Abstract
TMEM16 Ca2+-activated phospholipid scramblases (CaPLSases) mediate rapid transmembrane phospholipid flip-flop and as such play essential roles in various physiological and pathological processes such as blood coagulation, skeletal development, viral infection, cell-cell fusion, and ataxia. Pharmacological tools specifically targeting TMEM16 CaPLSases are urgently needed to understand these novel membrane transporters and their contributions to health and disease. Tannic acid (TA) and epigallocatechin gallate (EGCG) were recently reported as promising TMEM16F CaPLSase inhibitors. However, our present study shows that TA and EGCG do not inhibit the phospholipid-scrambling or ion conduction activities of the dual-functional TMEM16F. Instead, we found that TA and EGCG mainly acted as fluorescence quenchers that rapidly suppress the fluorophores conjugated to annexin V, a phosphatidylserine-binding probe commonly used to report on TMEM16 CaPLSase activity. These data demonstrate the false positive effects of TA and EGCG on inhibiting TMEM16F phospholipid scrambling and discourage the use of these polyphenols as CaPLSase inhibitors. Appropriate controls as well as a combination of both fluorescence imaging and electrophysiological validation are necessary in future endeavors to develop TMEM16 CaPLSase inhibitors.
Keywords: TMEM16F, ANO6, phospholipid scramblase, CaPLSase, ion channels, pharmacology, tannic acid, epigallocatechin gallate (EGCG), membrane transport, fluorescence, ion channel, membrane lipid, phospholipid, polyphenol, scramblase
The TMEM16 membrane protein family consists of Ca2+-activated chloride channels and CaPLSases (1, 2). Upon binding of intracellular Ca2+, CaPLSases rapidly flip-flop phospholipids across the lipid bilayer, resulting in a collapse of the asymmetric phospholipid distribution across the membrane. This consequently leads to extracellular exposure of phosphatidylserine (PS), a phospholipid that is normally restricted in the inner leaflet of the membrane (3–10). A number of TMEM16 CaPLSases have been linked to various human diseases. Mutations of TMEM16K endoplasmic reticulum CaPLSase have been identified in patients with spinocerebellar ataxia type 10 and autosomal recessive cerebellar ataxia type (11, 12). Additionally, mutations of TMEM16E, another CaPLSase, have been detected in patients with muscular dystrophy (9, 13–15), distal Miyoshi myopathy (13–15), and gnathodyaphyseal dysplasia (16). Loss-of-function mutations of TMEM16F, a dual-functional CaPLSase and nonselective ion channel, lead to Scott syndrome, a rare bleeding disorder characterized by deficient PS exposure in activated platelets, reduced thrombin generation, and prolonged bleeding time (8, 17). Interestingly, TMEM16F knockout mice exhibit an anticoagulant trait (18), suggesting that inhibiting TMEM16F CaPLSase might be a promising therapeutic option for preventing thrombosis-related diseases such as stroke, cardiac attack, and deep vein thrombosis. Despite their importance in physiology, potent and specific pharmacological reagents targeting TMEM16 CaPLSases have not been developed.
TA and EGCG are natural polyphenolic compounds (Fig. 1A) that are abundantly present in plant-based foods and drinks, such as fruits, vegetables, green tea, and red wine (19–21). Recently, these polyphenolic compounds were reported to potently inhibit TMEM16F CaPLSase based on the observations that TA and EGCG could efficiently prevent fluorescently tagged phospholipids and annexin V (AnV) probes from reporting TMEM16F-mediated lipid scrambling (22, 23). In agreement with numerous reported beneficial effects of TA and EGCG on human health (19–21, 24), the findings that these compounds could potently inhibit TMEM16F CaPLSase thus raised enthusiasm to apply these natural polyphenols as research and therapeutic tools to target TMEM16 CaPLSases.
Figure 1.
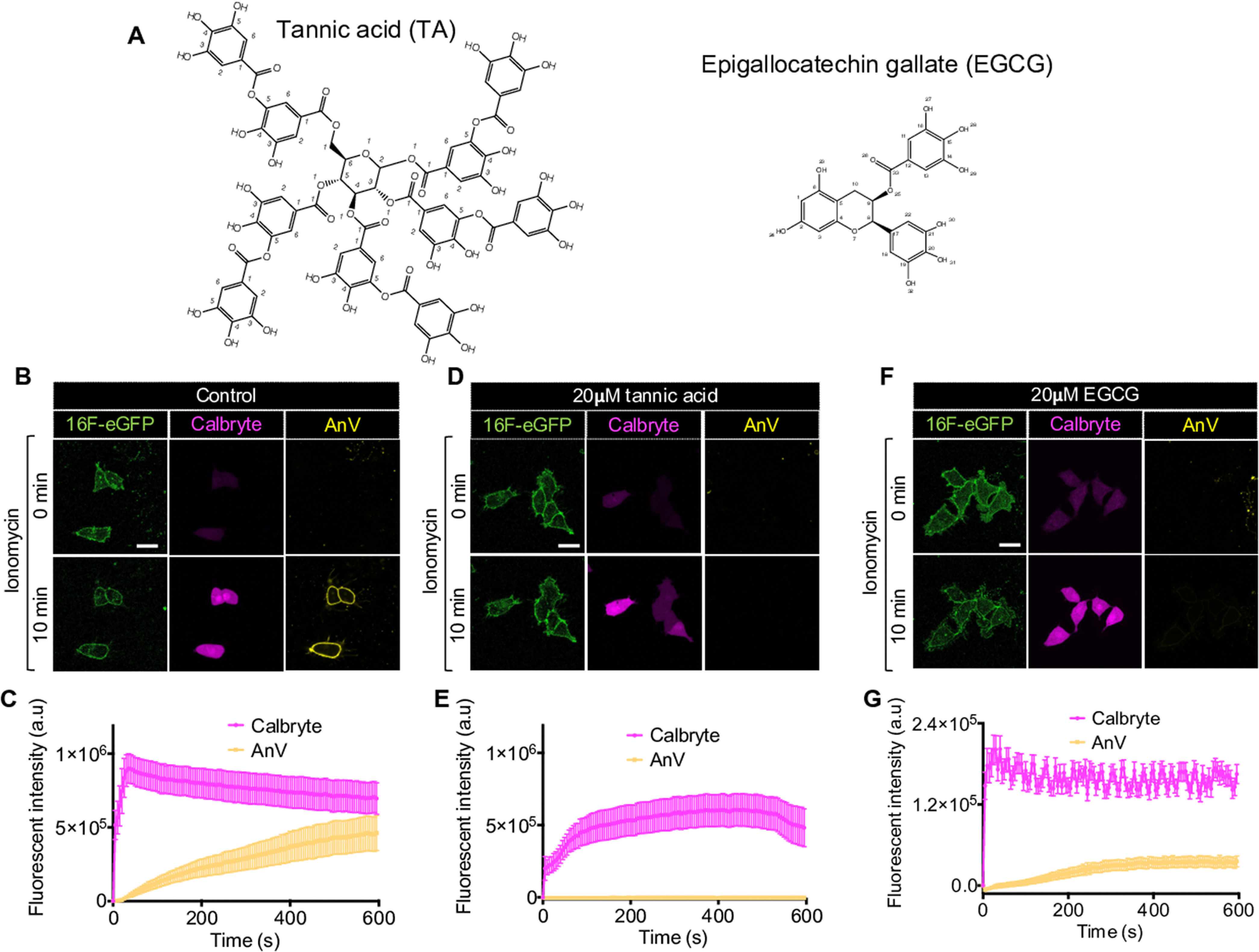
TA and EGCG diminish fluorescently tagged AnV-CF640R signal. A, chemical structures of TA and EGCG. B, D, and F, representative images show that TA (D) and EGCG (F) treatments do not affect ionomycin-induced intracellular Ca2+ influx (labeled by Calbryte (magenta)), but the polyphenols completely suppress AnV-CF640R signal (yellow) on HEK293 cells stably expressing mouse TMEM16F (green) even after 10 min of ionomycin treatment. Scale bars, 25 μm. Without TA and EGCG, the cells have normal AnV signal accumulating on their membrane after ionomycin stimulation (B). C, E, and G, fluorescence intensity changes of Calbryte and AnV over 10 min of ionomycin treatment for the cells in B, D, and F, respectively. n = 3–5 cells. Error bars, S.E. All images are representative of at least three independent biological replicates. a.u., arbitrary units.
