Abstract
Objective:
The most widely used diagnostic technique for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is real-time reverse transcriptase-polymerase chain reaction (RT-PCR). It can be done on different samples: nasopharyngeal swabs (NPS) or oropharyngeal swabs (OPS), and self-collected saliva. However, negative findings do not rule out infection.
Methods:
A review was conceived to discuss advantages and limitations of the available diagnostic modalities for nonserologic diagnosis of SARS-CoV-2 based on RT-PCR; the article also proposes some practical suggestions to improve diagnostic reliability.
Results:
A total of 16 papers (corresponding to 452 patients) of the 56 initially identified were included. Most of the papers describe findings from different samples obtained in limited case series; comparative studies are missing.
Conclusions:
Diagnostic accuracy of NPS and OPS is suboptimal and the risk of contaminated aerosol dispersal is not negligible. The SARS-CoV-2 RNA can be found in self-collected saliva specimens of many infected patients within 7 to 10 days after symptom onset. There is an urgent need for comparative trials to define the diagnostic modality of choice. Adequate education and training of health care personnel is mandatory.
Keywords: COVID-19, swab, nasopharynx, emergency, infection
Introduction
Since its outbreak in China in December 2019, coronavirus disease 2019 (COVID-19) resulting from primary infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global concern leading to more than 7 145 500 confirmed diagnoses and more than 408 000 deaths all over the world (data as of June 10, 2020), 1 with a rapid evolution of the epidemiological profile over time.
Diagnosis of SARS-CoV-2 infection is currently based on real-time reverse transcriptase-polymerase chain reaction (RT-PCR) performed on either nasopharyngeal swabs (NPS) or oropharyngeal swabs (OPS). 2 Despite suboptimal detection rates, 3 collection of secretions from the upper airway by means of NPS/OPS still represents the first-line diagnostic modality to test patients and otherwise asymptomatic population for COVID-19, provided that it is early and adequately performed after onset of symptoms. 2 As a fact, reduced detection rates reflect analytical sensitivity of RT-PCR test and the epidemiologic characteristics of COVID-19, given that a false negative RT-PCR result could be possibly obtained both in the initial phase of the disease (ie, a few days before symptom onset) and at the “tail end” of SARS-CoV-2 infection (ie, from 20 days after symptom onset) due to a low viral load and a viral shedding below analytical RT-PCR sensitivity threshold. 3
On the basis of the reported detection rates, 4 the US Center for Disease Control and Prevention (US-CDC) has recommended the collection of sole upper respiratory NPS, 2 but the US Food and Drug Administration pointed out that a negative RT-PCR test result does not completely rule out SARS-CoV-2 infection and it shall not be used as a single element for patient management decisions. 5 As a fact, it has been described that a negative NPS or OPS does not rule out COVID-19 as in some cases consistent diagnosis occurs from bronchoalveolar lavage fluid after repeated negative testing at NPS and OPS. 6 This could be partially related to the fact that SARS-CoV-2 RNA titer in the upper respiratory tract peaks between days 7 and 10 after the clinical onset. 6 Reduced detection rate could be also related to either inadequacy of sample collection into the nasopharynx (the risk that collection of secretions from the nasal cavity rather than the nasopharynx is unneglectable, given the incomplete patient cooperation during this unpleasant maneuver) or a limited viral local tropism due to the low expression of angiotensin-converting enzyme 2 receptors in the epithelial cells of the nasopharyngeal/oropharyngeal surface. 7
All the currently available diagnostic techniques are reported and described in Table 1.
Table 1.
