Abstract
The aim of the present study was to examine adherence and acceptance of a home-based program to promote physical activity (PA) in older persons with cognitive impairment (CI) following inpatient rehabilitation. Sixty-three older persons (≥ 65 years) with mild to moderate CI (Mini-Mental State Examination score 17–26), allocated to the intervention group of a randomized, controlled intervention trial underwent a 12-week home-based PA intervention including (1) physical training and outdoor walking to improve functional fitness and (2) motivational strategies (goal-setting, pedometer-based self-monitoring, social support delivered by home visits, phone calls) to promote PA. Training logs were used to assess adherence to physical training, outdoor walking and to motivational strategies (goal-setting, pedometer-based self-monitoring). Acceptance (subjective feasibility and effectiveness) of the program components was assessed by a standardized questionnaire. Mean adherence rates over the intervention period were 63.6% for physical training, 57.9% for outdoor walking, and between 40.1% (achievement of walking goals), and 60.1% (pedometer-based self-monitoring) for motivational strategies. Adherence rates significantly declined from baseline to the end of intervention (T1: 43.4–76.8%, T2: 36.1–51.5%, p values<.019). Most participants rated physical training, outdoor walking, goal-setting, and pedometer self-monitoring as feasible (68.2–83.0%) and effective (63.5–78.3%). Highest ratings of self-perceived effectiveness were found for home visits (90.6%) and phone calls (79.2%). The moderate to high adherence to self-performed physical training and motivational strategies proved the feasibility of the home-based PA program in older persons with CI following inpatient rehabilitation.
Keywords: Adherence, Feasibility, Physical activity, Geriatrics, Transitional care, Cognitive impairment
Introduction
Cognitive impairment (CI) is a crucial factor for rehabilitation success in geriatric patients, as individuals with CI are at a high risk for poor functional recovery during rehabilitation (McGilton et al. 2016) and experience more often caught in the downward spiral of decreased outdoor mobility after discharge (Brown et al. 2009), loss of autonomy, and functional independence (Portegijs et al. 2014). Despite the urgent necessity to increase functional fitness and to promote physical activity (PA), this vulnerable group is mostly excluded from interventions targeting care continuity in the community following ward-based rehabilitation (Chenoweth et al. 2015).
High adherence to exercise is a prerequisite of successfully implemented interventions in mobility impaired older adults (Fairhall et al. 2012; Taylor et al. 2017), but is reduced in persons with CI participating in home-based rehabilitation compared to their cognitively intact counterparts (Moseley et al. 2009). Nonetheless, high adherence to physical training was achieved in home-based and supervised programs with caregiver support in cognitively impaired elderly (Prick et al. 2016; Suttanon et al. 2013; Teri et al. 2003; Wesson et al. 2013). A previous home-based study demonstrated high adherence to autonomously performed physical training by cognitively and functionally restricted individuals following inpatient rehabilitation (Hauer et al. 2017), although deteriorating health is known to mitigate adherence to home exercise (Fairhall et al. 2012). Decreasing adherence to individually tailored training over time indicate the challenge of achieving a long-lasting increase in PA in this target group (Taylor et al. 2017). This may be hampered by CI-specific barriers, such as impaired executive function, reduced awareness of own deficits, and apathetic behavior (David et al. 2012; Jacus 2017).
With respect to these multiple barriers, interventions are needed that promote PA as part of daily routine (Heyn et al. 2004). To facilitate a change of activity-related behavioral patterns, behavioral change techniques (BCT; Abraham and Michie 2008) are implemented across PA interventions in diverse adult populations. Two reviews narratively synthesized the efficacy of a variety of BCT (named as motivational strategies in the following) on levels of PA in intervention studies among persons with CI (Nyman et al. 2018; van der Wardt et al. 2017). Within the audited studies, there is cautious evidence for the impact of goal-setting and self-monitoring in terms of high adherence to training and elevated levels of PA. Goal-setting and self-monitoring of own activity behavior led to gains of step counts among individuals without CI (Rosenberg et al. 2012), whereas both strategies were associated with limited efficacy and higher dropout in individuals with CI (Kerse et al. 2008; Vidoni et al. 2016). Home visits and phone calls provided by professionals are useful sources for social support and essential components for the success of activity promotion in elderly (Suttanon et al. 2012). Particularly, dementia-specific communication was beneficial in PA promotion among elderly with CI (Hauer et al. 2012).
Most of the interventions which aimed to change PA behavior using a motivational concept followed a continuously supervised approach. This supervision comprised either the use of caregivers as training partners (Taylor et al. 2017; Teri et al. 2003) or group-based sessions within a nursing care setting (Olsen et al. 2015; Phillips and Flesner 2013). Programs without continuous supervision, fostering the autonomous engagement in physical training and walking, were exclusively implemented in elderly with subjective memory complaints or very mild CI (Cox et al. 2013; Dannhauser et al. 2014; Lautenschlager et al. 2008). Interventions to promote PA in cognitively impaired individuals solely reported adherence to the exercise regimen, but did not provide information about the adoption of motivational strategies for PA promotion. Only Cox et al. (2013), examining the completion of worksheets, reported modest adherence to the general behavior counseling concepts to promote home-based PA in adults with memory complaints. This approach does not allow testing the feasibility of the unique motivational strategies.
By use of qualitative designs, a few studies conducted in cognitively impaired samples described participants’ and coaches’ experiences of participating in activity promotion programs rather than testing the feasibility of motivational strategies underlying the PA promotion (Olsen et al. 2015; Phillips and Flesner 2013; Suttanon et al. 2012). Within an intervention to promote independent walking intervention, elderly without CI were satisfied with motivational program components, also including pedometer-monitoring, as evaluated by use of a questionnaire (Rosenberg et al. 2012). However, none of those studies used quantitative methods in terms of evaluating adherence to autonomously performed training and motivational strategies over time as well as testing the subjective feasibility as a measure of participants’ acceptance in a sample of individuals with motor and cognitive impairment. The measurement of adherence to PA promotion programs might help to understand participants’ attitude toward exercise and PA (Hawley-Hague et al. 2016).
Therefore, the aim of this study was to examine the feasibility, adherence over time, and acceptance of a home-based program to promote PA in older persons with CI following geriatric rehabilitation.
