Abstract
Neonatal cholestasis is characterized by conjugated hyperbilirubinemia in the newborn and young infant and is a sign common to over 100 hepatobiliary and/or metabolic disorders. A timely evaluation for its etiology is critical in order to quickly identify treatable causes such as biliary atresia, many of which benefit from early therapy. An expanding group of molecularly defined disorders involving bile formation, canalicular transporters, tight junction proteins and inborn errors of metabolism are being continuously discovered because of advances in genetic testing and bioinformatics. The advent of next generation sequencing has transformed our ability to test for multiple genes and whole exome or whole genome sequencing within days to weeks, enabling rapid and affordable molecular diagnosis for disorders that cannot be directly diagnosed from standard blood tests or liver biopsy. Thus, our diagnostic algorithms for neonatal cholestasis are undergoing transformation, moving genetic sequencing to earlier in the evaluation pathway once biliary atresia, “red flag” disorders and treatable disorders are excluded. Current therapies focus on promoting bile flow, reducing pruritus, ensuring optimal nutrition, and monitoring for complications, without addressing the underlying cause of cholestasis in most instances. Our improved understanding of bile formation and the enterohepatic circulation of bile acids has led to emerging therapies for cholestasis which require appropriate pediatric clinical trials. Despite these advances, the cause and optimal therapy for biliary atresia remain elusive. The goals of this review are to outline the etiologies, diagnostic pathways and current and emerging management strategies for neonatal cholestasis.
Keywords: Neonatal Cholestasis, Biliary Atresia, Progressive Familial Intrahepatic Cholestasis, Alagille Syndrome, Genomics
Introduction
A subset of infants who present with jaundice have elevated serum direct or conjugated bilirubin concentrations, which represents impaired bile formation or flow (cholestasis) and is not physiological (normal). Conditions causing neonatal cholestasis include surgical and non-surgical disorders, some of which require rapid diagnosis and institution of treatment to avoid irreversible injury to other organs and to allow for best possible outcomes from surgical intervention for biliary atresia (BA). In this review, newer genetic causes of neonatal cholestasis will be outlined, current and future diagnostic paradigms will be suggested, and emerging therapeutic approaches will be described.
Definition of Cholestasis in Neonates
Cholestasis in infancy is defined as serum conjugated/direct bilirubin level >1 mg/dL and > 20% of the total bilirubin[1]. Although historically 2 mg/dL had been arbitrarily used as a threshold for conjugated/direct bilirubin, this has recently been reduced to 1 mg/dL to more accurately reflect clinical experience[2–4]. Recent reports suggest that during the first 5 days of life lower direct/conjugated bilirubin levels (>0.3–0.5 mg/dl) and direct/conjugated bilirubin >10% of total bilirubin are abnormal and should raise the suspicion for cholestasis at this age and require further evaluation[2–4]. Jaundice beyond 2 weeks of age in a breast-fed infant or at 2 weeks of age in a formula-fed infant should elicit fractionation of the serum bilirubin to differentiate the much more common breast milk-associated indirect hyperbilirubinemia from cholestasis. If conjugated/direct hyperbilirubinemia is identified, further evaluation for hepatobiliary causes should proceed immediately. The diagnostic pathway for neonatal cholestasis has evolved in recent years, largely because of the expanding identification of new genetic causes of cholestasis, the advent of next generation sequencing to identify genetic variants, the recognition of best outcomes for BA when hepatoportoenterostomy (HPE) is performed before 30–45 days of life[5–7], and emerging new potential therapies.
Causes of Neonatal Cholestasis
The frequency of neonatal cholestasis has been estimated to be 1 in every 2,500 live births[8, 9]. Etiologic categories include both extrahepatic and intrahepatic disorders, including anatomic obstruction of the biliary system (BA, choledochal cyst, cholelithiasis), neonatal infections, genetic and inborn errors of metabolism, endocrine disorders, toxin and drug exposures, hypoxia/ischemia, idiopathic neonatal hepatitis (INH) now referred to as transient neonatal cholestasis (TNC), and other miscellaneous causes[10] (Table 1). Although there are over 100 conditions that can present with cholestasis, establishing a prompt diagnosis is critical for optimal outcomes so focusing on the most common etiologies may be of benefit. BA accounts for 25–45% of cases; Alagille syndrome (arteriohepatic dysplasia; syndromic paucity of interlobular bile ducts) in 2–14%; genetic and metabolic diseases including α1-antitrypsin (A1AT) deficiency, cystic fibrosis, tyrosinemia, galactosemia, progressive familial intrahepatic cholestasis (PFIC), bile acid metabolism disorders, Niemann-Pick type C and citrin deficiency in 10–20%; hypopituitarism up to 5%; inspissated bile syndrome; parenteral nutrition associated cholestasis; and INH/TNC[11–13] Urinary tract infection, bacteremia, herpes simplex virus, and cytomegalovirus are uncommon but important treatable etiologies. In preterm infants, cholestasis may occur in 10–20% because of immaturity of the enterohepatic circulation, absence of feedings, intestinal inflammation or dysfunction, repeated bacterial or fungal infections, and use of parenteral nutrition[14–17].
Table 1.
Causes of Neonatal Cholestasis
| Anatomic Obstruction |
| Biliary atresia, choledochal cyst, cholelithiasis, biliary sludge, inspissated bile, spontaneous perforation of common bile duct, tumor |
| Infections |
| Viral, bacterial, spirochete, parasites |
| Toxins |
| Drugs, endotoxin, total parenteral nutrition associated cholestasis, herbal products, ? biliatresone |
| Endocrine |
| Hypothyroidism, panhypopituitarism |
| Immune |
| Gestational alloimmune liver disease |
| Genetic and Inborn Errors of Metabolism* |
| Alpha-1-antitrypsin deficiency (SERPINA1) |
| Alagille syndrome (JAGGED1, NOTCH2) |
| Arthrogryposis/Renal/Cholestasis (VPS33B, VIPAR) |
| Congenital hepatic fibrosis (PKHD1) |
| Citrin Deficiency (Adult citrullinemia type 2) (SLC25A13) |
| Cystic fibrosis (CFTR) |
| Bile acid synthesis defects (AKR1D1, AMACR, CYP7B1, HSD3B7, CYP7A1, CYP27A1) |
| Bile acid conjugation defects (BAAT, SLC27A5) |
| Fatty acid oxidation defects (SCAD, LCAD) |
| Galactosemia (GALT) |
| Glycogen storage disease type IV (GBE1) |
| Hereditary fructose intolerance (ALDOB) |
| Mitochondrial respiratory chain disorders (DGUOK, MPV17, POLG) |
| Neonatal ichthyosis sclerosing cholangitis syndrome (CLDN1) |
| Neonatal sclerosing cholangitis (DCDC2) |
| Niemann Pick type C disease (NPC1, NPC2) |
| Peroxisomal disorders (PEX1, PEX6, PEX10, PEX11B, PEX12, PEX13, PEX14, PEX16, PEX19, PEX2, PEX26, PEX3, PEX5, PEX7) |
| Progressive Familial Intrahepatic Cholestasis |
| Bile transport defects (ATP8B1, ABCB11, ABCB4, NR1H4, OSTα/β, WDR83OS, ABCC12) |
| Cytoskeleton defects (TJP2, MYO5B, UNC45, USP53, KIF12, PLEC) |
| Other defects (LSR, PPM1F) |
| Smith Lemni Opitz syndrome (DHCR7) |
| Lipid Storage Diseases (SCP2) |
| Tyrosinemia type 1 (FAH) |
| Urea cycle defects |
| Other |
| Idiopathic neonatal hepatitis (transient neonatal cholestasis) |
| Ischemia, Hypoxia, Hepatic Congestion |
| Hemophagocytic lymphohistiocytosis (HLH) |
| Malignancy |
Causative genes in parentheses
Adapted from Feldman AG, Sokol RJ, Nat Rev Gastroenterol Hepatol, 2019: Box 1
A better understanding of genetic disorders of bile formation and biliary physiology has been clarified over the past 10 years primarily because of technological advances in gene sequencing, advanced microscopy and cell biology. Many sites involved in the enterohepatic circulation of bile acids within the hepatocyte, cholangiocyte and enterocyte that regulate bile acid synthesis, transport and metabolism can lead to neonatal cholestasis (Figure 1). For example, many infants previously labeled as INH/TNC have been found to harbor disease-causing variants (either one or two alleles) in recently discovered genes, such as the those coding for canalicular transporters: ATP8B1 coding for FIC1, ABCB11 coding for bile salt export protein (BSEP), and ABCB4 coding for multidrug resistance protein 3 (MDR3), a phospholipid flippase[11],[18–21]. Moreover, the bile acid synthesis and conjugation enzymatic pathways have been fully defined, with mutations in many of the genes coding for these enzymes now identified to cause neonatal cholestasis (AKR1D1, AMACR, CYP7B1, HSD3B7, CYP7A1, CYP27A1, BAAT, SLC27A5). PFIC was originally a clinical description for idiopathic neonatal cholestatic conditions associated with progressive cholestasis and hepatic fibrosis, generally with normal/low serum γ-glutamyl transpeptidase (GGTP) and cholesterol concentrations and familial occurrence. It is now clear that there are over 10 genes (Table 1) that can cause a progressive cholestatic disease beginning in infancy consistent with PFIC, straining the original classification of PFIC 1 (ATP8B1), PFIC 2 (ABCB11) and PFIC 3 (ABCB4). Many of these genes have enlightened our understanding of the role of the cytoskeleton and tight junctions in bile formation (TJP2, MYO5B, UNC45, USP53, KIF12, PLEC, DCDC2), of the critical role of nuclear hormone receptors in the regulation of bile secretion (NR1H4) and of the role of mitochondrial energy production in promoting bile acid transport (DGUOK, MPV17, POLG). In addition to achieving a precise diagnosis and being able to provide families with genetic testing, defining the genetic etiology of each cholestatic infant will potentially allow for application of newer therapies based on specific gene mutations, promoting precision medical treatment to achieve optimal outcomes.