In this study, we attempted to assess the inhibitory effects of TA and EGCG on TMEM16F CaPLSase and ion channel activities by fluorescence imaging and electrophysiology. To our surprise, TA and EGCG displayed minimal effects on inhibiting TMEM16F channel activity. This prompted us to re-examine the effects of these polyphenols on TMEM16F lipid scramblase activity. We found that TA and EGCG indeed quenched various fluorophores, including those that are tagged to the AnV probes. We further demonstrated that the previous inhibitory effects of TA and EGCG on TMEM16F were mainly due to the fluorescence-suppressing effects of the polyphenols on the fluorophores conjugated to the extracellular AnV probes and thus prevent AnV from reporting the scrambling activity. We therefore conclude that TA and EGCG do not function as TMEM16 CaPLSase inhibitors. These results also suggest that precautions need to be taken in developing inhibitors targeting TMEM16 CaPLSases.
Results
Extracellular fluorescently tagged AnV cannot report TMEM16F lipid-scrambling activity in the presence of TA and EGCG
We applied a previously established fluorescence imaging–based scrambling assay (10, 25) to characterize the effects of TA and EGCG on TMEM16F-CaPLSase. In this assay, fluorescently tagged AnV (henceforth referred to as AnV) was included in the extracellular solution to monitor scramblase activity of HEK293 cells stably expressing murine TMEM16F. Upon its activation by ionomycin-induced Ca2+ influx, TMEM16F CaPLSase rapidly translocates PS from the inner to outer leaflet of the plasma membrane. Because the externalized PS recruits AnV to the cell surface, the gradual accumulation of fluorescence signal from AnV on the cell membrane is indicative of TMEM16F scrambling activity (Fig. 1, B and C). Consistent with previous reports (22, 23), there was no AnV fluorescence signal on the surface of TMEM16F-expressing HEK293 cells when TA (20 μm) and EGCG (20 μm) were present (Fig. 1, D–G). On the other hand, robust AnV signal was observed on the cell surface in the control experiment without TA or EGCG (Fig. 1, B and C). Furthermore, Ca2+ influx via ionomycin was not affected by the polyphenols as intracellular Ca2+ was instantly mobilized following Ca2+ ionophore stimulation regardless of the presence of TA or EGCG (Fig. 1, B–G). All of these observations imply that the polyphenols could impair TMEM16F lipid-scrambling activity. It is worth noting that 20 μm TA completely abolishes AnV surface signal, whereas 20 μm EGCG still allows weak AnV accumulation on the cell membrane, suggesting that EGCG may have a weaker inhibitory effect than TA (Fig. 1, D–G).
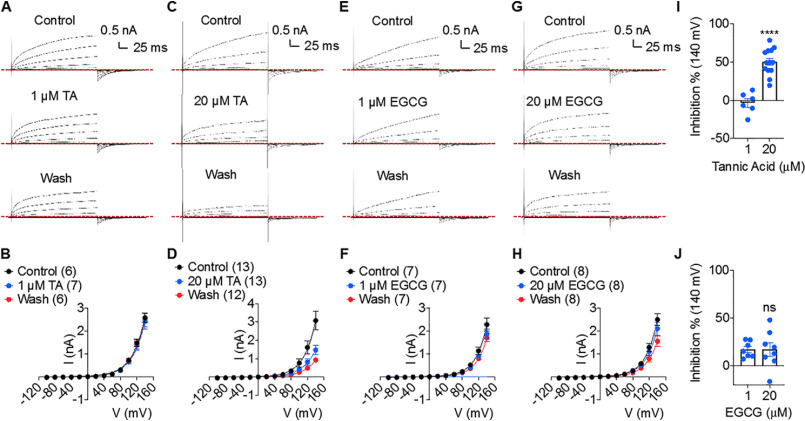
TA and EGCG do not specifically block TMEM16F ion channel activity
TMEM16F is a dual-functional moonlighting protein with both CaPLSase and ion channel activities (8, 18, 25, 26). Several lines of structural and functional evidence have suggested that in TMEM16F, phospholipids and ions share the same activation gates and Ca2+-dependent activation mechanism (18, 25, 27–29). As Ca2+-dependent activation of scrambling activity accompanies ion channel activity (25), we tested the effects of extracellular TA and EGCG on TMEM16F ion channel function using whole-cell patch clamp. Surprisingly, high concentration of TA (20 μm) only inhibited about 50% of TMEM16F current, and 20 μm EGCG had a negligible effect on TMEM16F current (Fig. 2, C, D, and I). Neither 1 μm TA nor EGCG affected TMEM16F channel function (Fig. 2, A, B, and E–J). As TA is a large molecule and previously known to have nonspecific effects on various proteins (30–34), we tested whether the inhibitory effect of 20 μm TA on TMEM16F's current could stem from its nonspecific effects by applying TA to the large-conductance, Ca2+-activated BK type K+ channel, which does not belong to TMEM16 family. Our outside-out patch clamp recording revealed that extracellular application of 20 μm TA significantly promoted BK channel activation, as seen by the left shift in the conductance-voltage (G-V) relationship toward negative voltages and prolonged deactivation kinetics (Fig. S1). Taken together, our electrophysiology results demonstrate that EGCG and low concentration of TA do not inhibit TMEM16F ion channel activity. High concentration of TA could partially inhibit TMEM16F channels. Given the massive chemical structure of TA (Fig. 1A), we speculate that its inhibitory effect is likely indirect and nonspecific.
