Abstract
Background
We report on predictors of adenovirus (ADV) viremia and correlation of ADV viral kinetics with mortality in ex vivo T-cell depleted (TCD) hematopoietic cell transplant (HCT).
Methods
T cell-depleted HCT recipients from January 1, 2012 through September 30, 2018 were prospectively monitored for ADV in the plasma through Day (D) +100 posttransplant or for 16 weeks after the onset of ADV viremia. Adenovirus viremia was defined as ≥2 consecutive viral loads (VLs) ≥1000 copies/mL through D +100. Time-averaged area under the curve (AAUC) or peak ADV VL through 16 weeks after onset of ADV viremia were explored as predictors of mortality in Cox models.
Results
Of 586 patients (adult 81.7%), 51 (8.7%) developed ADV viremia by D +100. Age <18 years, recipient cytomegalovirus seropositivity, absolute lymphocyte count <300 cells/µL at D +30, and acute graft-versus-host disease were predictors of ADV viremia in multivariate models. Fifteen (29%) patients with ADV viremia died by D +180; 8 of 15 (53%) died from ADV. Peak ADV VL (hazard ratio [HR], 2.25; 95% confidence interval [CI], 1.52–3.33) and increasing AAUC (HR, 2.95; 95% CI, 1.83–4.75) correlated with mortality at D +180.
Conclusions
In TCD HCT, peak ADV VL and ADV AAUC correlated with mortality at D +180. Our data support the potential utility of ADV viral kinetics as endpoints in clinical trials of ADV therapies.
Keywords: adenovirus infection, hematopoietic cell transplant, viral kinetics
Adenovirus viral burden was examined as predictors of mortality in multivariate models. Adenovirus time-averaged area under the curve (AAUC) and peak adenovirus viral load were independent predictors for mortality in ex vivo T cell-depleted hematopoietic cell transplant recipients.
Adenovirus (ADV) infection is associated with significant morbidity and mortality in allogeneic hematopoietic cell transplant (HCT) recipients [1, 2]. Risk factors for ADV infection include young age, T-cell depletion (TCD), lymphopenia, mismatched donor, and graft-versus-host disease (GVHD) [1, 3–6]. In pediatric HCT, routine ADV monitoring and preemptive therapy has led to improved outcomes [7] and has become standard practice for high-risk patients [8–11]. In contrast, routine monitoring for ADV is less common for adult HCT recipients [12]. Thus data on the correlation of ADV viral kinetics with outcomes of ADV infection are limited for adult HCT recipients.
An association between the magnitude of ADV viral load (VL) and severity of infection has been described. Limitations of the published studies include lack of routine monitoring for ADV [1], heterogeneity with regards to ADV risk [13–15], or focusing exclusively in pediatric HCT [15]. The time-averaged area under the curve (AAUC) of ADV viremia accounts for the severity and persistence of ADV infection and has been used as a marker of ADV viral burden [15].
At our center, we implemented routine ADV monitoring in adult and pediatric recipients of TCD HCT in 2012. The objectives of the present study are to (1) report the incidence of ADV viremia in adult and pediatric HCT, (2) identify risk factors for ADV viremia, and (3) explore the correlation between ADV peak VL or AAUC and mortality.
METHODS
Study Patients
The cohort consists of adult and pediatric recipients of first TCD HCT between January 2012 and September 2018 at Memorial Sloan Kettering Cancer Center (MSKCC). Data were extracted from the hospital databases and medical record review. The study was reviewed and approved by the MSKCC Institutional Review and Privavy Board and granted a waiver of authorization.
Conditioning Regimens and Standards of Care
For peripheral blood allografts, TCD was performed by the CliniMACS CD34 Reagent System (Miltenyi Biotec, Gladbach, Germany). For marrow allografts, TCD was performed by soybean lectin agglutination followed by E-rosetting [16]. Conditioning regimens and supportive care have been described [17–19]. Acute GVHD was scored by standard criteria [20].
All patients received acyclovir prophylaxis starting on day 7. Cytomegalovirus (CMV) seropositive recipients (R+) were monitored routinely for CMV and treated preemptively until November 2017. Since December 2017, CMV R+ recipients older than 12 years received letermovir prophylaxis for CMV [21]. Pediatric patients under the age of 12 years continued’ with preemptive initiation of anti-CMV therapy.
Adenovirus Screening and Management
Routine monitoring for ADV in the plasma was done by quantitative polymerase chain reaction assays. From January 2012 through November 2016, testing was performed by Viracor Eurofins (Lee’s Summit, MO). The linear range of quantitation (LOQ) was 100 copies/mL – 1 × 1010 copies/mL between January 2012 and April 2013, and 190 copies/mL – 1 × 1010 copies/mL between May 2013 and December 2016. Since January 2017, testing was performed by the Clinical Microbiology Laboratory at MSKCC. The LOQ was 200 – 2 × 106 copies/mL.
Adenovirus monitoring started on Day +14 posttransplant (D +14) and continued weekly through D +60 and at least once every 2 weeks through D +100. Patients with ADV viremia by D +100 continued to be monitored weekly for ADV for 16 weeks after the onset of ADV viremia.