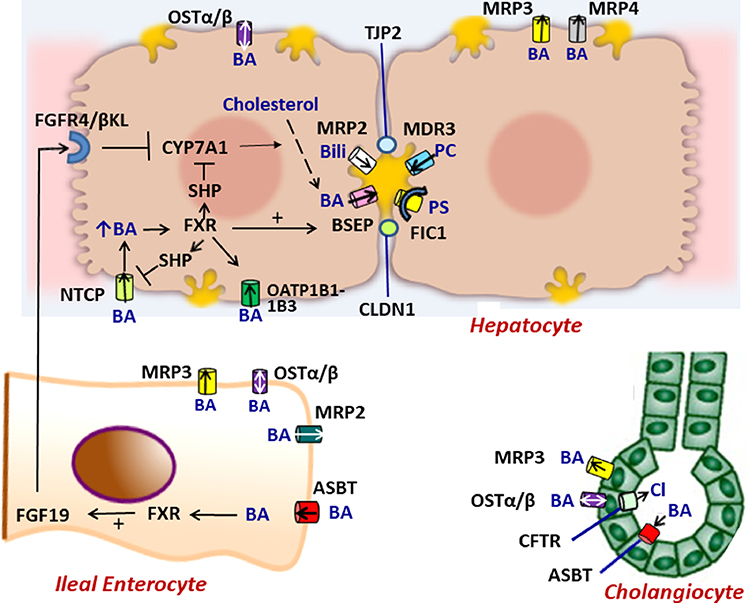
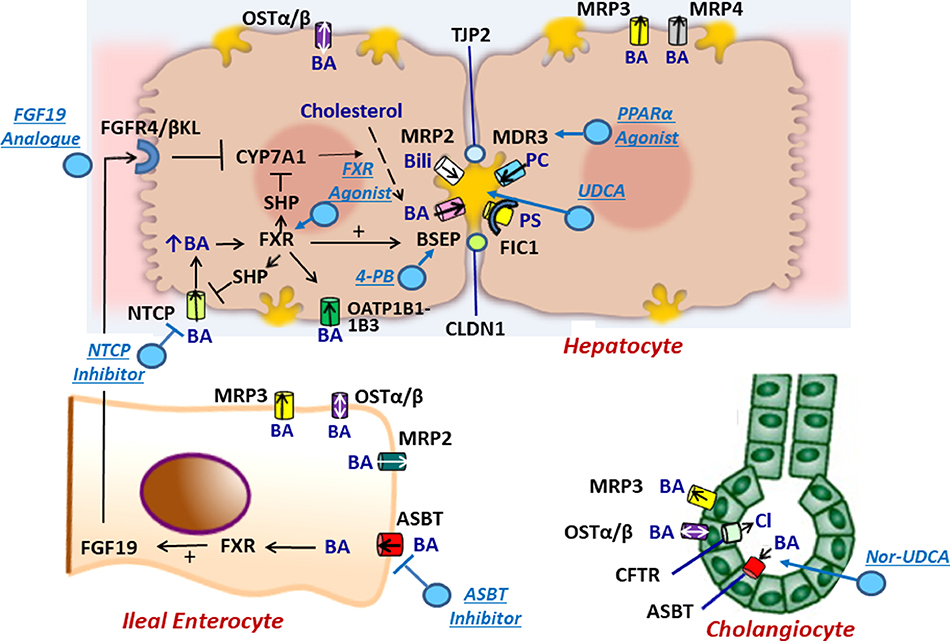
Figure 1.
Molecular regulation of the enterohepatic circulation of bile acids.In hepatocytes, bile acids are taken up by NTCP, OSTα/β or OATP1B1–1B3 on the basolateral membrane or are synthesized from cholesterol by CYP7A1. The ATP-dependent BSEP transports bile acids into bile across the canalicular membrane. FXR, a nuclear hormone receptor, is a master switch that upregulates mRNA expression of BSEP and downregulates NTCP and CYP7A1 (through SHP). MRP2 (conjugated bilirubin transporter), MDR3 (phospholipid transporter) and FIC1 (phosphotidylserine flippase) participate in bile formation across the canalicular membrane. MRP3 and MRP4 transport bile acids from the hepatocyte to the sinusoids. Tight junction proteins (TJP2 and CLDN1) maintain the boundary between canalicular bile and the hepatocyte, preventing the toxicitiy of secreted bile acids. In ileal enterocytes, ASBT promotes absorption of luminal bile acids across the enterocyte brush border which are transported by OSTα/β or MRP3 into portal blood to return to the liver where they interact with FXR to suppress further bile acid synthesis. Within the enterocyte, absorbed bile acids may also activate FXR which upregulates synthesis and secretion of FGF19 into the portal circulation. When it reaches the liver FGF19 binds to the FGFR4/ βKL receptor on the hepatocyte basolateral membrane and triggers suppression of CYP7A1. In cholangiocytes, ASBT in the apical membrane may transport bile acids from bile back into the cell and subsequently into the portal circulation by OSTα/β or MRP3 in the basolateral membrane. CTFR actively secretes chloride into bile. Perturbations of bile formation can result from genetic variants in many of these proteins (FIC1, BSEP, MDR3, MRP2, CFTR, TJP2, CLDN1) and from adaptive changes in transporter expression and function in response to inflammatory, obstructive, or drug-induced insults. . BA = bile acid, PC = phosphatidylcholine, Bili = conjugated bilirubin, Cl = chloride, PS= phosphatidylserine.
Diagnostic Evaluation
Is this jaundice cholestasis?
The timing of initial differentiation of indirect from direct/conjugated hyperbilirubinemia is the first question that must be addressed in the evaluation of a jaundiced infant. Breast milk-associated jaundice (indirect hyperbilirubinemia) can be present in 10–20% of two-week old breast milk-fed infants[22], thus being >300 times more common than cholestasis at this age. For this reason, cholestasis frequently goes unnoticed at this age and the breast-fed infant is labeled as having breast milk-associated jaundice. As the indirect hyperbilirubinemia improves over the next few weeks, the jaundice appears to improve despite the possibility of underlying cholestasis. Thus, many cholestatic infants who are breast-fed are discovered to still be jaundiced at their 2 month well child visit to a caregiver. This common sequence is an unfortunate missed opportunity to diagnose BA and other causes of cholestasis within the first 30–45 days of life, when surgery for BA has its best outcomes[5–7]. To prevent this delay in diagnosis of BA, it is recommended that breast-fed infants who remain jaundiced undergo serum bilirubin fractionation at 2–3 weeks of age. If conjugated or direct hyperbilirubinemia is identified, immediate referral is indicated for expedited evaluation by a pediatric gastroenterologist or hepatologist.
Initial Biochemical Testing
The current approach for evaluating an infant with cholestasis (Figure 2) centers on initially excluding BA (and A1AT deficiency in appropriate populations), following “red flags” that indicate the likelihood of a specific etiology (e.g., extrahepatic features of Alagille syndrome), and searching for other treatable conditions (Table 2 and Table 3). If this evaluation is not fruitful, then specific testing for less common and rarer conditions proceeds. The initial evaluation should include serum alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, GGT, and albumin, although these rarely discriminate among the etiologies[8]. However, a significantly elevated GGT (>150–200 U/L) is suggestive of BA, mechanical bile duct obstruction, paucity of interlobular bile ducts, A1AT deficiency, cystic fibrosis, neonatal sclerosing cholangitis or PFIC type 3. A normal or low GGT (<125 U/L) suggests PFIC type 1, 2, 4–6, bile acid synthesis or metabolism disorders, panhypopituitarism or INH/TNC. Elevated prothrombin time or international normalized ratio suggests either vitamin K malabsorption and deficiency or synthetic liver failure (particularly if unresponsive to parenteral vitamin K administration), metabolic disease or sepsis. Low serum albumin may indicate malnutrition or hepatic synthetic dysfunction. Serum α-fetoprotein levels can be elevated in tyrosinemia type 1 and other conditions [23, 24].
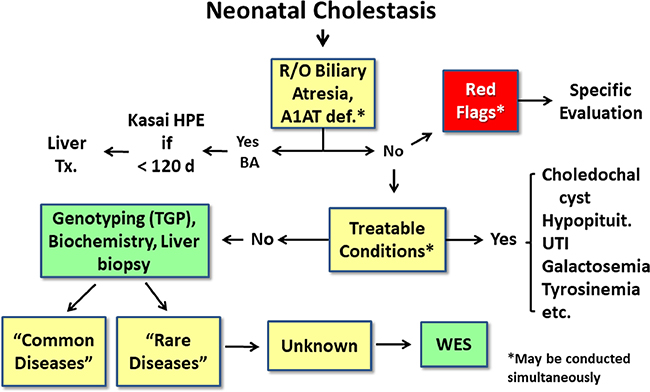
Figure 2.
Current paradigm for evaluation of neonatal cholestasis.In the current sequential evaluation of neonatal cholestasis, alpha-1 antitrypsin deficiency (A1AT), biliary atresia, and treatable causes of cholestasis (such as choledochal cyst or urinary tract infection) are excluded in a timely fashion, which may require liver biopsy and intraoperative cholangiogram to determine if biliary atresia is present. If a “red flag” points to a specific diagnosis (Table 2), evaluation for that disease should proceed promptly.If no diagnosis if found, the next tier of testing may include multiple blood and urine tests to evaluate for infectious, genetic and metabolic causes. If no diagnosis is confirmed, targeted gene panels or specific gene testing is performed and whole exome sequencing (WES) is reserved for those cases without a diagnosis after this exhaustive evaluation. R/O= rule out, HPE= hepatoportoenterostomy, UTI= urinary tract infection, PFIC= progressive familial intrahepatic cholestasis, TGP= targeted gene panel, WES= whole exome sequencing.
Table 2.