Diagnostic Test for SARS-CoV-2 Detection.
| Diagnostic test and description | Specimen | How to perform |
|---|---|---|
| RT-PCR: two separate oligonucleotide primers/probes selected from regions of the virus nucleocapsid N gene; an additional set targeting the human RNase P gene. All 3 assays must match in order to report presumptive positivity for SARS-CoV-2. 8 | NPS 9 |
|
| OPS 9 |
|
|
| Self-collected saliva 9 |
|
|
| BAL (Wang, Pascarella) | ||
Serological diagnosis: to be performed by means of binding or
neutralizing antibody detection
11
|
Blood sample (obtained by fingerstick and/or venipuncture) | |
Radiological diagnosis (chest CT). Typical findings are:
Number of segment involved and tendency to opacity confluence proportional to disease severity. 10 Unusual findings: pleural effusion, masses, cavitations, lymphadenopathies. 10 |
||
Abbreviations: BAL, bronchoalveolar lavage fluid; CIA, chemiluminescent immunoassay; CT, computed tomography; ELISA, enzyme-linked immunosorbent assay; NPS, nasopharyngeal swab; OPS, oropharyngeal swab; pVNT, pseudovirus neutralization tests; RT-PCR, real-time reverse transcriptase polymerase chain reaction; VNT, virus neutralization tests.
Some concerns should be also raised about safety, as during diagnostic maneuver dispersal of infected aerosol could occur with possible nosocomial transmission 12 : at the time of writing, more than 28 600 cases have been documented among Italian health care workers, 13 with 167 deaths among physicians. 14
This article aims at discussing advantages and limitations of the available diagnostic sampling modalities for nucleic acid amplification-based diagnostics of SARS-CoV-2 infection, including NPS, OPS, and self-collected saliva specimens, and proposing some practical suggestions to improve diagnostic reliability.
Patients and Methods
Eligibility Criteria
Consideration was given to any paper published in the English language in peer-reviewed journals and specifically concerning the issue (ie, diagnostic accuracy, advantages, and limitations of each procedure, as well as any possible standard procedure which could be adopted to increase effectiveness and safety) in human subjects (both adults and children). Animal studies were excluded. Case reports, reviews, letters, opinion, and perspective papers were included too. Unpublished materials were not considered.
Primary outcome measures were detection rates of SARS-CoV-2 nucleic acid by means of RT-PCR performed on different sampling obtained from the upper airways. Papers not clearly reporting this specific outcome were excluded. Secondary outcomes were related to identify advantages and limitations of each procedure, as well as any possible standard procedure which could be adopted to increase effectiveness and safety.
Information Sources and Search Strategy
English language papers concerning sampling procedures from the upper airways for nonserologic diagnosis of COVID-19 were selected after a MEDLINE search (accessed via PubMed on April 7, 2020) based on the following terms: “COVID-19 AND nasopharyngeal swab”, “COVID-19 AND oropharyngeal swab”, “COVID-19 AND saliva”, “COVID-19 AND swab.” Separate literature searches were made for NPS, OPS, and saliva specimen collection.
Study Selection
The reference lists were reviewed to ensure that all of the selected papers were truly relevant and to identify any that had possibly been overlooked. Eligibility assessment and data collection process were performed independently in an unblinded standardized manner by 2 review authors. Data extraction sheet was prepared as follows: Review author #1 extract information, and Review author #2 checked it. Disagreement between reviewers was solved by consensus or by a third author consultation in case of no agreement.
