real-world population of China- From Beijing Vascular Disease Patients Evaluation Study (BEST) Study. J Atheroscler Thromb, 2018; 25: 254-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76). Nagayama D, Yamaguchi T, Saiki A, Imamura H, Sato Y, Ban N, Kawana H, Nagumo A, Shirai K, Tatsuno I: High serum uric acid is associated with increased cardioankle vascular index (CAVI) in healthy Japanese subjects: a cross-sectional study. Atherosclerosis, 2015; 239: 163-168 [DOI] [PubMed] [Google Scholar]
- 77). Zheng X, Wei Q, Long J, Gong L, Chen H, Luo R, Ren W, Wang Y: Gender-specific association of serum uric acid levels and cardio-ankle vascular index in Chinese adults. Lipids Health Dis, 2018; 17: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78). Kumagai T, Kasai T, Kato M, Naito R, Maeno K, Kasagi S, Kawana F, Ishiwata S, Narui K: Establishment of the cardio-ankle vascular index in patients with obstructive sleep apnea. Chest, 2009; 36: 779-786 [DOI] [PubMed] [Google Scholar]
- 79). Alberto EC, Tanigawa T, Maruyama K, Kawasaki Y, Eguchi E, Mori H, Yoshimura K, Tanno S, Sakurai S, Hitsumoto S, Saito I: Relationships between nocturnal intermittent hypoxia, arterial stiffness and cardiovascular risk factors in a community-based population: The Toon Health Study. J Atheroscler Thromb, 2014; 21: 1290-1297 [DOI] [PubMed] [Google Scholar]
- 80). Kasai T, Inoue K, Kumagai T, Kato M, Kawana F, Sagara M, Ishiwata S, Ohno M, Yamaguchi T, Momomura S, Narui K: Plasma pentraxin3 and arterial stiffness in men with obstructive sleep apnea. Am J Hypertens, 2011; 24: 401-407 [DOI] [PubMed] [Google Scholar]
- 81). Noike H, Nakamura K, Sugiyama Y, Iizuka T, Shimizu K, Takahashi M, Hirano K, Suzuki M, Mikamo H, Nakagami T, Shirai K: Changes in cardio-ankle vascular index in smoking cessation. J Atheroscler Thromb, 2010; 17: 517-525 [DOI] [PubMed] [Google Scholar]
- 82). Kubozono T, Miyata M, Uegama K, Hamasaki S, Kusano K, Kubosono O, Tei C: Acute and chronic effects of smoking on arterial stiffness. Circ J, 2011; 95: 698-702 [DOI] [PubMed] [Google Scholar]
- 83). Nagayama D, Imamura H, Sato Y, Yamaguchi T, Ban N, Kawana H, Ohira M, Saiki A, Shirai K, Tatsuno I: Inverse relationship of cardioankle vascular index with BMI in healthy Japanese subjects: a cross-sectional study. Vasc Health Risk Manag, 2016; 13: 1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84). Satoh N, Shimatsu A, Kato Y, Araki K, Koyama K, Okajima T, Tanabe M, Ooishi M, Kotani K, Ogawa Y: Evaluation of the cardio-ankle vascular index, a new indicator of arterial stiffness independent of blood pressure, in obese and metabolic syndrome. Hypertens Res, 2008; 31: 1921-1930 [DOI] [PubMed] [Google Scholar]
- 85). Tonhajzerova I, Mestanikova A, Jurko A, Jr, Grendar M, Langer P, Ondrejka I, Jurko T, Hrtanek I, Cesnekova D, Mestanik M: Arterial stiffness and haemodynamic regulation in adolescent anorexia nervosa vs. obesity. Appl Physiol Nutr Metab, 2020; 45: 81-90 [DOI] [PubMed] [Google Scholar]
- 86). Yue M, Liu H, He M, Wu F, Li X, Pang Y, Yang X, Zhou G, Ma J, Liu M, Gong P, Li J, Zhang X: Gender-specific association of metabolic syndrome and its components with arterial stiffness in the general Chinese population. PLoS One, 2017; 12: e0186863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87). Kawada T, Andou T, Fukumitsu M: Relationship between cardio-ankle vascular index and components of metabolic syndrome in combination with sex and age. Diabetes Metab Syndr, 2014; 8: 242-244 [DOI] [PubMed] [Google Scholar]
- 88). Nagayama D, Endo K, Ohira M, Yamaguchi T, Ban N, Kawana H, Nagumo A, Saiki A, Oyama T, Miyashita Y, Shirai K: Effects of body weight reduction on cardioankle vascular index (CAVI). Obes Res Clin Pract, 2013; 7: e139-e145 [DOI] [PubMed] [Google Scholar]
