Abstract
Introduction
Strategies are needed to curb the increasing HIV incidence in young men who have sex with men (YMSM) and transgender women (YTGW) worldwide. We assessed the impact of youth‐friendly services (YFS) and a mobile phone application (app) on adherence to pre‐exposure prophylaxis (PrEP) in YMSM and YTGW in Thailand.
Methods
A randomized control trial was conducted in YMSM and YTGW aged 15 to 19 years. Participants were provided daily oral tenofovir disoproxil fumerate/emtricitabine (TDF/FTC), condoms and randomized to receive either YFS or YFS plus a PrEP app (YFS + APP), whose features included self‐assessment of HIV acquisition risk, point rewards and reminders for PrEP and clinic appointments. Clinic visits occurred at zero, one, three and six months and telephone contact at two, four and five months. HIV testing was performed at every clinic visit. PrEP adherence was evaluated with intracellular tenofovir diphosphate (TFV‐DP) concentrations in dried blood spot (DBS) samples at months 3 and 6. The primary endpoint assessed was “PrEP adherence” defined as TFV‐DP DBS concentrations ≥700 fmol/punch (equivalent to ≥4 doses of TDF/week).
Results
Between March 2018 and June 2019, 489 adolescents were screened at three centres in Bangkok. Twenty‐seven (6%) adolescents tested positive for HIV and 200 (41%) adolescents participated in the study. Of these, 147 were YMSM (74%) and 53 YTGW (26%). At baseline, median age was 18 years (IQR 17 to 19), 66% reported inconsistent condom use in the past month. Sexually transmitted infection prevalence was 23%. Retention at six months was 73%. In the YFS + APP arm, median app use duration was three months (IQR 1 to 5). PrEP adherence at month 3 was 51% in YFS and 54% in YFS + APP (p‐value 0.64) and at month 6 was 44% in YFS and 49% in YFS + APP (p‐value 0.54). No HIV seroconversions occurred during 75 person years of follow‐up.
Conclusions
Youth‐friendly PrEP services enabled good adherence among half of adolescent PrEP users. However, the mobile phone application tested did not provide additional PrEP adherence benefit in this randomized trial. Adolescent risk behaviours are dynamic and require adaptive programmes that focus on “prevention‐effective adherence.”
Keywords: adolescents, HIV prevention, pre‐exposure prophylaxis (PrEP) adherence, men who have sex with men (MSM), transgender women (TGW)
1. INTRODUCTION
Adolescents and young men who have sex with men (YMSM) and young transgender women (YTGW) bear disproportionate burdens of HIV globally [1, 2, 3, 4]. In Thailand, the HIV incidence among YMSM/YTGW has been consistently high, reported at 4 to 11 per 100 person years [3, 4, 5, 6, 7], similar to the United States where an estimated 14% of TGW are living with HIV (US CDC 2019) and over a third of all new HIV infections are in YMSM [8, 9]. The Thai epidemic among YMSM and YTGW is perpetuated by low rates of HIV test uptake, poor HIV knowledge and rates of condom use below 50% [10, 11]. To achieve the ending of AIDS, comprehensive HIV prevention packages must be delivered to this key population [12]. Pre‐exposure prophylaxis (PrEP) awareness in Thailand is low, reflected in national figures from December 2018, where only an estimated 4% of PrEP‐eligible YMSM and YTGW in Thailand received it, with one‐quarter and one‐third, respectively, accessing PrEP in Bangkok [13, 14]. Only 2% of all PrEP prescribed in 2017 were to under 20s despite this age group having the highest national HIV incidence of 10 per 100 person years [13].
YMSM and YTGW at risk of HIV face a multitude of barriers in accessing and adhering to PrEP, including financial, third party consent laws, low perceived HIV risk, gender‐based stigma and discrimination and lack of services that address their psychosocial needs [15, 16, 17, 18, 19]. Research studies from the United States emphasize the importance of regular contact to ensure good PrEP adherence in adolescents, with adherence dropping to less than half of PrEP users after monthly visits are extended to quarterly visits [20, 21].
Thai YMSM and YTGW face considerable issues with low PrEP uptake and adherence. Among the 2,986 Thai adolescents and young adults aged 15 to 24 years who received counselling about PrEP between 2016 and 2020, only 24% accepted PrEP [22]. Among the 714 who have cumulatively initiated PrEP nationwide, only 22% continue to take PrEP since 2016 [22]. These uptake and retention challenges are similar to those seen with previous adolescent trials network (ATN) studies in adolescents aged 15 to 19 years, with PrEP discontinuation during ongoing HIV acquisition risk more likely (0.82 per 10‐year increase in age) in younger individuals [23, 24].
Mobile health (mHealth) technologies can facilitate task‐shifting by utilizing its automation, widen availability, non‐reliance on infrastructure development and ability to deliver personalized care [25]. As the information‐motivation‐behavioural (IMB) skills model has been validated and used previously for various HIV reduction interventions, it was utilized in this study to support engagement of adolescents in increasing self‐risk awareness and consequently motivate behavioural risk reduction through tailored information: feedback of a calculated self‐HIV risk level based on information of weekly risk behaviours, and motivation: point accumulation for mobile application use [26, 27, 28]. The goal of this study was to investigate whether use of a mobile phone application (app) in conjunction with youth‐friendly services would improve PrEP adherence in YMSM and YTGW in Thailand.
2. METHODS
2.1. Study design and participants
This study was a prospective randomized controlled trial among young adolescents at risk of HIV acquisition in Bangkok, Thailand. Enrolment criteria included: (1) being YMSM or YTGW (assigned male sex at birth, self‐reported sex with men, self‐defined gender identity of being MSM or TGW for any period of time); (2) 15 to 19 years old; (3) self‐reported risk behaviours, including inconsistent condom use; (4) being HIV negative. Participants could be new, previous or ongoing PrEP users, and were screened and recruited into the study at voluntary HIV testing centres at the Thai Red Cross AIDS Research Centre (TRCARC) and its community‐based organization (CBO) branches, Rainbow Sky Association Thailand (RSAT) and Service Workers in Group Foundation (SWING), all located in Bangkok, Thailand via counsellors, online advertising, peer recruiters and word of mouth. Institutional Review Board approval was granted by the Faculty of Medicine, Chulalongkorn University with a waiver for parental consent granted. This study was registered with ClinicalTrials.gov Identifier: NCT03778892.
2.2. Study procedures
Participants were randomized (1:1) to receive youth friendly services (YFS) or YFS plus use of a PrEP adherence supporting mobile phone app (YFS + APP). Clinic visits occurred at months 0, 1, 3, 6 and telephone contact was made at months 2, 4 and 5. All participants were provided PrEP counselling, condoms and lubricants and daily oral tenofovir disoproxil fumarate (TDF) 300mg and emtricitabine (FTC) 200mg fixed‐dose combination tablets; TenoEm (Thai Government Pharmaceutical Organization), Tenof‐Em (Hetero Healthcare) or Ricovir‐EM (Mylan). Sexually transmitted infection (STI) screening (Neisseria gonorrhea, Chlamydia trachomatis) was performed on urine and anal swab samples and syphilis on blood samples at baseline and month 6 [29]. Surveys on sexual risk behaviours and perception were administered at baseline and monthly thereafter.
2.3. Youth‐friendly services only arm
YFS provided included ongoing counselling or support provided outside scheduled visits through online instant messaging or telephone calls with responses provided within 24 hours. Trained counsellors provided motivational interviewing focusing on risk reduction and adolescent self‐empowerment through collaborative, non‐judgemental discussions and positive reinforcement throughout the study [30, 31]. Visits focused on building a fun and friendly atmosphere to build rapport with participants. Clinics were available out‐of‐office hours. Topics covered in counselling sessions were tailored to individual needs and included educational issues, lesbian, gay, bisexual, transgender (LGBT) stigma and discrimination, mental health and substance abuse issues, with specialist referrals where necessary. All clients had access to transgender counselling and gender‐affirming related blood testing, and hormonal therapy.
