INTRODUCTION
There is a rise in popularity of the use of e-cigarettes (vape pens), termed “vaping,” in which liquid is vaporized by a battery-powered heating device and then inhaled. In recent months, e-cigarette, or vaping, product use–associated lung injury (EVALI) has become prominent in both lay press and in social media with an associated rapid proliferation of scholarly and public health publications. Attention to this topic skyrocketed when the Centers for Disease Control and Prevention (CDC) released a health advisory on August 30, 2019, warning consumers against the danger of e-cigarettes and their association with “severe pulmonary disease”.1 According to the CDC, as of January 7, 2020, over 2,602 cases of EVALI have been reported from at least 49 states. Fifty-seven patients have died from EVALI as of the submission of this manuscript.2 We present a case of EVALI in a previously healthy young woman for three purposes: to highlight to providers the importance of an accurate, thorough social history; to discuss the presentation of a patient with EVALI; and to describe the currently understood pathophysiology of this entity.
CASE DESCRIPTION
A 24-year-old woman presented to the Emergency Department (ED) a chief complaint of 3 days of intractable nausea and vomiting. Review of systems was positive for intermittent fever, diarrhea, headache, dyspnea on exertion, and chest tightness. Three days before presenting to the ED, an urgent care clinic diagnosed her with community-acquired pneumonia (CAP) and started her on moxifloxacin. She then developed worsening nausea and vomiting such that she could not tolerate oral intake (including her antibiotics). She also had worsening fevers to 38.9 °C and severe fatigue. Recent sick contacts included the patient’s grandmother and a friend, who both had gastroenteritis. She denied any recent travel outside of Pennsylvania. She acknowledged daily vaping for the last 5 months. She denied alcohol, tobacco, or other drug use.
In the ED, she had a temperature of 39.6 °C, a heart rate of 107 beats/min, a normal blood pressure, a respiratory rate of 20 breaths/min, and an oxygen saturation of 97% on room air. Initial laboratory results showed leukocytosis (white blood cell (WBC) count of 19,240 cells/mm3) with neutrophil predominance and procalcitonin of 0.62 ng/mL (Table 1). Initial chest radiograph showed patchy opacities in the bilateral mid and lower lung fields, concerning for multilobar pneumonia. The patient was subsequently started on intravenous (IV) ceftriaxone and IV azithromycin for empiric coverage of CAP, as well as normal saline for volume depletion. Inhaled albuterol and ondansetron were provided for symptom management.
Table 1.
Laboratory Values on Admission
| Laboratory values | Patient value | Normal range |
|---|---|---|
| White blood cells (WBC) | 19,240 | 4,000–11,000 cells per mm3 |
| C-reactive protein (CRP) | 30.56 | < 10 mg/L |
| Erythrocyte sedimentation rate (ESR) | 124 | < 20 mm/h |
| Procalcitonin | 0.62 | < 0.08 ng/mL |
| Potassium (serum) | 2.9 | 3.6–5.0 mmol/L |
Over the next 48 h, the patient’s signs and symptoms persisted. Her vomiting severely limited her ability to tolerate oral intake. She maintained an elevated WBC count (17,020 cells/mm3) and remained tachycardic as well. A non-contrast chest CT was obtained to further characterize the findings seen on initial radiograph (Fig. 1). Patchy bilateral, asymmetric ground-glass opacities, and patchy consolidation were present in all lobes. Subpleural sparing was found in the lingula and left lower lobe, with opacities extending to the lung periphery in other areas. Mild septal thickening was seen in the left lung apex. The differential now included worsening CAP, perhaps from an antibiotic-resistant organism, opportunistic infection from undiagnosed immune deficiency, pneumonitis, and granulomatosis with polyangiitis. Further laboratory investigation—including testing for Legionella urine antigen, Streptococcus pneumoniae urine antigen, rapid viral panel including mycoplasma, serum antinuclear antibody, antineutrophil cytoplasmic antibodies, HIV antibodies and p24 antigen, and blood cultures—all returned negative.
Figure 1.