Treatable causes of neonatal cholestasis
| Disorder | Treatment |
|---|---|
| Infection (viral, bacterial, spirochete, parasite) | Antimicrobials |
| Galactosemia | Galactose-free diet |
| Tyrosinemia type 1 | NTBC (2-(2- nitro-4-trifluoromethylbenzol)-1,3-cyclohexanedione), low tyrosine or phenylalanine diet |
| Hereditary fructose intolerance | Fructose or sucrose free diet |
| Hypothyroidism | Thyroid hormone replacement |
| Cystic fibrosis | Pancreatic enzymes |
| Hypopituitarism | Thyroid, growth hormone, cortisol replacement |
| Bile acid synthesis defects | Cholic acid or ursodeoxycholic acid supplementation |
| Biliary atresia | Hepatoportoenterostomy (Kasai procedure) |
| Choledochal cyst | Mucosectomy and Choledochoenterostomy |
| Spontaneous perforation of the common bile duct | Surgical drainage |
| Inspissated bile, common bile duct stone | Biliary tract irrigation |
| Parenteral nutrition associated cholestasis (intestinal failure associated cholestasis) | Intravenous lipid emulsion modification, advance enteral feedings |
Adapted from Feldman AG, Sokol RJ, Nat Rev Gastroenterol Hepatol, 2019: Table 153
Table 3:
“Red flag” findings for Neonatal Cholestasis
| Red Flag | Disease to Consider |
|---|---|
| Maternal History | |
| Prenatal Ultrasound abnormality | Choledochal cyst, cystic biliary atresia, gallstone |
| Intrahepatic cholestasis of pregnancy | PFIC, mitochondrial disease |
| Acute fatty liver of pregnancy | LCHAD |
| Maternal infection during pregnancy | Congenital infection |
| Physical Finding | |
| Acholic stool | Biliary atresia, choledochal cyst, gallstone, biliary sludge |
| Palpable mass in the right upper quadrant | Choledochal cyst |
| Heart murmur | Alagille syndrome or biliary atresia |
| Butterfly vertebrae | Alagille syndrome |
| Ascites | Spontaneous perforation of the bile duct |
| Dysmorphic facies | Alagille syndrome, Zellweger syndrome, chromosomal abnormality |
| Microcephaly | |
| Posterior embryotoxon | Congenital infection |
| Chorioretinitis | Alagille syndrome |
| Cataracts | Congenital infection |
| Vision abnormalities | Congenital infection, galactosemia |
| Splenomegaly | Panhypopituitarism, septo-optic-dysplasia |
| Multiple joint contractures | Nieman Pick type C |
| Purpura, thrombocytopenia | Arthrogryposis |
| Hypotonia | Congenital infection, Congenital lupus |
| Neurologic abnormalities (irritability, lethargy, poor feeding, hypotonia, or seizures | Mitochondrial disorder, peroxisomal disorder |
| Sepsis, intracranial hemorrhage, metabolic and mitochondrial disorders, panhypopituitarism | |
| Family History | |
| Early emphysema | Alpha-1-antitrypsin deficiency |
| Liver disease in siblings or stillbirths | Genetic disease or inborn error of metabolism |
| Consanguinity | Autosomal recessive genetic liver disease |
Adapted from Feldman AG, Sokol RJ, Nat Rev Gastroenterol Hepatol, 2019: Table 253
Tiers of Testing for Etiology of Neonatal Cholestasis
Testing for common and treatable etiologies of cholestasis should include a first tier of initial tests, which can be modified by historical and physical findings. A1AT deficiency should be excluded before exploratory surgery for BA by normal serum A1AT levels, the absence of protease inhibitor (PI) ZZ or SZ on electrophoresis, or by rapid genotyping the SERPINA1 gene, whichever can be performed within 24–48 hours. The newborn metabolic screen should be carefully reviewed (or repeated) to exclude hypothyroidism, tyrosinemia type 1, or galactosemia (treatable causes of cholestasis), or specific testing should be performed (thyroid function tests, urine succinylacetone for tyrosinemia, urinary-reducing substances or red blood cell galactose-1-phosphate uridyl transferase drawn before administration of any blood products for galactosemia). If infection is suspected, blood and urine cultures should be obtained in addition to appropriate viral cultures and IgM serologies. In low GGT patients, total serum bile acids (which are paradoxically low or normal in the majority of cases of bile acid synthesis disorders) can be measured[25]. Receiving oral ursodeoxycholic acid (UDCA) can elevate serum bile acid levels so this must be withheld for 5 days before total serum bile acid testing. A second tier of testing can include: serum amino acids (for citrin deficiency), urine organic acids, acylcarnitine profile (for fatty acid oxidation defects); very long-chain fatty acid levels (for peroxisomal disorders); and a sweat test, serum immunoreactive trypsinogen, or CFTR genotyping (for cystic fibrosis). If clinical, family history or biochemical ‘red flags’ (Table 3) are suggestive of a specific disorder (Figure 2), evaluation for that disease should proceed early in the evaluation process.
Imaging Evaluation
Early in the diagnostic evaluation, BA, the most common cause of neonatal cholestasis, must be excluded in virtually every cholestatic infant and imaging plays a major role in this determination. Abdominal ultrasonography should be performed to assess for liver size, position and composition, spleen number and size, ascites, and presence of a choledochal cyst, mass, gallstone or obstructing sludge. Several sonographic findings are associated with BA, including absent or abnormal gallbladder, triangular cord sign, hepatic artery–portal vein ratio, increased hepatic subcapsular blood flow, or findings of heterotaxia and laterality defects[8, 26]. Hepatobiliary scintigraphy with a technetium-labeled iminodiacetic acid analogue may be helpful in identifying patency of bile ducts from liver to intestine, excluding BA[8, 27], however the specificity of a non-excreting scan for BA is low (33–80%)[27, 28]. Endoscopic retrograde cholangiopancreatography, magnetic resonance cholangiopancreatography or percutaneous transhepatic cholecystocholangiography have been used in selected centers to define bile duct patency[29–32], however intraoperative cholangiography (IOC) remains the definitive diagnostic test for BA in most centers.
Liver Biopsy
Percutaneous liver biopsy may assist in differentiating BA from other etiologies and histopathology may point to other specific diagnoses. It has been used classically early in the evaluation of cholestasis in order to direct the evaluation to IOC if characteristic BA findings were present. The accuracy of liver biopsy histology for predicting the diagnosis of BA has been in the range of 85–95%[33, 34]. Histological features of BA include portal bile duct plugs, ductal reaction, and portal fibrosis. However, other causes of cholestasis can mimic BA histology, including A1AT deficiency, parenteral nutrition-associated cholestasis, MDR3 deficiency, DCDC2 disease and cystic fibrosis. Liver biopsy can also be helpful in identifying other diseases causing cholestasis (Table 4). Early in the course of BA (before age 4 weeks), not all of the characteristic histologic findings may be present, so liver histology should not be overinterpreted at that age. Recent diagnostic algorithms (Figure 2, Figure 3) have placed less emphasis on liver biopsy and more on genotyping in evaluating the cholestatic infant.
Table 4.
Histological Features of Specific Causes of Neonatal Cholestasis
| Disorder | Histology |
|---|---|
| Biliary obstruction | Portal tract bile duct plugs, ductular reaction, portal tract fibrosis |
| A1AT deficiency | Periodic acid Schiff-positive, diastase-resistant hepatocyte globules |
| Alagille syndrome | Bile duct paucity |
| Neonatal sclerosing cholangitis | Necro-inflammatory bile duct lesions |
| Inborn Errors of Metabolism | Hepatic steatosis and pseudo-acinar formation of hepatocytes |
| PFIC types 1, 2 | Electron microscopy - abnormal canalicular bile |
| Storage diseases | Electron microscopy - lysosomal storage material |
| Transient Neonatal Cholestasis | Multinucleated giant cells, extramedullary hematopoiesis, and hepatocellular cholestasis without portal tract involvement |
| Viral infections | CMV inclusions and HSV, CMV on immunohistochemistry |
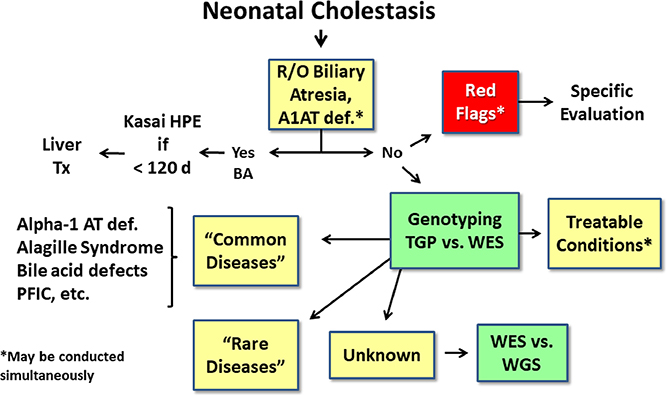
Figure 3.
Future evaluation of neonatal cholestasis. In the near future, it is anticipated that following exclusion of biliary atresia and A1AT deficiency, investigation of “red flags” pointing to specific etiologies, and testing for treatable conditions, rapid turn-around genotyping via whole exome sequencing (WES) or whole genome sequencing (WGS) will be utilized to evaluate for common and rare genetic causes. R/O= rule out, HPE= hepatoportoenterostomy, UTI= urinary tract infection, PFIC= progressive familial intrahepatic cholestasis, TGP= targeted gene panel, WES= whole exome sequencing, WGS= whole genome sequencing.
Liver Elastography
Noninvasive measures of liver stiffness fibrosis (such as acoustic radiation force impulse [ARFI], transient elastography, sheer wave elastography, or magnetic resonance elastography) as surrogate markers for the degree of hepatic fibrosis have achieved widespread use in adult hepatology. Because elastography measures are significantly higher in BA than other neonatal cholestatic conditions[35], it has been proposed that measuring liver stiffness could be used to speed the diagnostic path to IOC, avoid liver biopsy and ultimately shorten the time to HPE. For example, in a study from Taiwan using transient elastography in 48 cholestatic infants, among which 15 had BA, liver stiffness above 7.7 kPa predicted BA (area under the receiver operated characteristic curve (AUC) of 0.85) with an odds ratio of 128 (P<0.001)[36]. The utility of these noninvasive procedures for distinguishing BA from other cholestatic disorders is promising but requires further study.