Methods
Study design
This feasibility study is part of a blinded, randomized-controlled 12-week intervention trial (RCT) with a 12-week follow-up period to improve functional fitness and to promote PA in older persons with CI after discharge from ward-rehabilitation (ISRCTN82378327). The RCT was approved by the ethics committee of the Medical Department of the University of Heidelberg and was conducted according to the Declaration of Helsinki. The present study uses patient-reported data of adherence and feasibility from participants allocated to the intervention group (IG).
Participants
Participants were consecutively recruited from geriatric rehabilitation wards of a German geriatric hospital. Inclusion criteria were: age ≥ 65 years, a score of 17–26 on the Mini-Mental State Examination (MMSE; Folstein et al. 1975) indicating mild to moderate cognitive impairment (O’Bryant et al. 2008), ability to walk ≥ 4 m without a walking aid, residence within 30 km of the study center, discharge to the patient’s home, no terminal disease, no delirium, German-speaking, and written informed consent.
Intervention
The home-based intervention program included (1) physical training to improve functional fitness and (2) a comprehensive motivational concept to promote PA behavior. The intervention was implemented by skilled trainers (sport scientists) within five home visits with decreasing frequency and weekly phone calls beginning after the second home visit.
Physical training
The physical training included strength (tiptoe stance, stair climbing, sit-to-stand transfers), static balance exercises (i.e., side by side, semi-tandem, tandem), and outdoor walking. During the first home visit, participants were introduced in the training program by the trainer. To set graded tasks, the physical exercises were adjusted to participants’ individual needs and functional abilities. To remind and instruct participants to execute the physical training, a large poster, containing pictures of the exercises and advices about safety, was fixed in a central position of the home. Participants were encouraged to autonomously perform the physical training and outdoor walking each day. More detailed information about the study design and the physical training has been published previously (Bongartz et al. 2017).
Motivational concept
To increase motivation and adherence to PA, theory-based behavioral change techniques (Abraham and Michie 2008) have been adapted to a CI-specific comprehensive motivational approach, which encompassed following strategies:
Provision of information about the benefits of regular training and PA (manual, trainer)
Encouragement to engage in daily activity (CI-specific communication by trainer within home visits)
Provision of instructions about how and where to perform the exercises (given by the trainer during home visits, poster)
Setting graded tasks (tailoring of the intervention to participants’ needs and functional abilities)
Goal-setting and planning of a walking path in participants’ home environment to transfer activity goals in specific and measurable behavior (structured procedure to set indoor and outdoor walking goals; planning and realization of the walking path during home visits)
Self-monitoring of behavioral outcomes (pedometer, training log)
Regular review of goals (pedometer, training log)
Barrier identification and problem solving (home visits, phone calls)
Feedback and positive reinforcement (home visits, phone calls)
These nine specific motivational strategies were conflated to the three core motivational components of goal-setting, self-monitoring, and social support. Since the strategy of setting graded tasks is an inherent feature of the individually tailored physical training, this strategy was described as part of the physical training program.
Goal-setting
To involve participants in the goal-setting process, a blinded assessor performed a modified version of Talking Mats (Murphy et al. 2005), which is a semi-structured and pictorial measurement tool developed for cognitively impaired individuals with communication disability. To identify individual meaningful activity goals, each participant was asked to rank prescribed indoor (cooking; self-care; daily activities/homework; self-formulated activity) and outdoor activities (gardening/letterbox; doctor/pharmacy; grocery/bakery/post office; self-formulated activity), which were visualized by pictures.
Since key motor functions such as standing, walking, sit-to-stand transfer, and walking stairs are major prerequisites of mobility, participants additionally evaluated their importance, self-perceived competence, and chance of success on a visual analog scale with range of 0–100. The evaluation of importance refers to the individual value of the key motor function, and the perceived competence captures beliefs about own abilities to perform the corresponding function. The chance of success reflects participants’ view to which degree they can improve based on the estimated levels of importance and competence.
Based on the evaluation of the predefined target activity (e.g., going to the bakery) and the key motor functions during the first home visit, trainer and participants collaboratively planned an individualized walking path in the environment around participants’ home. As part of the training sessions, the walking path was practiced by participants under the supervision of the trainer and its feasibility was tested with respect to the distance, environmental characteristics (e.g., slippery ground, stairs), and participants’ need for assistive devices.
Self-monitoring
To provide feedback about walking behavior and to increase the motivation to engage in daily walking, participants were encouraged to monitor daily step counts by use of simple pedometers (YAMAX Digiwalker CW 700). The trainer instructed participants how to use the pedometer and gave haptic support for attaching it on the waist. The training log was also applied to derive information about personal progress and daily execution of physical training and walking. If participants failed to achieve daily execution of training and the defined walking path, trainer and participant collaboratively tried to identify barriers hampering regular walking and solutions to overcome these barriers during the phone calls and home visits.
Social support
Social support in our study was delivered by trainers within the home visits and phone calls. The professionals served as credible source for information about benefits of regular training and PA by means of a CI-specific communication, which involved simple and repetitively used instructions, haptic support, and picturesque language to encourage persons to integrate training in daily routines (Hauer et al. 2012). Positive feedback was given by the trainer to reinforce participants to maintain their efforts (9). Participation in a local exercise group in a geriatric hospital was offered to each participant as an additional option for regular training and social support by peers.
Measurements
Outcome measures
Main outcomes of the present study were the a) feasibility of goal-setting and professional social support (home visits, phone calls), b) adherence to training and the motivational strategies of goal-setting and self-monitoring by pedometers, and c) patient-rated feasibility and effectiveness of the program.
Feasibility of social support and goal-setting
The feasibility of goal-setting was defined as the proportion of participants (in %), who were able to nominate at least one activity. Comprehensive feasibility was achieved, if participants nominated both indoor and outdoor goals. Partial feasibility was achieved, if participants nominated at least one activity goal. To determine the feasibility of professional social support provided by the research team, the number of performed home visits and phone calls were documented by trainers in standardized protocols.
Adherence to training and motivational strategies
Adherence was documented by participants throughout the 12-week intervention by use of training logs in a simple calendar format. Participants were instructed to daily record the execution of physical training sessions, outdoor walking sessions, achievement of walking goals, and execution of self-monitoring via the pedometer. The amount of step counts was not included as a measure of adherence due to possible inaccuracy regarding the detection of step counts by this simple piezoelectric pedometer. The training logs were returned at the end of the intervention period. The mean weekly adherence rates (in %) to physical training, outdoor walking, achieved walking goals, and pedometer-use were calculated as: number of sessions executed / number of possible sessions * 100. These adherence rates are reported at week 2, 12, and for the total intervention period. As in some cases, the introduction of the pedometer and the implementation of the walking path were performed in the second training session, we defined week 2 as the baseline reference of the intervention. Based on adherence rates, participants were classified as persons with (i) low adherence (0.0–33.3%), (ii) moderate adherence (33.4–66.6%), and (iii) high adherence (66.7–100%).