Results
Study Selection and Characteristics
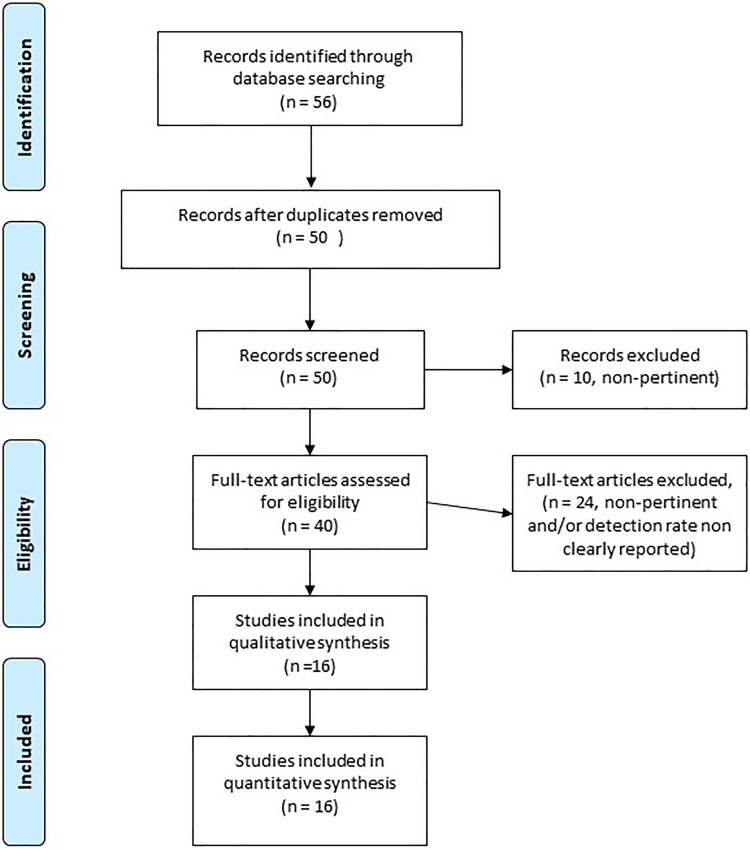
A total of 16 papers (including 9 case series, 3 letters, 2 opinion/perspective papers, 1 state of the art paper, and 1 case report) of the 56 initially identified papers were included in this minireview, corresponding to 452 patients (Figure 1).
Figure 1.
Flowchart of article selection.
Most of the papers describe findings from different samples obtained in limited case series; original studies specifically designed for comparative purposes on sampling techniques for collection from the upper airways are missing.
Syntheses of Results
During evaluation of the selected papers, we decided to perform separate literature analysis for NPS/OPS and saliva specimens, on the basis of the fact that in many case series diagnosis of SARS-CoV-2 infection was performed through appeal to NPS and OPS in a combined modality.
Under this circumstance, 12 papers were deemed pertinent to assess the diagnostic role of NPS/OPS in patients with COVID-19. They include 1 state of the art article, 15 1 opinion paper, 3 1 case report, 16 2 letters (1 with a single-case description 6 and 1 presenting results obtained in 28 patients 17 ), and 7 original papers performed on limited case series of adult patients ranging between 5 and 292 patients. 18 -24 Among these, the only comparative study was the one published by Xie et al, 22 who compared SARS-CoV-2 RNA detection rates from different samples including OPS, blood, urine, and stool samples with different fluorescent RT-PCR kits. The remaining original papers incidentally reported virologic results from specimens collected through OPS or NPS. 18 -24 Reported detection rates range between 40% and 100% and 32% and 100%, respectively, for NPS and OPS, and peak in early stage disease (up to 100% when tested by day 5). Corresponding figures when sampling is performed after day 5 drop to ≈40% for NPS and 25% to 40% for OPS (Table 2).
Table 2.
Advantages and Limitations of Sampling Proceduresa From the Upper Respiratory Tract for Diagnosis of SARS-CoV-2 Infection.
| Sampling procedures | |||
|---|---|---|---|
| NPS | OPS | Saliva specimen (self-test) | |
| Advantages |
|
|
|
| Limitations |
|
|
|
| Detection rate | 40%-100% (≈100% within day 5; ≈40% after day 5) 4,19 | 32%-100% (32%-100% within day 5; 25%-40% after day 5) 4,18,20 -22,30 | 33%-92% (87%-92% within day 7; ≈33% after day 20)
26,27,30,31
|
| Cost (per patient) | ≈US$120-US$150 | ≈US$120-US$150 | US$15 |
Abbreviations: NPS, nasopharyngeal swabs; OPS, oropharyngeal swabs; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
a NPS, OPS, and saliva specimens.