Figure 2.
Effects of TA and EGCG on TMEM16F's channel activity. A, C, E, and G, representative current whole-cell recordings of HEK293 cells stably expressing mouse TMEM16F showing the effects on channel block by extracellular application of 1 μm TA (A), 20 μm TA (C), 1 μm EGCG (E), and 20 μm EGCG (G). B, D, F, and H, quantifications of TMEM16F's peak currents in control, 1 μm TA (B), 20 μm TA (D), 1 μm EGCG (F), and 20 μm EGCG (H) and after TA/EGCG washout. Numbers in parentheses denote the number of interindividual recordings. I, inhibitory effect of 1 and 20 μm TA on TMEM16F current. J, inhibitory effect of 1 and 20 μm EGCG on TMEM16F current. Unpaired two-tailed student's t test was used; p values are <0.0001 in I and 0.9912 in J (not significant (ns)). Data are presented as mean ± S.E. (error bars).
In vitro assay shows that TA and EGCG quench fluorescence signal of the PS probes and other dyes
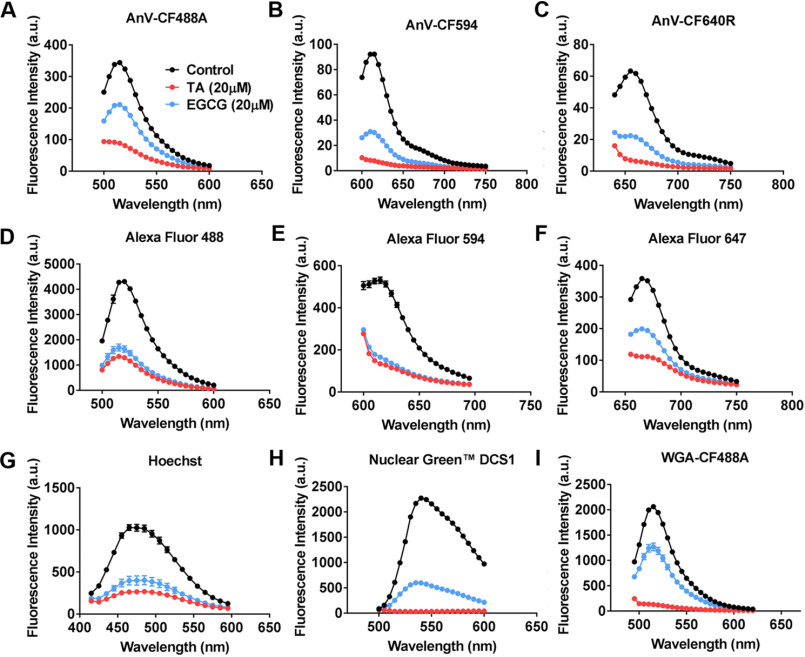
TA and EGCG are known to be able to quench various fluorophores (24, 35–38). Therefore, we suspected that the polyphenols might hinder the ability of the extracellular AnV to correctly report TMEM16F CaPLSase activity through their quenching effects on the fluorophores conjugated to AnV. To test this hypothesis, we measured the fluorescence signals of the AnV probes tagged with various fluorophores (CF488A, CF594, and CF640R) in the presence or absence of polyphenols in vitro using the spectrometry mode of a SpectraMax plate reader. In the presence of 20 μm TA or EGCG, the fluorescence signal of all AnV probes was significantly attenuated, indicating that the polyphenols indeed quench the PS probes in aqueus solution (Fig. 3, A–C). Furthermore, our in vitro experiments also corroborated that TA and EGCG could suppress the fluorescence intensities of various fluorescence probes/dyes, including the Alexa Fluor–conjugated IgGs (Alexa Fluor 488, 594, and 647; Fig. 3, D–F), nucleic acid dyes (Hoechst and Nuclear Green DCS1; Fig. 3, G and H), and CF488A-conjugated wheat germ agglutinin (WGA) (Fig. 3I).
Figure 3.
Effects of TA and EGCG on fluorescence signal of different fluorophores and fluorescence probes in aqueous solution. 20 μm EGCG (blue traces) and 20 μm TA (red traces) were applied to various fluorescence probes/fluorophores, and the intensities of the emission spectra of the fluorophores were measured by the spectrometry function of a plate reader (see “Experimental procedures” for details). Controls (black traces) are fluorescence probes/fluorophores in the absence of TA and EGCG. A, 0.75 µg/ml AnV-CF488A; B, 0.75 µg/ml AnV-CF594; C, 0.75 µg/ml AnV-CF640R; D, 4 µg/ml IgG-Alexa Fluor 488; E, 4 µg/ml IgG-Alexa Fluor 594; F, 4 µg/ml IgG-Alexa Fluor 647; G, 16 μm Hoechst in the presence of 10 μm nucleotide; H, 1:500 Nuclear Green DCS1 in the presence of 10 μm nucleotide; I, 4 mg/ml WGA-CF488A. Data are presented as mean ± S.E. (error bars). a.u., arbitrary units.
TA and EGCG quench the fluorescence signal of the extracellular PS probes accumulating on the cell membrane
As the cell-free results evinced that TA and EGCG could quench the AnV probes in aqueous solution, we verified whether this was the case on our cell-based assays. First, we allowed AnV to accumulate on the surface of TMEM16F-expressing HEK293 cells after ionomycin stimulation (Fig. 4, A, C, and E, middle rows). Next, we introduced TA and EGCG to the medium after a significant amount of AnV had accumulated on the cell surface (2.5 min after ionomycin application). AnV fluorescence signal on the cell membrane was instantaneously decreased upon polyphenol applications (Fig. 4, B, D, and F). 20 μm TA completely abolished AnV signal, whereas 1 μm TA and 20 μm EGCG eliminated more than 80% of AnV signal.
Figure 4.
