Abstract
Cryopreservation and thawing of PBMCs are inevitable processes in expanding the scale of experiments in human immunology. Here, we carried out a fundamental study to investigate the detailed effects of PBMC cryopreservation and thawing on transcriptomes. We sorted Tregs from fresh and cryopreserved/thawed PBMCs from an identical donor and performed single-cell RNA-sequencing (scRNA-seq). We found that the cryopreservation and thawing process minimally affects the key molecular features of Tregs, including FOXP3. However, the cryopreserved and thawed sample had a specific cluster with up-regulation of genes for heat shock proteins. Caution may be warranted in interpreting the character of any cluster of cells with heat shock-related properties when cryopreserved and thawed samples are used for scRNA-seq.
Keywords: Cryopreservation; Single-cell analysis; Transcriptome; T-lymphocytes, regulatory
INTRODUCTION
No matter how sophisticated the experimental techniques of modern immunology, a batch effect originates from non-biological factors (1,2). The batch effect may corrupt the data produced by experiments, and it is difficult to correct later. A large-scale and simultaneous experiment under identical conditions is an effective approach to remove batch effects. In translational research using specimens originating from a defined cohort with human subjects, the cryopreservation and thawing process is inevitable in conducting a large-scale and simultaneous experiment (3,4). When PBMCs are tested in protein-based ex vivo techniques, such as enzyme-linked immune absorbent spot and flow cytometry, the cryopreservation and thawing protocols provide stable and reproducible immunophenotype data (5,6). However, significant gene expression changes are occasionally detected after the cryopreservation and thawing process using comprehensive methods, such as transcriptome analysis (7).
Single-cell RNA-sequencing (scRNA-seq) is a powerful experimental technique for characterizing heterogeneous immune cell types at the molecular level (8). In human immunology studies, scRNA-seq is widely applied for various specimens, including normal tissues, tumor tissues, bone marrow, and PBMCs (9,10,11,12). However, current single-cell sequencing using a water-in-oil microdroplet platform also has a significant batch effect among different sets of experiments, and it has been attempted to correct the batch effect by a bioinformatics approach (13). To minimize the batch effect, cryopreservation and thawing of specimens for scRNA-seq analysis has been introduced (14). Previous studies comparing fresh cells and cryopreserved/thawed cells showed that DMSO based cryopreservation is a reliable method without significant transcriptomic changes (15).
During immune landscape analysis of Tregs, we had a chance to compare the scRNA-seq data of Tregs sorted from fresh and cryopreserved/thawed PBMCs from an identical human subject. Our results suggest that unique transcriptomic features of Tregs are conserved after a cryopreservation and thawing process, but a specific cluster with heat shock protein gene expression was found only in the cryopreserved/thawed sample.
MATERIALS AND METHODS
Study subject
Peripheral blood was obtained from a single healthy donor twice at a 1-week interval for preparation of cryopreserved/thawed and fresh PBMCs. PBMCs were isolated from whole blood via standard Ficoll-Paque (GE Healthcare, Uppsala, Sweden) density gradient centrifugation. PBMCs were cryopreserved and thawed using a standard protocol (15). The freezing media comprised 70% FBS, 20% Roswell Park Memorial Institute (RPMI) 1640 media, and 10% DMSO. This study protocol was reviewed and approved by the Institutional Review Boards (KH2018-118). Written informed consent was obtained from the subject prior to the study.
Isolation of Tregs from PBMCs
Untouched CD4+CD25+CD127- Tregs were isolated from PBMCs using the EasySep Human CD4+CD127lowCD25+ Treg Isolation Kit (STEMCELL Technologies, Vancouver, Canada) according to the manufacturer's instructions.
scRNA-seq
We generated scRNA-seq libraries using the Chromium Single Cell 3′ Library & Gel Bead Kit v2 (10X Genomics, Pleasanton, CA, USA) according to the manufacturer's instructions. Briefly, thousands of cells were separated into nanoliter-scale droplets. In each droplet, cDNA was produced by reverse transcription, adding a cell barcoding sequence and unique molecular identifier (UMI) to each cDNA molecule. Libraries were constructed and sequenced at a depth of approximately 50,000 reads per cell by the Novaseq 6000 platform (Illumina, San Diego, CA, USA).