2.4. Youth‐friendly services plus mobile phone application arm
In addition to YFS, the YFS + APP arm received an app for use, named “Project Raincoat” produced by Focal Intelligence Co., Ltd., which was designed based on use of the IMB skills model, specifically with information tailored to promote PrEP adherence and reduction of risk behaviours [26, 28]. We conducted two adolescent YTGW and YMSM focus group discussions (FGDs) to inform design of the app. Focus groups of participants aged 15 to 19 years were conducted using a semi‐structured interview guide with topics on desirable app functions, aesthetic designs and potential barriers and motivators for use. Key themes and subthemes were identified from a content analysis and utilized in informing app design, which included features supporting self‐risk evaluation where users could input data once weekly on number of sex acts, sex partners, pills taken and condom use which was then used to calculate a feedback HIV risk of low, medium, high and very high risk. Points were rewarded in real time for data input (maximum reward of 21 points per week) as well as responding to staff follow‐up calls (5 points each), attendance of clinic visits (10 points each) and negative anti‐HIV test results (50 points each). Points were exchangeable for cash, 100 points being exchangeable for 100 Thai Baht (3 USD). Users were able to customize self‐reminders for medication and appointment reminders. Due to budgetary restraints the app was available on the Android operating system (OS) only and those using other OSs were loaned Android OS mobile phones.
2.5. Data collection process
Behavioural surveys were completed by participants on paper forms at clinic visits. During scheduled telephone follow‐up sessions, these were administered by staff. Participants completed monthly surveys asking about numbers of sexual partners, numbers of sex acts, condom use, numbers of PrEP pills taken each week and self‐perceived HIV risk level. Self‐perceived risk was determined by asking participants “in the last month, how would you rate your own risk of getting HIV?”. Participants were able to select 1 from the 4 following possible responses; “low risk,” “moderate risk,” “high risk” and “extremely high risk.” PrEP adherence was evaluated using tenofovir‐diphosphate (TFV‐DP) concentrations in dried blood spot (DBS) samples at months 3 and 6; with ≥700 fmol/punch equivalent to ≥4 TDF doses/week. App paradata including first and last entry into the app were collected [32].
2.6. Laboratory assays
HIV antibody testing was performed using a chemiluminescent immunoassay (Architect HIV Ag/Ab Combo Reagent, Abbott Laboratories, Wiesbaden, Germany or Cobas HIV Combi Principle CMIA, 4th Generation). HIV testing at CBOs was performed using a rapid strip test (Alere Determine HIV‐1/2, Alere International Limited, Ballybrit Galway, Ireland). STI screening was performed on urine and anal swab specimens for Neisseria gonorrhea (NG) and Chlamydia trachomatis (CT) with nucleic acid amplification (NAAT) testing using in vitro polymerase chain reaction (PCR) assays (Abbott RealTime CT/NG, Abbott Molecular, Inc., IL, USA). Syphilis testing at the TRCAC was performed using the electrochemiluminescence immunoassay analyser (ECLIA) and chemiluminescent magnetic microparticle immunoassay (CMIA). Syphilis testing at CBOs was performed using a solid phase immunochromatographic assay for the qualitative detection of antibodies of all isotypes (IgG, IgM, IgA) against Treponema pallidum (SD BIOLINE Syphilis 3.0, Standard Diagnostics, Inc., Kyunggi‐do, Korea). TFV‐DP concentrations were measured from dried blood spot (DBS) samples collected onto Whatman Protein Saver 903 cards. Dried blood spots were stored at −70°C until analysis. TFV‐DP was analysed using liquid chromatography mass spectrometry (LC‐MS/MS). The TFV‐DP calibration curve range was 200 to 10,000 fmol/3mm punch [33, 34].
2.7. Sample size estimation
Estimating from results of previous adolescent PrEP trials, presuming that 45% of those in the YFS arm and 65% of those in the YFS + APP would have TFV‐DP levels ≥700 fmol/punch with a power of test of >80% and presumed 20% loss of cases to follow‐up, a sample size of 100 for each arm was calculated, equating to a total of 200 participants needed for the trial [21, 35].
2.8. Statistical analysis
Primary outcome was PrEP adherence as measured via TFV‐DP at months 3 and 6 follow‐up. PrEP adherence was defined as those who had TFV‐DP levels of ≥700 fmol/punch [36]. Secondary outcomes included rates of HIV infection, rates of study retention at month 6, associated factors with PrEP adherence and overall HIV acquisition risk protection (combined period with PrEP adherence or 100% consistent condom use).
Consistent condom use was defined as 100% condom use during all episodes of sexual intercourse. Substance use was defined as substances taken recreationally, including alcohol, sildenafil citrate, poppers and other illicit drugs (including amphetamine, ketamine and marijuana). Loss to follow‐up was defined as no attendance for clinic visits or response to scheduled telephone calls for at least two consecutive months.
Associations of PrEP adherence with factors at enrolment was examined using univariable and multivariable logistic regression models. Associations were presented using odds ratios and 95% CI with p‐values calculated utilizing the Z‐test (Wald test). Factors where an association of p < 0.1 in univariable analysis were selected for further multivariable analysis.
Age analysis was divided into 15 to 17 to represent “adolescence” and 18 to 19 to represent “young adulthood” [37]. To assess level of HIV protection from either PrEP adherence and/or 100% condom use, behavioural risk data were summarized into three‐month blocks of risk periods prior to TFV‐DP collection. Stata/SE 13.0 was used for all data analyses.
3. RESULTS
3.1. Characteristics at baseline and month 6
Between March 2018 and June 2019, 489 adolescents were screened, 27 (6%) tested positive for HIV and 200 (41%) enrolled to the study. There were 147 were YMSM (74%) and 53 YTGW (26%). Baseline characteristics between the YFS and YFS + APP arms were similar (Table 1). Median (IQR) age was 18 years (17‐19) at enrolment. Median (IQR) age of sexual debut was 16 (15‐17) years. Of those sexually active within the past month, 34% reported consistent condom use. Laboratory diagnosed STI prevalence at enrolment was 23%. Thirteen percent of participants overall reported substance use in the last three months. Self‐reported substance use included alcohol (6%), amphetamines or methamphetamines (4%), ketamine (0.5%), poppers (volatile alkyl nitrites) (5%), marijuana (2%) and sildenafil citrate (4%).
Table 1.
Baseline characteristics of adolescents taking once daily HIV pre‐exposure prophylaxis
| Overall, n = 200 (%) | YFS, n = 100 | YFS + APP, n = 100 | |
|---|---|---|---|
| Gender identity | |||
| MSM | 147 (74%) | 71 (71%) | 76 (76%) |
| TGW | 53 (26%) | 29 (29%) | 24 (24%) |
| Age at enrolment median (IQR) | 18 (17, 19) | 18 (17, 19) | 18 (17,19) |
| 15 to 17 years | 63 (32%) | 32 (32%) | 31 (31%) |
| 18 to 19 years | 137 (68%) | 68 (68%) | 69 (69%) |
| Age sexual debut (years) | 16 (15, 17) | 16 (15, 17) | 16 (15, 17) |
| Sexually transmitted infections a | 45 (23%) | 19 (19%) | 26 (26%) |
| No. of sex partners in the past month | |||
| 0 | 57 (29%) | 29 (29%) | 28 (28%) |
| 1 | 87 (44%) | 41 (41%) | 46 (46%) |
| ≥2 | 56 (28%) | 30 (30%) | 26 (26%) |
| Condom use in the past month in sexually active (N = 143) | |||
| Inconsistent | 94 (66%) | 46 (65%) | 48 (67%) |
| Consistent | 49 (34%) | 25 (35%) | 24 (33%) |
| Substance use in the past 3 months b | 26 (13%) | 8* (8%) | 18* (18%) |
| Self‐perceived risk (N = 197) | |||
| Low risk | 121 (61%) | 62 (63%) | 59 (60%) |
| Moderate risk | 66 (34%) | 29 (30%) | 37 (37%) |
| High‐ extremely high risk | 10 (5%) | 7 (7%) | 3 (3%) |
Consistent condom use = 100% condom use. MSM, men who have sex with men; TGW, transgender women; YFS, youth‐friendly services; YFS + APP, youth‐friendly services plus pre‐exposure prophylaxis adherence motivation mobile phone application use.