- 89). Kirkham FA, Bunting E, Fantin F, Zamboni M, Rajkumar C: Independent association between cardio-ankle vascular index and sarcopenia in older U.K. adults. J Am Geriatr Soc, 2019; 67: 317-322 [DOI] [PubMed] [Google Scholar]
- 90). Im IJ, Choi HJ, Jeong SM, Kim HJ, Son JS, Oh HJ: The association between muscle mass deficits and arterial stiffness in middle-aged men. Nutr Metab Cardiovasc Dis, 2017; 27: 1130-1135 [DOI] [PubMed] [Google Scholar]
- 91). Xue Q, Qin MZ, Jia J, Liu JP, Wang Y: Association between frailty and the cardio-ankle vascular index. Clin Interv Aging, 2019; 14: 735-742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92). Yukutake T, Yamada M, Fukutani N, Nishiguchi S, Kayama H, Tanigawa T, Adachi D, Hotta T, Morino S, Tashiro Y, Arai H, Aoyama T: Arterial stiffness determined according to the cardio-ankle vascular index (CAVI) is associated with mild cognitive decline in community-dwelling elderly subjects. J Atheroscler Thromb, 2014; 21: 49-55 [DOI] [PubMed] [Google Scholar]
- 93). Yamamoto N, Yamanaka G, Ishikawa M, Takasugi E, Murakami S, Yamanaka T, Ishine M, Matsubayashi K, Hanafusa T, Otsuka K: Cardio-ankle vascular index as a predictor of cognitive impairment in community-dwelling elderly people: four-year follow-up. Dement Geriatr Cogn Disord, 2009; 28: 153-158 [DOI] [PubMed] [Google Scholar]
- 94). Sugiura T, Dohi Y, Takagi Y, Yoshikane N, Ito M, Suzuki K, Nagami T, Iwase M, Seo Y, Ohte N: Impacts of lifestyle behavior and shift work on visceral fat accumulation and the presence of atherosclerosis in middle-aged male workers. Hypertens Res, 2020; 43: 235-245 [DOI] [PubMed] [Google Scholar]
- 95). Hata K, Nakagawa T, Hasegawa M, Kitamura H, Hayashi T, Ogami A: Relationship between overtime work hours and cardio-ankle vascular index (CAVI): A cross-sectional study in Japan. J Occup Health, 2014; 56: 271-278 [DOI] [PubMed] [Google Scholar]
- 96). Morita N, Kambayashi I, Okuda T, Oda S, Takada S, Nakajima T, Shide N, Shinkaiya H, Okita K: Inverse relationship between sleep duration and cardio-ankle vascular index in children. J Atheroscler Thromb, 2017; 24: 819-826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97). Shimizu K, Takahashi M, Shirai K: A huge earthquake hardened arterial stiffness monitored with cardio-ankle vascular index. J Atheroscler Thromb, 2013; 20: 503-511 [DOI] [PubMed] [Google Scholar]
- 98). Nagayama D, Imamura H, Endo K, Saiki A, Sato Y, Yamaguchi T, Watanabe Y, Ohira M, Shirai K, Tatsuno I: Marker of sepsis severity is associated with the variation in cardio-ankle vascular index (CAVI) during sepsis treatment. Vasc Health Risk Manag, 2019; 15: 509-516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99). Spinelli FR, Pecani A, Ciciarello F, Colasanti T, Di Franco M, Miranda F, Conti F, Valesini G, Alessandri C: Association between antibodies to carbamylated proteins and subclinical atherosclerosis in rheumatoid arthritis patients. BMC Musculoskelet Disord, 2017; 18: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100). Carlucci PM, Purmalek MM, Dey AK, Temesgen-Oyelakin Y, Sakhardande S, Joshi AA, Lerman JB, Fike A, Davis M, Chung JH, Playford MP, Naqi M, Mistry P, Gutierrez-Cruz G, Dell'Orso S, Naz F, Salahuddin T, Natarajan B, Manna Z, Tsai WL, Gupta S, Grayson P, Teague H, Chen MY, Sun HW, Hasni S, Mehta NN, Kaplan MJ: Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight, 2018; 3: e99276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101). Matsushita K, Ding N, Kim ED, Budoff M, Chirinos JA, Fernhall B, Hamburg NM, Kario K, Miyoshi