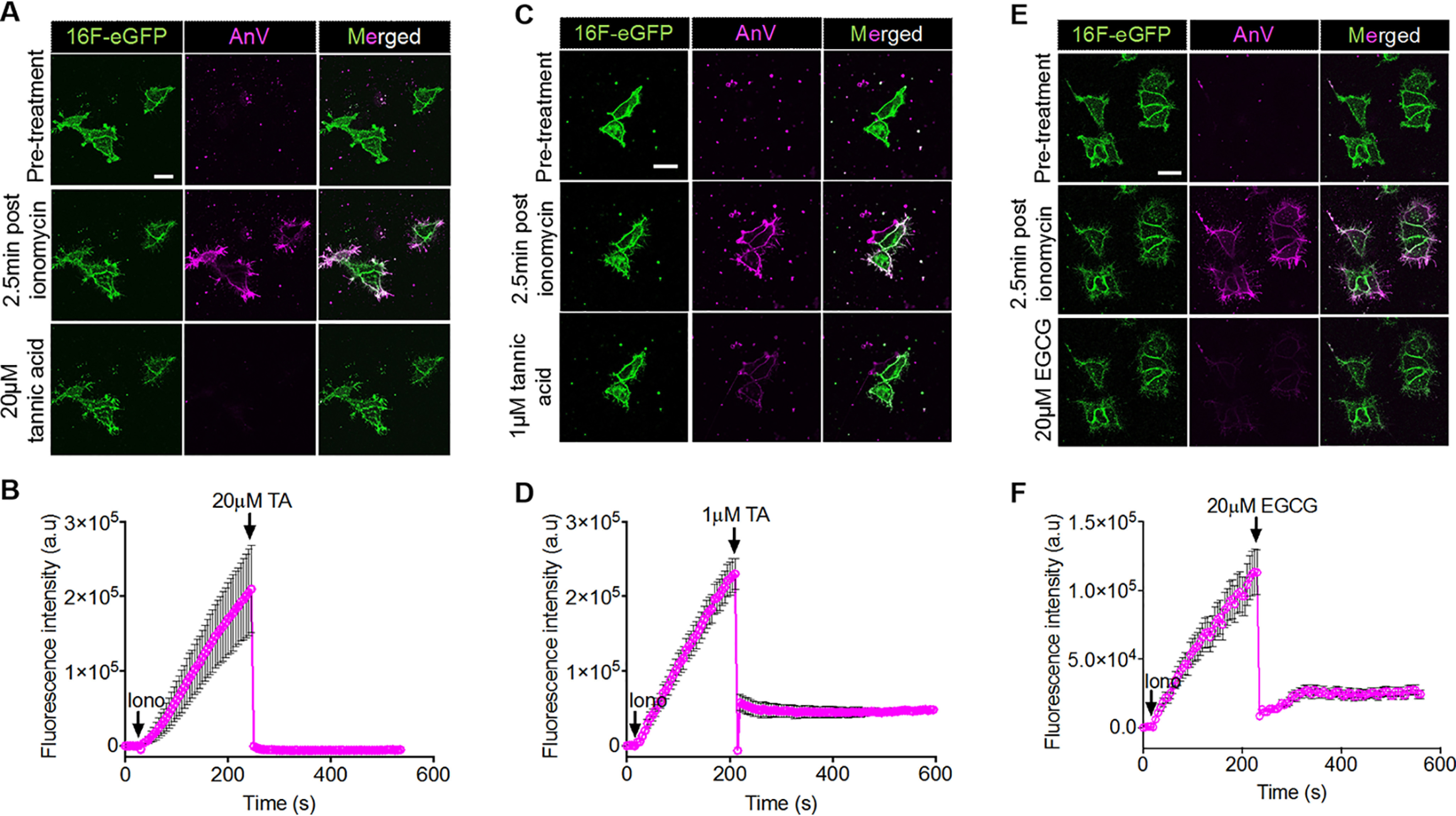
TA and EGCG suppress fluorescence signal of fluorescently tagged AnV-CF594 instead of inhibiting TMEM16F-mediated phospholipid-scrambling activity. A, C, and E, representative images showing 20 μm TA (A), 1 μm TA (C), and 20 μm EGCG (E) immediately deplete fluorescence signal of AnV-CF594 (magenta) of scrambling cells (HEK293 cells stably expressing mouse TMEM16F; green). Scale bars, 25 μm. B, D, and F, mean fluorescence intensity change of AnV fluorescence over 10 min of ionomycin treatment for each cell in A, C, and E, respectively. Applications of ionomycin, TA, and EGCG are marked by a downward arrow and chemical abbreviations. n = 3–8 cells. Error bars, S.E. All images are representative of at least three independent replicates. a.u., arbitrary units.
To further validate the fluorescence-quenching effects of TA and EGCG on the AnV probes and verify that loss of AnV signal was not due to defects in TMEM16F functions, we tested whether the polyphenols also quenched the fluorescence signal of the AnV probes binding to apoptotic cells, which also have PS-exposed cell surface but are independent of TMEM16F-CaPLSase (39). Staurosporine (STS) was used to induce apoptosis, which resulted in PS being exposed to the cell surface through caspase-dependent phospholipid scramblases (40), as evidenced by AnV signal on cell membrane and strong cytosolic caspase dye (TF3-DEVD-FMK) staining (Fig. S2, Before treatment). Application of TA and EGCG immediately abolished the AnV signal on the apoptotic cell surface (Fig. S2, C–H), suggesting that the polyphenols indeed quenched the fluorophores of the AnV probes. Interestingly, the intensity of the intracellular caspase dye was also instantly reduced upon TA and EGCG addition, suggesting that the compromised membrane, as a result of apoptosis, allowed TA and EGCG to leak into the cytosol and partially quench the caspase dye (Fig. S2, C–H). Based on the above observations, we conclude that TA and EGCG can rapidly quench the extracellular fluorescence signal from fluorophore-conjugated AnV.
Additionally, to further corroborate that TA and EGCG treatments have minimal effects on TMEM16F scrambling activity, we first stimulated TMEM16F scrambling and then reprobed the ionomycin-stimulated TMEM16F-stably expressed HEK293 cells with AnV-CF594. Prior to ionomycin treatment, the cells were stained with AnV-CF594 to verify that the cell surface was PS-negative (Fig. S3, left). The cells were first treated with either ionomycin alone or ionomycin in combination with TA or EGCG. After 10-min treatment and extensive washes to eliminate ionomycin, TA, and EGCG, the cells were reprobed with AnV-CF594 to observe surface-exposed PS. We found that the polyphenol-treated cells did not show obvious inhibitory effect on AnV-CF594 binding to the cell surface (Fig. S3, right). This observation is consistent with the lack of polyphenol inhibitory effect on TMEM16F current (Fig. 2). As TMEM16F is a moonlighting protein with both CaPLSase and ion channel activities (8, 18) and its activation leads to simultaneous phospholipid and ion permeation (25), our electrophysiology and fluorescence lipid scrambling results explicitly demonstrate that TA and EGCG do not inhibit TMEM16F.