Acceptance
After the intervention, participants completed a questionnaire specifically designed to document participants’ acceptance of the program components (training, outdoor walking, self-monitoring by pedometers, walking-related goal-setting, training group, home visits, phone calls) capturing the two subscales of perceived feasibility (e.g., “Were you able to use the pedometer?”) and effectiveness (e.g., “Did the pedometer help you to improve yourself?”). Subjects responded on a 4-point Likert scale with a range of 1 (“not”), 2 (“rather not”), 3 (“rather yes”), and 4 (“yes”). The components’ feasibility/effectiveness was classified according to the amount of participants with a score ≥ 3 for the respective component and expressed in percentage.
Descriptive variables
Socio-demographic and clinical characteristics including age, gender, body mass index (BMI), number of medications, number of diagnoses, and education were documented from patient charts or by standardized interviews. Education was categorized as primary school or none (low educational level), vocational or other secondary school (middle educational level), and university or vocational postsecondary school (high educational level). Functional fitness was assessed by the Short Physical Performance Battery (SPPB) (Guralnik et al. 1994). For PA behavior, the number of steps was measured within 48 h using an established, ambulatory sensor system (PAMSys™, BioSensics, Cambridge, MA, USA), based on an algorithm validated among older adults (Najafi et al. 2003). To capture different aspects of psychological status, apathy was assessed via the Apathy Evaluation Scale-Clinical Version (AES-C) (Marin et al. 1991) and depression by the 15-item Geriatric Depression Scale (GDS) (Allgaier et al. 2011; Greenberg 2007). To describe the proportion of persons with clinically relevant apathetic syndrome, a cutoff score (>40.5; range 18–72) was used which has been ascertained within a cognitively impaired sample of community-dwelling elderly (Clarke et al. 2007).
Statistical analyses
Descriptive data were presented as frequencies and percentages for categorical variables, means (M), and standard deviations (SD) or medians and interquartile ranges (IQR) for continuous variables. Unpaired t tests, Mann–Whitney U-tests, and Chi-square tests were used for baseline comparison between dropouts and completers according to the data distribution. Paired t tests were performed to test for differences regarding mean adherence rates between beginning (week 2) and end of intervention (week 12).
Results
Participant characteristics
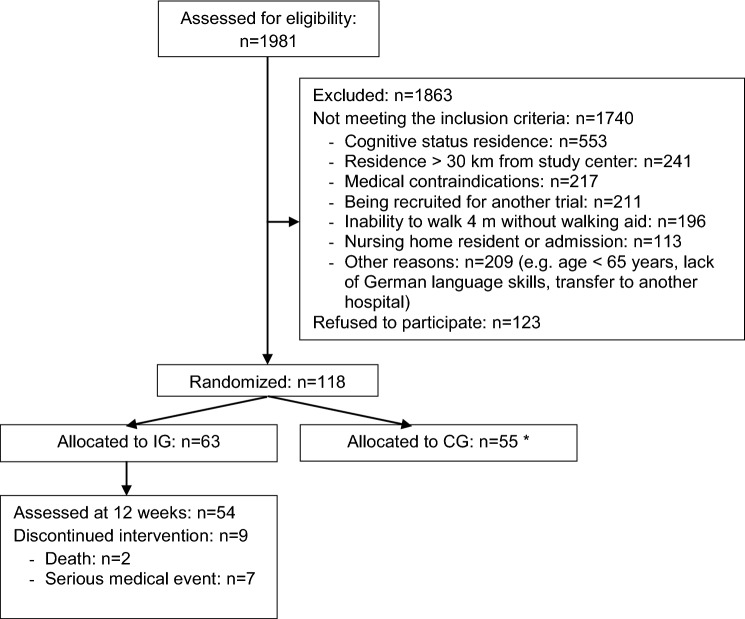
Out of 1981 patients screened for eligibility, 118 individuals were enrolled to the original RCT according to predefined inclusion criteria, of whom 63 participants were allocated to IG (see Fig. 1). The present sample comprised multi-morbid, sedentary, cognitively, and physically impaired older adults with more than the half showing apathetic symptoms (for sample description see Table 1).
Fig. 1.
Flowchart of the recruitment process and course of the intervention trial. IG intervention group. CG control group, *As the participants in the CG did not receive the physical training and motivational strategies to promote physical activity as the core of the present article, the CG was not included in the analysis
Table 1.
Baseline characteristics of intervention group
| Characteristics | Intervention group (N = 63) |
|---|---|
| Age (years), M (SD) | 82.2 (5.8) |
| Sex, females, n (%) | 48 (76.2) |
| Educational level (low/middle/high), % | 33.3/47.6/19.0 |
| MMSE, score (range 0–30), M (SD) | 23.3 (2.7) |
| Number of diagnoses, M (SD) | 11.3 (3.6) |
| Number of medication, M (SD) | 9.4 (3.3) |
| SPPB, total score (range 0–12), M (SD) | 5.4 (2.1) |
| Habitual gait velocity (m/s), M (SD) | 0.50 (0.21) |
| Number of steps/day, Mdn (IQR)a | 2662.5 (1175.1–4686.5) |
| Geriatric Depression Scale, score (range 0–15), M (SD) | 5.2 (3.0) |
| The presence of apathetic symptoms (AES-C score > 40.5), n (%) | 33 (52.4) |
M mean, SD standard deviation, Mdn median, IQR interquartile range, MMSE mini-mental state examination; SPPB short physical performance battery; m/s meters per second, AES-C apathy evaluation score clinical version
an = 62, missing sensor-based activity data for one person
Fifty-four subjects completed the intervention program and were therefore eligible for analyses of adherence and acceptance of the program. Reasons for dropout were death, fall-related fractures, and other serious medical events unrelated to the intervention program. When dropouts (n = 9) were compared with those participants who stayed in the study until the end of the intervention (n = 54), no significant differences in baseline characteristics were found (p > .05), except for age (M = 86.2, SD = 4.0 years [dropouts] vs. M = 81.5, SD = 5.9 years [completers]; p < .025) and physical performance (SPPB total score, M = 4.0, SD = 1.3 [dropouts] vs. M = 5.7, SD = 2.2 [completers]; p < .030).