scRNA-seq data processing
The sequenced data were de-multiplexed using mkfastq (Cellranger, v3.0.2; 10X Genomics) to generate fastq files. After de-multiplexing, the reads were aligned to the human reference genome (GRCh38; 10X Cellranger reference GRCh38 v3.0.0) and feature-barcode matrices generated using the cellranger count, and then aggregated by cellranger aggr using default parameters. Subsequent analysis was performed using Seurat R package v3.1.5 (R Foundation, Vienna, Austria). Mitochondrial read counts were collected using PercentageFeatureSet function with the following option: pattern = ‘^MT-’. In each cell, the gene expression was normalized based on the total read count and log-transformed. After log-transformation, principal component analysis was performed and PC1 to PC6 applied following clustering and uniform manifold approximation and projection (UMAP).
Identification of differentially expressed genes (DEGs)
To identify DEGs for any 2 transcriptome profiles, we utilized the Wilcoxon rank sum test and receiver operating characteristics method in Seurat's implementation based on a Bonferroni-adjusted p<0.05 and log2 fold change >0.25.
Gene Ontology analysis for biological pathways (GO-BP)
The Gene Ontology analysis was performed using Enrichr (https://amp.pharm.mssm.edu/Enrichr/).
RESULTS AND DISCUSSION
Single-cell transcriptomes of fresh and cryopreserved/thawed cells
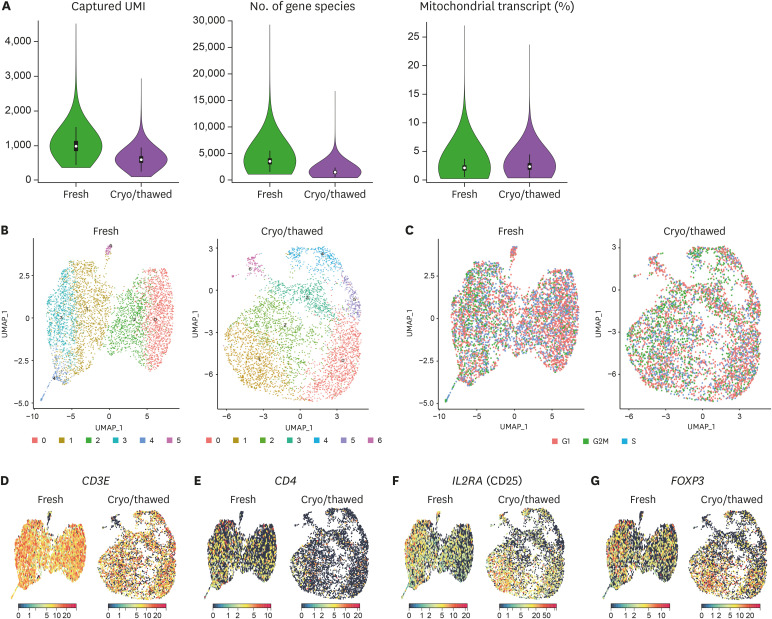
Applying a 10X Genomics scRNA-seq platform, we analyzed 4,990 cells from the fresh sample and 4,495 cells from the cryopreserved/thawed sample. The fresh sample had 18,449 median genes per cell, and the cryopreserved/thawed sample had 17,227 median genes per cell. Captured UMI, which represents the number of genes per cell, was relatively higher in the fresh sample (median 986; 1st–3rd quartile, 518–691) than the cryopreserved/thawed sample (median 600; 1st–3rd quartile, 858–1,131; Fig. 1A). The number of gene species was also relatively higher in the fresh sample (median, 3,503; 1st–3rd quartile, 3,011–4,019) than the cryopreserved/thawed sample (median 1,435; 1st–3rd quartile, 1,229–1,674; Fig. 1A). The proportion of mitochondrial transcripts, which represents contamination by dead or dying cells, was similar in the fresh sample (median 2.1; 1st–3rd quartile, 1.7–2.5) and the cryopreserved/thawed sample (median 2.3; 1st–3rd quartile, 1.8–2.4; Fig. 1A).