The ADV VL threshold for treatment initiation, type, dosing regimen, and duration of treatment were at the discretion of the treating physicians. During the study period, cidofovir was available per standards of care. Brincidofovir was intermittently available at our institution through participating in clinical trials (ADV HALT trial [ClinicalTrials.gov Identifier NCT01241344], a randomized, placebo-controlled, multicenter, phase II study to evaluate the safety and efficacy of preemptive treatment with brincidofovir (CMX001) for the prevention of ADV disease in pediatric and adult allogeneic HCT recipients; and AdVise trial [ClinicalTrials.gov Identifier NCT02087306], an open-labeled, multicenter study of the safety and efficacy of brincidofovir in the treatment of early versus late ADV infection). In addition, patients may have received brincidofovir through emergency Investigational New Drug application or the BCV expanded access program.
Definitions
Patients were categorized into 2 groups as pediatric (<18 years) or adult (≥18 years). Transient ADV viremia was defined as maximum ADV VL <1000 copies/mL. Adenovirus viremia was defined as ≥2 consecutive VL ≥1000 copies/mL by D +100 posttransplant. Peak ADV viremia was defined as maximum VL over 16 weeks after first ADV viremia ≥1000 copies/mL. Adenovirus disease was scored by the European Group for Bone Marrow Transplantation guidelines [9]. Death was attributed to ADV as previously described using hierarchy for cause of death. For patients with GVHD, death was attributed to GVHD. For patients with ADV infection and concomitant fatal opportunistic infections (disseminated toxoplasmosis or mucormycosis), mortality was attributed to the latter [22].
Statistics
Descriptive analyses were used to summarize patient demographic, clinical, and transplant characteristics. Mann-Whitney rank-sum tests were performed to compare continuous variables. The χ 2 tests and Fisher’s exact tests (if cell counts were less than 5) were used to compare categorical variables. The AAUC was calculated as the log10 of the sum of the trapezoids representing the AUC of ADV viremia each week from the onset of ADV viremia through 16 weeks or week of last follow up (whichever occurred first) and divided by the number of weeks of follow up [15]. The incidence of ADV viremia was estimated by the cumulative incidence function. Death, relapse, and second transplant were treated as competing risks. A landmark survival analysis from D +100 until D +365 was performed by the Kaplan-Meier method, and relevant groups were compared by the log-rank test. Cox proportional hazard models were used to evaluate the association between ADV viremia, peak ADV viremia, and AAUC and overall mortality. Age, sex, underlying disease, recipient CMV serology, donor type, acute GVHD, and absolute lymphocyte count (ALC) at D +30 posttransplant (ALC +30) were examined as predictors of ADV viremia in competing risk regressions and multivariate regressions. Adjusted hazard ratios (HRs) of AAUC and mortality at D +180 and at D +365 were obtained from final multivariate models respectively, after adjusting for the abovementioned potential covariates. Forward selection was used for model selection. Covariates with P < .3 were entered in the multivariate model and those with P < .1 stayed in the final model. P < .05 was considered statistically significant. All statistical analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Characteristics of Patients
The study cohort consisted of 586 patients including 107 (18.3%) pediatric and 479 (81.7%) adult HCT recipients. The clinical characteristics of patients are shown on Table 1. The median ALC +30 was 500 cells/µL (interquartile range [IQR], 300–650) in pediatric HCT recipients and 500 cells/µL (IQR, 400–800) in adult HCT recipients.
Table 1.
Demographics and Characteristics of the Cohort (Total, n = 586)
| Adult Total n = 479 | Pediatric Total n = 107 | ||||
|---|---|---|---|---|---|
| Characteristic | n | % | n | % | |
| Age (years) | Median (IQR) | 54 (41–63) | 8 (2–13) | ||
| Sex | Female | 200 | 41.8% | 39 | 36.4% |
| Male | 279 | 58.2% | 68 | 63.6% | |
| Underlying disease | Acute leukemia/myelodysplastic syndrome | 348 | 72.6% | 68 | 63.6% |
| Multiple myeloma | 88 | 18.4% | 0 | 0% | |
| Chronic leukemia/myeloproliferative disorder | 33 | 6.9% | 0 | 0% | |
| Immune deficiencya,b | 3 | 0.6% | 26 | 24.3% | |
| Other nonmalignant conditionsc,d | 7 | 1.5% | 13 | 12.1% | |
| Donor type | Matched related | 149 | 31.1% | 15 | 14.0% |
| Matched unrelated | 243 | 50.8% | 37 | 34.6% | |
| Mismatched related | 4 | 0.8% | 16 | 15.0% | |
| Mismatched unrelated | 83 | 17.3% | 39 | 36.4%% | |
| Conditioning intensity | Myeloablative | 475 | 99.2% | 102 | 95.3% |
| Reduced intensity | 4 | 0.8% | 2 | 1.9% | |
| Nonablative | 0 | 0% | 3 | 2.8% | |
| CMV serostatus | R+/D+ | 162 | 33.8% | 43 | 40.2% |
| R+/D− | 104 | 21.7% | 18 | 16.8% | |
| R−/D+ | 63 | 13.2% | 20 | 18.7% | |
| R−/D− | 150 | 31.3% | 26 | 24.3% | |
| Acute GVHD | 0–1 | 396 | 82.7% | 93 | 86.9% |
| 2–4 | 81 | 16.9% | 12 | 11.2% | |
| 3–4 | 7 | 1.5% | 5 | 4.7% | |
| Not evaluable | 2 | 0.4% | 2 | 1.9% | |
| ALC +30 (cells/µL) | Median (IQR) | 500 (300–650) | 500 (400–800) |
Abbreviations: ALC +30, absolute lymphocyte count at day +30 after hematopoietic cell transplant; CMV, cytomegalovirus; D, donor; GVHD, graft-versus-host disease; IQR, interquartile range; R, recipient.