Feasibility of social support and goal-setting
In those participants completing the study, in median 5 (IQR = 5–5) home visits and 9 (IQR = 8–10) telephone calls were conducted. Goal-setting was feasible for 62 participants. Comprehensive feasibility was achieved in 57 individuals and partial feasibility in 4 persons. Due to cognitive deficits, one participant was not able to engage in goal-setting. Mean time to complete goal-setting was 11.3 min (SD = 3.1), with a range of 6–19 min.
More than half of the participants (n = 35, 55.6%) rated “washing and having a shower” the most important indoor activity, followed by “cooking a meal” (n = 21, 33.3%) and “doing housework” (n = 4; 6.3%). Going to the “doctor/pharmacy” was the most rated out-of-home-activity (n = 24, 38.1%). Just slightly fewer participants ranked “doing shopping” the highest priority outdoor walking (n = 18, 28.6%). Key motor functions were rated as highly important, while the level of perceived competence was comparatively low. The chance of success to improve present abilities was in between the level of perceived competence and the level of importance (see Table 2).
Table 2.
Subjective importance, competence, and chance of success of key motor functions
| Importance (range 0–100) | Competence (range 0–100) | Chance of success (range 0–100) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | M | (SD) | n | M | (SD) | n | M | (SD) | |
| Standing | 56 | 83.0 | (17.2) | 62 | 55.6 | (22.0) | 61 | 73.4 | (20.2) |
| Walking | 59 | 84.2 | (20.4) | 62 | 53.0 | (23.0) | 58 | 72.5 | (19.2) |
| Sit-to-stand transfer | 59 | 86.6 | (13.4) | 62 | 61.6 | (21.4) | 58 | 79.2 | (17.4) |
| Walking stairs | 59 | 82.7 | (23.3) | 62 | 52.0 | (25.0) | 58 | 72.1 | (21.1) |
M mean, SD standard deviation
Adherence
Of the 54 study completers, three subjects were excluded from analyses of adherence to training and motivational strategies due to impaired vision (n = 1), not able to write in German language without support by others (n = 1), and loss of training log (n = 1).
Adherence to physical training and outdoor walking
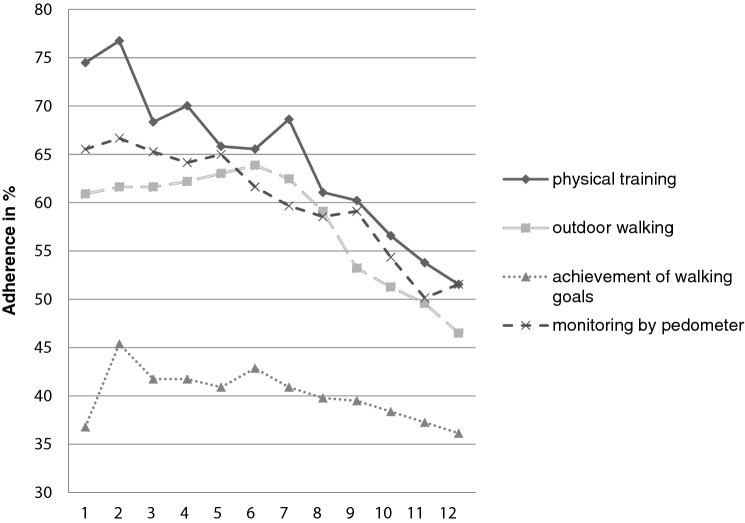
Mean adherence rates over the total 12-week intervention period were 63.6% (SD = 33.8) for physical training and 57.9% (SD = 35.2) for outdoor walking, with about half of the participants exhibiting high (≥ 66.7%) adherence rates (physical training: n = 26, 51.0%; outdoor walking: n = 23, 45.1%). Adherence to physical training and outdoor walking was highest at the beginning of the intervention, showing significant declines over the intervention period (p ≤ .001–.019) (see Fig. 1; see Table 3).
Table 3.
Adherence to physical training, outdoor walking, and motivational strategies over the total intervention period, at week 2, and week 12
| Adherence Parameters | Total (Week 1–12) | Week 2 | Week 12 | Difference week 2 vs. 12 |
|---|---|---|---|---|
| M % (SD) | M % (SD) | M % (SD) | p value | |
| Training | 63.6 (33.8) | 76.8 (32.7) | 51.5 (47.9) | < .001*** |
| Outdoor walking | 57.9 (35.2) | 61.6 (38.2) | 46.5 (45.1) | .019* |
| Achievement of walking goals | 40.1 (38.5) | 43.4 (40.7) | 36.1 (46.4) | .098 |
| Self-monitoring by pedometers | 60.1 (37.7) | 66.7 (41.8) | 51.5 (49.5) | .017* |
M mean, SD standard deviation; adherence as mean percentage during total intervention, week 2 and week 12 in intervention group with complete adherence data based on training log (n=51); *p < .05, **p < .01, ***p < .001 based on paired t tests to test for differences between week 2 and 12
Adherence to motivational strategies
Mean adherence rates over the intervention period were 40.1% (SD = 38.5) for achievement of walking goals and 60.1% (SD = 37.7) for self-monitoring by pedometers, with half of the participants displaying high adherence to self-monitoring (n = 26, 51.0%) and 18 individuals (35.4%) highly adhering to goal achievement. Adherence rates to self-monitoring by pedometers (M = 66.7%, SD = 41.8) and goal achievement (M = 43.4, SD = 40.7) were also highest at the intervention start and decreased over time, whereas the decline was significant for self-monitoring by pedometers (p = .017) and was close to level of significance for goal achievement (p = .098) (Fig. 2).
Fig. 2.
Weekly adherence to the home-based physical activity promotion program
Acceptance
Questionnaire data to evaluate the patient-rated acceptance of the program components were available for 53 participants, as one study completer did not send back the questionnaire. Regarding the feasibility, the highest rated program component was physical training (M = 3.3, SD = 0.9, 83%).