Other Biomarkers
Recent studies have indicated that mRNA expression of matrix metalloproteinase 7 (MMP7) is strongly upregulated in liver biopsies obtained at the time of diagnosis of BA compared to similarly age intrahepatic cholestasis controls[37, 38] and in the newborn mouse viral model of BA[39, 40], and that the protein is highly expressed intrahepatic bile ducts. This led to several groups reporting that elevated serum MMP7 at 1– 2 months of age discriminates those with BA from all others with neonatal cholestasis, with positive predictive values exceeding 90% and negative predictive values exceeding 95%[37, 41, 42]. A potential role of rapidly measuring serum MMP7 by ELISA early in the evaluation of neonatal cholestasis to guide the further work-up for BA has been proposed and remains to be tested prospectively. Similarly, IL-33 appears to be overexpressed in the bile ducts of patients with BA[43] leading to a proposed used of serum IL-33 to differentiate BA from non-BA in a similar manner as MMP7[44].
Next Generation Sequencing (NGS)
New gene sequencing technologies have allowed for rapid testing of large numbers of genes, including an individual’s entire genome, within days to weeks, thus challenging our prior use of genotyping late in the traditional paradigm for evaluation of cholestasis[11, 45–48]. NGS also allows for the possibility of discovering new genetic causes of neonatal cholestasis. NGS is performed by several high-throughput platforms using massively parallel processing of spatially separated amplified DNA templates[47]. Targeted gene panels (TGPs) analyzing dozens of genes known to cause cholestatic disorders, whole exome sequencing (WES), and whole genome sequencing (WGS) are now clinically available tools (as costs have decreased substantially) in many centers and countries that can provide an opportunity to identify all known gene variants that have been associated with cholestatic diseases in a single test. Various bioinformatics platforms can make it possible to obtain genetic results in a few days although the turnaround time is generally measured in weeks. Most TGP panels include at least JAGGED1 and NOTCH2 (Alagille syndrome), ATP8B1 (PFIC type 1), ABCB11 (PFIC type 2), ABCB4 (PFIC type 3), SERPINA1 (A1AT deficiency), ABCC2 (Dubin–Johnson syndrome), and SLC25AJ3 (neonatal or infantile intrahepatic cholestasis caused by citrin deficiency), as well as many other rarer conditions[45, 49–52].
There are several published reports of the utility of using NGS panels in the evaluation of neonatal cholestasis. One study used a multi-gene panel that sequenced 61 cholestasis-related genes in 141 patients and made a potential genetic diagnosis in 22%[50]. A second study of gene sequencing in 51 infants with cholestasis without a etiology, a molecular diagnosis was established in 27%[45]. A Japanese study using NGS in 109 patients with neonatal cholestasis \reported a molecular diagnosis in 26%[51]. A North and south American study using a 66 gene panel in 716 infants and older children with cholestasis or liver disease of unknown etiology reported a positive or likely positive molecular diagnosis in 11.7% and a single pathogenic or likely pathogenic variant in another 12.7%[52]. It needs to be emphasized that variants of unknown significance (VOUS) need to be interpreted cautiously and should not be used to establish a molecular diagnosis. Viewed in total, these reports indicate that a molecular diagnosis can be made in a significant number of cholestatic infants using TGPs. The possible use of WES expands these capabilities and opens the window for discovery of new genetic causes of cholestasis.
The advents of more rapid turn-around time, decreasing cost, and automation of bioinformatics for TGP and WES genotyping begs the question of whether multigenic sequencing may become an early diagnostic test in the evaluation of neonatal cholestasis, replacing the current diagnostic algorithm (Figure 3). Thus, molecular diagnostics may become a cost- and time-effective means to determine the etiology in patients for whom BA has been excluded and while other treatable or “red flag” indicated disorders are being evaluated. This shift in the paradigm for evaluation of neonatal cholestasis is being implemented at selected centers around the world and may become the standard in coming years.
Management of Neonatal Cholestasis
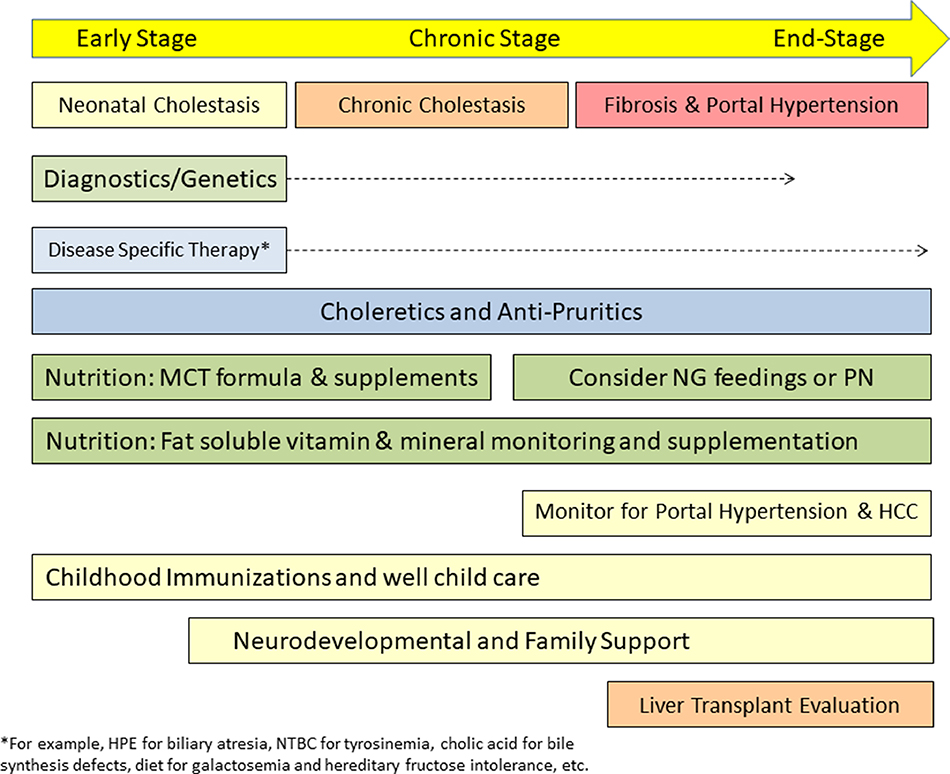
As outlined in a recent review article[53], therapy of neonatal cholestasis can be tailored to the three stages of disease (Figure 4): early, chronic and end-stage, each with specific goals for management. Treatable etiologies should be identified and specific therapy initiated expeditiously (Table 2). This is best illustrated by BA in which early timing (at < 30–45 days of life) of HPE dictates best short- and long-term outcomes[5, 6]. Early diagnosis and initiation of therapy is beneficial in hypopituitarism, tyrosinemia type 1, and infections, among other conditions[54]. Targeted pharmacologic, dietary or surgical treatments are available for a few genetic etiologies of cholestasis (Tables 2), including oral cholic acid which replaces the primary bile acid pool for several disorders of bile acid synthesis[55]; and nitisinone which prevents accumulation of toxic intermediates (maleylacetoacetic acid and fumarylacetoacetic acid) in tyrosinemia type 1[56]. Lactose and galactose-restricted diets are required for galactosemia and fructose, sucrose and sorbitol-restricted diets for hereditary fructose intolerance[57].
Figure 4.
Holistic Management of Neonatal Cholestasis. Different stages of neonatal cholestasis are defined by the rate of progression from early (neonatal) to chronic (> 6 months of cholestasis) to end-stage liver disease. The latter is characterized by progressive portal fibrosis and synthetic liver failure or complications of portal hypertension. Diagnostic testing may be continued as new etiologies are discovered over time. Disease-specific therapy (if available) should be instituted. Medical therapies can be utilized to improve or treat pruritus, cholangitis and portal hypertension. In the future, new choleretic, anti-fibrotic, anti-inflammatory and bile acid-modifying agents might become available. Medium-chain triglyceride containing infant formula and fat-soluble vitamin supplementation are essential for most infants who remain cholestatic. Monitoring and therapy are initiated for complications of portal hypertension; screening for hepatocellular carcinoma is initiated as indicated. Immunization schedules are accelerated if liver transplantation is planned. Developmental services and family support are provided as needed. MCT= medium chain triglycerides, NG = nasogastric, PN = parenteral nutrition, HCC = hepatocellular carcinoma, HPE = hepatoportoenterostomy, NTBC = 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione
To promote bile flow and reduce the severity of cholestasis-induced pruritus, UDCA is commonly prescribed at doses of 15–20 mg/kg/day in many of the cholestatic diseases of infancy, despite a paucity of efficacy data in most of these diseases. UDCA is generally well tolerated without significant side effects. If pruritus does not respond to UDCA, antihistamines, rifampicin (20 mg/kg/day), naltrexone (1–2 mg/kg/day), sertraline (1–4 mg/kg/day), bile acid binding resins (e.g, cholestyramine) and other measures are frequently used off label, often in combination, to reduce pruritus and improve the quality of life for the infant and their family. Surgical interruption of the enterohepatic circulation of bile acids (partial external or internal biliary diversion or ileal exclusion) may be effective in a subset of patients with PFIC type 1, PFIC type 2 and Alagille syndrome, with improvement in pruritus, stabilization of cholestasis and improved growth[58].
To promote growth and development and prevent the consequences of malnutrition, maximizing nutrition is an essential component of treatment for all cholestatic infants. Since intraluminal bile acid concentrations above the critical micellar concentration are required for intestinal absorption of dietary fat and fat-soluble vitamins (vitamins A, D, E and K), cholestatic infants are at high risk for steatorrhea and fat-soluble vitamin malabsorption and deficiencies. In the early stage of cholestasis, breast milk or standard infant formulas may be adequately absorbed with milder degrees of cholestasis or after successful bile drainage for BA[59]. However, more severe cholestasis will require infant formulas containing larger amounts of medium-chain triglycerides (MCT) and adequate amounts of essential fatty acids or MCT oil supplements, fat that is absorbed despite poor bile flow[60]. Infants with chronic cholestasis often require at least 125–140% of the recommended caloric requirement based on ideal body weight because of increased oxygen consumption compounding the fat malabsorption[61]. To achieve this goal, supplemental nasogastric tube nocturnal drip feedings or home parenteral nutrition[62] may be necessary, especially in end-stage cholestasis when children are placed on waiting lists for liver transplantation. The importance of achieving adequate nutrition is underscored by the fact that liver transplantation graft and patient survival are directly related to nutritional status [63, 64].