a Immune deficiency in adult patients: combined immune deficiency (n = 1), Wiskott-Aldrich syndrome (n = 1), hemophagocytic lymphohistiocytosis (n = 1).
bImmune deficiency in pediatric patients: chronic granulomatous disease (n = 4), familial hemophagocytic lymphohistiocytosis (n = 2), homozygous interferon gamma receptor deficiency (n = 1), Kostmann’s neutropenia (n = 1), lymph adhesion deficiency (n = 1), severe combined immunodeficiency (n = 16), X-linked hyperimmunoglobulin M syndrome (n = 1).
cNonmalignant conditions in adult patients: aplastic anemia (n = 3), mast cell activation disorder (n = 1), paroxysmal nocturnal hemoglobinuria (n = 3).
dNonmalignant conditions in pediatric patients: aplastic anemia (n = 12), leukodystrophy (n = 1).
Adenovirus Viremia
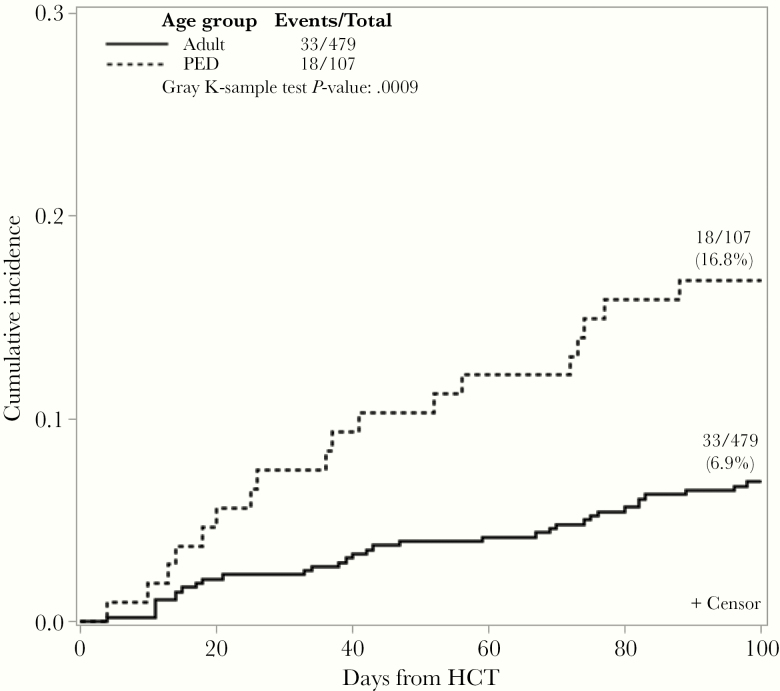
By D +100, 27 (25.0%) and 46 (9.6%) of pediatric and adult HCT recipients, respectively, had ADV detected in the plasma. Transient ADV viremia (maximum VL <1000 copies/mL) occurred in 9 (8.4%) and 13 (2.7%) of pediatric and adult patients, respectively. Adenovirus viremia (≥2 consecutive VL ≥1000 copies/mL) developed in 18 (16.8%) pediatric patients and 33 (6.9%) adult patients, respectively (P = .0009) (Figure 1). Adenovirus viremia occurred at a median of 42 days (IQR, 16–77) and 50 days (IQR, 26–74) post-HCT in pediatric and adult HCT, respectively. The peak ADV VL was a median of 4.7 log10 copies/mL (IQR, 4–6) and 4.9 log10 copies/mL (IQR, 4–6) in pediatric and adult recipients, respectively.
Figure 1.
Cumulative incidence of adenovirus viremia at Day +100 posttransplant by age group. Adenovirus viremia was defined as ≥2 consecutive viral loads ≥1000 copies/mL by Day +100 posttransplant. Pediatric (PED) patients were (<18 years); Adult (≥18 years). HCT, hematopoietic cell transplant.
Adenovirus Disease
Among pediatric patients, 5 (5% of total, 28% of patients with ADV viremia) developed ADV disease including the following: colitis (2), hepatitis (1), pneumonitis (3), and hemorrhagic cystitis/nephritis (1). Two patients developed ADV disease in 2 sites (pneumonitis and hemorrhagic cystitis in 1 patient and hepatitis and pneumonitis in 1 patient). Adenovirus disease occurred at a median of 52 days post-HCT (IQR, 43–84). The peak ADV VL among those with disease was a median of 7 log10 copies/mL (IQR, 6–7).
Among adult patients, 16 patients (3% in total, 48% of patients with ADV viremia) developed ADV disease including colitis (8), pneumonitis (4), and hemorrhagic cystitis/nephritis (4). Adenovirus disease occurred at a median of 44 days (IQR, 24–66) post-HCT. The peak ADV VL was a median of 6 log10 copies/mL (IQR, 5–7).