The components walking-related goal-setting (M = 3.0, SD = 1.2, 73.0%), self-monitoring by pedometers (M = 3.1, SD = 1.2, 68.2%), and outdoor walking (M = 2.9, SD = 1.1, 67.9%) were also rated to be feasible by the majority of the present sample. The participation in the training group was only rated as feasible by six persons (M = 1.3, SD = 1.0, 11.8%), but those who were able to participate in the center-based group rated this component as effective (M = 3.7, SD = 0.8, 85.7%). Home visits (M = 3.6, SD = 0.8, 90.6%) and phone calls (M = 3.1, SD = 1.1, 79.2%), which were delivered by professionals, achieved highest rates for effectiveness. Self-monitoring by pedometers was the component rated as the least effective (M = 2.9, SD = 1.2, 63.5%) (see Table 4).
Table 4.
Subjective feasibility and efficacy of program components
| Intervention component | M (SD) (range 1–4) | % of participants with feasibility score ≥ 3 | M (SD) (range 1–4) | % of participants with effectiveness score ≥ 3 |
|---|---|---|---|---|
| Physical training | 3.3 (1.0) | 83.0a | 3.4 (1.0) | 78.3a |
| Outdoor walking | 2.9 (1.1) | 67.9a | 3.1 (1.1) | 75.0b |
| Goal-Setting | 3.0 (1.1) | 73.0c | 3.1 (1.1) | 75.0b |
| Self-monitoring by pedometers | 3.1 (1.2) | 68.2d | 2.9 (1.2) | 63.5d |
| Training group | 1.3 (1.0) | 11.3e | 3.7 (0.8) | 85.7f |
| Home visits | n.a. | n.a. | 3.6 (0.8) | 90.6b |
| Phone calls | n.a. | n.a. | 3.1 (1.1) | 79.2b |
Presented are mean scores and standard deviations on the feasibility and effectiveness of the implemented program components as well as the proportion of participants with scores ≥ 3
M mean, SD standard deviation, n.a. not applied
an = 53; bn = 52, cn = 48; dn = 50, en = 47; fn = 7
Discussion
The present results describe a successful home-based program to promote PA in cognitively and physically restricted community-dwelling older persons post-discharge from ward-rehabilitation. All program components achieved high acceptance leading to an initially high adherence to autonomously performed physical training, outdoor walking, and to the frequent uptake of the motivational strategies of goal-setting and self-monitoring which declined over time.
Physical training and outdoor walking
Adherence to home-based, self-performed physical training was initially high, indicating good feasibility of the individually tailored home-based approach. Within a previous home-based physical exercise program following discharge from rehabilitation including a mixed sample concerning cognitive status, the adherence in individuals with CI was lower compared to their cognitively intact counterparts (Moseley et al. 2009). This emphasizes the challenge to ensure high adherence to autonomously performed physical training in this vulnerable group.
Study results were comparable to the adherence rates of an exercise in sample of cognitively impaired and non-impaired individuals following discharge from rehabilitation (Salpakoski et al. 2014), to caregiver-supported functional training (Prick et al. 2016; Suttanon et al. 2013; Taylor et al. 2017), and walking programs in community-dwelling persons with CI (Lowery et al. 2014; McCurry et al. 2005).
We assume that the individual tailoring of the physical training and planning of a walking path respective to participants’ physical abilities and environmental conditions as applied in the present study potentially facilitated high initial adherence. Study participants lived in the urban area in and around the city of Heidelberg, a university town with no major environmental barriers, easy access to public transportation and a wide variety of green areas. These environmental characteristics refer to a high perceived walkability which is associated with low concerns to fall and higher levels of PA (Harada et al. 2017). The initially high adherence to training declined over time simultaneously with the decreasing frequency of our home visits confirming decreasing adherence rates in previous PA studies in diverse samples of older adults (Cox et al. 2013; Taylor et al. 2017). This finding might indicate the need of continuous supervision of training especially in multi-morbid populations.
Motivational strategies
The autonomous engagement in PA without any external supervision has so far been promoted only in populations with subjective memory complaints, representing cognitively less affected individuals (Cox et al. 2013; Dannhauser et al. 2014; Lautenschlager et al. 2008). Only one of these studies tested the adherence to a comprehensive motivational approach by reporting the completion rate of worksheets in a PA promotion program, but not the adherence to the specific strategies, documenting a modest adoption of a motivational concept into participants’ daily routines (Cox et al. 2013). In contrast, the present study allows a detailed insight into different motivational strategies, documenting a comparatively high feasibility and adherence considering the sample of sedentary older adults with low functional fitness, moderate depressive, and high apathetic symptoms. Deteriorating physical function was identified as main barrier for adherence to a PA program in older adults after discharge from ward-rehabilitation (Fairhall et al. 2012). Depressive symptoms and apathy are psychological correlates showing close associations with decreased levels of PA among individuals with CI (David et al. 2012; Yuenyongchaiwat et al. 2018). In consideration of these PA-related barriers in our vulnerable sample, the adherence to motivational strategies over the intervention period can be rated as high.
Goal-setting
The comprehensive approach of goal-setting, including the identification of goals as measure of feasibility and the achievement of goals as measure of adherence, showed heterogeneous results in the present study. The high rate of identified goals indicated feasibility of the structured goal-setting approach, while the rate of achieved goals was considerably lower. These results are in line with the studies testing the feasibility and the adherence of goal-setting in diverse older populations including persons with CI (Kerse et al. 2008) and without CI (Fairhall et al. 2012; Smit et al. 2018; van Seben et al. 2019).
The present positive results with respect to the identification of goals in the present study indicate that individuals with CI may have profited from the use of talking mats as previously demonstrated (Murphy et al 2005), representing a structured, nonverbal strategy, while more complex instruments have shown limited feasibility in patients with CI (Stevens et al. 2013). With the talking mat method also problems in goal-setting were avoided as experienced by stroke patients with communicative and cognitive problems, which reported the goal-setting process be driven by the therapist rather than participants (Smit et al. 2018). Considering those findings, it was remarkable that the participants of the present study were able to give reliable information about the priority of goals and the subjective importance, control, and chance of success of key motor functions. Study results contrast to a previous study which reported problems to formulate specific and realistic behavioral goals in reports of geriatric patients without CI (van Seben et al. 2019).