Figure 1. Single-cell transcriptome of Tregs from fresh and cryopreserved/thawed PBMCs. (A) Assessment of the quality of scRNA-seq results. Captured UMI (left), number of gene species (middle), and proportion of mitochondrial transcript (right) in the fresh sample and cryopreserved/thawed sample. Data are given as the median value and 1st–3rd quartile range for each parameter. (B) UMAPs of 4,990 cells for the fresh sample and 4,495 cells for the cryopreserved/thawed sample, colored by cluster. (C) Normalized expression of cell cycle genes on UMAP plots, colored by stage of the cell cycle. (D-G) Normalized expression of representative marker genes from Tregs on UMAP plots. CD3E (CD3) (D), CD4 (E), IL2RA (CD25) (F), and FOXP3 (FoxP3) (G).
Next, we subjected all cells in each group to UMAP using the highly variable genes in each group and the Seurat package (16). We found six different clusters for the fresh sample (clusters 0–5) and seven different clusters for the cryopreserved/thawed sample (clusters 0–6; Fig. 1B). Cell cycle-associated gene expression was uniformly distributed among clusters (Fig. 1C). Moreover, the expression levels of key molecules defining Tregs, including CD3E (CD3), CD4, IL2RA (CD25), and FOXP3 were homogenous and not polarized to specific clusters in both the fresh and cryopreserved/thawed samples (Fig. 1D-G). This result indicates that the molecular identity of Tregs is still conserved across the different clusters after a cryopreservation and thawing process at the single-cell transcriptome level, even though the amount and variability of gene expression are reduced after cryopreservation and thawing.
Cluster-specific up-regulated genes in fresh and cryopreserved/thawed cells
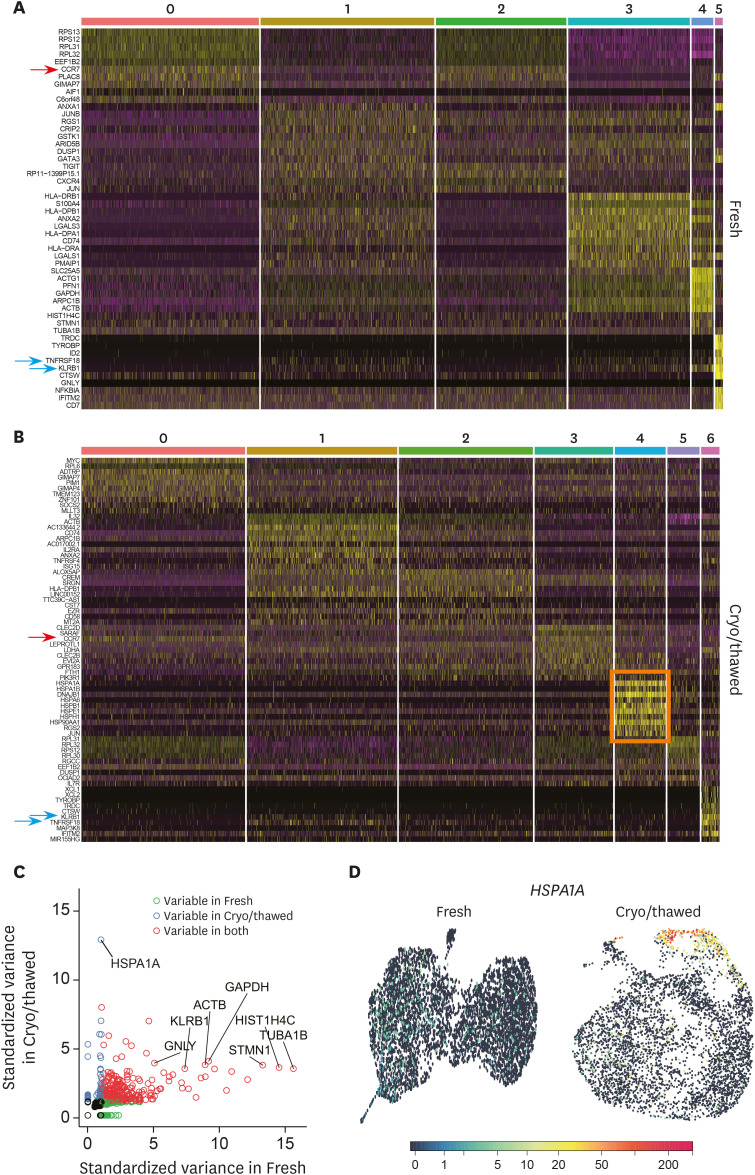
To characterize each cluster identified in the fresh and cryopreserved/thawed samples, we investigated DEGs among different clusters in the fresh and cryopreserved/thawed samples. Representative up-regulated genes from each cluster were noted on a heatmap for the fresh sample (Fig. 2A) and the cryopreserved/thawed sample (Fig. 2B). A detailed list of genes up-regulated in each cluster is given in Supplementary Tables 1 and 2.