Sexually transmitted infections were laboratory confirmed and included syphilis, Neisseria gonorrhoea and Chlamydia trachomatis, the latter 2 from anal swabs and urine samples
Substance use included alcohol, amphetamines, methamphetamines, ketamine, poppers (volatile alkyl nitrates), marijuana and sildenafil citrate
p < 0.05 with Pearson’s chi‐squared test.
An overall six‐month retention rate of 73% was observed at six‐month follow‐up, 72% in the YFS arm and 73% in the YFS + APP arm (p = 0.87). No characteristics were found to be associated with month 6 retention (Table 2). Consistent condom use in the past month among sexually active adolescents increased from 34% (95% CI, 25 to 43) at baseline to 58% (95% CI, 49 to 68) at month 3 (p < 0.001) and remained a similar level at month 6 (52%; 95% CI, 41 to 62). No evidence of reduced condom use was seen with good PrEP adherence (Table 3). STI incidence was 25.2 per 100 patient years during the study. In the 75 person‐years of follow‐up, there were no incidence cases of HIV seroconversion.
Table 2.
Characteristics associated with retention a at 6 months
| Characteristics | No | Retention (%) | Unadjusted odds ratio (95% CI) | Wald test p‐value |
|---|---|---|---|---|
| Gender identity | ||||
| MSM | 147 | 109 (74.2) | 1.35 (0.68, 2.69) | 0.39 |
| TGW | 53 | 36 (67.9) | 1 | |
| Age at enrolment (years) | ||||
| 15 to 17 | 63 | 44 (69.8) | 1 | |
| 18 to 19 | 137 | 101 (73.7) | 1.21 (0.63, 2.34) | 0.57 |
| Education | ||||
| ≤Junior high school | 87 | 65 (74.7) | 1 | |
| ≥Senior high school | 113 | 80 (70.8) | 0.82 (0.44, 1.54) | 0.54 |
| Number of sex partners in last month | ||||
| 0 | 57 | 43 (75.4) | 1 | |
| 1 | 87 | 63 (72.4) | 0.85 (0.04, 1.84) | 0.69 |
| ≥2 | 56 | 39 (69.6) | 0.75 (0.33, 1.71) | 0.49 |
| Condom use in last month among sexually active (N = 143) | ||||
| 100% condom use | 49 | 34 (69.4) | 1 | |
| Inconsistent condom use | 94 | 68 (72.3) | 1.15 (0.54, 2.46) | 0.71 |
| Substance use in last three months b | ||||
| No | 174 | 122 (70.1) | 1 | 0.06 |
| Yes | 26 | 23 (88.5) | 3.27 (0.94, 11.36) | |
| Self‐perceived risk (N = 197) | ||||
| Felt at low risk | 121 | 92 (76.0) | 1 | |
| Felt at moderate‐extremely high risk | 76 | 53 (69.7) | 0.72 (0.38, 1.38) | 0.33 |
| Intervention arm | ||||
| YFS | 100 | 72 (72.0) | 1 | |
| YFS + APP | 100 | 73 (73.0) | 1.05 (0.56, 1.96) | 0.87 |
MSM, men who have sex with men; TGW, transgender women; YFS, youth‐friendly services; YFS + APP, youth‐friendly services and mobile phone application.
Retention defined as engagement with PrEP prevention services throughout six‐month study period with no more than 1 period of disengagement for no longer than 2 consecutive months. PrEP, pre‐exposure prophylaxis
Substance use defined as alcohol, amphetamines, methamphetamines, ketamine, poppers (volatile alkyl nitrates), marijuana and sildenafil citrate.
Table 3.
Proportions of adolescents with 100% condom use over time stratified by PrEP adherence at months 3 and 6
| Baseline a | Month 3 visit | p‐value | Baseline a | Month 6 visit | p‐value | |
|---|---|---|---|---|---|---|
| Overall b , c | 38 (34%, 25 to 43) | 66 (58%, 49 to 68) | 0.0002 | 33 (33%, 24 to 42) | 48 (52%, 41 to 62) | 0.008 |
| PrEP adherent d | 21/67 (31%, 20 to 42) | 36/60 (60%, 48 to 72) | 17/50 (34%, 21 to 47) | 27/49 (55% 41 to 69) | ||
| Non‐PrEP adherent | 17/45 (38%, 24 to 52) | 30/53 (57%, 43 to 70) | 16/50 (32% 19 to 45) | 21/44 (48%, 33 to 62) |
Baseline figures based on availability of TFV‐DP results to match with self‐reported condom use data for corresponding follow‐up month analysed
p‐value of Z‐test for proportions comparing % of consistent condom use between month 3 versus month 6 = 0.329
No p‐values calculated from Z‐test for proportions comparing % of consistent condom use between PrEP adherent versus non‐adherent groups within visits reached 0.05
PrEP adherent defined as tenofovir‐diphosphate levels ≥700 fmol/punch.
3.2. Mobile application use
Of the 100 participants assigned to use the app, 62% had their own phone and the remaining 38% were loaned phones. Eighty‐seven percent of participants used the app more than once and median duration of app use was three months (IQR 1 to 5). Approximately 75% of participants completed self‐risk assessments in the first two weeks, dropping to 50% in six weeks and 25% by week 12. For point rewards, 60% of adolescents earned 100 to 199 points in the app, with a median of 120 points earned (IQR 60 to 205). One third (32%) redeemed their cash rewards at their month 3 visit and the remaining at their month 6 closing visit.
3.3. Assessment of TDF adherence using TFV‐DP DBS concentrations
A total of 294 TFV‐DP samples were collected from 164 participants, 155 at month 3 and 139 at month 6. An additional seven timepoints (5 clients at month 3 and 2 clients at month 6) were added in the analysis as undetectable TFV‐DP levels, where blood samples were not taken due to self‐reporting of no PrEP use in the preceding month. A total of 301 risk periods were therefore analysed. PrEP adherence by TFV‐DP level was 52% (95% CI 45 to 60) at month 3 and 48% (95% CI 39 to 55) at month 6. PrEP adherence at month 3 was 51% in the YFS arm and 54% in the YFS + APP arm (p = 0.64) and at month 6 was 44% in the YFS arm and 49% in the YFS + APP arm (p = 0.54) (Figure 1).
Figure 1.
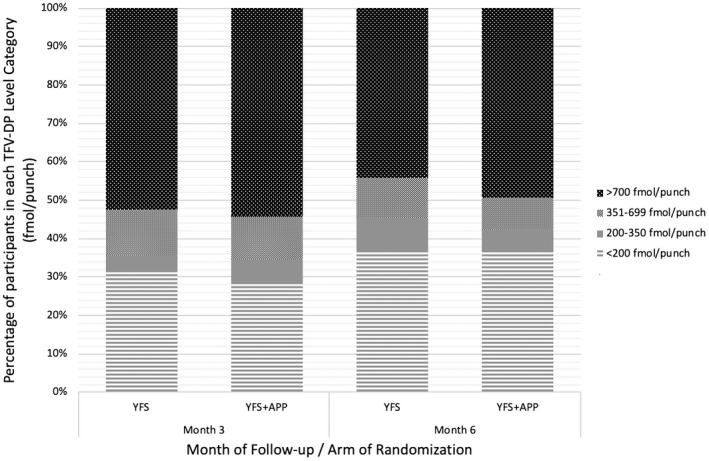
Adherence to once daily oral HIV pre‐exposure 3 prophylaxis at months 3 and 6 of follow‐up by randomization arm.
YFS, Youth‐friendly services; YFS + APP, Youth‐friendly services plus mobile phone application use.