While the identification of subjective goals represents a relevant but not sufficient objective, it is the transfer into action which is crucial to test the effectiveness of motivational strategies. In the present study, the rate of achieved walking goals was moderate, confirming previous studies on adherence to walking goals in older adults without (Fairhall et al. 2012) and with CI (Kerse et al. 2008). The discrepancy between high rate of identified goals and a moderate rate of achieved walking goals may emphasize the difficulty to implement the initially set goals into daily routines due to the high physical restrictions in the group of geriatric patients with CI. The high presence of functional deficits in the transitional stage after admission to home environment may have led to a shift of goals over time, as some individuals tended to choose too ambitious long-term goals after discharge from ward-rehabilitation given their physical abilities (van Seben et al. 2019). These individuals may have set their activity goals based on their prior abilities to perform activities of daily living before hospitalization, which resulted in these goals being unrealistic in the stage post-discharge from ward-rehabilitation. This could also be true for some individuals in the present study who highly ranked outdoor goals (e.g., going to the doctor), which were not viable for them in consideration of their degree of functional restrictions.
The only moderate rate of adherence to walking goals over the intervention period might explain the limited effects of goal-setting on physical function during geriatric inpatient rehabilitation, as demonstrated by a recent meta-analysis (Smit et al. 2019). Therefore, future interventions might incorporate standardized instruments to continuously monitor the adaption of initially set goals, which could help to optimize strategies to realize walking goals into practice and therefore to increase adherence to goal-setting over time.
Self-monitoring by pedometers
In the present study, self-monitoring of activity behavior by pedometers was shown to be a useful tool to increase motivation to PA for most participants as indicated by high adherence and acceptance.
Previous pedometer-based interventions to promote PA in persons with CI showed heterogeneous results with respect to the impact on PA, albeit they did not test the daily adherence (Logsdon et al. 2009; Vidoni et al. 2016). In one study, a high rate of dropouts limited the efficacy of a technology-assisted pedometer driven intervention, also leading to limited efficacy (Vidoni et al. 2016). The study by Logsdon et al (2009) also reported a restricted manageability of such technical advices due the lack of fine motor skills and memory complaints in some participants with more severe CI, whereas usability was adequate for the majority of participants leading to increased PA-levels. Since the primary purpose of the pedometer was to provide feedback and to increase the participant’s motivation rather than measuring the true walking distance, we selected simple to use and robust pedometers to achieve high self-perceived feasibility.
Social support
In the present study, professional social support as documented for home visits and telephone calls was highly appreciated in the vulnerable group of participants, confirming results from previous qualitative studies conducted in persons with CI (Olsen et al. 2015; Phillips and Flesner 2013; Suttanon et al. 2012). The face-to-face contact within home visits was rated as the most effective program component in this PA program. This finding indicates that health care professionals may serve as a valuable source to provide information how to perform training and which benefits can be expected (Olsen et al. 2015). High subjective effectiveness of phone calls also supports previous results which also demonstrated high satisfaction with phone calls in older adults without CI (Rosenberg et al. 2012) and with subjective CI (Cox et al. 2013). The trainers in our program were skilled in CI-specific communication, which might be a key strategy of the successful implementation of home visits as rated by participants. Positively framed messages focusing on the benefits of PA behavior may have induced elevated motivation to PA compared to negatively framed messages informing about the risk of being non-active (Notthoff et al. 2016). Such supportive communication, including positive reinforcements, has the potential to strengthen the perceived competence to exercise in a group of cognitively impaired persons (Tortosa-Martínez et al. 2017).
The provision of frequent external sources of motivation as provided by a more continuous supervision might be a key factor for the maintenance of adherence to PA in this trial. The program implementation by peers or lay trainers as trustful training partners and motivators is recommended as a promising strategy to increase the participant’s self-efficacy and to ensure maintenance of PA within older adults (Matz-Costa et al. 2019). A regular frequency of contact with professionals was effective in PA promotion in nursing home residents (Jansen et al. 2015) as well as in sedentary community-dwelling women (Poulsen et al. 2007).
Limitations
One limitation of the present study is the relatively small sample size explicitly including participants of the intervention group. As the control group was not included in the intervention program, no information about adherence was available in the control condition. Our analysis of adherence was based on self-reports which may represent a limiting factor for the accuracy of adherence to PA in elderly with CI (Visser et al. 2014). As adherence to training and outdoor walking only included the daily frequency of exercise, physical training, and outdoor activities executed several times per day were not considered, with the potential consequence of underreporting actually performed PA.
Conclusion
Study results indicated a comparatively high adherence to the present PA promotion program proving the feasibility of the individually tailored program in vulnerable, multi-morbid persons with relevant motor and cognitive impairment. Successful participation was further documented by high patient-rated feasibility and effectiveness of the program components. Continuous supervision delivered by professionals might constitute an essential ingredient for maintaining adherence to PA promotion in elderly with multiple restrictions, as this study revealed a decline of adherence to the intervention program concomitant with decreasing frequency of home visits.
Acknowledgments
This work was funded by the Social and Private Long-Term Care Insurance (Soziale und Private Pflegeversicherung) and the Municipal Association for Youth and Social Affairs in Baden-Württemberg (Kommunalverband für Jugend und Soziales Baden-Württemberg) (Grant No: 80221-208-009-01-01). Funders had no role in study concept and design, data collection, analysis and interpretation, and preparation of the manuscript.
Compliance with ethical standards
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Responsible editor: Matthias Kliegel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tobias Eckert, Email: tobias.eckert@bethanien-heidelberg.de.
Martin Bongartz, Email: bongartz@nar.uni-heidelberg.de.
Phoebe Ullrich, Email: phoebe.koepp@bethanien-heidelberg.de.
Bastian Abel, Email: bastian.abel@bethanien-heidelberg.de.
Werner Christian, Email: christian.werner@bethanien-heidelberg.de.
Rainer Kiss, Email: rainer.kiss@posteo.de.
Klaus Hauer, Email: khauer@bethanien-heidelberg.de.