From the early stage of neonatal cholestasis, infants require fat-soluble vitamin supplementation and careful monitoring vitamin status to prevent and treat deficiencies. Deficiency of vitamin K has been fatal in infants with cholestasis as presentation with intracranial hemorrhage from vitamin K deficiency coagulopathy has been reported multiple times. Vitamin D deficiency can lead to severe rickets and bone fractures, vitamin A deficiency to corneal and retinal abnormalities that may lead to blindness, and vitamin E deficiency to irreversible neurologic and muscular morbidities[61]. Thus, monitoring is essential each 2–3 months during the first year of life by obtaining serum 25-hydroxy vitamin D, serum retinol and the retinol: retinol binding protein ratio, prothrombin time and international normalized ratio, and serum alpha tocopherol normalized to serum lipids. For those in the end-stage of cholestasis, it is essential to accelerate the childhood immunization schedule to achieve full immunization before liver transplantation, particularly for the live vaccines which should not be administered during significant immunosuppression[65–68]. Under immunization of pediatric liver transplant recipients remains a significant problem[69]. Through the chronic and end-stages of cholestasis monitoring is advised for signs of portal hypertension, ascites, pruritus and hepatocellular carcinoma. Attention should also be directed to the motor and cognitive development and emotional status of the child and the well-being of the family since developmental delays and impaired quality of life are common[70, 71].
Emerging Potential Therapies for Cholestasis
Although there are currently no U.S. Food and Drug Administration approved drugs for treatment of cholestasis in children, UDCA and 6-ethylchenodeoxcholic acid or obetacholic acid (OCA) are approved for treatment of adults with the cholestatic disease primary biliary cholangitis (PBC)[72–77]. New potential therapeutic opportunities for cholestatic disorders have emerged in recent years based on recent insights into our understanding of bile formation and secretion, bile acid signaling along the enterohepatic circulation and regulatory networks (Figure 5). Pharmacologic agents have been developed to either block or stimulate many of these pathways and have advanced to early or late phase therapeutic trials in adults or children with chronic cholestasis. In this section, we will review these agents with knowledge that they may not all be effective or safe and that carefully conducted clinical trials in children will be needed.
Figure 5.
Emerging therapeutic targets for the treatment of cholestasis. Inhibitors of NTCP may reduce the bile acid uptake by hepatocytes and subsequent toxicity. FXR agonists may upregulate hepatocyte efflux of bile constituents through BSEP, MRP2, MRP3, MRP4, and OSTα/β. UCDA, molecular chaperones such as 4-phenylbutyrate or other agents may reduce the toxic bile acid burden of the hepatocyte and improve bile flow and fat absorption. PPARα agonists may induce canalicular MDR3 expression and phospholipid secretion protecting cholangiocytes against bile acid toxicity. Alternatively, another strategy that would reduce hepatocyte bile acid levels is the inhibition of bile acid synthesis through suppressing CYP7A1 by FGF19 agonists, FXR agonists, or short interfering RNAs. In the ileal enterocyte, inhibiting ASBT will increase fecal excretion of bile acids, lower the bile acid pool size, change bile acid composition and alter enterocyte FXR signaling. In the cholangiocyte, nor-UCDA may protect the cholangiocyte from bile acid-induced injury by altering bile pH. 4-PB = 4-phenylbutyrate, BA = bile acid, PC = phosphatidylcholine, Bili = conjugated bilirubin, Cl = chloride, PS= phosphotidylserine, UDCA = ursodeoxycholic acid.
A final common pathway leading to cholestatic liver injury and fibrosis is the accumulation of excess amounts of hydrophobic bile acids in the cholestatic hepatocyte[78]. Thus, efforts to reduce or prevent hepatocyte retention of bile acids would theoretically be beneficial. UDCA, a hydrophilic largely non-toxic bile acid, may improve cholestasis by reducing hepatocyte bile acid concentrations through stimulating canalicular secretion through MRP2 and BSEP and basolateral export through MRP3 and MRP4, and by dilution of the pool of toxic bile acids. Other proposed mechanisms include cytoprotective, anti-inflammatory and antifibrotic properties of UDCA[79–81]. Despite its common use in pediatric cholestatic disorders there is a paucity of clear evidence of clinical effectiveness in this age group, although UCDA is generally well tolerated. It has been shown that excessively large doses of UDCA (>28 mg/kg/day) in adults with primary sclerosing cholangitis were associated with worse clinical outcomes[82].
Nor-UDCA is a side chain shortened derivative of UCDA that is resistant to amidation and enhances shunting of secreted bile acids from the bile duct lumen back to the hepatocyte[83]. This process induces a bicarbonate rich hypercholeresis that may counteract intrinsic bile acid toxicity to biliary epithelia[84] and could be of potential benefit in a number of cholestatic disorders, particularly cystic fibrosis liver disease. Nor-UDCA also has anti-inflammatory, anti-lipotoxic, antiproliferative and anti-fibrotic properties[83, 85–87] and is currently undergoing trials in several adult cholestatic liver diseases.
The farnesoid X receptor (FXR) agonists are a new class of drugs developed for the treatment of cholestasis. FXR is a nuclear hormone receptor within hepatocytes (and enterocytes) which is activated by bile acids (most strongly by chenodeoxycholic acid and cholic acid), which then transactivates expression of BSEP and MRP2 in the hepatocyte and FGF19 in the ileal enterocyte. FXR also indirectly down regulates bile acid synthesis by activating expression of the small heterodimer partner (SHP) in the hepatocyte which then inhibits expression of the rate limiting enzyme for bile acid synthesis, cholesterol 7-alpha hydroxylase (CYP7A1), as well as CYP8B1, and also downregulates NTCP to prevent uptake of circulating conjugated bile acids by the hepatocyte[88]. The combined effect is to reduce hepatocyte bile acid concentrations which may protect the hepatocyte from potential bile acid mediated toxicity. Thus it has been proposed that FXR agonists could activate these pathways to stimulate bile flow and reduce hepatocyte bile acid concentrations and cholestatic hepatocyte injury[89, 90]. Activation of FXR may alter intestinal bile acid concentrations and composition, change the intestinal microbiome, and modify the gut–liver axis that is believed to potentiate cholestatic liver injury[91–93]. FXR agonists have yet to undergo clinical trial testing in neonatal cholestatic conditions or children with other liver disorders.
Agonists for other nuclear receptor (for example, PXR, CAR, and PPARα) and the membrane bound G-protein coupled bile acid receptor TGR5 are under development as these pathways have properties that regulate bile acid homeostasis, and may also have potential hepatic anti-inflammatory properties[94]. PPARα agonists (e.g., bezafibrate) can increase MDR3 expression and its redistribution to the canalicular membrane which would stimulate biliary phospholipid secretion that may provide biliary epithelial protection against bile acid toxicity. PPARα agonists may also suppress CYP7A1 and CYP27A1 and thus reduce bile acid synthesis, induce CYP3A4 which may aid in bile acid detoxification, and may have anti-inflammatory, anti-fibrotic and antipruritic actions[93]. TGR5 is not expressed in hepatocytes, but is in cholangiocytes, gallbladder epithelium, endothelial cells and Kupffer cell. It may be involved in biliary HCO3- secretion and has anti-inflammatory properties and hepatoprotective effects from bile acid retention[48, 95, 96]. This, together with TGR5 polymorphisms in PSC[97],makes it an attractive therapeutic target.
Another attractive pharmacological target for treatment of cholestasis (based on the effectiveness of surgical biliary diversion in PFIC and Alagille syndrome) is blocking ileal bile acid uptake by highly selective, non-absorbed apical sodium–bile acid transporter (ASBT) inhibitors (for example, maralixibat)[58, 98]. These drugs bind to ASBT, interrupting the enterohepatic circulation of bile acids by increasing fecal excretion and reducing portal venous bile acid levels, thereby reducing hepatic uptake of bile acids which, in turn, reduces hepatic FXR activation and increases bile acid synthesis in the liver, with alterations in the composition of bile[90, 99, 100]. In addition, blocking ileal bile acid uptake reduces ligand binding and activation of ileal enterocyte FXR, thereby reducing intestinal FGF19 secretion into the portal circulation[101]. Since FGF19, through binding to FGFR4/βKL on the hepatocyte basolateral membrane, normally suppresses CYP7A1, the effect of ASBT blockade is to release this hepatocyte suppression of bile acid synthesis. Thus, ASBT inhibitors both reduces the bile acid pool size and increases bile acid synthesis and secretion by the hepatocyte. In maralixibat and A4250 trials in Alagille syndrome and PFIC, preliminary reports demonstrate reduction of pruritus, serum bile acid levels or both in a subset of patients [98, 102, 103]. Similarly, inhibitors of the hepatocyte basolateral bile acid uptake protein, NTCP, such as myrcludex B, could also reduce the bile acid burden in the liver[104], although NTCP is already downregulated to some extent in models of cholestasis. Bile acid sequestrants that are already in clinical use to prevent hypercholesterolaemia and pruritus (for example, cholestyramine or colesevelam) might also be beneficial in enhancing fecal bile acid excretion, although they have not been shown to alter the course of the liver disease itself and palatability is an issue in small children[105]. A major difference between ASBT inhibitors and sequestrants is that ASBT inhibitors and not sequestrants allow free bile acids to enter the colon, where they can still signal through FXR and may induce secretion of GLP-1, which can have hepatoprotective effects[48].
A number of the genetic causes of cholestasis result in perturbations of the synthesis or structure of bile transport proteins or their intracellular trafficking to the canalicular membrane. As in other genetic diseases, small-molecule chemical chaperones that influence the folding or stability of the malformed proteins to enhance their trafficking to the canalicular membrane may be an effective therapeutic strategy[106]. Early testing of potential chaperones, such as 4-phenylbutyrate, has been performed for selective cases of PFIC types 1 and 2 with missense mutations that result in cholestasis[107–110]. It is hoped that more chaperones can be developed for specific genetic variants that allow for precision medical treatments based on genotype.