3.4. Associated factors with PrEP adherence
Variables with significant association (p < 0.1) in univariable analysis to PrEP adherence were included in a further multivariable analysis, which included: gender identity, age, number of sex partners, self‐perceived risk for HIV infection and study arm. Education was not included due to its collinearity with age. Multivariable analysis showed only gender identity remained significantly associated with PrEP adherence at month 3, with YMSM being associated with greater odds of PrEP adherence compared to YTGW (OR 3.1, 95% CI 1.3 to 7.6, p < 0.01), but not at month 6 (OR = 2.4, 95% CI 1.0 to 6.2, p = 0.06) (Table 4).
Table 4.
Associated factors with PrEP adherence a , 4A at month 3 of follow‐up 4B at month 6
| N |
PrEP adherent a N % |
Unadjusted odds ratio (95% CI) | p‐value | Adjusted odds ratio (95% CI) | p‐value | ||
|---|---|---|---|---|---|---|---|
| Characteristics (Total N = 160) | |||||||
| Month 3 follow‐up | |||||||
| Total | 160 | 84 | 52.5 | ||||
| Gender identity | |||||||
| MSM | 120 | 74 | 61.7 | 4.83 (2.16, 10.79) | 0.003 | 3.13 (1.30, 7.55) | 0.01 |
| TGW | 40 | 10 | 25.0 | 1 | |||
| Age at enrolment(years) | |||||||
| 15 to 17 | 49 | 17 | 34.7 | 1 | |||
| 18 to 19 | 111 | 67 | 60.4 | 2.87 (1.42, 5.77) | 0.003 | 1.91 (0.87, 4.20) | 0.11 |
| Education | |||||||
| ≤Junior high school | 70 | 30 | 42.9 | 1 | |||
| ≥Senior high school | 90 | 54 | 60.0 | 2.00 (1.06, 3.77) | 0.03 | ||
| No. of sex partners in past month | |||||||
| 0 | 48 | 17 | 35.4 | 1 | |||
| 1 | 70 | 40 | 57.1 | 2.43 (1.14, 5.20) | 0.02 | 1.62 (0.69, 3.79) | 0.26 |
| ≥2 | 42 | 27 | 64.3 | 3.28 (1.38, 7.80) | 0.007 | 1.74 (0.66, 4.63) | 0.26 |
| Condom use in past month (N = 112) | |||||||
| Consistent use | 38 | 21 | 55.3 | 1 | |||
| Inconsistent use | 74 | 46 | 62.2 | 1.33 (0.66, 2.94) | 0.48 | ||
| Substance use in past three months b | |||||||
| No | 137 | 70 | 51.1 | 1 | |||
| Yes | 23 | 14 | 60.9 | 1.49 (0.60, 3.67) | 0.39 | ||
| Self‐perceived HIV acquisition risk (N = 157) | |||||||
| Not at risk | 96 | 42 | 43.8 | 1 | |||
| At risk | 61 | 40 | 65.6 | 2.45 (1.26, 4.76) | 0.008 | 1.74 (0.84, 3.63) | 0.14 |
| Laboratory diagnosed STI at baseline | |||||||
| No | 123 | 63 | 51.2 | 1 | |||
| Yes | 37 | 21 | 56.8 | 1.25 (0.60, 2.62) | 0.56 | ||
| Intervention | |||||||
| YFS | 79 | 40 | 50.6 | 1 | |||
| YFS + APP | 81 | 44 | 54.3 | 1.16 (0.62, 2.16) | 0.64 | 1.08 (0.54, 2.16) | 0.82 |
| Characteristics (total N = 141) | |||||||
| Month 6 follow‐up | |||||||
| Total | 141 | 66 | 46.8 | ||||
| Gender identity | |||||||
| MSM | 105 | 57 | 54.3 | 3.56 (1.53, 8.30) | 0.003 | 2.44 (0.96, 6.20) | 0.06 |
| TGW | 36 | 9 | 25.0 | 1 | |||
| Age at enrolment (years) | |||||||
| 15 to 17 | 42 | 12 | 28.6 | 1 | |||
| 18 to 19 | 99 | 54 | 54.6 | 3.00 (1.38, 6.53) | 0.006 | 1.89 (0.80, 4.50) | 0.15 |
| Education | |||||||
| ≤Junior high school | 63 | 23 | 36.5 | 1 | |||
| ≥Senior high school | 78 | 43 | 55.1 | 2.14 (1.08, 4.22) | 0.03 | ||
| No. of sex partners in past month | |||||||
| 0 | 41 | 16 | 39.0 | 1 | |||
| 1 | 62 | 25 | 40.3 | 1.06 (0.47, 2.37) | 0.90 | 0.80 (0.33, 1.91) | 0.61 |
| ≥2 | 38 | 25 | 65.8 | 3.00 (1.20, 7.52) | 0.02 | 1.94 (0.71, 5.30) | 0.20 |
| Condom use in past month (N = 100) | |||||||
| Consistent use | 33 | 17 | 51.5 | 1 | |||
| Inconsistent use | 67 | 33 | 49.2 | 0.91 (0.40, 2.10) | 0.83 | ||
| Substance use in past three months b | |||||||
| No | 118 | 57 | 48.3 | 1 | |||
| Yes | 23 | 9 | 39.1 | 0.69 (0.28, 1.71) | 0.42 | ||
| Self‐perceived HIV acquisition risk | |||||||
| Not at risk | 89 | 37 | 41.6 | 1 | |||
| At risk | 52 | 29 | 55.8 | 1.77 (0.89, 3.54) | 0.10 | 1.28 (0.60, 2.76) | 0.52 |
| Laboratory diagnosed STI at baseline | |||||||
| No | 107 | 51 | 47.7 | 1 | |||
| Yes | 34 | 15 | 44.1 | 0.87 (0.40, 1.88) | 0.72 | ||
| Intervention | |||||||
| YFS | 68 | 30 | 44.1 | 1 | |||
| YFS + APP | 73 | 36 | 49.3 | 1.23 (0.64, 2.39) | 0.54 | 1.18 (0.58, 2.42) | 0.64 |
Bold value indicates p < 0.1. fmol/punch, femtomoles per 3 mm punch; MSM, men who have sex with men; TGW, transgender women; STI, sexually transmitted infection, laboratory confirmed and included syphilis, Neisseria gonorrhoea and Chlamydia trachomatis, the latter 2 from anal swab and urine samples; TFV‐DP DBS, tenofovir diphosphate dried blood spots; YFS, Youth‐friendly services; YFS + APP, Youth‐friendly services plus mobile phone application.
“PrEP adherent” defined as TFV‐DP DBS concentrations ≥700 fmol/punch [equivalent to ≥4 doses of TDF/week].
“substance use” defined as alcohol, sildenafil citrate and other recreational and illicit drug use including alcohol, amphetamines, methamphetamines, ketamine, poppers (volatile alkyl nitrates), marijuana and sildenafil citrate.
3.5. Overall HIV acquisition risk protection
A total of 296 risk period blocks for analysis were available (145 in YFS, 151 in YFS + APP, 5 of 301 available TFV levels had risk data missing so were excluded from this part of the analysis). In addition to the 51% of risk periods protected from HIV with PrEP, an additional 18% of risk periods were protected with self‐reported consistent condom use, achieving a presumed total of 69% protection against HIV (Figure 2). Of the 67 % of risk periods with inconsistent condom use reported, 54% of these were protected with PrEP.
Figure 2.