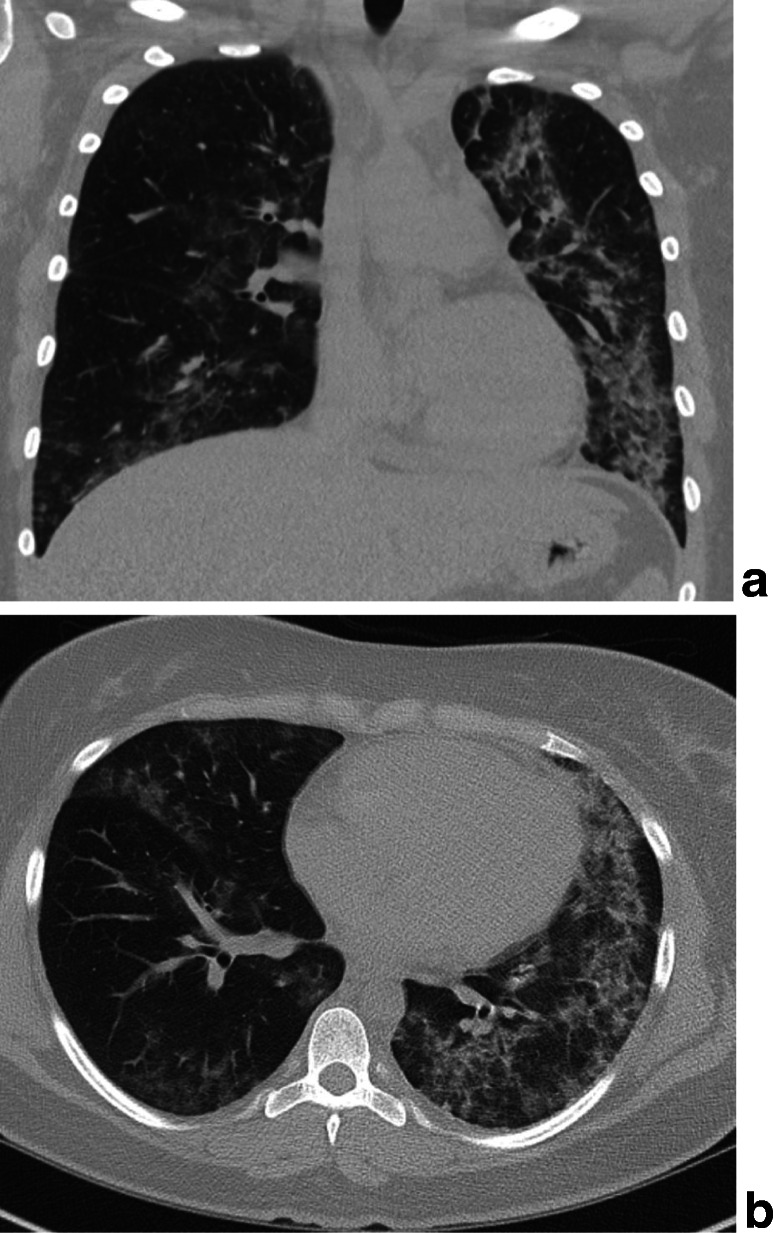
Noncontrast chest computed tomography images of the chest in coronal (a) and axial (b) sections show bilateral, asymmetric ground glass opacities, and patchy consolidation. These findings were present in all lobes, predominantly involving the lingula and left lower lobe. Subpleural sparing seen in the lingula and left lower lobe—with opacities extending to the lung periphery in other areas—was also noted. Mild septal thickening was seen in the left lung apex.
Antibiotics were broadened to IV vancomycin and piperacillin/tazobactam to cover for antibiotic-resistant organisms, and a 5-day course of azithromycin was completed. Due to the findings on chest CT, the social history was revisited, which expanded the history of vaping that was ascertained at the time of admission. The patient reported that she began smoking marijuana at age 18. Her use gradually increased over 5 years, until she and her significant other switched to using a vape pen approximately 5 months before she fell ill. She only used cartridges containing tetrahydrocannabinol (THC). She never used nicotine cartridges or smoked cigarettes. Approximately 2 weeks before the onset of symptoms, she switched THC vape cartridges to one labeled with “Dank Vape” branding. With this information, EVALI was added to our differential.
By day 5, she was still having fevers and her WBC count remained elevated at 15,000 cells/mm3. A bronchoscopy was pursued to establish an etiology for her unremitting pulmonary illness. Bronchoscopy cultures were negative. Tissue samples were negative, including for lipid-containing macrophages, eosinophils, or findings that would be consistent with vasculitis. Based on this information and the CT scan, she had EVALI with an organizing pneumonia predominant pattern. The patient continued on antibiotics for 2 more days before spontaneously improving. She was discharged home on hospital day 8 with explicit counseling to cease vaping as it was likely the cause of her current hospital visit. She abstained for 2 weeks and reported at her pulmonology follow-up visit that all symptoms had resolved. She has since resumed vaping with the THC-containing cartridge brand she used prior to using Dank Vape brand cartridges.