Discussion
In this study, we carefully examined the potential inhibitory effects of TA and EGCG on TMEM16F. Through a series of experiments, including patch clamp, cell-free, and cell-based fluorescence assays, we failed to find evidence to support that the polyphenols would be specific inhibitors for TMEM16F. Instead, these polyphenols quench the fluorophores of the AnV probes as well as other fluorescence dyes/probes. The quenching artifacts prevent the AnV probes from reporting TMEM16F-mediated PS exposure on the cell surface, thereby giving the impression that TA and EGCG “inhibit” TMEM16F activation.
Polyphenols TA and EGCG have been reported as potent inhibitors of TMEM16F scramblase. Due to their well-known beneficial effects on health (19–21, 24), TA and EGCG are seemingly promising candidates to treat TMEM16F scramblase-related diseases, such as stroke and heart attack. They also have been increasingly used as pharmacological tools to understand the biology of TMEM16 scramblases over the past several years (41–43). Our current study casts caution on utilizing these polyphenols as research and therapeutic tools to study and target TMEM16 CaPLSases. Furthermore, precautions need to be taken when developing inhibitors for TMEM16 CaPLSases using fluorescence-based assays. Given the dual functionality of TMEM16F as both phospholipid scramblase and ion channel, we recommend combining fluorescence imaging–based scrambling assays with electrophysiology when trying to identify bona fide inhibitors for TMEM16 scramblases.
It is worth noting that higher concentration of TA can exert nonspecific effects on TMEM16F as evidenced by the partial inhibitory effect on TMEM16F current (Fig. 2I) and stimulating effect on different types of ion channels, such as the BK channel shown in this study (Fig. S1) or TRPM4, TRPC4, and TRPC5 channels in a previous report (33). Therefore, the nonspecific nature of TA prevents it from serving as an ideal pharmacological tool for mechanistic studies.
Experimental procedures
Cell lines and cell culture
HEK293T was purchased from the Duke Cell Culture Facility. C-terminally enhanced GFP–tagged murine (m) TMEM16F-stably expressed HEK293 cells (mTMEM16F-stable HEK293) were a generous gift from Dr. Min Li. All cells were maintained in Dulbecco's modified Eagle's medium (Gibco, catalog no. 11995-065) containing 1% penicillin-streptomycin and 10% fetal bovine serum. To reselect and maintain the homogeneous population of mTMEM16F-stable HEK293 cells, 100 µg ml−1 of hygromycin was occasionally added to the culture medium. All cells were cultured in a temperature-controlled (37 °C) incubator supplemented with 5% CO2.
Transfection
Plasmid carrying the cDNAs of mouse BK channel α subunit (Addgene no. 113566) was used in this study. After cells were seeded on PLL-coated coverslips for at least 24 h, X-tremeGENE9 transfection reagent (Millipore-Sigma) was used to transiently transfect the plasmid into the cells. After 5 h of transfection, culture supernatant was changed to fresh medium. Experiments were performed 24–48 h post-transfection.
Fluorescence microscopy
mTMEM16F-stable HEK293 cells were seeded on PLL-coated #0 coverslips overnight prior to the experiments. Extracellular solution in all imaging assays (imaging solution) contained 0.5 µg ml−1 fluorescently tagged AnV, 10 mm HEPES, 140 mm NaCl, 2.5 mm CaCl2, pH 7.4. Stock solution (10 mm) of TA and EGCG were prepared using ultrapure water and stored at 4 °C. Working concentrations of TA and EGCG were established by further diluting the stock TA and EGCG solutions in the imaging solution. All experiments were independently repeated at least three times.
Ca2+ imaging and scrambling assay
2 mm stock solution of calcium indicator CalbryteTM 590 AM (AAT Bioquest, catalog no. 20701) was prepared by using DMSO and stored at −20 °C. The stock solution was diluted to a working concentration of 1 μm by using Hanks' balanced salt solution (HBSS; Gibco, catalog no. 14025-092). The cells were incubated with 1 μm CalbryteTM 590 AM for 20–30 min in the incubator supplied with 5% CO2 at 37 °C. After washing the cells with HBSS, the coverslips were mounted to our customized imaging chamber containing the imaging solution that has CF 640R–tagged annexin V (AnV-CF640R) (Biotium, catalog no. 29014), and we proceeded with our scrambling assay as described previously (10, 27). In brief, a final concentration of 5 μm ionomycin was added to the imaging chamber to induce Ca2+ influx and subsequent scrambling activity of the cells. In TA or EGCG treatment, a 20 μm concentration of either TA or EGCG was added together with ionomycin into the imaging chamber. Changes in fluorescence intensity of Calbryte and AnV-CF640R were recorded using a ×63/1.4 NA Oil Plan-Apochromat DIC in a Zeiss 780 inverted confocal microscope. This experiment was executed using time-lapse imaging with 5-s intervals.
Cell-based AnV quenching assay
mTMEM16F stable HEK293 cells were stimulated with 5 μm ionomycin, and the scrambling activity was monitored using CF594-tagged AnV (AnV-CF594) for 2–3 min. After that, either 1 μm TA, 20 μm TA, or 20 μm EGCG was added into the imaging chamber. Changes in the fluorescence intensity of AnV-CF594 were recorded using time-lapse imaging with 5-s intervals. This experiment was executed using a ×63/1.4 NA Oil Plan-Apochromat DIC in a Zeiss 780 inverted confocal microscope.
Reprobing of the scrambling cells with AnV after TA/EGCG treatment
mTMEM16F-stable HEK293 cells were seeded on PLL-coated #0 coverslips for 48 h. The cells were first stained and imaged with 0.5 µg ml−1 AnV-CF594 prior to any treatments. The coverslips were then transferred to a chamber containing either 5 μm ionomycin alone, 5 μm ionomycin with 1 μm TA, or 5 μm ionomycin with 20 μm EGCG. After a 10-min stimulation, the ionomycin solutions with or without TA or EGCG in the chamber were removed, and the cells were thoroughly washed twice with a large volume of HBSS. The cells were then briefly incubated with AnV-CF594 solution for 3–5 min, and AnV-CF594 signal was captured by a Prime 95B Scientific CMOS camera (Photometrics) connected to an Olympus IX73 inverted epifluorescent microscope. A ×40 objective with NA of 0.75 was used for imaging. The image acquisition was managed by MetaFluor software (Molecular Devices). At least 3 fields of view/sample were imaged.