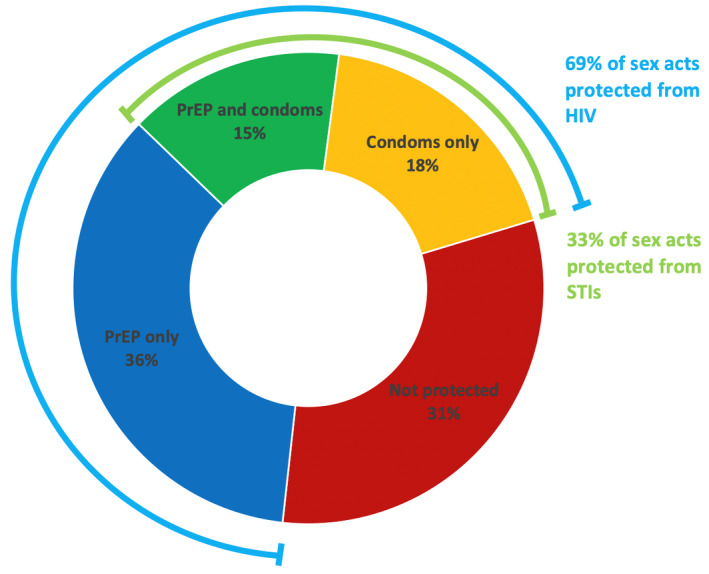
Mode of protection of risk periods against HIV and STIs seen in Thai adolescents.
“PrEP protection” defined as ≥700 fmol/punch, “condom protection” defined as 100% condom use. PrEP, pre‐exposure prophylaxis; STIs, sexually transmitted infections.
4. DISCUSSION
To our knowledge, this is the first study in the Asia Pacific region that provides insights of youth‐friendly PrEP service implementation in YMSM and YTGW aged 15 to 19 years with an innovative approach using a mobile phone application to support adherence. This study demonstrated that youth‐friendly service integration to a PrEP programme enabled retention of three‐quarters of adolescent clients and HIV protective TFV‐DP levels of ≥700 fmol/punch among half of adolescent PrEP users during six months of follow‐up. However, the mobile application used did not provide additional PrEP adherence benefit in this randomized trial. For Thailand, lessons learned in this study are particularly valuable in supporting nationwide PrEP services for key populations including youth under its universal health coverage scheme as of October 2019, considerable progress from Thailand’s first fee‐based PrEP services in 2014 [29, 38, 39, 40, 41].
TFV‐DP DBS concentrations ≥700 fmol/punch, deemed to be protective from HIV for the preceding 30 days [42] in this study was found in 46% to 52% of participants at both three and six months, similar figures to those seen in US adolescent studies conducted between 2013 and 2014 (30% to 50%) at the same time points [21, 35]. An earlier adolescent study in 2010 that saw protective levels of only 20% by six months [43], a trend of improved adherence in newer studies also observed in adult studies, presumed to be due to higher general knowledge in PrEP efficacy and better informed and more motivated study participants in PrEP studies [44, 45]. The fact that all aforementioned studies including this study did not see any HIV seroconversions despite imperfect PrEP adherence may have been due to PrEP use being effectively used in an event‐driven fashion during risky events. Encouraging “prevention‐effective adherence,” the use of PrEP during periods of risk exposure to effectively protect against HIV acquisition is important because it acknowledges the dynamic pattern of HIV risk acquisition behaviours and the use of alternate HIV prevention strategies [46]. This may be a more appropriate goal to aim for in adolescents where the expectation of perfect adherence may become a barrier for those who would otherwise benefit from PrEP [44, 46]. We believe that in this study, a mix of few sex acts, use of alternate HIV protection methods such as condoms and reduced perceived risk of situations driven by incomplete neurocognitive development typical of the adolescence phase of growth contributed to the inconsistent PrEP use seen [44, 45, 47]. This emphasises the need to combine PrEP with condoms and other HIV prevention modalities as a package to protect adolescents against HIV. It is encouraging that approximately 50% of participants had “high” adherence based on TFV‐DP DBS concentrations of ≥700 fmol/punch and in combination with self‐reported consistent condom use increased the overall protection to 68%. This cohort showed that with service delivery of an HIV prevention package, an increase in proportion of condom use was seen, contrary to speculated concerns of PrEP reducing condom use [48]. However, it is acknowledged that condom use can only be measured by self‐report. We believe that recall bias was minimized by surveys at monthly intervals, although it should be kept in mind this may still have been offset with a degree of reporting bias due to social desirability bias.
The vulnerability to HIV infection of the population investigated was reflected by the high rates of self‐reported inconsistent condom use (66%) and laboratory diagnosed STIs at baseline of 23%, similar figures to those seen in the ATN studies 110 and 113 [21, 35]. This was in contrast to the majority of participants who felt at low or moderate risk of HIV infection. This suggests that HIV prevention services for adolescents may be better justified to offer services based on risk behaviours rather than self‐perceived risk. This cohort of adolescents had a relatively lower number of sex acts (2 to 3/month) and partners in the preceding month compared to much higher numbers (8 to 10/month) in the seminal ANRS Ipergay trial in adult MSM. This highlights the unique context of young people being at high risk of HIV acquisition for reasons such as lower rates of condom use and access to PrEP services, rather than number of sexual exposures [49]. For this reason, event‐driven PrEP may be more suited to adolescents, further studies are needed [50].
There are studies in adolescent HIV prevention research with preliminary data suggesting the feasibility and acceptability of apps in supporting PrEP adherence, however, trial results on efficacy are pending [51, 52, 53, 54]. The “Raincoat” app used in this trial did not show efficacy in PrEP adherence or service retention. The median time of app use was only three months out the total six months of the study, substantially lower than in an adult study where >60% continued to use a PrEP adherence app at by six months [55]. This may have been due to (1) lack of social networking features such as chat boards or a leader board, (2) lack of ongoing new features over the six‐month period contributing to boredom (3) availability on only one operating system (Android), meaning one‐third of participants had to borrow phones to use the app, which may have been inconvenient. Retention could further be addressed with more adaptive designs to service delivery, which include (1) convenience – more accessibility and flexibility of services, self‐testing for HIV and postal delivery of PrEP, (2) adaptability to medication needs – adolescent sexual activity changes over time and may be more suited to event‐driven PrEP, (3) technological developments to add interactive activity such as leader boards, and chat boards to increase engagement or sense of community.
There are some data to suggest risk perception influences risk behaviours in adult MSM [56, 57], and has similarly been described in a Thai YMSM study. We designed “Project Raincoat” based on the assumption that self‐risk assessment would lead to increased adherence. Findings from studies have been mixed with some evidence to suggest risk perception has some relation to reduced risk behaviours [58, 59], whereas in others despite risk perception, risk reduction was unaffected [60]. It could be argued that although there is evidence to suggest low perceived risk is associated with poor adherence in YMSM [61], this does not mean the opposite is necessarily true. It is important to point out that risk perception was part of a spectrum of other factors influencing risk taking behaviours not explicitly addressed by the intervention in this arm, such as addressing of barriers to condom use and alcohol use which may explain the outcomes observed in this study [62]. At the time of this study, there were no universally accepted validity tools available for the effectiveness of mobile applications prior to taking them to randomized trials. There is acknowledgement that multiple factors influence whether a mobile application is effective, including participant demographics, intervention factors (intensity, components), technology used (video, infographics, text) and behavioural change intervention used, only some of which were possible to investigate in this trial [63]. More understanding is also needed on whether and how point rewards influence YMSM behaviour. A qualitative component of this study is underway.
Retention in HIV prevention services was fairly high at 73% by six months, similar to figures seen in the ATN 110/113 studies, with no clear differences between YMSM and YTGW in contrast to previous experience in Thailand suggesting TGW have poorer retention rates in HIV prevention care [64]. This may have been related to comprehensive care including gender affirming hormone therapy [65]. High overall retention rates suggest that the approach to counselling and multidisciplinary care taken in this study were important in engaging and retaining adolescents in HIV prevention services as has been previously found [12].
The strength of this study was it provided youth‐friendly services that focused on adolescents between 15 and 19 years. This study was made possible through Thailand’s policies that allow adolescents aged 13 to 18 years to access to HIV and STI testing and treatment without parental consent and approval of parental consent exemption by an ethics committee. It was further supported by the Princess PrEP Program led by HRH Princess Somsawali, recently appointed UNAIDS Goodwill Ambassador for HIV Prevention in Asia and the Pacific (2019) that funded our PrEP supply in accordance with HIV acquisition risks regardless of age or gender identity [66]. These strengths of Thailand exemplify the advances possible in HIV prevention efforts through giving importance to the overcoming of legal and structural barriers. Finally, this study is continuing with qualitative data collection on the mobile phone application used, which will provide information to improve future designs.