DISCUSSION
Several cases of EVALI have been reported describing previously healthy adults with recent vaping histories contracting a non-infectious pulmonary illness similar to this case.3–14 These cases have proven valuable—not only to help warn e-cigarette users of previously unforeseen consequences but also to develop four currently accepted patterns seen in EVALI: acute eosinophilic pneumonia, organizing pneumonia, lipoid pneumonia, and diffuse alveolar hemorrhage. However, a recent case series suggests that further investigation is needed before fully determining that these four distinct patterns represent specific injury patterns for EVALI.15–17
To the authors’ knowledge, two previous reports showed a significant link between specific types of vape cartridge brand and lung illness.18, 19 Greater than 80% of the patients in both reports used THC-containing cartridges; between 40 and 60% of interviewed patients used “Dank Vape” cartridges—the brand of THC-containing liquid used by our patient—though no studies supporting direct causation exist. To our knowledge, ours is the first case reported in Pennsylvania to have a link to this specific black-market brand. Even with this new-found association between a specific brand of black-market cartridges and recent cases of EVALI, there are reports of EVALI in patients who smoke other brands of THC-containing cartridges. A recent report of over 1,900 patients with EVALI showed that 82% of patients used THC-containing cartridges and 78% of those patients obtained the product from non-commercial sources (friends, family, dealers).20 This data strengthens the association between EVALI and THC-containing e-cigarette, or vaping, products, particularly those products sold on the black-market.
In September 2019, the New York Department of Health began investigating their own cases of EVALI and found high concentrations of Vitamin E acetate oil in the THC-containing cartridges, all of which were obtained from patients who were hospitalized for lung injury.21 Vitamin E is a well-known additive to black-market cartridge liquid and is used as a thickening agent. These liquids are not regulated by government oversight bodies and are widely available in smoke shops, in addition to being sold on the internet.22 The Utah Department of Health also found evidence of vitamin E acetate in samples from likely EVALI patients.19 More recently, bronchoalveolar lavage (BAL) specimens from 29 patients across the US were positive for vitamin E acetate, supporting the theory that this toxin is somehow involved in the mechanism of injury.23 The direct impacts of aerosolized vitamin E on lung function and surfactant stability have been brought into question, but little has been published in the medical literature.
Researchers analyzed e-cigarette liquids for other ingredients that could cause lung injury. Hess et al. tested several popular brands of e-cigarette liquid for metal concentration, and found evidence of nickel and chromium in many samples. Chronic bronchitis, decreased lung immune cell function, and lung cancer are associated with these metals.24 Allen et al. tested 51 flavors of e-cigarette liquids and found measurable levels of diacetyl, 2,3-pentandedione, and acetoin—chemicals which are linked to lung damage (specifically, bronchiolitis obliterans), and upon which the Occupational Safety and Health Association has set occupational exposure and inhalation limits—in 47 of them.25
E-cigarette devices have also been linked to pathologic lung changes. Madison et al. showed that chronic e-cigarette use, independent of nicotine, blunts lung immune cell function in mice.26 Specifically, this study found that lipid homeostasis in alveolar macrophages and type II pneumocytes was disrupted, causing persistent lung inflammation when compared to non-device-using subjects. Ghosh et al. suggest that e-cigarette users may be at risk for chronic obstructive lung disease—previously thought to affect those who smoke traditional cigarettes only27 —believed to be caused by a nicotine-dependent imbalance between antiproteases and proteases leading to alveolar elastase damage.
Making the diagnosis of EVALI requires an extensive, detailed social history. The method of substance inhalation (vaping versus smoking)—as well as the specific substance and brand name that is used—may help shed light on a patient’s clinical presentation. Traditionally, social history has involved asking questions about tobacco use, alcohol use, and illicit drugs. This case illustrates that deeper exploration regarding marijuana use is now required, particularly when discussing vaping products. Our patient was vaping THC-containing liquid for 5 months, a practice which is unregulated and illegal in Pennsylvania. Two weeks prior to her presentation, she changed cartridge suppliers and her new supplier carried only the “Dank Vape” brand THC-containing product. These were details that were not asked about at admission, but came to light later in the case and only after an extensive workup had been pursued.