In vitro fluorescence quenching assay
To examine the effects of TA and EGCG on different fluorophores, aqueous solution of 0.75 µg/ml fluorescently tagged AnV (either CF488A, CF594, or CF640R) (Biotium), 4 µg/ml IgG (Alexa Fluor 488, Alexa Fluor 594, and Alexa Fluor 647, Thermo Fisher Scientific), 16 μm Hoechst (Life Technologies), 1:500 Nuclear Green DCS1 (AAT Bioquest), and 4 mg/ml WGA-CF488A (Biotium) were prepared using PBS. Hoechst and Nuclear Green DCS1 solutions were supplemented with 10 μm nucleotide to boost the fluorescence signals of these two nucleic dyes. TA and EGCG were added into each of these fluorophores at a final concentration of 20 μm. After mixing using a pipette, 100 μl of each of these fluorophores containing 20 μm TA or EGCG were added to a clear-bottom 96-well plate. The emissions of the fluorophores were measured using the spectrometry mode of a Spectramax M5 plate reader (Molecular Devices) with a 5-nm increment. The same set of fluorophore solutions without TA and EGCG was also examined as a control. Each sample was done in three replicates.
STS-induced apoptosis and active caspase-3/7 staining
STS was diluted in culture medium and applied to the cells at a final concentration of 10 μm. The cells were incubated with STS for 4 h. Next, medium containing STS was removed and replaced with fresh medium containing TF3-DEVD-FMK (caspase-3/7 dye) from the Live Cell Caspase 3/7 binding assay kit (AAT Bioquest, catalog no. 20101). The detailed staining procedure was described previously (27). Only cells that were positive with TF3-DEVD-FMK and AnV-CF640R were focused for imaging. The control, TA, or EGCG solutions at appropriate concentrations were added to the apoptotic cells. Fluorescence changes of AnV-CF640R and caspase dye were captured with confocal time-lapse imaging at 1-s intervals. This experiment was executed using a ×63/1.4 NA Oil Plan-Apochromat DIC in a Zeiss 780 inverted confocal microscope.
Electrophysiology
Voltage-clamp recordings were low-pass filtered at 5 kHz (Axopatch 200B) and digitally sampled at 10 kHz (Axon Digidata 1550A) and digitized by Clampex 10 (Molecular Devices). Electrodes were pulled from borosilicate capillaries (Sutter Instruments) and had initial resistances of 2–5 megaohms. Pipette electrodes were made from borosilicate capillaries (Sutter Instruments) and fire-polished with a microforge (Narishige). All experiments were performed at room temperature.
TA and EGCG were diluted into bath buffer at desired concentrations using aqueous stock solutions of 20 and 10 mm, respectively. External application of TA or EGCG was performed via local focal perfusion using a pressurized perfusion apparatus (ALA-VM8, ALA Scientific Instruments). For whole-cell recording, the perfusion outlet was positioned close to the patched cell before forming the whole-cell configuration for recordings. For outside-out recording, the patches were moved next to the perfusion outlet before recording.
Whole-cell TMEM16F channel recordings were performed on the mTMEM16F-stable HEK293 cells. The cells were trypsinized and plated on PLL-coated coverglass (Assistent) 1–2 h before electrophysiology. Bath solution contained 140 mm NaCl, 10 mm HEPES, 2 mm MgCl2, pH 7.3 (adjusted with NaOH). Pipette solution contained 140 mm CsCl, 10 mm HEPES, 1 mm CaCl2, pH 7.3 (adjusted with NaOH). Upon formation of whole-cell configuration, TMEM16F channels were activated by a current-voltage (I-V) protocol in which the membrane was held at −60 mV and test voltage steps from −100 to +140 mV. Typically, with the presence of 1 mm intracellular Ca2+, considerable TMEM16F currents were observed within 30–60 s after breaking in. Following acquisition of a control I-V recording, TA or EGCG at the desired concentrations was focally perfused to the cell for 2–3 s before recording acquisition. Next, the cell was followed by focal perfusion of a control bath solution to wash off TA or EGCG for 3–4 s before another recording acquisition.
To test TA and EGCG effects on BK channels, an outside-out configuration was used. The pipette solution contained 140 mm KCl, 10 mm HEPES, 1 mm MgCl2, 0.1 mm free Ca2+, pH 7.3 with KOH. Extracellular solution contained 140 mm KCl, 10 mm HEPES, 1 mm MgCl2, pH 7.3 with KOH. The smooth curves represent Boltzmann fits, G/Gmax = 1/(1 + exp(−ze(V − V½)/kT), where Gmax represents tail current amplitude elicited by depolarization of +200 mV, z is the number of equivalent gating charges, V is the membrane voltage, V½ is the half-activation voltage, F is Faraday's constant, R is the gas constant, and T is the absolute temperature.
Data analysis
All fluorescence imaging data analysis was performed in Zeiss, MetaFluor, ImageJ, Mathlab, Microsoft Excel, Origin, and GraphPad Prism. All electrophysiology data analysis was performed in Clampfit, Microsoft Excel, and GraphPad Prism. Structures of TA and EGCG were depicted using ChemDraw 18.2 (PerkinElmer Life Sciences).
Data availability
All data are contained within the article.
Supplementary Material
Acknowledgments
We thank Dr. Ping Dong for help preparing the manuscript.
This article contains supporting information.
Author contributions—T. L., S. C. L., Y. Z., and P. L. formal analysis; T. L., S. C. L., and H. Y. funding acquisition; T. L., S. C. L., Y. Z., and P. L. validation; T. L., S. C. L., Y. Z., and P. L. investigation; T. L. and Y. Z. methodology; T. L. and H. Y. writing-original draft; T. L. and H. Y. writing-review and editing; Y. Z. software; Y. Z. visualization; H. Y. conceptualization; H. Y. resources; H. Y. supervision; H. Y. project administration.
Funding and additional information—This work was supported by National Institutes of Health Grant NIH-DP2-GM126898 (to H. Y.) and American Heart Association Predoctoral Fellowships 20PRE35120162 (to T. L.) and 19PRE3438456 (to S. C. L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- CaPLSase
- Ca2+-activated phospholipid scramblase
- PLL
- poly-l-lysine
- PS
- phosphatidylserine
- TA
- tannic acid
- EGCG
- epigallocatechin gallate
- AnV
- annexin V
- WGA
- wheat germ agglutinin
- STS
- staurosporine
- HBSS
- Hanks' balanced salt solution
- NA
- numerical aperture
- DIC
- differential interference contrast.