Our study also had a number of limitations. The mobile application was only available on the Android platform. Additionally, this study was not specifically designed to focus on YTGW, who are increasingly recognised as a distinct group with unique needs and therefore it could be argued that a separate gender‐specific study with tailored interventions for YTGW is more appropriate [67]. The relatively short six‐month duration of follow‐up was also a limitation. Although this study looked at PrEP “execution,” further study is needed to address PrEP “persistence” in order to study ongoing HIV risk adolescents have over time [46]. We currently have an ongoing open‐label extension study following up participants to look at PrEP persistence. It must also be noted that the comprehensive YFS offered in this study is not routinely offered across Thailand and other middle‐income countries in the region, which may have negated any effects the mobile app had on PrEP adherence on the intervention arm of this study. Self‐reported measures regarding sex acts, sex partners or condom use may have been subject to reporting bias. Finally, this study may have been underpowered to detect differences in study arms due to a higher than expected dropout rate.
5. CONCLUSIONS
Using youth‐friendly service delivery, we were able to achieve retention in three‐quarters of our clients at six months and good adherence among half of adolescent PrEP users, with no seroconversions. However, the mobile phone application tested did not provide additional PrEP adherence benefit to comprehensive YFS in this randomized trial. Adolescent HIV risk behaviours are dynamic and require adaptive programmes that focus on “prevention‐effective adherence.” Further research is required on how improved adherence may be programmatically supported in adolescents both short and long‐term through further qualitative and PrEP persistence studies.
COMPETING INTERESTS
The authors have no conflicts of interest to declare.
AUTHORS’ CONTRIBUTIONS
WNS was responsible for writing the initial version of the manuscript. SK did the data analysis. TP supervised the overall manuscript writing process and content. NP and TC oversaw PrEP operations at the TRCARC. TRC led TFV‐DP laboratory analysis. PW coordinated this study and did the data collection. CS oversaw holistic care of study participants. SJ and DL oversaw PrEP operations at community‐based organisations. All authors have read and approved the final version of this manuscript.
FUNDING
This study was supported by the CIPHER Grant ID 2017/472‐SON; The Princess PrEP Fund; FHI360; LINKAGES; The Chulalongkorn University Ratchadapisek Sompotch Fund, Faculty of Medicine (Grant number 61/037); The Chulalongkorn University Ratchadapisek Sompotch Fund (2019), Telehealth Cluster Grant; The Chulalongkorn University Ratchadapisek Sompotch Fund, Postdoctoral Fellowship Fund; The Center of Excellence in Transgender Health, Chulalongkorn University.
ACKNOWLEDGEMENTS
The CE‐PID – TRC Adolescent Study Team:
Praphan Phanuphak, Chitsanu Pancharoen, Somsong Teeratakulpisarn, Reshmie Ramautarsing, Rena Janamnuaysook, Kritima Samitpol, Jiratchaya Kongkapan, Artsanee Chancham, Pravit Mingkwanrungruang, Narukjaporn Thammajaruk, Nuttawut Teachatanawat, Krittaporn Termvanich, Tippawan Pankam, Thantip Sungsing, Sorawit Amatavete, Chotika Prabjantuek, Phubet Panpet, Jureeporn Jantarapakde, Tuangtip Theerawit,Watsamon Jantarabenjakul, Suvaporn Anugulruengkitt, Nattapong Jitrunruengnit, Noppadol Wacharachaisurapol, Pintip Suchartlikitwong, Pakpoom Janewongwirot, Juthamanee Moonwong, Rachaneekorn Nadsasarn, Patchareeyawan Srimuan, Siwanart Thammasala, Sasiwimol Ubolyam, Patcharin Eamyoung, Jiratchaya Sophonphan, Stephen Kerr, Taninee Petwijit, Chansuda Bongsebandhu‐phubakdi, Buajoom Raksakul, Pathranis Meekrua, Orawan Fungfoosri, Prapaipan Plodgratoke, Pakitta Kidsumret, Kamolchanok Chumyen, Sahakun Chintanakarn, Sirinthon Yenjit, Chanin Suksom, Tanunjit Rugphan, Somsri Tantipaibulvut, Prakaipech Kaw‐In, Penprapa Chanachon, Chanjiraporn Pondet.
Special thanks to: Marissa Vicari from CIPHER for her support of this project, Emeritus Professor Praphan Phanuphak for his guidance and support of this project at the Thai Red Cross AIDS Research Center, Professor Linda‐Gail Bekker for her guidance and support of this project, Dr Chitsanu Pancharoen for his support training staff in adolescent counselling for this project, Anuchit Vasionta from Focal Intelligence Co. Ltd and his team Rachapong Pornwiriyangkura, Mathawee Tusjunt, Patcharamon Siriphongpiphat, Rawisara Loekiatthamrong and Sitthiphong Achavanfor their support in designing and producing “Project Raincoat,” and Yardpiroon Tawon for her hard work in supporting TFV‐DP assay analyses.
Songtaweesin, W. N. , Kawichai, S. , Phanuphak, N. , Cressey, T. R. , Wongharn, P. , Saisaengjan, C. , Chinbunchorn, T. , Janyam, S. , Linjongrat, D. and Puthanakit, T. ; the CE‐PID – TRC Adolescent Study Team . Youth‐friendly services and a mobile phone application to promote adherence to pre‐exposure prophylaxis among adolescent men who have sex with men and transgender women at‐risk for HIV in Thailand: a randomized control trial. J Int AIDS Soc. 2020; 23(S5):e25564
ClinicalTrials.gov Identifier: NCT03778892.
Contributor Information
Wipaporn Natalie Songtaweesin, Email: wipaporn.n@chula.ac.th.