T, Tanaka H, Townsend R: Cardio-ankle vascular index and cardiovascular disease: Systematic review and metaanalysis of prospective and cross-sectional studies. J Clin Hypertens, 2019; 21: 16-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102). Tanaka A, Tomiyama H, Maruhashi T, Matsuzawa Y, Miyoshi T, Kabutoya T, Kario K, Sugiyama S, Munakata M, Ito H, Ueda S, Vlachopoulos C, Higashi Y, Inoue T, Node K, Physiological Diagnosis Criteria for Vascular Failure Committee : Physiological diagnostic criteria for vascular failure. Hypertension, 2018; 72(5): 1060-1071 [DOI] [PubMed] [Google Scholar]
- 103). Miyoshi T, Ito H, Horinaka S, Shirai K, Higaki J, Orimio H: Protocol for evaluating the cardio-ankle vascular index to predict cardiovascular events in Japan: a prospective multicenter cohort study. Pulse, 2017; 4(Suppl 1): 11-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104). Kabutoya T, Kario K: Comparative assessment of cutoffs for the cardio-ankle vascular index and brachial-ankle pulse wave velocity in a nationwide registry — a cardiovascular prognostic coupling study -. Pulse, 2018; 6: 131-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105). Schillaci G, Battista F, Settimi L, Anastasio F, Pucci G: Cardio-ankle vascular index and subclinical heart disease. Hypertens Res, 2015; 38: 68-73 [DOI] [PubMed] [Google Scholar]
- 106). Zhang C, Ohira M, Iizuka T, Mikamo H, Nakagami T, Suzuki M, Hirano K, Takahashi M, Shimizu K, Sugiyama Y, Yamaguchi T, Kawana H, Endo K, Saiki A, Oyama T, Kurosu T, Tomaru T, Wang H, Noike H, Shirai K: Cardio-ankle vascular index relates to left ventricular ejection fraction in patients with heart failure. A retrospective study. Int Heart J, 2013; 54: 216-221 [DOI] [PubMed] [Google Scholar]
- 107). Sakane K, Miyoshi T, Doi M, Hirohata S, Kaji Y, Kamikawa S, Ogawa H, Hatanaka K, Kitawaki T, Kusachi S, Yamamoto K: Association of new arterial stiffness parameter, the cardio-ankle vascular index, with left ventricular diastolic function. J Atheroscler Thromb, 2008; 15: 261-268 [DOI] [PubMed] [Google Scholar]
- 108). Namba T, Masaki N, Matsuo Y, Sato A, Kimura T, Horii S, Yasuda R, Yada H, Kawamura A, Takase B, Adachi T: Arterial stiffness is significantly associated with left ventricular diastolic dysfunction in patients with cardiovascular disease. Int Heart J, 2016; 57: 729-735 [DOI] [PubMed] [Google Scholar]
- 109). Ogawa A, Shimizu K, Nakagami T, Maruoka H, Shirai K: Physical function and cardio-ankle vascular index in elderly heart failure patients. International Heart Journal, 2020, in press [DOI] [PubMed] [Google Scholar]
- 110). Kawasaki T, Sasayama S, Yagi S, Asakawa T, Hirai T: Noninvasive assessment of the age related changes in stiffness of major branches of the human arteries. Cardiovasc Res, 1987; 21: 678-687 [DOI] [PubMed] [Google Scholar]
- 111). Shirai K, Shimizu K, Takata M, Suzuki K: Independency of the cardio-ankle vascular index from blood pressure at the time of measurement. J Hypertens, 2017; 35: 1521-1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112). Shirai K, Suzuki K, Tsuda S, Shimizu K, Takata M, Yamamoto T, Maruyama M, Takahashi K: Comparison of cardio-ankle vascular index (CAVI) and CAVI0 in large healthy and hypertensive populations. J Atheroscler Thromb, 2019; 26: 603-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113). Luo ZX, Zeng Q, Luo R, Wang Y, Ge Q: Relative contributions of ectopic liver and abdominal fat accumulation to arterial stiffness. Endocr Pract, 2015; 21: 574-580 [DOI] [PubMed] [Google Scholar]