References
- 1. Pedemonte N., and Galietta L. J. (2014) Structure and function of TMEM16 proteins (anoctamins). Physiol. Rev. 94, 419–459 10.1152/physrev.00039.2011 [DOI] [PubMed] [Google Scholar]
- 2. Whitlock J. M., and Hartzell H. C. (2017) Anoctamins/TMEM16 proteins: chloride channels flirting with lipids and extracellular vesicles. Annu. Rev. Physiol. 79, 119–143 10.1146/annurev-physiol-022516-034031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bevers E. M., and Williamson P. L. (2016) Getting to the outer leaflet: physiology of phosphatidylserine exposure at the plasma membrane. Physiol. Rev. 96, 605–645 10.1152/physrev.00020.2015 [DOI] [PubMed] [Google Scholar]
- 4. Brunner J. D., Lim N. K., Schenck S., Duerst A., and Dutzler R. (2014) X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 516, 207–212 10.1038/nature13984 [DOI] [PubMed] [Google Scholar]
- 5. Gyobu S., Miyata H., Ikawa M., Yamazaki D., Takeshima H., Suzuki J., and Nagata S. (2016) A role of TMEM16E carrying a scrambling domain in sperm motility. Mol. Cell Biol. 36, 645–659 10.1128/MCB.00919-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malvezzi M., Chalat M., Janjusevic R., Picollo A., Terashima H., Menon A. K., and Accardi A. (2013) Ca2+-dependent phospholipid scrambling by a reconstituted TMEM16 ion channel. Nat. Commun. 4, 2367 10.1038/ncomms3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suzuki J., Fujii T., Imao T., Ishihara K., Kuba H., and Nagata S. (2013) Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J. Biol. Chem. 288, 13305–13316 10.1074/jbc.M113.457937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suzuki J., Umeda M., Sims P. J., and Nagata S. (2010) Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468, 834–838 10.1038/nature09583 [DOI] [PubMed] [Google Scholar]
- 9. Whitlock J. M., Yu K., Cui Y. Y., and Hartzell H. C. (2018) Anoctamin 5/TMEM16E facilitates muscle precursor cell fusion. J. Gen. Physiol. 150, 1498–1509 10.1085/jgp.201812097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Le T., Le S. C., and Yang H. (2019) Drosophila Subdued is a moonlighting transmembrane protein 16 (TMEM16) that transports ions and phospholipids. J. Biol. Chem. 294, 4529–4537 10.1074/jbc.AC118.006530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balreira A., Boczonadi V., Barca E., Pyle A., Bansagi B., Appleton M., Graham C., Hargreaves I. P., Rasic V. M., Lochmüller H., Griffin H., Taylor R. W., Naini A., Chinnery P. F., Hirano M., et al. (2014) ANO10 mutations cause ataxia and coenzyme Q10 deficiency. J. Neurol. 261, 2192–2198 10.1007/s00415-014-7476-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Renaud M., Anheim M., Kamsteeg E. J., Mallaret M., Mochel F., Vermeer S., Drouot N., Pouget J., Redin C., Salort-Campana E., Kremer H. P., Verschuuren-Bemelmans C. C., Muller J., Scheffer H., Durr A., et al. (2014) Autosomal recessive cerebellar ataxia type 3 due to ANO10 mutations: delineation and genotype-phenotype correlation study. JAMA Neurol. 71, 1305–1310 10.1001/jamaneurol.2014.193 [DOI] [PubMed] [Google Scholar]
- 13. Hicks D., Sarkozy A., Muelas N., Köehler K., Huebner A., Hudson G., Chinnery P. F., Barresi R., Eagle M., Polvikoski T., Bailey G., Miller J., Radunovic A., Hughes P. J., Roberts R., et al. (2011) A founder mutation in Anoctamin 5 is a major cause of limb-girdle muscular dystrophy. Brain 134, 171–182 10.1093/brain/awq294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bolduc V., Marlow G., Boycott K. M., Saleki K., Inoue H., Kroon J., Itakura M., Robitaille Y., Parent L., Baas F., Mizuta K., Kamata N., Richard I., Linssen W. H., Mahjneh I., et al. (2010) Recessive mutations in the putative calcium-activated chloride channel Anoctamin 5 cause proximal LGMD2L and distal MMD3 muscular dystrophies. Am. J. Hum. Genet. 86, 213–221 10.1016/j.ajhg.2009.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Penttilä S., Palmio J., Suominen T., Raheem O., Evilä A., Muelas Gomez N., Tasca G., Waddell L. B., Clarke N. F., Barboi A., Hackman P., and Udd B. (2012) Eight new mutations and the expanding phenotype variability in muscular dystrophy caused by ANO5. Neurology 78, 897–903 10.1212/WNL.0b013e31824c4682 [DOI] [PubMed] [Google Scholar]
- 16. Tsutsumi S., Kamata N., Vokes T. J., Maruoka Y., Nakakuki K., Enomoto S., Omura K., Amagasa T., Nagayama M., Saito-Ohara F., Inazawa J., Moritani M., Yamaoka T., Inoue H., and Itakura M. (2004) The novel gene encoding a putative transmembrane protein is mutated in gnathodiaphyseal dysplasia (GDD). Am. J. Hum. Genet. 74, 1255–1261 10.1086/421527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castoldi E., Collins P. W., Williamson P. L., and Bevers E. M. (2011) Compound heterozygosity for 2 novel TMEM16F mutations in a patient with Scott syndrome. Blood 117, 4399–4400 10.1182/blood-2011-01-332502 [DOI] [PubMed] [Google Scholar]
- 18. Yang H., Kim A., David T., Palmer D., Jin T., Tien J., Huang F., Cheng T., Coughlin S. R., Jan Y. N., and Jan L. Y. (2012) TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell 151, 111–122 10.1016/j.cell.2012.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crozier A., Jaganath I. B., and Clifford M. N. (2009) Dietary phenolics: chemistry, bioavailability and effects on health. Nat. Prod. Rep. 26, 1001–1043 10.1039/b802662a [DOI] [PubMed] [Google Scholar]
- 20. Serrano J., Puupponen-Pimia R., Dauer A., Aura A. M., and Saura-Calixto F. (2009) Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 53, S310–S329 10.1002/mnfr.200900039 [DOI] [PubMed] [Google Scholar]
- 21. Chakrawarti L., Agrawal R., Dang S., Gupta S., and Gabrani R. (2016) Therapeutic effects of EGCG: a patent review. Expert Opin. Ther. Pat. 26, 907–916 10.1080/13543776.2016.1203419 [DOI] [PubMed] [Google Scholar]