References
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008;27:379. doi: 10.1037/0278-6133.27.3.379. [DOI] [PubMed] [Google Scholar]
- Allgaier AK, Kramer D, Mergl R, Fejtkova S, Hegerl U. Validity of the geriatric depression scale in nursing home residents: comparison of GDS-15, GDS-8, and GDS-4. Psychiatr Prax. 2011;38:280–286. doi: 10.1055/s-0030-1266105. [DOI] [PubMed] [Google Scholar]
- Bongartz M, Kiss R, Ullrich P, Eckert T, Bauer J, Hauer K. Development of a home-based training program for post-ward geriatric rehabilitation patients with cognitive impairment: study protocol of a randomized-controlled trail. BMC Geriatr. 2017;17:214. doi: 10.1186/s12877-017-0615-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Roth DL, Allman RM, Sawyer P, Ritchie CS, Roseman JM. Trajectories of life-space mobility after hospitalization. Ann Intern Med. 2009;150:372–378. doi: 10.7326/0003-4819-150-6-200903170-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth L, Kable A, Pond D. Research in hospital discharge procedures addresses gaps in care continuity in the community, but leaves gaping holes for people with dementia: a review of the literature. Australas J Ageing. 2015;34:9–14. doi: 10.1111/ajag.12205. [DOI] [PubMed] [Google Scholar]
- Clarke DE, Reekum R, Simard M, Streiner DL, Freedman M, Conn D. Apathy in dementia: an examination of the psychometric properties of the apathy evaluation scale. J Neuropsychiatry Clin Neurosci. 2007;19:57–64. doi: 10.1176/jnp.2007.19.1.57. [DOI] [PubMed] [Google Scholar]
- Cox KL, et al. The FABS trial: a randomised control trial of the effects of a 6-month physical activity intervention on adherence and long-term physical activity and self-efficacy in older adults with memory complaints. Prev Med. 2013;57:824–830. doi: 10.1016/j.ypmed.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Dannhauser TM, Cleverley M, Whitfield TJ, Fletcher BC, Stevens T, Walker Z. A complex multimodal activity intervention to reduce the risk of dementia in mild cognitive impairment–ThinkingFit: pilot and feasibility study for a randomized controlled trial. BMC Psychiatry. 2014;14:129. doi: 10.1186/1471-244x-14-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R, et al. Decreased daytime motor activity associated with apathy in Alzheimer disease: an actigraphic study. Am J Geriatr Psychiatry. 2012;20:806–814. doi: 10.1097/JGP.0b013e31823038af. [DOI] [PubMed] [Google Scholar]
- Fairhall N, Sherrington C, Kurrle SE, Lord SR, Lockwood K, Cameron ID. Effect of a multifactorial interdisciplinary intervention on mobility-related disability in frail older people: randomised controlled trial. BMC Med. 2012;10:120. doi: 10.1186/1741-7015-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Greenberg SA (2007) How to try this: the geriatric depression scale: short form. Am J Nurs 107:60–69; quiz 69–70. doi:10.1097/01.NAJ.0000292204.52313.f3 [DOI] [PubMed]
- Guralnik JM, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- Harada K, Park H, Lee S, Shimada H, Yoshida D, Anan Y, Suzuki T. Joint association of neighborhood environment and fear of falling on physical activity among frail older adults. J Aging Phys Act. 2017;25(1):140–148. doi: 10.1123/japa.2016-0082. [DOI] [PubMed] [Google Scholar]
- Hauer K, Schwenk M, Zieschang T, Essig M, Becker C, Oster P. Physical training improves motor performance in people with dementia: a randomized controlled trial. J Am Geriatr Soc. 2012;60:8–15. doi: 10.1111/j.1532-5415.2011.03778.x. [DOI] [PubMed] [Google Scholar]
- Hauer K, Ullrich P, Dutzi I, Beurskens R, Kern S, Bauer J, Schwenk M. Effects of standardized home training in patients with cognitive impairment following geriatric rehabilitation: a randomized controlled pilot study. Gerontology. 2017;63:495–506. doi: 10.1159/000478263. [DOI] [PubMed] [Google Scholar]
- Hawley-Hague H, Horne M, Skelton DA, Todd C. Review of how we should define (and measure) adherence in studies examining older adults’ participation in exercise classes. BMJ Open. 2016;6:e011560. doi: 10.1136/bmjopen-2016-011560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Jacus JP. Awareness, apathy, and depression in Alzheimer’s disease and mild cognitive impairment. Brain Behav. 2017;7:e00661. doi: 10.1002/brb3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen CP, Classen K, Wahl HW, Hauer K. Effects of interventions on physical activity in nursing home residents. Eur J Ageing. 2015;12:261–271. doi: 10.1007/s10433-015-0344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerse N, et al. Does a functional activity programme improve function, quality of life, and falls for residents in long term care? Cluster randomised controlled trial. BMJ. 2008;337:a1445. doi: 10.1136/bmj.a1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager NT, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- Logsdon RG, McCurry SM, Pike KC, Teri L. Making physical activity accessible to older adults with memory loss: a feasibility study. Gerontologist. 2009;49(Suppl 1):S94–S99. doi: 10.1093/geront/gnp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery D, et al. The effect of exercise on behavioural and psychological symptoms of dementia: the EVIDEM-E randomised controlled clinical trial. Int J Geriatr Psychiatry. 2014;29:819–827. doi: 10.1002/gps.4062. [DOI] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-V. [DOI] [PubMed] [Google Scholar]
- Matz-Costa C, Howard EP, Castaneda-Sceppa C, Diaz-Valdes Iriarte A, Lachman ME. Peer-based strategies to support physical activity interventions for older adults: A typology, conceptual framework, and practice guidelines. The Gerontologist. 2019;59(6):1007–1016. doi: 10.1093/geront/gny092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry SM, Gibbons LE, Logsdon RG, Vitiello MV, Teri L. Nighttime insomnia treatment and education for Alzheimer’s disease: a randomized, controlled trial. J Am Geriatr Soc. 2005;53:793–802. doi: 10.1111/j.1532-5415.2005.53252.x. [DOI] [PubMed] [Google Scholar]
- McGilton KS, Chu CH, Naglie G, van Wyk PM, Stewart S, Davis AM. Factors influencing outcomes of older adults after undergoing rehabilitation for hip fracture. J Am Geriatr Soc. 2016;64:1601–1609. doi: 10.1111/jgs.14297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley AM, Sherrington C, Lord SR, Barraclough E, St George RJ, Cameron ID. Mobility training after hip fracture: a randomised controlled trial. Age Ageing. 2009;38:74–80. doi: 10.1093/ageing/afn217. [DOI] [PubMed] [Google Scholar]
- Murphy J, Tester S, Hubbard G, Downs M, MacDonald C. Enabling frail older people with a communication difficulty to express their views: the use of talking mats as an interview tool. Health Soc Care Community. 2005;13:95–107. doi: 10.1111/j.1365-2524.2005.00528.x. [DOI] [PubMed] [Google Scholar]