Extrapulmonary symptoms are common in EVALI. According to the CDC’s national EVALI surveillance cohort of 339 patients through October 2019, 262 (77%) patients had gastrointestinal complaints, while 289 (85%) had constitutional symptoms (self-reported fever, chills or weight loss) at presentation.28 Therefore, patients with minimal medical comorbidities who present with signs and symptoms similar to our patient—fever (particularly with a failure to respond to appropriate antimicrobial therapy), shortness of breath, increased WBC, nausea, and diarrhea—should have a thorough social history taken to establish a full differential diagnosis. If there is a history of vape pen use, EVALI should be high on the differential list. Due to the large overlap between EVALI and CAP—including elevated WBC and procalcitonin levels as well as infiltrates on lung imaging—empiric treatment with antibiotics is reasonable until infection is ruled out or the patient begins to improve. If the clinical picture is unclear, bronchoscopy can be used to aid in ruling out other causes of lung injury, as EVALI is a diagnosis of exclusion. Lung biopsies can also be taken to aid in complete characterization of disease if needed. If the clinical picture is suggestive, a diagnosis of EVALI can be made and supportive care can be given. For patients who recover, long-term health consequences of continued vaping following resolution of the acute EVALI episodes are not yet known.
CONCLUSION
EVALI is a rapidly evolving clinical entity that presents with a wide range of severity and symptoms. It is becoming clearer that certain types of vape cartridges are associated with precipitating EVALI. It is critical for medical providers to understand the clinical presentation and social history background that helps define this emerging entity. This case illustrates how exploring a more detailed social history helps establish EVALI as a diagnosis, as there is currently no confirmatory testing. Counseling should be provided on the risks of vape pen use, including the risk association that certain cartridge brands carry. Further case reporting will allow better understanding of etiologic agents contained in vape cartridges which may be precipitating EVALI, so that medical and regulatory communities can reduce the public health crisis at hand.
Compliance with ethical standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.CDC. Health advisory: severe pulmonary disease associated with use of e-cigarette products. Atlanta, GA: US Department of Health and Human Services, CDC; 2019. https://emergency.cdc.gov/han/han00421.asp. Accessed January 14, 2020.
- 2.CDC. Outbreak of lung injury associated with e-cigarette use, or vaping. Atlanta, GA: US Department of Health and Human Services, CDC; 2019. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html. Accessed January 14, 2020.
- 3.Davidson K, Brancato A, Heetderks P, et al. Outbreak of Electronic-Cigarette–Associated Acute Lipoid Pneumonia — North Carolina, July–August 2019. MMWR Morb Mortal Wkly Rep. 2019;68:784–786. doi: 10.15585/mmwr.mm6836e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agustin M, Yamamoto M, Cabrera F, Eusebio R. Diffuse Alveolar Hemorrhage Induced by Vaping. Case Rep Pulmonol. 2018;2018:1–3. doi: 10.1155/2018/9724530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arter Z, Wiggins A, Hudspath C, Kisling A, Hostler DC, Hostler JM. Acute eosinophilic pneumonia following electronic cigarette use. Respir Med Case Rep. 2019;27:100825. doi: 10.1016/j.rmcr.2019.100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He T, Oks M, Esposito M, Steinberg H, Makaryus M. “Tree-in-Bloom”: Severe Acute Lung Injury Induced by Vaping Cannabis Oil. Ann Am Thorac Soc. 2017;14(3):468–470. doi: 10.1513/AnnalsATS.201612-974LE. [DOI] [PubMed] [Google Scholar]
- 7.Henry T, Kanne J, Kligerman S, et al. Imaging of Vaping-Associated Lung Disease. N Engl J Med. 2019;381:1486–1487. doi: 10.1056/NEJMc1911995. [DOI] [PubMed] [Google Scholar]
- 8.Itoh M, Aoshiba K, Herai Y, Nakamura H, Takemura T. Lung injury associated with electronic cigarettes inhalation diagnosed by transbronchial lung biopsy. Respirol Case Rep. 2017;6(1):e00282. doi: 10.1002/rcr2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan MS, Khateeb F, Akhtar J, et al. Organizing pneumonia related to electronic cigarette use: A case report and review of literature. Clin Respir J. 2018;12(3):1295–1299. doi: 10.1111/crj.12775. [DOI] [PubMed] [Google Scholar]
- 10.Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019;381(15):1488–1489. doi: 10.1056/NEJMc1912038. [DOI] [PubMed] [Google Scholar]
- 11.Modi S, Sangani R, and Alhajhusain A. Acute Lipoid Pneumonia Secondary to E-Cigarettes Use: An Unlikely Replacement for Cigarettes [Abstract]. Chest. 2015;148:382A.