the CE‐PID – TRC Adolescent Study Team:
Praphan Phanuphak, Chitsanu Pancharoen, Somsong Teeratakulpisarn, Reshmie Ramautarsing, Rena Janamnuaysook, Kritima Samitpol, Jiratchaya Kongkapan, Artsanee Chancham, Pravit Mingkwanrungruang, Narukjaporn Thammajaruk, Nuttawut Teachatanawat, Krittaporn Termvanich, Tippawan Pankam, Thantip Sungsing, Sorawit Amatavete, Chotika Prabjantuek, Phubet Panpet, Jureeporn Jantarapakde, Tuangtip Theerawit, Watsamon Jantarabenjakul, Suvaporn Anugulruengkitt, Nattapong Jitrunruengnit, Noppadol Wacharachaisurapol, Pintip Suchartlikitwong, Pakpoom Janewongwirot, Juthamanee Moonwong, Rachaneekorn Nadsasarn, Patchareeyawan Srimuan, Siwanart Thammasala, Sasiwimol Ubolyam, Patcharin Eamyoung, Jiratchaya Sophonphan, Stephen Kerr, Taninee Petwijit, Chansuda Bongsebandhu‐phubakdi, Buajoom Raksakul, Pathranis Meekrua, Orawan Fungfoosri, Prapaipan Plodgratoke, Pakitta Kidsumret, Kamolchanok Chumyen, Sahakun Chintanakarn, Sirinthon Yenjit, Chanin Suksom, Tanunjit Rugphan, Somsri Tantipaibulvut, Prakaipech Kaw‐In, Penprapa Chanachon, and Chanjiraporn Pondet
REFERENCES
- 1. van Griensven F, Guadamuz Thomas E, de Lind van Wijngaarden JW, Phanuphak N, Solomon SS, Lo Y‐R. Challenges and emerging opportunities for the HIV prevention, treatment and care cascade in men who have sex with men in Asia Pacific. Sex Transmit Infect. 2017;93(5):356–62. [DOI] [PubMed] [Google Scholar]
- 2. Baral SD, Grosso A, Holland C, Papworth E. The epidemiology of HIV among men who have sex with men in countries with generalized HIV epidemics. Curr Opin HIV AIDS. 2014;9(2):156–67. [DOI] [PubMed] [Google Scholar]
- 3. van Griensven F, Thienkrua W, McNicholl J, Wimonsate W, Chaikummao S, Chonwattana W, et al. Evidence of an explosive epidemic of HIV infection in a cohort of men who have sex with men in Thailand. AIDS. 2013;27(5):825–32. [DOI] [PubMed] [Google Scholar]
- 4. Thienkrua W, van Griensven F, Mock PA, Dunne EF, Raengsakulrach B, Wimonsate W, et al. Young men who have sex with men at high risk for HIV, Bangkok MSM Cohort Study, Thailand 2006–2014. AIDS Behav. 2018;22(7):2137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Griensven F, Varangrat A, Wimonsate W, Tanpradech S, Kladsawad K, Chemnasiri T, et al. Trends in HIV prevalence, estimated hiv incidence, and risk behavior among men who have sex with men in Bangkok, Thailand, 2003–2007. J Acquir Immune Defic Syndr. 2010;53(2):234–9. [DOI] [PubMed] [Google Scholar]
- 6. Centers for Disease C, Prevention . HIV and syphilis infection among men who have sex with men–Bangkok, Thailand, 2005–2011. MMWR Morb Mortal Wkly Rep. 2013;62(25):518–20. [PMC free article] [PubMed] [Google Scholar]
- 7. Wasantioopapokakorn M, Manopaiboon C, Phoorisri T, Sukkul A, Lertpiriyasuwat C, Ongwandee S, et al. Implementation and assessment of a model to increase HIV testing among men who have sex with men and transgender women in Thailand, 2011–2016. AIDS Care. 2018;30(10):1239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garofalo R, Hotton AL, Kuhns LM, Gratzer B, Mustanski B. Incidence of HIV infection and sexually transmitted infections and related risk factors among very young men who have sex with men. J Acquir Immune Defic Syndr. 2016;72(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elopre L, McDavid C, Brown A, Shurbaji S, Mugavero MJ, Turan JM. Perceptions of HIV pre‐exposure prophylaxis among young, black men who have sex with men. AIDS Patient Care STDS. 2018;32(12):511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chemnasiri T, Netwong T, Visarutratana S, Varangrat A, Li A, Phanuphak P, et al. Inconsistent condom use among young men who have sex with men, male sex workers, and transgenders in Thailand. AIDS Educ Prev. 2010;22(2):100–9. [DOI] [PubMed] [Google Scholar]
- 11. Musumari PM, Tangmunkongvorakul A, Srithanaviboonchai K, Yungyuankul S, Techasrivichien T, Suguimoto SP, et al. Prevalence and Correlates of HIV testing among young people enrolled in non‐formal education centers in urban Chiang Mai, Thailand: A cross‐sectional study. PLoS One. 2016;11:e0153452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pettifor A, Nguyen NL, Celum C, Cowan FM, Go V. Tailored combination prevention packages and PrEP for young key populations. J Int AIDS Soc. 2015;18:19434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cumulative number of Thai PrEP users: PrEP‐30 and Princess PrEP estimated number as of December 2018, Thai Red Cross AIDS Research Center. [Internet]. 2018. [cited 2020 Apr 7]. Available from: www.PrEPthai.net
- 14. Zablotska I, Grulich AE, Phanuphak N, Anand T, Janyam S, Poonkasetwattana M, et al. PrEP implementation in the Asia‐Pacific region: opportunities, implementation and barriers. J Int AIDS Soc. 2016;19:21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Society for Adolescent H, Medicine . HIV Pre‐exposure prophylaxis medication for adolescents and young adults: a position paper of the society for adolescent health and medicine. J Adolesc Health. 2018;63(4):513–6. [DOI] [PubMed] [Google Scholar]
- 16. UNAIDS . Ending the AIDS epidemic for adolescents, with adolescents. A practical guide to meaningfully engage adolescents in the AIDS response. 2016. [cited 2020 Apr 7]. Available from: http://www.unaids.org/en/resources/documents/2016/ending‐AIDS‐epidemic‐adolescents
- 17. Avert . HIV prevention programmes overview [Internet]. [cited 2020 Apr 7]. https://www.avert.org/professionals/hiv‐programming/prevention/overview
- 18. Baggaley R, Armstrong A, Dodd Z, Ngoksin E, Krug A. Young key populations and HIV: a special emphasis and consideration in the new WHO Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations. J Int AIDS Soc. 2015;18:19438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bekker LG, Gill K, Wallace M. Pre‐exposure prophylaxis for South African adolescents: what evidence? S Afr Med J. 2015;105(11):907–11. [DOI] [PubMed] [Google Scholar]
- 20. Organization WH . WHO Implementation tool for pre‐exposure prophylaxis (PrEP) of HIV infection. Module 1: Clinical. 2017.
- 21. Hosek SG, Landovitz RJ, Kapogiannis B, Siberry GK, Rudy B, Rutledge B, et al. Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States. JAMA Pediatr. 2017;171(11):1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bureau of AIDS TaS . Report on HIV PrEP Provision Thailand: Bureau of AIDS, TB and STIs. 2020. [cited 2020 Jan 7]. Available from: http://www.prepthai.net/ViewAGroup.aspx
- 23. Schueler K, Ferreira M, Nikolopoulos G, Skaathun B, Paraskevis D, Hatzakis A, et al. Pre‐exposure prophylaxis (PrEP) awareness and use within high HIV transmission networks. AIDS Behav. 2019;23(7):1893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spinelli MA, Scott HM, Vittinghoff E, Liu AY, Gonzalez R, Morehead‐Gee A, et al. Missed visits associated with future preexposure prophylaxis (PrEP) discontinuation among PrEP users in a municipal primary care health network. Open Forum Infect Dis. 2019;6:ofz101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. eHealth WGOf . mHealth: New horizons for health through mobile technologies: second global survey on eHealth. 2011.
- 26. Aliabadi N, Carballo‐Dieguez A, Bakken S, Rojas M, Brown W 3rd, Carry M, et al. Using the information‐motivation‐behavioral skills model to guide the development of an HIV prevention smartphone application for high‐risk MSM. AIDS Educ Prev. 2015;27(6):522–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ybarra ML, Korchmaros JD, Prescott TL, Birungi R. A randomized controlled trial to increase HIV preventive information, motivation, and behavioral skills in Ugandan adolescents. Ann Behav Med. 2015;49(3):473–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hawkins RP, Kreuter M, Resnicow K, Fishbein M, Dijkstra A. Understanding tailoring in communicating about health. Health Educ Res. 2008;23(3):454–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thai Ministry of Public Health . Thailand National Guidelines on Pre‐Exposure Prophylaxis: HIV‐PrEP. 2018. ISBN: 978‐616‐11‐3732‐8. http://aidssti.ddc.moph.go.th/medias/view/126/549
- 30. Palacio A, Garay D, Langer B, Taylor J, Wood BA, Tamariz L. Motivational interviewing improves medication adherence: a systematic review and meta‐analysis. J Gen Intern Med. 2016;31(8):929–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller WR, Rollnick S. Meeting in the middle: motivational interviewing and self‐determination theory. Int J Behav Nutr Phys Act. 2012;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Couper MP, Alexander GL, Zhang N, Little RJ, Maddy N, Nowak MA, et al. Engagement and retention: measuring breadth and depth of participant use of an online intervention. J Med Internet Res. 2010;12:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anderson PL, Liu AY, Castillo‐Mancilla JR, Gardner EM, Seifert SM, McHugh C, et al. Intracellular tenofovir‐diphosphate and emtricitabine‐triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother. 2018;62:e01710‐17 10.1128/AAC.01710-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre‐exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hosek SG, Rudy B, Landovitz R, Kapogiannis B, Siberry G, Rutledge B, et al. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74(1):21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine‐tenofovir concentrations and pre‐exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hergenrather KC, Emmanuel D, Durant S, Rhodes SD. Enhancing HIV prevention among young men who have sex with men: a systematic review of HIV behavioral interventions for young gay and bisexual men. AIDS Educ Prev. 2016;28(3):252–71. [DOI] [PubMed] [Google Scholar]
- 38. Thailand NNBo . PrEP HIV‐prevention med makes progress in Thailand 2019 [updated 29 October 2019. Available from: http://thainews.prd.go.th/en/news/detail/TCATG191029161049997
- 39. Office NHS .Top 10 performance of the Universal Coverage Scheme (UCS) Fund in 2019. National Health Security Office. [cited 2020 Apr 30]. Available from: http://eng.nhso.go.th/view/1/Description_news/NewsAndEvents/4/EN‐US
- 40. Health MoP . Thailand National guidelines on HIV/AIDS treatment and prevention 2017. Bangkok, Thailand; 2017.