There is a great deal of interest in developing novel therapies to reduce inflammation and fibrosis in pediatric as well as adult cholestatic diseases[111]. As an anti-inflammatory, high-dose corticosteroid therapy in BA did not demonstrate a short-term or long-term benefit after HPE in the START trial conducted by the NIH-funded Childhood Liver Disease Research Network[112]. Similarly, in an attempt to block innate immune injury in BA, a phase 1/2A clinical trial of intravenous immunoglobulin was of no benefit[113]. Some believe that a subset of patients with BA, who perhaps will be identified in the future through cellular and molecular immunotyping, could benefit from anti-inflammatory agents. This approach to precision pediatric hepatology awaits confirmation. Several anti-inflammatory or anti-fibrotic agents currently under investigation in adult liver diseases (e.g., the CCR2 or CCR5 antagonist, cenicriviroc) may prove to be beneficial and would be attractive agents for trials in pediatric cholestasis. Gene-editing and gene transfer strategies as potential cures for genetic causes of cholestasis may revolutionize how these diseases are treated in the future. These approaches are under investigation in a number of animal models; safety in children with underlying cholestasis will first need to be established if these therapies reach the human clinical trial stage.
Conclusions
The evaluation and management of neonatal cholestatic disorders is in the midst of a transformation driven largely by advances in genetics, technology and new and emerging pharmacotherapeutics. With the identification of molecular causes for many of these disorders, new categorization schemes are likely to be developed to replace soon outmoded terminology, such as PFIC (how many PFIC types will we have?). Incorporating genomics into the cholestasis evaluation paradigm has already begun, and only cost, availability and turn-around time of whole genome sequencing is keeping it from replacing many of the biochemical tests and the use of liver biopsy in the current diagnostic paradigm. For many diseases, through molecular definition of each patient, it is hoped that precision medicine approaches can be developed in the future that will allow each patient to receive the best therapy tailored to their own personal pathophysiology. Defining the etiology and pathogenesis of BA still remains a challenge as is the development of newer effective therapies. The next decade will see many clinical trials of newer agents (Figure 5) in infants and children with cholestasis, hopefully providing families with new hope for a better quality of life for their affected children.
Acknowledgements
R.J.S. is supported in part by NIH grants U01DK062453 and UL1T002535. A.G.F. is supported by an AHRQ K08 Award HS026510–01A1.
Abbreviations
- A1AT
α-1-antitrypsin deficiency
- BA
Biliary atresia
- BSEP
Bile salt export protein
- FXR
Farnesoid X receptor
- GGT
γ-glutamyl transpeptidase
- HPE
Hepatoportoenterostomy
- INH
Idiopathic neonatal hepatitis
- IOC
Intraoperative cholangiography
- MCT
Medium chain triglycerides
- MDR3
Multidrug resistance protein 3
- MMP 7
Matrix metalloproteinase 7
- NGS
Next generation sequencing
- OCA
Obetacholic acid
- PBC
Primary biliary cholangitis
- PFIC
Progressive familial intrahepatic cholestasis
- TGP
Targeted gene panel
- TNC
Transient neonatal cholestasis
- UDCA
Ursodeoxycholic acid
- WES
Whole exome sequencing
- WGS
Whole genome sequencing
Footnotes
Declaration of Competing Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Feldman A, Suchy FJ. Approach to the infant with cholestasis In: Suchy FJ, Sokol RJ, Balistreri WF, editors. Liver Disease in Children New York City: Cambridge University Press; 2014, p. 101–10. [Google Scholar]
- [2].Harpavat S, Finegold MJ, Karpen SJ. Patients with biliary atresia have elevated direct/conjugated bilirubin levels shortly after birth. Pediatrics 2011;128(6):e1428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harpavat S, Garcia-Prats JA, Shneider BL. Newborn Bilirubin Screening for Biliary Atresia. N Engl J Med 2016;375(6):605–6. [DOI] [PubMed] [Google Scholar]
- [4].Harpavat S, Ramraj R, Finegold MJ, Brandt ML, Hertel PM, Fallon SC, et al. Newborn Direct or Conjugated Bilirubin Measurements As a Potential Screen for Biliary Atresia. J Pediatr Gastroenterol Nutr 2016;62(6):799–803. [DOI] [PubMed] [Google Scholar]
- [5].Chardot C, Buet C, Serinet MO, Golmard JL, Lachaux A, Roquelaure B, et al. Improving outcomes of biliary atresia: French national series 1986–2009. Journal of hepatology 2013;58(6):1209–17. [DOI] [PubMed] [Google Scholar]
- [6].Serinet MO, Wildhaber BE, Broue P, Lachaux A, Sarles J, Jacquemin E, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics 2009;123(5):1280–6. [DOI] [PubMed] [Google Scholar]
- [7].Schreiber RA, Barker CC, Roberts EA, Martin SR, Alvarez F, Smith L, et al. Biliary atresia: the Canadian experience. The Journal of pediatrics 2007;151(6):659–65, 65 e1. [DOI] [PubMed] [Google Scholar]
- [8].Fawaz R, Baumann U, Ekong U, Fischler B, Hadzic N, Mack CL, et al. Guideline for the Evaluation of Cholestatic Jaundice in Infants: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017;64(1):154–68. [DOI] [PubMed] [Google Scholar]
- [9].Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007;46(2):566–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Suchy FJ. Neonatal cholestasis. Pediatr Rev 2004;25(11):388–96. [PubMed] [Google Scholar]
- [11].Balistreri WF, Bezerra JA. Whatever happened to “neonatal hepatitis”? Clin Liver Dis 2006;10(1):27–53, v. [DOI] [PubMed] [Google Scholar]
- [12].Yerushalmi B, Sokol RJ, Narkewicz MR, Smith D, Ashmead JW, Wenger DA. Niemann-pick disease type C in neonatal cholestasis at a North American Center. J Pediatr Gastroenterol Nutr 2002;35(1):44–50. [DOI] [PubMed] [Google Scholar]
- [13].Lu YB, Peng F, Li MX, Kobayashi K, Saheki T. [Progresses and perspectives in the study on citrin deficiency]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2006;23(6):655–8. [PubMed] [Google Scholar]
- [14].Hoang V, Sills J, Chandler M, Busalani E, Clifton-Koeppel R, Modanlou HD. Percutaneously inserted central catheter for total parenteral nutrition in neonates: complications rates related to upper versus lower extremity insertion. Pediatrics 2008;121(5):e1152–9. [DOI] [PubMed] [Google Scholar]
- [15].Hsieh MH, Pai W, Tseng HI, Yang SN, Lu CC, Chen HL. Parenteral nutrition-associated cholestasis in premature babies: risk factors and predictors. Pediatr Neonatol 2009;50(5):202–7. [DOI] [PubMed] [Google Scholar]
- [16].Lee WS, Sokol RJ. Intestinal Microbiota, Lipids, and the Pathogenesis of Intestinal Failure-Associated Liver Disease. J Pediatr 2015;167(3):519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Satrom K, Gourley G. Cholestasis in Preterm Infants. Clin Perinatol 2016;43(2):355–73. [DOI] [PubMed] [Google Scholar]
- [18].Gonzales E, Davit-Spraul A, Baussan C, Buffet C, Maurice M, Jacquemin E. Liver diseases related to MDR3 (ABCB4) gene deficiency. Front Biosci (Landmark Ed) 2009;14:4242–56. [DOI] [PubMed] [Google Scholar]
- [19].Liu LY, Wang XH, Lu Y, Zhu QR, Wang JS. Association of variants of ABCB11 with transient neonatal cholestasis. Pediatr Int 2013;55(2):138–44. [DOI] [PubMed] [Google Scholar]
- [20].Goldschmidt ML, Mourya R, Connor J, Dexheimer P, Karns R, Miethke A, et al. Increased frequency of double and triple heterozygous gene variants in children with intrahepatic cholestasis. Hepatol Res 2016;46(4):306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Balistreri WF, Bezerra JA, Jansen P, Karpen SJ, Shneider BL, Suchy FJ. Intrahepatic cholestasis: summary of an American Association for the Study of Liver Diseases single-topic conference. Hepatology 2005;42(1):222–35. [DOI] [PubMed] [Google Scholar]
- [22].Kelly DA, Stanton A. Jaundice in babies: implications for community screening for biliary atresia. BMJ 1995;310(6988):1172–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chinsky JM, Singh R, Ficicioglu C, van Karnebeek CDM, Grompe M, Mitchell G, et al. Diagnosis and treatment of tyrosinemia type I: a US and Canadian consensus group review and recommendations. Genet Med 2017;19(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Demir H, Hizal G, Uslu Kizilkan N, Gurakan F, Talim B, Coskun T, et al. Serum alpha-fetoprotein levels in neonatal cholestasis. Turk J Pediatr 2013;55(2):152–7. [PubMed] [Google Scholar]
- [25].Sundaram SS, Bove KE, Lovell MA, Sokol RJ. Mechanisms of disease: Inborn errors of bile acid synthesis. Nat Clin Pract Gastroenterol Hepatol 2008;5(8):456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mittal V, Saxena AK, Sodhi KS, Thapa BR, Rao KL, Das A, et al. Role of abdominal sonography in the preoperative diagnosis of extrahepatic biliary atresia in infants younger than 90 days. AJR Am J Roentgenol 2011;196(4):W438–45. [DOI] [PubMed] [Google Scholar]
- [27].Kianifar HR, Tehranian S, Shojaei P, Adinehpoor Z, Sadeghi R, Kakhki VR, et al. Accuracy of hepatobiliary scintigraphy for differentiation of neonatal hepatitis from biliary atresia: systematic review and meta-analysis of the literature. Pediatric radiology 2013;43(8):905–19. [DOI] [PubMed] [Google Scholar]
- [28].Yang JG, Ma DQ, Peng Y, Song L, Li CL. Comparison of different diagnostic methods for differentiating biliary atresia from idiopathic neonatal hepatitis. Clinical imaging 2009;33(6):439–46. [DOI] [PubMed] [Google Scholar]