- 12.Sommerfeld CG, Weiner DJ, Nowalk A, Larkin A. Hypersensitivity Pneumonitis and Acute Respiratory Distress Syndrome From E-Cigarette Use. Pediatrics. 2018;141(6):e20163927. doi: 10.1542/peds.2016-3927. [DOI] [PubMed] [Google Scholar]
- 13.Thota D, Latham E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J Emerg Med. 2014;47(1):15–7. doi: 10.1016/j.jemermed.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Viswam D, Trotter S, Burge P, Walters G. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep. 2018;2018:bcr-2018-224350. [DOI] [PMC free article] [PubMed]
- 15.Schier JG, Meiman JG, Layden J, CDC et al. Lung Injury Response Group. Severe Pulmonary Disease Associated with Electronic-Cigarette–Product Use — Interim Guidance. MMWR Morb Mortal Wkly Rep. 2019;68:787–790. doi: 10.15585/mmwr.mm6836e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butt YM, Smith ML, Tazelaar HD, et al. Pathology of Vaping-Associated Lung Injury. N Engl J Med. 2019;381(18):1780–1781. doi: 10.1056/NEJMc1913069. [DOI] [PubMed] [Google Scholar]
- 17.More on the Pathology of Vaping-Associated Lung Injury. N Engl J Med. November 2019:NEJMc1914980.
- 18.Layden JE, Ghana I, Pray I, et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin - Preliminary Report. September: N Engl J Med; 2019. [DOI] [PubMed] [Google Scholar]
- 19.Lewis N, McCaffrey K, Sage K, et al. E-cigarette Use, or Vaping, Practices and Characteristics Among Persons with Associated Lung Injury — Utah, April–October 2019. MMWR Morb Mortal Wkly Rep. 2019;68:953–956. doi: 10.15585/mmwr.mm6842e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellington S, Salvatore PP, Ko J, et al. Update: Product, Substance-Use, and Demographic Characteristics of Hospitalized Patients in a Nationwide Outbreak of E-cigarette, or Vaping, Product Use–Associated Lung Injury — United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. ePub: 14 January 2020. 10.15585/mmwr.mm6902e2 [DOI] [PMC free article] [PubMed]
- 21.New York State Department of Health Announces Update on Investigation into Vaping-Associated Pulmonary Illnesses. Albany, NY: New York State Department of Health, Sept. 2019 (www.health.ny.gov/press/releases/2019/2019-09-05_vaping.htm.) Accessed November 26, 2019.
- 22.Betuel E. A Thickener Used in THC Vapes Is a Hugely Popular Scam on the Black Market.Inverse, 13 Sept. 2019 (www.inverse.com/article/59207-vitamin-e-acetate-thc-vapes) Accessed November 26, 2019.
- 23.Blount BC, Karwowski MP, Morel-Espinosa M, et al. Evaluation of bronchoalveolar lavage fluid from patients in an outbreak of e-cigarette, or vaping, product use–associated lung injury—10 states, August–October 2019. MMWR Morb Mortal Wkly Rep. 2019;68:1040–1. doi: 10.15585/mmwr.mm6845e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hess C, Olmedo P, Navas-Acien A, Goessler W, Cohen JE, Rule AM. E-Cigarettes as a Source of Toxic and Potentially Carcinogenic Metals. Environ Res. 2017;152:221–225. doi: 10.1016/j.envres.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen J, Flanagan S, LeBlanc M, et al. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ Health Perspect. 2016;124(6):733–739. doi: 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madison M, Landers C, Gu B, et al. Electronic Cigarettes Disrupt Lung Lipid Homeostasis and Innate Immunity Independent of Nicotine. J Clin Invest. 2019;129(10):4290–4304. doi: 10.1172/JCI128531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh A, Coakley R, Ghio A, et al. Chronic E-Cigarette Use Increases Neutrophil Elastase and Matrix Metalloprotease Levels in the Lung. Am J Respir Crit Care Med. August 2019:rccm.201903-0615OC. [DOI] [PMC free article] [PubMed]
- 28.Siegel DA, Jatlaoui TC, Koumans EH, et al. Update: Interim Guidance for Health Care Providers Evaluating and Caring for Patients with Suspected E-cigarette, or Vaping, Product Use Associated Lung Injury — United States, October 2019. MMWR Morb Mortal Wkly Rep. 2019;68:919–927. doi: 10.15585/mmwr.mm6841e3. [DOI] [PMC free article] [PubMed] [Google Scholar]