- 41. Colby D, Srithanaviboonchai K, Vanichseni S, Ongwandee S, Phanuphak N, Martin M, et al. HIV pre‐exposure prophylaxis and health and community systems in the Global South: Thailand case study. J Int AIDS Soc. 2015;18:19953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baxi SM, Vittinghoff E, Bacchetti P, Huang Y, Chillag K, Wiegand R, et al. Comparing pharmacologic measures of tenofovir exposure in a U.S. pre‐exposure prophylaxis randomized trial. PLoS One. 2018;13:e0190118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hosek SG, Siberry G, Bell M, Lally M, Kapogiannis B, Green K, et al. The acceptability and feasibility of an HIV preexposure prophylaxis (PrEP) trial with young men who have sex with men. J Acquir Immune Defic Syndr. 2013;62(4):447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haberer JE. Current concepts for PrEP adherence in the PrEP revolution: from clinical trials to routine practice. Current Opin HIV AIDS. 2016;11(1):10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pyra MN, Haberer JE, Hasen N, Reed J, Mugo NR, Baeten JM. Global implementation of PrEP for HIV prevention: setting expectations for impact. J Int AIDS Soc. 2019;22:e25370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haberer JE, Bangsberg DR, Baeten JM, Curran K, Koechlin F, Amico KR, et al. Defining success with HIV pre‐exposure prophylaxis: a prevention‐effective adherence paradigm. AIDS. 2015;29(11):1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cohn LD, Macfarlane S, Yanez C, Imai WK. Risk‐perception: differences between adolescents and adults. Health Psychol. 1995;14(3):217–22. [DOI] [PubMed] [Google Scholar]
- 48. Maljaars LP, Gill K, Smith PJ, Gray GE, Dietrich JJ, Gomez GB, et al. Condom migration after introduction of pre‐exposure prophylaxis among HIV‐uninfected adolescents in South Africa: a cohort analysis. South Afr J HIV Med. 2017;18:712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sagaon‐Teyssier L, Suzan‐Monti M, Demoulin B, Capitant C, Lorente N, Préau M, et al. Uptake of PrEP and condom and sexual risk behavior among MSM during the ANRS IPERGAY trial. AIDS Care. 2016;28 Sup1:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Organization WH . What’s the 2+1+1? Event‐driven oral pre‐exposure prophylaxis to prevent HIV for men who have sex with men: Update to WHO’s recommendation on oral PrEP. Geneva: World Health Organization; 2019. [Google Scholar]
- 51. LeGrand S, Knudtson K, Benkeser D, Muessig K, McGee A, Sullivan PS, et al. Testing the efficacy of a social networking gamification app to improve pre‐exposure prophylaxis adherence (P3: Prepared, Protected, emPowered): protocol for a randomized controlled trial. JMIR Res Protoc. 2018;7:e10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mitchell JT, LeGrand S, Hightow‐Weidman LB, McKellar MS, Kashuba AD, Cottrell M, et al. Smartphone‐based contingency management intervention to improve pre‐exposure prophylaxis adherence, Pilot Trial. JMIR mHealth and uHealth. 2018;6:e10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Biello KB, Marrow E, Mimiaga MJ, Sullivan P, Hightow‐Weidman L, Mayer KH. A Mobile‐based app (MyChoices) to increase uptake of HIV testing and pre‐exposure prophylaxis by young men who have sex with men: protocol for a pilot randomized controlled trial. JMIR Res Protoc. 2019;8:e10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu A, Coleman K, Bojan K, Serrano PA, Oyedele T, Garcia A, et al. Developing a Mobile App (LYNX) to support linkage to HIV/sexually transmitted infection testing and pre‐exposure prophylaxis for young men who have sex with men: protocol for a randomized controlled trial. JMIR Res Protoc. 2019;8:e10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Finkenflügel RNN, Hoornenborg E, Achterbergh RCA, Marra E, Davidovich U, de Vries HJC, et al. A mobile application to collect daily data on preexposure prophylaxis adherence and sexual behavior among men who have sex with men: use over time and comparability with conventional data collection. Sex Transm Dis. 2019;46(6):400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Downing MJ Jr. Perceived likelihood of HIV and sexually transmitted infection acquisition among men who have sex with men. J Assoc Nurses AIDS Care. 2014;25(1):98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van der Snoek EM, de Wit JBF, Mulder PGH, van der Meijden WI. Incidence of sexually transmitted diseases and hiv infection related to perceived HIV/AIDS threat since highly active antiretroviral therapy availability in men who have sex with men. Sex Transm Dis. 2005;32(3):170–5. [DOI] [PubMed] [Google Scholar]
- 58. Corneli A, Wang M, Agot K, Ahmed K, Lombaard J, Van Damme L. Perception of HIV risk and adherence to a daily, investigational pill for HIV prevention in FEM‐PrEP. J Acquir Immune Defic Syndr. 2014;67(5):555–63. [DOI] [PubMed] [Google Scholar]
- 59. Bauermeister JA, Pingel ES, Jadwin‐Cakmak L, Harper GW, Horvath K, Weiss G, et al. Acceptability and preliminary efficacy of a tailored Online HIV/STI testing intervention for young men who have sex with men: the get connected! Program. AIDS Behav. 2015;19(10):1860–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Corneli AL, McKenna K, Headley J, Ahmed K, Odhiambo J, Skhosana J, et al. A descriptive analysis of perceptions of HIV risk and worry about acquiring HIV among FEM‐PrEP participants who seroconverted in Bondo, Kenya, and Pretoria, South Africa. J Int AIDS Soc. 2014;17:19152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Arrington‐Sanders R, Wilson CM, Perumean‐Chaney SE, Patki A, Hosek S. Brief report: role of sociobehavioral factors in subprotective TFV‐DP levels among YMSM enrolled in 2 PrEP trials. J Acquir Immune Defic Syndr. 2019;80(2):160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Khumsaen N, Stephenson R. Beliefs and Perception about HIV/AIDS, self‐efficacy, and HIV sexual risk behaviors among young thai men who have sex with men. AIDS Educ Prev. 2017;29(2):175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, et al. The Effectiveness of mobile‐health technology‐based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10:e1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Seekaew P, Nguyen E, Sungsing T, Jantarapakde J, Pengnonyang S, Trachunthong D, et al. Correlates of nonadherence to key population‐led HIV pre‐exposure prophylaxis services among Thai men who have sex with men and transgender women. BMC Public Health. 2019;19(1):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sevelius JM, Patouhas E, Keatley JG, Johnson MO. Barriers and facilitators to engagement and retention in care among transgender women living with human immunodeficiency virus. Ann Behav Med. 2014;47(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Phanuphak N, Sungsing T, Jantarapakde J, Pengnonyang S, Trachunthong D, Mingkwanrungruang P, et al. Princess PrEP program: the first key population‐led model to deliver pre‐exposure prophylaxis to key populations by key populations in Thailand. Sex Health. 2018;15(6):542–55. [DOI] [PubMed] [Google Scholar]
- 67. Poteat T, German D, Flynn C. The conflation of gender and sex: Gaps and opportunities in HIV data among transgender women and MSM. Glob Public Health. 2016;11(7–8):835–48. [DOI] [PMC free article] [PubMed] [Google Scholar]