- [29].Liu B, Cai J, Xu Y, Peng X, Zheng H, Huang K, et al. Three-dimensional magnetic resonance cholangiopancreatography for the diagnosis of biliary atresia in infants and neonates. PloS one 2014;9(2):e88268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Meyers RL, Book LS, O’Gorman MA, White KW, Jaffe RB, Feola PG, et al. Percutaneous cholecysto-cholangiography in the diagnosis of obstructive jaundice in infants. Journal of pediatric surgery 2004;39(1):16–8. [DOI] [PubMed] [Google Scholar]
- [31].Jensen MK, Biank VF, Moe DC, Simpson PM, Li SH, Telega GW. HIDA, percutaneous transhepatic cholecysto-cholangiography and liver biopsy in infants with persistent jaundice: can a combination of PTCC and liver biopsy reduce unnecessary laparotomy? Pediatric radiology 2012;42(1):32–9. [DOI] [PubMed] [Google Scholar]
- [32].Shanmugam NP, Harrison PM, Devlin J, Peddu P, Knisely AS, Davenport M, et al. Selective use of endoscopic retrograde cholangiopancreatography in the diagnosis of biliary atresia in infants younger than 100 days. J Pediatr Gastroenterol Nutr 2009;49(4):435–41. [DOI] [PubMed] [Google Scholar]
- [33].Zerbini MC, Gallucci SD, Maezono R, Ueno CM, Porta G, Maksoud JG, et al. Liver biopsy in neonatal cholestasis: a review on statistical grounds. Mod Pathol 1997;10(8):793–9. [PubMed] [Google Scholar]
- [34].Russo P, Magee JC, Boitnott J, Bove KE, Raghunathan T, Finegold M, et al. Design and validation of the biliary atresia research consortium histologic assessment system for cholestasis in infancy. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 2011;9(4):357–62 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Haafiz AB. Liver fibrosis in biliary atresia. Expert Rev Gastroenterol Hepatol 2010;4(3):335–43. [DOI] [PubMed] [Google Scholar]
- [36].Wu JF, Lee CS, Lin WH, Jeng YM, Chen HL, Ni YH, et al. Transient elastography is useful in diagnosing biliary atresia and predicting prognosis after hepatoportoenterostomy. Hepatology 2018;68(2):616–24. [DOI] [PubMed] [Google Scholar]
- [37].Jiang J, Wang J, Shen Z, Lu X, Chen G, Huang Y, et al. Serum MMP-7 in the Diagnosis of Biliary Atresia. Pediatrics 2019;144(5). [DOI] [PubMed] [Google Scholar]
- [38].Lertudomphonwanit C, Mourya R, Fei L, Zhang Y, Gutta S, Yang L, et al. Large-scale proteomics identifies MMP-7 as a sentinel of epithelial injury and of biliary atresia. Sci Transl Med 2017;9(417). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nadler EP, Li X, Onyedika E, Greco MA. Differential expression of hepatic fibrosis mediators in sick and spontaneously recovered mice with experimental biliary atresia. J Surg Res 2010;159(2):611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nadler EP, Patterson D, Violette S, Weinreb P, Lewis M, Magid MS, et al. Integrin alphavbeta6 and mediators of extracellular matrix deposition are up-regulated in experimental biliary atresia. J Surg Res 2009;154(1):21–9. [DOI] [PubMed] [Google Scholar]
- [41].Wu JF, Jeng YM, Chen HL, Ni YH, Hsu HY, Chang MH. Quantification of Serum Matrix Metallopeptide 7 Levels May Assist in the Diagnosis and Predict the Outcome for Patients with Biliary Atresia. J Pediatr 2019;208:30–7 e1. [DOI] [PubMed] [Google Scholar]
- [42].Yang L, Zhou Y, Xu PP, Mourya R, Lei HY, Cao GQ, et al. Diagnostic Accuracy of Serum Matrix Metalloproteinase-7 for Biliary Atresia. Hepatology 2018;68(6):2069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dong R, Dong K, Wang X, Chen G, Shen C, Zheng S. Interleukin-33 overexpression is associated with gamma-glutamyl transferase in biliary atresia. Cytokine 2013;61(2):433–7. [DOI] [PubMed] [Google Scholar]
- [44].Behairy OG, Elsadek AE, Behiry EG, Elhenawy IA, Shalan NH, Sayied KR. Clinical Value of Serum Interleukin-33 Biomarker in Infants with Neonatal Cholestasis. J Pediatr Gastroenterol Nutr 2019. [DOI] [PubMed] [Google Scholar]
- [45].Matte U, Mourya R, Miethke A, Liu C, Kauffmann G, Moyer K, et al. Analysis of gene mutations in children with cholestasis of undefined etiology. J Pediatr Gastroenterol Nutr 2010;51(4):488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wagner M, Trauner M. Recent advances in understanding and managing cholestasis. F1000Res 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet 2010;11(1):31–46. [DOI] [PubMed] [Google Scholar]
- [48].Trauner M, Fuchs CD, Halilbasic E, Paumgartner G. New therapeutic concepts in bile acid transport and signaling for management of cholestasis. Hepatology 2017;65(4):1393–404. [DOI] [PubMed] [Google Scholar]
- [49].Herbst SM, Schirmer S, Posovszky C, Jochum F, Rodl T, Schroeder JA, et al. Taking the next step forward - Diagnosing inherited infantile cholestatic disorders with next generation sequencing. Mol Cell Probes 2015;29(5):291–8. [DOI] [PubMed] [Google Scholar]
- [50].Wang NL, Lu YL, Zhang P, Zhang MH, Gong JY, Lu Y, et al. A Specially Designed Multi-Gene Panel Facilitates Genetic Diagnosis in Children with Intrahepatic Cholestasis: Simultaneous Test of Known Large Insertions/Deletions. PloS one 2016;11(10):e0164058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Togawa T, Sugiura T, Ito K, Endo T, Aoyama K, Ohashi K, et al. Molecular Genetic Dissection and Neonatal/Infantile Intrahepatic Cholestasis Using Targeted Next-Generation Sequencing. J Pediatr 2016;171:171–7 e4. [DOI] [PubMed] [Google Scholar]
- [52].Karpen S, Kamath B, Alexander J, Ichetovkin I, Rosenthal P, Soliman W, et al. Use of a comprehensive 66 gene panel to diagnose the causes of cholestasis in >700 individuals. Hepatology 2017;66(S1):655A Abstract 1213.28120420 [Google Scholar]
- [53].Feldman AG, Sokol RJ. Neonatal cholestasis: emerging molecular diagnostics and potential novel therapeutics. Nat Rev Gastroenterol Hepatol 2019;16(6):346–60. [DOI] [PubMed] [Google Scholar]
- [54].Feldman AG, Sokol RJ. Neonatal Cholestasis . Neoreviews 2013;14(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Heubi JE, Bove KE, Setchell KDR. Oral Cholic Acid Is Efficacious and Well Tolerated in Patients With Bile Acid Synthesis and Zellweger Spectrum Disorders. J Pediatr Gastroenterol Nutr 2018;66(2):e57–e9. [DOI] [PubMed] [Google Scholar]
- [56].Santra S, Baumann U. Experience of nitisinone for the pharmacological treatment of hereditary tyrosinaemia type 1. Expert Opin Pharmacother 2008;9(7):1229–36. [DOI] [PubMed] [Google Scholar]
- [57].Demirbas D, Brucker WJ, Berry GT. Inborn Errors of Metabolism with Hepatopathy: Metabolism Defects of Galactose, Fructose, and Tyrosine. Pediatr Clin North Am 2018;65(2):337–52. [DOI] [PubMed] [Google Scholar]
- [58].Wang KS, Tiao G, Bass LM, Hertel PM, Mogul D, Kerkar N, et al. Analysis of surgical interruption of the enterohepatic circulation as a treatment for pediatric cholestasis. Hepatology 2017;65(5):1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lane E, Murray KF. Neonatal Cholestasis. Pediatr Clin North Am 2017;64(3):621–39. [DOI] [PubMed] [Google Scholar]
- [60].Sundaram SS, Mack CL, Feldman AG, Sokol RJ. Biliary atresia: Indications and timing of liver transplantation and optimization of pretransplant care. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society 2017;23(1):96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Feranchak AP, Suchy FJ, Sokol RJ. In: Suchy FJ, Sokol RJ, Balistreri WF, editors. Liver Disease in Children. Cambridge University Press; 2014, p. 111–40. [Google Scholar]
- [62].Sullivan JS, Sundaram SS, Pan Z, Sokol RJ. Parenteral nutrition supplementation in biliary atresia patients listed for liver transplantation. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society 2012;18(1):120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Utterson EC, Shepherd RW, Sokol RJ, Bucuvalas J, Magee JC, McDiarmid SV, et al. Biliary atresia: clinical profiles, risk factors, and outcomes of 755 patients listed for liver transplantation. The Journal of pediatrics 2005;147(2):180–5. [DOI] [PubMed] [Google Scholar]
- [64].DeRusso PA, Ye W, Shepherd R, Haber BA, Shneider BL, Whitington PF, et al. Growth failure and outcomes in infants with biliary atresia: a report from the Biliary Atresia Research Consortium. Hepatology 2007;46(5):1632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Campbell AL, Herold BC. Immunization of pediatric solid-organ transplantation candidates: immunizations in transplant candidates. Pediatric transplantation 2005;9(5):652–61. [DOI] [PubMed] [Google Scholar]
- [66].Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger, United States, 2017, https://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf; 2017. [DOI] [PMC free article] [PubMed]
- [67].Feldman AG, Feudtner C, Kempe A. Reducing the Underimmunization of Transplant Recipients. JAMA Pediatr 2017. [DOI] [PubMed] [Google Scholar]
- [68].Feldman AG, Sundaram SS, Beaty BL, Kempe A. Hospitalizations for Respiratory Syncytial Virus and Vaccine-Preventable Infections in the First 2 Years After Pediatric Liver Transplant. J Pediatr 2017;182:232–8 e1. [DOI] [PubMed] [Google Scholar]
- [69].Feldman AG, Sundaram SS, Beaty BL, Torres R, Curtis DJ, Kempe A. Immunization Status at the Time of Liver Transplant in Children and Adolescents. Jama 2019;322(18):18224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Alonso EM, Martz K, Wang D, Yi MS, Neighbors K, Varni JW, et al. Factors predicting health-related quality of life in pediatric liver transplant recipients in the functional outcomes group. Pediatric transplantation 2013;17(7):605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].de Vries W, Lind RC, Sze YK, van der Steeg AF, Sieders E, Porte RJ, et al. Overall Quality of Life in Adult Biliary Atresia Survivors with or without Liver Transplantation: Results from a National Cohort. European journal of pediatric surgery: official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift fur Kinderchirurgie 2016;26(4):349–56. [DOI] [PubMed] [Google Scholar]