- 22. Suzuki T., Suzuki J., and Nagata S. (2014) Functional swapping between transmembrane proteins TMEM16A and TMEM16F. J. Biol. Chem. 289, 7438–7447 10.1074/jbc.M113.542324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watanabe R., Sakuragi T., Noji H., and Nagata S. (2018) Single-molecule analysis of phospholipid scrambling by TMEM16F. Proc. Natl. Acad. Sci. U. S. A. 115, 3066–3071 10.1073/pnas.1717956115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu X., Zhang W., Yang C., Yao Y., Huang L., Li S., Wang J., and Ji Y. (2019) Rapid and selective fluorometric determination of tannic acid using MoO3-x quantum dots. Mikrochim. Acta 186, 247 10.1007/s00604-019-3311-2 [DOI] [PubMed] [Google Scholar]
- 25. Yu K., Whitlock J. M., Lee K., Ortlund E. A., Cui Y. Y., and Hartzell H. C. (2015) Identification of a lipid scrambling domain in ANO6/TMEM16F. Elife 4, e06901 10.7554/eLife.06901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scudieri P., Caci E., Venturini A., Sondo E., Pianigiani G., Marchetti C., Ravazzolo R., Pagani F., and Galietta L. J. (2015) Ion channel and lipid scramblase activity associated with expression of TMEM16F/ANO6 isoforms. J. Physiol. 593, 3829–3848 10.1113/JP270691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Le T., Jia Z., Le S. C., Zhang Y., Chen J., and Yang H. (2019) An inner activation gate controls TMEM16F phospholipid scrambling. Nat. Commun. 10, 1846 10.1038/s41467-019-09778-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feng S., Dang S., Han T. W., Ye W., Jin P., Cheng T., Li J., Jan Y. N., Jan L. Y., and Cheng Y. (2019) Cryo-EM studies of TMEM16F calcium-activated ion channel suggest features important for lipid scrambling. Cell Rep. 28, 567–579.e4 10.1016/j.celrep.2019.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alvadia C., Lim N. K., Clerico Mosina V., Oostergetel G. T., Dutzler R., and Paulino C. (2019) Cryo-EM structures and functional characterization of the murine lipid scramblase TMEM16F. Elife 8, e44365 10.7554/eLife.44365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shrager P. G., Macey R. I., and Strickholm A. (1969) Internal perfusion of crayfish, giant axons: action of tannic acid, DDT, and TEA. J. Cell. Physiol. 74, 77–90 10.1002/jcp.1040740111 [DOI] [PubMed] [Google Scholar]
- 31. Namkung W., Thiagarajah J. R., Phuan P. W., and Verkman A. S. (2010) Inhibition of Ca2+-activated Cl− channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. FASEB J. 24, 4178–4186 10.1096/fj.10-160648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Namkung W., Phuan P. W., and Verkman A. S. (2011) TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J. Biol. Chem. 286, 2365–2374 10.1074/jbc.M110.175109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsai T. Y., Leong I. L., Shiao L. R., Wong K. L., Shao L., Chan P., and Leung Y. M. (2020) Tannic acid, a vasodilator present in wines and beverages, stimulates Ca2+ influx via TRP channels in bEND.3 endothelial cells. Biochem. Biophys. Res. Commun. 526, 117–121 10.1016/j.bbrc.2020.03.078 [DOI] [PubMed] [Google Scholar]
- 34. Herz F., and Kaplan E. (1968) Effects of tannic acid on erythrocyte enzymes. Nature 217, 1258–1259 10.1038/2171258a0 [DOI] [PubMed] [Google Scholar]
- 35. Chen R. L., Lin C. H., Chung C. Y., and Cheng T. J. (2005) Determination of tannin in green tea infusion by flow-injection analysis based on quenching the fluorescence of 3-aminophthalate. J. Agric. Food Chem. 53, 8443–8446 10.1021/jf051077f [DOI] [PubMed] [Google Scholar]
- 36. Ahmed G. H., Laíño R. B., Calzón J. A., and García M. E. (2015) Fluorescent carbon nanodots for sensitive and selective detection of tannic acid in wines. Talanta 132, 252–257 10.1016/j.talanta.2014.09.028 [DOI] [PubMed] [Google Scholar]
- 37. Zhang L., Wang Y., Xu M., and Hu X. (2017) Galloyl moieties enhance the binding of (−)-epigallocatechin-3-gallate to β-lactoglobulin: a spectroscopic analysis. Food Chem. 237, 39–45 10.1016/j.foodchem.2017.05.048 [DOI] [PubMed] [Google Scholar]
- 38. Zhang L., Liu Y., and Wang Y. (2019) Interaction between an (−)-epigallocatechin-3-gallate-copper complex and bovine serum albumin: fluorescence, circular dichroism, HPLC, and docking studies. Food Chem. 301, 125294 10.1016/j.foodchem.2019.125294 [DOI] [PubMed] [Google Scholar]
- 39. Suzuki J., Imanishi E., and Nagata S. (2014) Exposure of phosphatidylserine by Xk-related protein family members during apoptosis. J. Biol. Chem. 289, 30257–30267 10.1074/jbc.M114.583419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suzuki J., Denning D. P., Imanishi E., Horvitz H. R., and Nagata S. (2013) Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 341, 403–406 10.1126/science.1236758 [DOI] [PubMed] [Google Scholar]
- 41. Öhlinger T., Müllner E. W., Fritz M., Sauer T., Werning M., Baron D. M., and Salzer U. (2020) Lysophosphatidic acid-induced pro-thrombotic phosphatidylserine exposure and ionophore-induced microvesiculation is mediated by the scramblase TMEM16F in erythrocytes. Blood Cells Mol. Dis. 83, 102426 10.1016/j.bcmd.2020.102426 [DOI] [PubMed] [Google Scholar]
- 42. Ousingsawat J., Wanitchakool P., Schreiber R., and Kunzelmann K. (2018) Contribution of TMEM16F to pyroptotic cell death. Cell Death Dis. 9, 300 10.1038/s41419-018-0373-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ousingsawat J., Wanitchakool P., Kmit A., Romao A. M., Jantarajit W., Schreiber R., and Kunzelmann K. (2015) Anoctamin 6 mediates effects essential for innate immunity downstream of P2X7 receptors in macrophages. Nat. Commun. 6, 6245 10.1038/ncomms7245 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the article.