Risk Factors of Adenovirus Viremia
To identify risk factors for ADV viremia, demographic and transplant characteristics were entered into univariate and multivariate models. In univariate analysis, age <18 years, CMV R+, ALC +30 <300 cells/µL, and acute GVHD grade 2–4 were associated with ADV viremia (Table 2). In multivariate models, age <18 years (HR, 2.82; 95% CI, 1.58–5.03; P = .0005), CMV R+ (HR, 2.56; 95% CI, 1.34–4.90; P = .0046), ALC +30 <300 cells/µL (HR, 2.28; 95% CI, 1.16–4.48; P = .0164), and acute GVHD grade 2–4 (HR, 2.76; 95% CI, 1.52–5.01; P = .0009) remained significant for risk factors of ADV viremia.
Table 2.
Univariate and Multivariate Risk Factors for Adenovirus Viremia
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Characteristic | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age | Adult (≥18 years) | ref | ref | ||
| Peds (<18 years) | 2.60 (1.46–4.61) | .0011 | 2.82 (1.58–5.03) | .0005 | |
| Sex | Female | ref | |||
| Male | 0.98 (0.56–1.71) | .9506 | |||
| Underlying disease | Acute leukemia/MDS | ref | |||
| Multiple myeloma | 0.99 (0.47–2.11) | .9734 | |||
| Chronic leukemia/MPD | 0.33 (0.04–2.40) | .2687 | |||
| Immune deficiency | 1.17 (0.35–3.89) | .7994 | |||
| Other nonmalignant conditions | - | ||||
| Donor type | Matched | ref | |||
| Mismatched | 1.20 (0.65–2.20) | .5614 | |||
| Recipient CMV serology | R− | ref | ref | ||
| R+ | 2.65 (1.39–5.07) | .0032 | 2.56 (1.34–4.90) | .0046 | |
| Acute GVHDb | 0–1 | ref | ref | ||
| 2–4 | 2.59 (1.42–4.68) | .0017 | 2.76 (1.52–5.01) | .0009 | |
| ALC + 30a (cells/µL) | ≥300 | ref | ref | ||
| <300 | 2.43 (1.26–4.68) | .0080 | 2.28 (1.16–4.48) | .0164 |
Abbreviations: ALC, absolute lymphocyte count; CI, confidence interval; CMV, cytomegalovirus; D+, days posttransplant; GVHD, graft-versus-host disease; HR, hazard ratio; MDS, myelodysplastic syndrome; MPD, myeloproliferative disorder; R, recipient; ref, reference.
aRepresents ALC value most proximal to D +30 (±7 days).
bAcute GVHD was treated categorical variable.
Treatment of Adenovirus Viremia
Thirty-one (61%) patients with ADV viremia received treatment. The median time from onset of ADV viremia to start of treatment was 11 days (IQR, 7–18). The median VL at the initiation of treatment was 4.2 log10 copies/mL (IQR, 3.9–5.2). Details of treatment administered are shown in Supplementary Table S1. In addition, 4 patients received ADV-directed cytotoxic T cells on compassionate use.
Twenty of 51 (39%) patients with ADV viremia cleared ADV without any antiviral treatment. In these patients, ADV viremia occurred at a median of 64 days posttransplant (IQR, 48–81). The peak VL was 5.6 log10 copies/mL (IQR, 4.6–6.7) and 3.6 log10 coipes/mL (IQR, 3–4) in patients with and without antiviral treatment, respectively (P < .0001).
Overall Survival
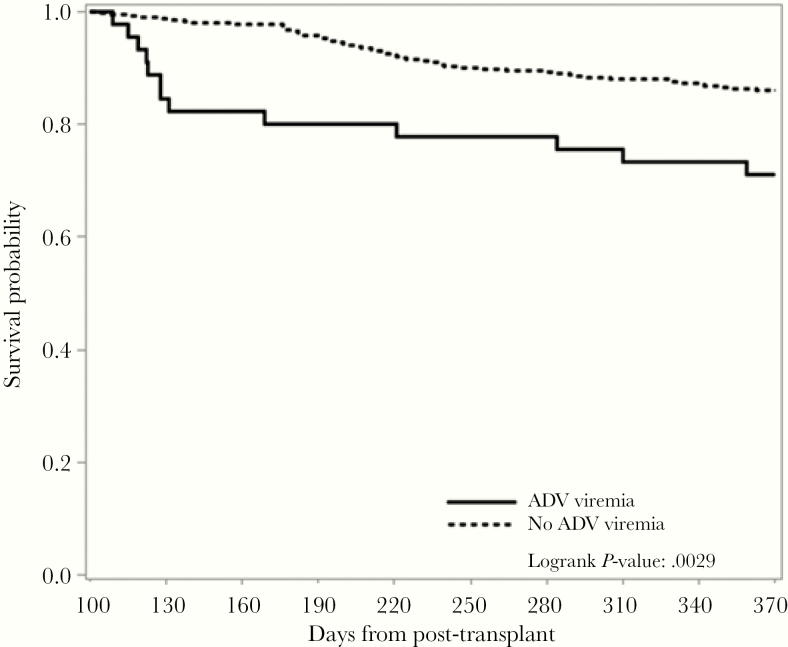
Six patients with ADV viremia and 28 patients without ADV viremia died before D +100. A landmark survival analysis was performed for 45 patients with ADV viremia and 507 patients without ADV viremia who were alive by D +100 (Figure 2).
Figure 2.