- 114). Masaki M, Komamura K, Goda A, Hirotani S, Otsuka M, Nakabo A, Fukui M, Fujiwara S, Sugahara M, Lee-Kawabata M, Tsujino T, Koshiba M, Masuyama T: Elevated arterial stiffness and diastolic dysfunction in subclinical hypothyroidism. Circ J, 2014; 78: 1494-1500 [DOI] [PubMed] [Google Scholar]
- 115). Hitsumoto T: Clinical impact of blood testosterone concentration on cardio-ankle vascular index in female patients with type 2 diabetes mellitus. Cardiol Res, 2019; 10: 9-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116). Choi BG, Kim MJ, Yang HS, Lee YW, Choe YB, Ahn KJ: Assessment of arterial stiffness in Korean patients with psoriasis by cardio-ankle vascular index. Angiology, 2017; 68: 608-613 [DOI] [PubMed] [Google Scholar]
- 117). Asai K, Yamori M, Yamazaki T, Yamaguchi A, Takahashi K, Sekine A, Kosugi S, Matsuda F, Nakayama T, Bessho K, Nagahama Study Group : Tooth loss and atherosclerosis: the Nagahama Study. J Dent Res, 2015; 94(3 Suppl): 52S-58S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118). Takenaka T, Hoshi H, Kato N, Kobayashi K, Takane H, Shoda J, Suzuki H: Cardio-ankle vascular index to screen cardiovascular diseases in patients with end-stage renal diseases. J Atheroscler Thromb, 2008; 15: 339-344 [DOI] [PubMed] [Google Scholar]
- 119). Hitsumoto T: Relationships between the cardio-ankle vascular index and pulsatility index of the common carotid artery in patients with cardiovascular risk factors. J Clin Med Res, 2019; 11: 593-599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120). Shimizu K, Yamamoto T, Takahashi M, Sato S, Noike H, Shirai K: Effect of nitroglycerin administration on cardio-ankle vascular index. Vasc Health Risk Manag, 2016; 12: 313-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121). Yamamoto T, Shimizu K, Takahashi M, Tatsuno I, Shirai K: The effect of nitroglycerin on arterial stiffness of the aorta and the femoral-tibial arteries. J Atheroscler Thromb, 2017; 24: 1048-1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122). Nagayama D, Ohira M, Saiki A, Shirai K, Tatsuno I: Sarpogrelate hydrochloride decreases cardio-ankle vascular index accompanied by increased serum lipoprotein lipase mass in type 2 diabetic patients. Int Heart J, 2014; 55: 337-341 [DOI] [PubMed] [Google Scholar]
- 123). Shiba T, Takahashi M, Yoshida I, Taniguchi H, Matsumoto T, Hori Y: Arteriosclerotic changes after intravitreal injections of anti-vascular endothelial growth factor drugs in patients with exudative age-related macular degeneration. Ophthalmologica, 2016; 235: 225-232 [DOI] [PubMed] [Google Scholar]
- 124). Shimizu N, Ban N, Watanabe Y, Rikitake A, Watanabe R, Tanaka S, Sato Y, Imamura H, Kawana H, Yamaguchi T, Saiki A, Tatsuno I, Shirai K: Elevation of cardio-ankle vascular index in a patient with malignant lymphoma treated with a combination therapy of rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisolone. J Clin Med Res, 2017; 9: 729-732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125). Alonso-Domínguez R, Recio-Rodríguez JI, Patino-Alonso MC, Sánchez-Aguadero N, García-Ortiz L, Gómez-Marcos MA: Acute effect of healthy walking on arterial stiffness in patients with type 2 diabetes and differences by age and sex: a pre-post intervention study. BMC Cardiovasc Disord, 2019; 19: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126). Streese L, Königstein K, Goricki L, Infanger D, Wölnerhanssen B, Peters T, Schmidt-Trucksäss A, Hanssen H: Short- and long-term effects of bariatric surgery on vascular phenotype. Obes Surg, 2019; 29: 1301-1308 [DOI] [PubMed] [Google Scholar]
- 127). Nishiwaki M, Kora N, Matsumoto N: Ingesting a small amount of beer reduces arterial stiffness in healthy humans. Physiol Rep, 2017; 5: e13381. [DOI] [PMC free article] [PubMed] [Google Scholar]