Figure 2. Cluster-specific up-regulated genes in fresh and cryopreserved/thawed cells. (A) Heatmaps presenting up-regulated genes from each cluster for the fresh sample and (B) the cryopreserved/thawed sample. The arrows indicate the commonly up-regulated genes in the clusters from both fresh and cryopreserved/thawed samples. The pink box indicates cluster 4 in the cryopreserved/thawed sample, with multiple up-regulated heat shock protein genes. (C) Plots of the variable genes from the fresh or cryopreserved/thawed samples, or both. (D) UMAP plot of the representative expression pattern for HSPA1A.
Immunologically well-known genes were found among representative up-regulated genes in each Treg cluster. For example, CCR7, a marker of naïve or central memory T cells, was significantly up-regulated in clusters 0 and 2 in the fresh sample and in clusters 0 and 3 in the cryopreserved/thawed sample (Fig. 2A and B, red arrows). KLRB1 and TNFRSF18 were commonly up-regulated in cluster 5 in the fresh sample and cluster 6 in the cryopreserved/thawed sample (Fig. 2A and B, blue arrows), which are the genes for surface protein CD161 and glucocorticoid-induced TNF receptor, respectively. Intriguingly, the genes associated with heat shock proteins, such as HSPA1A, HSPA1B, HSPA6, HSPB1, HSPE1, HSPH1, and HSP90AA1, were exclusively up-regulated in cluster 4 in the cryopreserved/thawed sample (Fig. 2B, pink box). When we plotted the variable genes in the fresh and cryopreserved/thawed samples, genes for heat shock proteins, including HSPA1A, were specifically variable in the cryopreserved/thawed samples (Fig. 2C). This cryopreservation/thawing-specific gene expression pattern was exemplified at single-cell resolution by HSPA1A and confirmed that cluster 4 was specifically found in Tregs from the cryopreserved/thawed sample (Fig. 2D).
Identification of unique transcriptional signatures in cryopreserved/thawed cells
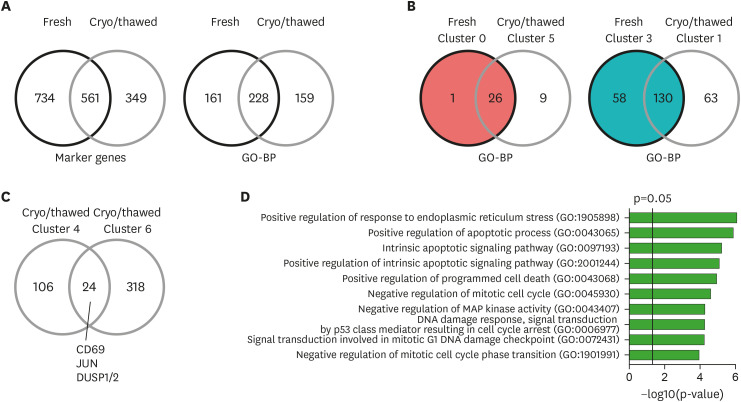
To further evaluate the gene expression changes associated with the cryopreservation and thawing process, we investigated the variably expressed genes in fresh and cryopreserved/thawed samples. Among 910 genes up-regulated in any cluster from the cryopreserved/thawed sample, 561 (61.6%) genes overlapped with the genes up-regulated in the clusters in the fresh sample (Fig. 3A). When we analyzed the gene lists for each cluster in GO-BP, 228 of 391 (58.3%) pathways in the cryopreserved/thawed sample overlapped with the pathways up-regulated in the clusters n the fresh sample (Fig. 3A; Supplementary Tables 3 and 4).