Landmark survival analysis from Day +100 until Day +365 post-HCT by adenovirus (ADV) viremia.
By D +180, 36 (80%) patients with ADV viremia and 489 (95%) patients without ADV viremia were alive (P < .0001). By D +365, 32 (71%) patients with viremia and 436 (86%) patients without ADV were alive (P = .0029).
Adenovirus Attributable Mortaliy
Of 15 patients with ADV viremia who died by D +180, 6 (40%) died of disseminated ADV disease. Adenovirus probably contributed to death in 2 additional patients. The causes of death in the remaining 7 patients were GVHD (2 patients), fatal opportunistic infections (mucormycosis 1 patient, toxoplasmosis 1 patient), bacterial sepis (1 patient), and veno-occulsive disease of the liver (1 patient). By D +365, 4 additional patients with ADV viremia died. None of these deaths were attributed to ADV. Three patients died of CMV and 1 patient died of GVHD.
Adenovirus Viremia as Predictor of Mortality
Adenovirus viremia was entered as a categorical variable in univariate and multivatiate models for mortality. Adenovirus viremia (HR, 3.63; 95% CI, 1.99–6.60; P < .0001) was an independent risk factor for mortality at D +180 along with HLA-mismatched donor and ALC +30 <300 cells/µL (Table 3). Adenovirus viremia remained a risk factor for mortality at D +365 post-HCT (HR, 2.69; 95% CI, 1.63–4.44; P = .0001). In contrast, age <18 years was associated with decreased risk at D +360.
Table 3.
Univariate and Multivariate Cox Proportional Hazard Model Evaluating ADV Viremia and Mortality at D +180 and at D +365 Posttransplanta
| Overall Mortality at D +180 | Overall Mortality at D +365 | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| Variable | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Age (years) | ||||||||
| Adult (≥18) | ref | ref | ref | |||||
| Pediatric (<18) | 0.89 (0.48– 1.68) | .7289 | 0.65 (0.38–1.12) | .1178 | 0.33 (0.18–0.6) | .0003 | ||
| HLA Match | ||||||||
| Matched | ref | ref | ref | ref | ||||
| Mismatched | 1.68 (0.99–2.85) | .0537 | 1.77 (1.03–3.07) | .0403 | 1.56 (1.07–2.31) | .0227 | 1.97 (1.31–2.95) | .001 |
| Acute GVHD | ||||||||
| 0–1 | ref | ref | ||||||
| 2–4 | 1.4 (0.74–2.66) | .2947 | 1.37 (0.87–2.16) | .1799 | ||||
| ALC +30 (cells/µL) | ||||||||
| ≥300 | ref | ref | ref | ref | ||||
| <300 | 5.51 (3.28–9.25) | <.0001 | 4.29 (2.48–7.42) | <.0001 | 3.09 (2.03–4.70) | <.0001 | 2.82 (1.81–4.39) | <.0001 |
| ADV Viremia | ||||||||
| No | ref | ref | ref | ref | ||||
| Yes | 3.77 (2.10–6.73) | <.0001 | 3.63 (1.96–6.60) | <.0001 | 2.36 (1.45–3.86) | .0006 | 2.69 (1.63–4.44) | .0001 |
Abbreviations: ADV, adenovirus; ALC, absolute lymphocyte count; CI, confidence interval; D, day; GVHD, graft-versus-host disease; HLA, human leukocyte antigen; HR, hazard ratio; ref, reference.
aAdenovirus viremia was entered as a categorical variable. Sex, underlying disease, and cytomegalovirus recipient serology were included in the model and were not significant.
Next, the log10 peak ADV VL was entered as a continuous variable in univariate and multivariate models for mortality (Supplementary Table S2a). Increasing peak ADV VL correlated with increased mortality at D +180 (HR, 2.25; 95% CI, 1.52–3.33; P < .0001) and at D +365 (HR, 1.92; 95% CI, 1.34–2.75; P = .0004).
Adenovirus AAUC is a viral marker that takes into consideration both severity and persistence of ADV infection. To assess the correlation of increasing AAUC with mortality, AAUC was entered as a continuous variable in multivariate models. An increase in AAUC was associated with increased mortality at D +180 (HR, 2.95; 95% CI, 1.83–4.75; P < .0001) and at D +365 (HR, 3.00; 95% CI, 1.93–4.65; P < .0001), respectively (Table 4).
Table 4.
Univariate and Multivariate Cox Proportional Hazard Model Evaluating AAUC and Mortality at D +180 and at D +365 Posttransplanta
| Overall Mortality at D +180 | Overall Mortality at D +365 | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| Predictors | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Age (years) | ||||||||
| Adult (≥18) | ref | ref | ||||||
| Pediatric (<18) | 0.42 (0.12–1.50) | .1812 | 0.30 (0.09–1.02) | .0546 | ||||
| HLA Match | ||||||||
| Matched | ref | ref | ||||||
| Mismatched | 0.96 (.31–3.03) | .9495 | 0.95 (0.34–2.64) | .925 | ||||
| CMV Serology | ||||||||
| R− | ref | ref | ref | ref | ||||
| R+ | 0.32 (0.10–1.08) | .0668 | 0.22 (0.06–0.87) | .0304 | 0.32 (0.11–0.93) | .0363 | 0.23 (0.07–0.76) | .016 |
| Acute GVHD | ||||||||
| 0–1 | ref | ref | ||||||
| 2–4 | 1.46 (0.52–4.13) | .467 | 2.08 (0.84–5.12) | .1128 | ||||
| ALC +30 (cells/µL) | ||||||||
| ≥300 | ref | ref | ||||||
| <300 | 0.25 (0.09–0.69) | .0074 | 3.61 (1.44–9.04) | .0061 | ||||
| AAUC log10 | 2.26 (1.24–5.12) | <.0001 | 2.95 (1.83–4.75) | <.0001 | 3.02 (1.95–4.67) | <.0001 | 3.00 (1.93–4.65) | <.0001 |
Abbreviation: AAUC, time-averaged area under the curve of ADV viremia; ALC, absolute lymphocyte count; CI, confidence interval; CMV, cytomegalovirus; D, day; GVHD, graft-versus-host disease; HLA, human leukocyte antigen; HR, hazard ratio; R, recipient; ref, reference.