- [72].Lindor KD, Dickson ER, Baldus WP, Jorgensen RA, Ludwig J, Murtaugh PA, et al. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology 1994;106(5):1284–90. [DOI] [PubMed] [Google Scholar]
- [73].Heathcote EJ, Cauch-Dudek K, Walker V, Bailey RJ, Blendis LM, Ghent CN, et al. The Canadian Multicenter Double-blind Randomized Controlled Trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology 1994;19(5):1149–56. [PubMed] [Google Scholar]
- [74].Combes B, Luketic VA, Peters MG, Zetterman RK, Garcia-Tsao G, Munoz SJ, et al. Prolonged follow-up of patients in the U.S. multicenter trial of ursodeoxycholic acid for primary biliary cirrhosis. Am J Gastroenterol 2004;99(2):264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Poupon RE, Balkau B, Eschwege E, Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med 1991;324(22):1548–54. [DOI] [PubMed] [Google Scholar]
- [76].Pares A, Caballeria L, Rodes J, Bruguera M, Rodrigo L, Garcia-Plaza A, et al. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double-blind controlled multicentric trial. UDCA-Cooperative Group from the Spanish Association for the Study of the Liver. J Hepatol 2000;32(4):561–6. [DOI] [PubMed] [Google Scholar]
- [77].Corpechot C, Abenavoli L, Rabahi N, Chretien Y, Andreani T, Johanet C, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology 2008;48(3):871–7. [DOI] [PubMed] [Google Scholar]
- [78].Cai SY, Ouyang X, Chen Y, Soroka CJ, Wang J, Mennone A, et al. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI insight 2017;2(5):e90780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Paumgartner G, Pusl T. Medical treatment of cholestatic liver disease. Clin Liver Dis 2008;12(1):53–80, viii. [DOI] [PubMed] [Google Scholar]
- [80].Zollner G, Wagner M, Fickert P, Silbert D, Gumhold J, Zatloukal K, et al. Expression of bile acid synthesis and detoxification enzymes and the alternative bile acid efflux pump MRP4 in patients with primary biliary cirrhosis. Liver Int 2007;27(7):920–9. [DOI] [PubMed] [Google Scholar]
- [81].Marschall HU, Wagner M, Zollner G, Fickert P, Diczfalusy U, Gumhold J, et al. Complementary stimulation of hepatobiliary transport and detoxification systems by rifampicin and ursodeoxycholic acid in humans. Gastroenterology 2005;129(2):476–85. [DOI] [PubMed] [Google Scholar]
- [82].Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology 2009;50(3):808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Halilbasic E, Fiorotto R, Fickert P, Marschall HU, Moustafa T, Spirli C, et al. Side chain structure determines unique physiologic and therapeutic properties of norursodeoxycholic acid in Mdr2−/− mice. Hepatology 2009;49(6):1972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hohenester S, Wenniger LM, Paulusma CC, van Vliet SJ, Jefferson DM, Elferink RP, et al. A biliary HCO3- umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology 2012;55(1):173–83. [DOI] [PubMed] [Google Scholar]
- [85].Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, et al. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 2006;130(2):465–81. [DOI] [PubMed] [Google Scholar]
- [86].Moustafa T, Fickert P, Magnes C, Guelly C, Thueringer A, Frank S, et al. Alterations in lipid metabolism mediate inflammation, fibrosis, and proliferation in a mouse model of chronic cholestatic liver injury. Gastroenterology 2012;142(1):140–51 e12. [DOI] [PubMed] [Google Scholar]
- [87].European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017;67(1):145–72. [DOI] [PubMed] [Google Scholar]
- [88].Ali AH, Carey EJ, Lindor KD. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med 2015;3(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem 2002;277(4):2908–15. [DOI] [PubMed] [Google Scholar]
- [90].Beuers U, Trauner M, Jansen P, Poupon R. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J Hepatol 2015;62(1 Suppl):S25–37. [DOI] [PubMed] [Google Scholar]
- [91].Huang L, Zhao A, Lew JL, Zhang T, Hrywna Y, Thompson JR, et al. Farnesoid X receptor activates transcription of the phospholipid pump MDR3. J Biol Chem 2003;278(51):51085–90. [DOI] [PubMed] [Google Scholar]
- [92].Moschetta A, Bookout AL, Mangelsdorf DJ. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med 2004;10(12):1352–8. [DOI] [PubMed] [Google Scholar]
- [93].Halilbasic E, Baghdasaryan A, Trauner M. Nuclear receptors as drug targets in cholestatic liver diseases. Clin Liver Dis 2013;17(2):161–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Tran M, Liu Y, Huang W, Wang L. Nuclear receptors and liver disease: Summary of the 2017 basic research symposium. Hepatol Commun 2018;2(7):765–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Keitel V, Cupisti K, Ullmer C, Knoefel WT, Kubitz R, Haussinger D. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology 2009;50(3):861–70. [DOI] [PubMed] [Google Scholar]
- [96].Keitel V, Donner M, Winandy S, Kubitz R, Haussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun 2008;372(1):78–84. [DOI] [PubMed] [Google Scholar]
- [97].Karlsen TH, Franke A, Melum E, Kaser A, Hov JR, Balschun T, et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology 2010;138(3):1102–11. [DOI] [PubMed] [Google Scholar]
- [98].Shneider BL, Spino C, Kamath BM, Magee JC, Bass LM, Setchell KD, et al. Placebo-Controlled Randomized Trial of an Intestinal Bile Salt Transport Inhibitor for Pruritus in Alagille Syndrome. Hepatol Commun 2018;2(10):1184–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Baghdasaryan A, Fuchs CD, Osterreicher CH, Lemberger UJ, Halilbasic E, Pahlman I, et al. Inhibition of intestinal bile acid absorption improves cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis. J Hepatol 2016;64(3):674–81. [DOI] [PubMed] [Google Scholar]
- [100].Miethke AG, Zhang W, Simmons J, Taylor AE, Shi T, Shanmukhappa SK, et al. Pharmacological inhibition of apical sodium-dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology 2016;63(2):512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ding L, Yang L, Wang Z, Huang W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B 2015;5(2):135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Thompson RJ, Kelly DA, McClean P, Miethke AG, Soufi N, Rivet C. Phase 2 open label efficacy and safety study of the apical sodium-depnedent bile acid transporter inhibitor maralixibat in children with progressive familial intrahepatic cholestasis: 48-week interim efficacy analysis. Hepatology 2017;66(1):57A.28295463 [Google Scholar]
- [103].Shneider BL, Spino C, Kamath BM, Magee JC, Whitington PF, Setchell KD, et al. Results of ITCH, A Multi-center Randomized Double-blind Placebo-controlled Trial of Maralixibat, an Ileal Apical Sodium-dependent Bile Acid Transporter Inhibitor (ASBTi), for Pruritus in Alagille Syndrome (ALGS). Hepatology 2017;66(1):84A.28195363 [Google Scholar]
- [104].Slijepcevic D, Roscam Abbing RLP, Katafuchi T, Blank A, Donkers JM, van Hoppe S, et al. Hepatic uptake of conjugated bile acids is mediated by both sodium taurocholate cotransporting polypeptide and organic anion transporting polypeptides and modulated by intestinal sensing of plasma bile acid levels in mice. Hepatology 2017;66(5):1631–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zema MJ. Colesevelam hydrochloride: evidence for its use in the treatment of hypercholesterolemia and type 2 diabetes mellitus with insights into mechanism of action. Core Evid 2012;7:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Cortez L, Sim V. The therapeutic potential of chemical chaperones in protein folding diseases. Prion 2014;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hayashi H, Sugiyama Y. 4-phenylbutyrate enhances the cell surface expression and the transport capacity of wild-type and mutated bile salt export pumps. Hepatology 2007;45(6):1506–16. [DOI] [PubMed] [Google Scholar]
- [108].Hasegawa Y, Hayashi H, Naoi S, Kondou H, Bessho K, Igarashi K, et al. Intractable itch relieved by 4-phenylbutyrate therapy in patients with progressive familial intrahepatic cholestasis type 1. Orphanet journal of rare diseases 2014;9:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].van der Velden LM, Stapelbroek JM, Krieger E, van den Berghe PV, Berger R, Verhulst PM, et al. Folding defects in P-type ATP 8B1 associated with hereditary cholestasis are ameliorated by 4-phenylbutyrate. Hepatology 2010;51(1):286–96. [DOI] [PubMed] [Google Scholar]
- [110].Gonzales E, Grosse B, Schuller B, Davit-Spraul A, Conti F, Guettier C, et al. Targeted pharmacotherapy in progressive familial intrahepatic cholestasis type 2: Evidence for improvement of cholestasis with 4-phenylbutyrate. Hepatology 2015;62(2):558–66. [DOI] [PubMed] [Google Scholar]
- [111].Verkade HJ, Bezerra JA, Davenport M, Schreiber RA, Mieli-Vergani G, Hulscher JB, et al. Biliary atresia and other cholestatic childhood diseases: Advances and future challenges. J Hepatol 2016;65(3):631–42. [DOI] [PubMed] [Google Scholar]
- [112].Bezerra JA, Spino C, Magee JC, Shneider BL, Rosenthal P, Wang KS, et al. Use of corticosteroids after hepatoportoenterostomy for bile drainage in infants with biliary atresia: the START randomized clinical trial. JAMA: the journal of the American Medical Association 2014;311(17):1750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sokol RJ, Spino C, Moore J, Bezerra JA, Whitington PF, Karpen SJ, et al. Intravenous Immunoglobulin (IVIG) Following Portoenterostomy in Infants with Biliary Atresia: a Phase 1/2A Trial. Hepatology 2016;64(Supplement):1123A. [Google Scholar]