Figure 3. Identification of unique transcriptional signatures in cryopreserved/thawed cells. (A) Venn diagram of marker genes up-regulated in any cluster (left), and of GO-BP enriched in any cluster (right) from the fresh and cryopreserved/thawed cells. (B) Venn diagrams of gene expression similarities between cluster 0 of the fresh sample and cluster 5 of the cryopreserved/thawed sample (left), and between cluster 3 of the fresh sample and cluster 1 of the cryopreserved/thawed sample (right) based on the enriched GO-BP. (C) Venn diagram of marker genes up-regulated in clusters 4 and 6 in the cryopreserved/thawed cells. (D) Bar plot showing enrichment p-values for the top 10 GO-BP enriched in commonly up-regulated genes (n=24) from both cluster 4 and 6 in the cryopreserved/thawed cells.
Next, we analyzed the similarity of each cluster between the fresh and cryopreserved/thawed samples based on GO-BP. Cluster 0 in the fresh sample had 27 enriched pathways and was most similar to cluster 5 in the cryopreserved/thawed sample, with 26 overlapping pathways (96.3%; Fig. 3B). Cluster 3 in the fresh sample had 188 enriched pathways and was most similar to cluster 1 in the cryopreserved/thawed sample, with 130 overlapping pathways (69.1%; Fig. 3B).
We evaluated the DEGs specifically up-regulated in cluster 4 in the cryopreserved/thawed sample, which demonstrated up-regulation of heat shock protein genes. T-cell activation-related genes, including CD69, JUN, DUSP1, and DUSP2, were commonly up-regulated in cluster 6 in the cryopreserved/thawed sample (Fig. 3C). Commonly up-regulated genes between clusters 4 and 6 (n=24) were analyzed by GO-BP, finding that pathways related to ER stress, apoptosis, cell cycle arrest, and DNA damage were significantly enriched (Fig. 3D; Supplementary Table 5). These data indicate that cluster 4 resembles cluster 6 in gene expression signatures, but acquires expression of heat shock protein genes in the cryopreservation and thawing process.
In conclusion, we show that cryopreservation and thawing minimally affect the key molecular features of Tregs although cell clustering results were not identical. However, the cells with up-regulated heat shock protein genes were clearly clustered in Tregs after cryopreservation and thawing, and this cluster may represent the signature of recent heat exposure applied during a cryopreservation and thawing process. Although cryopreserved and thawed samples can be used for scRNA-seq, caution might be warranted in interpreting the character of any cluster of cells with heat shock-related properties.
ACKNOWLEDGEMENTS
This work was funded by Samsung Science and Technology Foundation under Project Number SSTF-BA1402-51 (to Shin EC).
Abbreviations
- DEG
differentially expressed gene
- GO-BP
Gene Ontology analysis for biological pathways
- scRNA-seq
single-cell RNA-sequencing
- UMAP
uniform manifold approximation and projection
- UMI
unique molecular identifier
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Lee JS, Yi K, Ju YS, Shin EC.
- Data curation: Lee JS, Yi K, Shin EC.
- Formal analysis: Lee JS, Yi K, Shin EC.
- Funding acquisition: Shin EC.
- Resources: Ju YS, Shin EC.
- Software: Yi K.
- Supervision: Ju YS, Shin EC.
- Validation: Yi K.
- Visualization: Lee JS.
- Writing - original draft: Lee JS, Yi K, Shin EC.
- Writing - review & editing: Lee JS, Yi K, Ju YS, Shin EC.