aSex and underlying disease were not significant.
To discern potential differences on the impact of AAUC on morality between adult and pediatric patients, we performed the analyses by age group. In pediatric patients, AAUC was associated with increased mortality at D +180 and D +365 posttransplant with HR 2.56 (95% CI, 1.52–4.31) and HR 2.71 (95% CI, 1.69–4.34), respectively. Likewise, in adult patients, AAUC was associated with mortality at D +180 and D +365 posttransplant with HR 2.96 (95% CI, 1.64–5.34) and HR 3.13 (95% CI, 1.83–5.36).
Next, we divided AAUC into 4 quartiles (Q1–Q4) and report mortality by quartiles. No death occurred in the lowest AAUC quartile (Q1). There was an increment increase in mortality for Q2 (8%), Q3 (31%), and Q4 (77%) by D +180 and for Q2 (8%), Q3 (54%), and Q4 (85%) by D +365 (Table 5). The mortality of Q2 was used as reference in multivariate models. Supplementary Table S3 showed HR for mortality for Q3 and Q4 after adjusting for covariates.
Table 5.
Overall Mortality by Quartiles of Time-Averaged Area Under the Curve of ADV Viremiaa
| Overall Mortality at D +180 | Overall Mortality at D +365 | |||||
|---|---|---|---|---|---|---|
| Quartiles | Mortality | HR (95% CI) | P Value | Mortality | HR (95% CI) | P Value |
| Q1 (0.04 ≤ AAUC < 0.86) | 0% | - | 0% | - | ||
| Q2 (0.86 ≤ AAUC < 2.58) | 8% | ref | 8% | ref | ||
| Q3 (2.58 ≤ AAUC < 3.42) | 31% | 4.96 (0.55–44.41) | .1522 | 54% | 7.33 (0.88–61.42) | .0663 |
| Q4 (3.42 ≤ AAUC < 5.65) | 77% | 16.32 (2.08–128.32) | .008 | 85% | 21.88 (2.75–174.07) | .0035 |
Abbreviation: AAUC, time-averaged area under the curve of ADV viremia; ADV, adenovirus; CI, confidence interval; HR, hazard ratio; R, recipient; ref, reference.
aHazard ratio and 95% CIs were obtained from multivariable Cox proportional hazards model (Supplementary Table S3). Age (adult vs pediatric), sex, underlying diseases, human leukocyte antigen match (matched vs mismatched), cytomegalovirus serology (R+ vs R−), acute graft-versus-host disease (grade 0–1 vs grade 2–4), ALC +30 (≥300 vs <300), and AAUC quartiles were entered as covariates. Only AAUC remained significant risk factor for mortality. Cytomegalovirus R+ was protective after adjusting for AAUC.
DISCUSSION
We analyzed a contemporary cohort of 586 TCD HCT recipients (81.7% adults) from a sinlge center to study the impact of ADV VL kinetics on mortality. The incidence of ADV viremia was higher in pediatric compared with adult HCT recipients (16.8% vs 6.9%, respectively) in agreement with reported literature [1, 23–27]. In multivatiate models, age, acute GVHD, ALC +30 <300 cells/µL, and CMV R+ were independent risk factors for ADV viremia. An association of CMV R+ and ADV viremia has been reported after in vivo TCD with alemtuzumab [23].
Five pediatric patients (28% of patients with ADV viremia) and 16 adult patients (48% of patients with ADV viremia) developed ADV disease. In a landmark survival analysis at D +100, ADV viremia was associated with lower survival at D +180 and at D +365. Patients with ADV viremia were 3.6 times more likely to die compared with patients without ADV viremia at D +180 (P < .0001). The ADV attributable mortality of 40% in the present study is lower than we have reported before the implementation of routince ADV monitoring [1]. However, because the 2 cohorts were not contemporaneous, additional factors may have contributed to the survival difference. The substantial rates of ADV disease among patients with ADV viremia, the ADV attributable mortality, and the negative impact of ADV in overall survival despite routine ADV monitoring, highlight the unmet need for effective treatment for ADV viremia in TCD HCT.