- Najafi B, Aminian K, Paraschiv-Ionescu A, Loew F, Bula CJ, Robert P. Ambulatory system for human motion analysis using a kinematic sensor: monitoring of daily physical activity in the elderly. IEEE Trans Biomed Eng. 2003;50:711–723. doi: 10.1109/tbme.2003.812189. [DOI] [PubMed] [Google Scholar]
- Notthoff N, Klomp P, Doerwald F, Scheibe S. Positive messages enhance older adults’ motivation and recognition memory for physical activity programmes. Eur J Ageing. 2016;13:251–257. doi: 10.1007/s10433-016-0368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman SR, Adamczewska N, Howlett N. Systematic review of behaviour change techniques to promote participation in physical activity among people with dementia. Br J Health Psycho. 2018;23:148–170. doi: 10.1111/bjhp.12279. [DOI] [PubMed] [Google Scholar]
- O’Bryant SE, Humphreys JD, Smith GE, Ivnik RJ, Graff-Radford NR, Petersen RC, Lucas JA. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol. 2008;65(7):963–967. doi: 10.1001/archneur.65.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CF, Telenius EW, Engedal K, Bergland A. Increased self-efficacy: the experience of high-intensity exercise of nursing home residents with dementia—a qualitative study. BMC Health Serv Res. 2015;15:379. doi: 10.1186/s12913-015-1041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LJ, Flesner M. Perspectives and experiences related to physical activity of elders in long-term-care settings. J Aging Phys Act. 2013;21:33–50. doi: 10.1123/japa.21.1.33. [DOI] [PubMed] [Google Scholar]
- Portegijs E, Rantakokko M, Mikkola TM, Viljanen A, Rantanen T. Association between physical performance and sense of autonomy in outdoor activities and life-space mobility in community-dwelling older people. J Am Geriatr Soc. 2014;62:615–621. doi: 10.1111/jgs.12763. [DOI] [PubMed] [Google Scholar]
- Poulsen T, Elkjaer E, Vass M, Hendriksen C, Avlund K. Promoting physical activity in older adults by education of home visitors. Eur J Ageing. 2007;4:115–124. doi: 10.1007/s10433-007-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prick A-E, de Lange J, Scherder E, Twisk J, Pot AM. The effects of a multicomponent dyadic intervention on the mood, behavior, and physical health of people with dementia: a randomized controlled trial. Clin Interv Aging. 2016;11:383. doi: 10.2147/cia.s95789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DE, Kerr J, Sallis JF, Norman GJ, Calfas K, Patrick K. Promoting walking among older adults living in retirement communities. J Aging Phys Act. 2012;20:379–394. doi: 10.1123/japa.20.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpakoski A, et al. Effects of a multicomponent home-based physical rehabilitation program on mobility recovery after hip fracture: a randomized controlled trial. J Am Med Dir Assoc. 2014;15:361–368. doi: 10.1016/j.jamda.2013.12.083. [DOI] [PubMed] [Google Scholar]
- Smit EB, Bouwstra H, van der Wouden JC, Wattel LM, Hertogh CM. Patient-centred goal setting using functional outcome measures in geriatric rehabilitation: is it feasible? Eur Geriatr Med. 2018;9:71–76. doi: 10.1007/s41999-017-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit EB, Bouwstra H, Hertogh CM, Wattel EM, van der Wouden JC. Goal-setting in geriatric rehabilitation: a systematic review and meta-analysis. Clin Rehabil. 2019;33:395–407. doi: 10.1177/0269215518818224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A, Beurskens A, Köke A, van der Weijden T. The use of patient-specific measurement instruments in the process of goal-setting: a systematic review of available instruments and their feasibility. Clin Rehabil. 2013;27(11):1005–1019. doi: 10.1177/0269215513490178. [DOI] [PubMed] [Google Scholar]
- Suttanon P, Hill KD, Said CM, Byrne KN, Dodd KJ. Factors influencing commencement and adherence to a home-based balance exercise program for reducing risk of falls: perceptions of people with Alzheimer’s disease and their caregivers. Int Psychogeriatr. 2012;24:1172–1182. doi: 10.1017/s1041610211002729. [DOI] [PubMed] [Google Scholar]
- Suttanon P, et al. Feasibility, safety and preliminary evidence of the effectiveness of a home-based exercise programme for older people with Alzheimer’s disease: a pilot randomized controlled trial. Clin Rehabil. 2013;27:427–438. doi: 10.1177/0269215512460877. [DOI] [PubMed] [Google Scholar]
- Taylor ME, et al. A home-based, carer-enhanced exercise program improves balance and falls efficacy in community-dwelling older people with dementia. Int Psychogeriatr. 2017;29:81–91. doi: 10.1017/S1041610216001629. [DOI] [PubMed] [Google Scholar]
- Teri L, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA. 2003;290:2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- Tortosa-Martínez J, Beltrán-Carrillo VJ, Caus N, Iglesias-Martínez MJ, Lozano-Cabezas I, Jimenez-Hernández S, Cortell-Tormo J (2017) Psychosocial benefits of exercise for older adults with amnestic mild cognitive impairment: innovative practice. Dementia:1471301217725895 [DOI] [PubMed]
- van der Wardt V, Hancox J, Gondek D, Logan P, Nair RD, Pollock K, Harwood R. Adherence support strategies for exercise interventions in people with mild cognitive impairment and dementia: a systematic review Prev Med Rep. 2017;7:38–45. doi: 10.1016/j.pmedr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Seben R, Smorenburg SM, Buurman BM. A qualitative study of patient-centered goal-setting in geriatric rehabilitation: patient and professional perspectives. Clin Rehabil. 2019;33:128–140. doi: 10.1177/0269215518791663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni ED, et al. Feasibility of a memory clinic-based physical activity prescription program. J Alzheimer’s Disease: JAD. 2016;53:161–170. doi: 10.3233/jad-160158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Brychta RJ, Chen KY, Koster A. Self-reported adherence to the physical activity recommendation and determinants of misperception in older adults. J Aging Phys Act. 2014;22(2):226–234. doi: 10.1123/japa.2012-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson J, Clemson L, Brodaty H, Lord S, Taylor M, Gitlin L, Close J. A feasibility study and pilot randomised trial of a tailored prevention program to reduce falls in older people with mild dementia. BMC Geriatr. 2013;13:89. doi: 10.1186/1471-2318-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuenyongchaiwat K, Pongpanit K, Hanmanop S. Physical activity and depression in older adults with and without cognitive impairment. Dement Neuropsychol. 2018;12(1):12–18. doi: 10.1590/1980-57642018dn12-010002. [DOI] [PMC free article] [PubMed] [Google Scholar]