SUPPLEMENTARY MATERIALS
Marker genes for each cluster from the fresh sample
Marker genes for each cluster from the cryopreserved/thawed sample
DEGs and associated biological pathways for each cluster from the fresh sample
DEGs and associated biological pathways for each cluster from the cryopreserved/thawed sample
DEGs and associated biological pathways for commonly up-regulated genes (n=24) from both cluster 4 and 6 in the cryopreserved/thawed cells
References
- 1.Cossarizza A, Chang HD, Radbruch A, Akdis M, Andrä I, Annunziato F, Bacher P, Barnaba V, Battistini L, Bauer WM, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur J Immunol. 2017;47:1584–1797. doi: 10.1002/eji.201646632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitcomb BW, Perkins NJ, Albert PS, Schisterman EF. Treatment of batch in the detection, calibration, and quantification of immunoassays in large-scale epidemiologic studies. Epidemiology. 2010;21(Suppl 4):S44–S50. doi: 10.1097/EDE.0b013e3181dceac2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim CG, Jang M, Kim Y, Leem G, Kim KH, Lee H, Kim TS, Choi SJ, Kim HD, Han JW, et al. VEGF-A drives TOX-dependent T cell exhaustion in anti-PD-1-resistant microsatellite stable colorectal cancers. Sci Immunol. 2019;4:eaay0555. doi: 10.1126/sciimmunol.aay0555. [DOI] [PubMed] [Google Scholar]
- 4.Kim HD, Song GW, Park S, Jung MK, Kim MH, Kang HJ, Yoo C, Yi K, Kim KH, Eo S, et al. Association between expression level of PD1 by tumor-infiltrating CD8(+) T cells and features of hepatocellular carcinoma. Gastroenterology. 2018;155:1936–1950.e17. doi: 10.1053/j.gastro.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Barcelo H, Faul J, Crimmins E, Thyagarajan B. A practical cryopreservation and staining protocol for immunophenotyping in population studies. Curr Protoc Cytom. 2018;84:e35. doi: 10.1002/cpcy.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreher CR, Dittrich MT, Guerkov R, Boehm BO, Tary-Lehmann M. CD4+ and CD8+ cells in cryopreserved human PBMC maintain full functionality in cytokine ELISPOT assays. J Immunol Methods. 2003;278:79–93. doi: 10.1016/s0022-1759(03)00226-6. [DOI] [PubMed] [Google Scholar]
- 7.Wagh V, Meganathan K, Jagtap S, Gaspar JA, Winkler J, Spitkovsky D, Hescheler J, Sachinidis A. Effects of cryopreservation on the transcriptome of human embryonic stem cells after thawing and culturing. Stem Cell Rev Rep. 2011;7:506–517. doi: 10.1007/s12015-011-9230-1. [DOI] [PubMed] [Google Scholar]
- 8.Papalexi E, Satija R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat Rev Immunol. 2018;18:35–45. doi: 10.1038/nri.2017.76. [DOI] [PubMed] [Google Scholar]
- 9.Madissoon E, Wilbrey-Clark A, Miragaia RJ, Saeb-Parsy K, Mahbubani KT, Georgakopoulos N, Harding P, Polanski K, Huang N, Nowicki-Osuch K, et al. scRNA-seq assessment of the human lung, spleen, and esophagus tissue stability after cold preservation. Genome Biol. 2019;21:1. doi: 10.1186/s13059-019-1906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szabo PA, Levitin HM, Miron M, Snyder ME, Senda T, Yuan J, Cheng YL, Bush EC, Dogra P, Thapa P, et al. Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat Commun. 2019;10:4706. doi: 10.1038/s41467-019-12464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tikhonova AN, Dolgalev I, Hu H, Sivaraj KK, Hoxha E, Cuesta-Domínguez Á, Pinho S, Akhmetzyanova I, Gao J, Witkowski M, et al. The bone marrow microenvironment at single-cell resolution. Nature. 2019;569:222–228. doi: 10.1038/s41586-019-1104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran HT, Ang KS, Chevrier M, Zhang X, Lee NY, Goh M, Chen J. A benchmark of batch-effect correction methods for single-cell RNA sequencing data. Genome Biol. 2020;21:12. doi: 10.1186/s13059-019-1850-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guillaumet-Adkins A, Rodríguez-Esteban G, Mereu E, Mendez-Lago M, Jaitin DA, Villanueva A, Vidal A, Martinez-Marti A, Felip E, Vivancos A, et al. Single-cell transcriptome conservation in cryopreserved cells and tissues. Genome Biol. 2017;18:45. doi: 10.1186/s13059-017-1171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wohnhaas CT, Leparc GG, Fernandez-Albert F, Kind D, Gantner F, Viollet C, Hildebrandt T, Baum P. DMSO cryopreservation is the method of choice to preserve cells for droplet-based single-cell RNA sequencing. Sci Rep. 2019;9:10699. doi: 10.1038/s41598-019-46932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36:411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Marker genes for each cluster from the fresh sample
Marker genes for each cluster from the cryopreserved/thawed sample
DEGs and associated biological pathways for each cluster from the fresh sample
DEGs and associated biological pathways for each cluster from the cryopreserved/thawed sample
DEGs and associated biological pathways for commonly up-regulated genes (n=24) from both cluster 4 and 6 in the cryopreserved/thawed cells