To correlate the ADV viral burden with mortality, we used peak ADV VL as a marker of severity of infection and AAUC as a marker of severity and persistence of infection [28, 29]. The AAUC has been previously used as a primary endpoint in Phase 2 and 3 studies of investigational antivirals [30–32]. In multivariate models, peak VL and AAUC were predictors of mortality. When adult and pediatric HCT recipients were analyzed separately, increasing AAUC was associated with increased mortality at D +180 and D +365 for both groups. A recent multicenter study of European pediatric HCT patients showed a similar association [15]. Hill et al [14] reported a correlation between persistent ADV viremia and mortality at 100 days but not at 1 year post-HCT; however, results of ADV viremia were not available for clinical decision making. In our cohort, ADV VL results were available to the clinicians in real time, yet AAUC remained a predictor of mortality, again highlighting the need for improved therapeutic strategies.
A limitation of our study is that initiation of treatment for ADV, dosing regimens, and duration were at clinicians’ discretion. In addition, treatment practices may have varied during the study period and between adult and pediatric services. Acknowledging this limitation, our analyses were focused on the correlation of ADV VL kinetics with mortality independently of potential modifiers (such as treatment). Our study provides real-world evidence on the impact of ADV infection on mortality in adult and pediatric TCD HCT recipients with current management strategies. We show that ADV viral burden is a predictor for mortality. Our data show that (1) interventions that reduce ADV viral burden are likely to reduce mortality and (2) AAUC can be a valuable virologic marker in clinical trials evaluating new therapeutics for ADV infection in HCT recipients.
Supplementary Material
Notes
Acknowledgments. M. A. P. thanks Theodore and Laura Hromadka for their generous support.
Disclaimer. The content is soley the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. The study was partially funded by a grant from Chimerix. This research was also funded in part by NIH award number P01 CA23766 and NIH/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Potential conflicts of interest. S. E. P. receives support for the conduct of industry sponsored trials through MSKCC from Atara Biotherapeutics and has patent related to selection of banked EBV specific T cells for PTLD. All rights assigned to MSKCC. S. A. G. has served an advisory borad for Amgen, Actinuum, Celgene, Johnson & Johnson, JAZZ pharmaceutical, Takeda, Novartis, KITE, and Spectrum pharma and has received research suppor from Amgen, Actinuum, Celgene, Johnson & Johnson, and Miltenyi, Takeda. G. A. P. has served as investigator for Chimerix, Inc., Astellas Pharma, Merck & Co., and Takeda and received research support and consulting and other fees from Chimerix, Inc., Astellas Pharma, and Merck & Co., and other fees from ADMA, Shionogi, Octapharma, Partners Therapeutics and Amplyx. M. A. P. reports honoraria from Abbvie, Bellicum, Celgene, Bristol-Myers Squibb, Incyte, Merck & Co., Novartis, Nektar Therapeutics, Omeros, and Takeda. He serves on data and safety monitoring boards for Cidara Therapeutics, Servier and Medigene, and the scientific advisory boards of MolMed and NexImmune. He has received research support for clinical trials from Incyte, Kite/Gilead, and Miltenyi Biotec. He serves in a volunteer capacity as a member of the Board of Directors of American Society for Transplantation and Cellular Therapy and Be The Match (National Marrow Donor Program), as well as on the CIBMTR Cellular Immunotherapy Data Resource Committee. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lee YJ, Chung D, Xiao K, et al. Adenovirus viremia and disease: comparison of T cell-depleted and conventional hematopoietic stem cell transplantation recipients from a single institution. Biol Blood Marrow Transplant 2013; 19:387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev 2014; 27:441–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Tol MJ, Claas EC, Heemskerk B, et al. Adenovirus infection in children after allogeneic stem cell transplantation: diagnosis, treatment and immunity. Bone Marrow Transplant 2005; 35Suppl 1:S73–6. [DOI] [PubMed] [Google Scholar]
- 4. Runde V, Ross S, Trenschel R, et al. Adenoviral infection after allogeneic stem cell transplantation (SCT): report on 130 patients from a single SCT unit involved in a prospective multi center surveillance study. Bone Marrow Transplant 2001; 28:51–7. [DOI] [PubMed] [Google Scholar]
- 5. Chakrabarti S, Mautner V, Osman H, et al. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood 2002; 100:1619–27. [DOI] [PubMed] [Google Scholar]
- 6. Heemskerk B, Lankester AC, van Vreeswijk T, et al. Immune reconstitution and clearance of human adenovirus viremia in pediatric stem-cell recipients. J Infect Dis 2005; 191:520–30. [DOI] [PubMed] [Google Scholar]
- 7. Grimley MS, Chemaly RF, Englund JA, et al. Brincidofovir for asymptomatic adenovirus viremia in pediatric and adult allogeneic hematopoietic cell transplant recipients: a randomized placebo-controlled phase II trial. Biol Blood Marrow Transplant 2017; 23:512–21. [DOI] [PubMed] [Google Scholar]
- 8. Hiwarkar P, Kosulin K, Cesaro S, et al. Management of adenovirus infection in patients after haematopoietic stem cell transplantation: state-of-the-art and real-life current approach: a position statement on behalf of the Infectious Diseases Working Party of the European Society of Blood and Marrow Transplantation. Rev Med Virol 2018; 28:e1980. [DOI] [PubMed] [Google Scholar]
- 9. Matthes-Martin S, Feuchtinger T, Shaw PJ, et al. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011). Transpl Infect Dis 2012; 14: 555–63. [DOI] [PubMed] [Google Scholar]
- 10. Matthes-Martin S, Boztug H, Lion T. Diagnosis and treatment of adenovirus infection in immunocompromised patients. Expert Rev Anti Infect Ther 2013; 11:1017–28. [DOI] [PubMed] [Google Scholar]
- 11. Lion T. Adenovirus persistence, reactivation, and clinical management. FEBS Lett 2019; 593:3571–82. [DOI] [PubMed] [Google Scholar]
- 12. Soriano G, Perales MA. Adenovirus viremia and infection after reduced-intensity allogeneic hematopoietic stem cell transplant: should we institute a routine screening program? Clin Infect Dis 2012; 55:1371–2. [DOI] [PubMed] [Google Scholar]
- 13. Sedláček P, Petterson T, Robin M, et al. Incidence of adenovirus infection in hematopoietic stem cell transplantation recipients: findings from the advance study. Biol Blood Marrow Transplant 2019; 25:810–8. [DOI] [PubMed] [Google Scholar]
- 14. Hill JA, Mayer BT, Xie H, et al. Kinetics of double-stranded DNA viremia after allogeneic hematopoietic cell transplantation. Clin Infect Dis 2018; 66:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zecca M, Wynn R, Dalle JH, et al. Association between adenovirus viral load and mortality in pediatric allo-HCT recipients: the multinational AdVance study. Bone Marrow Transplant 2019; 54:1632–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cho C, Perales MA. Expanding therapeutic opportunities for hematopoietic stem cell transplantation: T cell depletion as a model for the targeted allograft. Annu Rev Med 2019; 70:381–93. [DOI] [PubMed] [Google Scholar]
- 17. Tamari R, Oran B, Hilden P, et al. Allogeneic stem cell transplantation for advanced myelodysplastic syndrome: comparison of outcomes between CD34+ selected and unmodified hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2018; 24:1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tamari R, Chung SS, Papadopoulos EB, et al. CD34-selected hematopoietic stem cell transplants conditioned with myeloablative regimens and antithymocyte globulin for advanced myelodysplastic syndrome: limited graft-versus-host disease without increased relapse. Biol Blood Marrow Transplant 2015; 21:2106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jakubowski AA, Small TN, Kernan NA, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Transplant 2011; 17:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol 1997; 97:855–64. [DOI] [PubMed] [Google Scholar]
- 21. Lin A, Maloy M, Su Y, et al. Letermovir for primary and secondary cytomegalovirus prevention in allogeneic hematopoietic cell transplant recipients: real-world experience. Transpl Infect Dis 2019; 21:e13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant 2007; 13:1469–76. [DOI] [PubMed] [Google Scholar]
- 23. Sive JI, Thomson KJ, Morris EC, Ward KN, Peggs KS. Adenoviremia has limited clinical impact in the majority of patients following alemtuzumab-based allogeneic stem cell transplantation in adults. Clin Infect Dis 2012; 55:1362–70. [DOI] [PubMed] [Google Scholar]
- 24. Öhrmalm L, Lindblom A, Omar H, et al. Evaluation of a surveillance strategy for early detection of adenovirus by PCR of peripheral blood in hematopoietic SCT recipients: incidence and outcome. Bone Marrow Transplant 2011; 46:267–72. [DOI] [PubMed] [Google Scholar]
- 25. Rustia E, Violago L, Jin Z, et al. Risk factors and utility of a risk-based algorithm for monitoring cytomegalovirus, epstein-barr virus, and adenovirus infections in pediatric recipients after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2016; 22:1646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hiwarkar P, Gaspar HB, Gilmour K, et al. Impact of viral reactivations in the era of pre-emptive antiviral drug therapy following allogeneic haematopoietic SCT in paediatric recipients. Bone Marrow Transplant 2013; 48:803–8. [DOI] [PubMed] [Google Scholar]
- 27. Mynarek M, Ganzenmueller T, Mueller-Heine A, et al. Patient, virus, and treatment-related risk factors in pediatric adenovirus infection after stem cell transplantation: results of a routine monitoring program. Biol Blood Marrow Transplant 2014; 20:250–6. [DOI] [PubMed] [Google Scholar]
- 28. Heim A. Advances in the management of disseminated adenovirus disease in stem cell transplant recipients: impact of adenovirus load (DNAemia) testing. Expert Rev Anti Infect Ther 2011; 9:943–5. [DOI] [PubMed] [Google Scholar]
- 29. Vegvari C, Hadjichrysanthou C, Cauët E, et al. How can viral dynamics models inform endpoint measures in clinical trials of therapies for acute viral infections? PLoS One 2016; 11:e0158237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. DeVincenzo JP, Whitley RJ, Mackman RL, et al. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med 2014; 371:711–22. [DOI] [PubMed] [Google Scholar]
- 31. Lamarca A, Clumeck N, Plettenberg A, et al. Efficacy and safety of a once-daily fixed-dose combination of abacavir/lamivudine compared with abacavir twice daily and lamivudine once daily as separate entities in antiretroviral-experienced HIV-1-infected patients (CAL30001 Study). J Acquir Immune Defic Syndr 2006; 41:598–606. [DOI] [PubMed] [Google Scholar]
- 32. Pulido F, Katlama C, Marquez M, et al. A randomized study investigating the efficacy and safety of amprenavir in combination with low-dose ritonavir in protease inhibitor-experienced HIV-infected adults. HIV Med 2004; 5:296–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.