Only 4 papers were found to be relevant to assess the possible diagnostic role of saliva sampling on COVID-19. 25 -28 They include 1 perspective paper without case description, 25 1 letter with one-case description (dealing with gargle lavage), 28 and 2 further studies performed on small case series (23 and 12 adult patients, respectively) from the same Chinese group. 26,27 Reported detection rates range between 33% and 92% with positive findings in 87% to 92% of affected patients when performed within 7 days (Table 2).
Discussion
Nasopharyngeal and Oropharyngeal Swabs
Consistent diagnosis of COVID-19 based on detection of SARS-CoV-2 RNA isolated from upper airways secretions is supported by the documented extensive viral tropism and its active high replication rate in the pharyngeal district. 19
Nevertheless, according to recent evidence, detection rate of SARS-CoV-2 RNA on the grounds of RT-PCR performed on both NPS and OPS would be lower than expected. 3 As a fact, it has been reported that 3% to 34.7% of patients with chest computed tomography (CT) findings suggestive for COVID-19 initially had negative swab tests. 18,32 Moreover, most of the patients with a precocious negative RT-PCR result but consistent CT findings subsequently developed RT-PCR positivity at serial examination after about 5 days as a mean. 32
Analytical sensitivity of RT-PCR is limited by the presence of at least 2 grey zones where false negative results attributable to reduced viral load may occur during the real first days of infections in patients mainly asymptomatic or with mild symptoms and in the ending phase of infection after clinical recovery despite persistence of virus shedding. 3
As a fact, it is well known that pharyngeal virus shedding peaks during the first week of symptoms, 19 then it would gradually decrease with a supposed shift from oral positivity (attested during early disease) to blood and gastrointestinal positivity during late infection. 21 This is supported by the study of Zhang et al 21 who compared virological results obtained in a cohort of 39 patients with COVID-19 in Wuhan. They found that none of the patients with viral RNA detected in the blood had concomitant positivity to RT-PCR on OPS. This means that such cases would be considered COVID-19 negative according to traditional surveillance although already actively viremic.
Diagnostic reliability significantly drops also in the case of improper execution: as a fact, swab advancement into the nasal cavity during NPS performance may be impaired by anatomic conditions, reduced patient cooperation, or execution by a not adequately trained examiner. Under these circumstances, collection may accidentally occur into the nasal cavity rather than the nasopharynx, resulting in failed retrieval of infected secretions placed in the nasopharynx. With regard to OPS, unintentional contamination from the oral cavity may occur. In addition, inadequate methods for swab performance (ie, collection from the nasal cavity during NPS or from the tonsillar complex as for detection of group A β-hemolytic streptococcus during OPS performance) have been proposed as standard collecting procedures. 23 For instance, a national guideline showed unclear graphical representations (documenting collection from the middle nasal meatus and the tonsillar complex) despite adequate explanations. 33
Collection of upper airway secretions by means of NPS and OPS for SARS-CoV-2 diagnostic purpose has been codified by the US-CDC 2 ; the sole use of swabs with a synthetic tip and shaft in aluminum or plastic materials is recommended.
Based on our experience, NPS should be performed as follows. 9 After having sterilely opened the outer case of the swab, the latter is inserted into nasal cavity by slightly elevating the tip of the nose, enlarging the nostril and thus reducing the risk of contamination of the nasal vestibule. The swab then flows over the floor of the nasal cavity in parallel with the line of the hard palate. After reaching the nasopharynx (whose distance generally corresponds to a notch on the stick), the swab needs to stay there for a few seconds, then rotated and extracted in order to enhance absorption of secretions useful for diagnosis. Oropharyngeal swab is performed by gently pressing the tongue depressor anteriorly over the tongue and avoiding to touch the tongue with the swab. The right position of the swab is nearby the posterior wall of the oropharynx achieving collection of secretions descending from the upper nasopharynx. 9 Figures 2 and 3 show adequate collection of nasopharyngeal (Figure 2A-C) and oropharyngeal (Figure 3A-C) secretions, respectively, during NPS and OPS execution performed by a trained ear, nose, and throat examiner and confirmed by means of transnasal videoendoscopic control.
Figure 2.
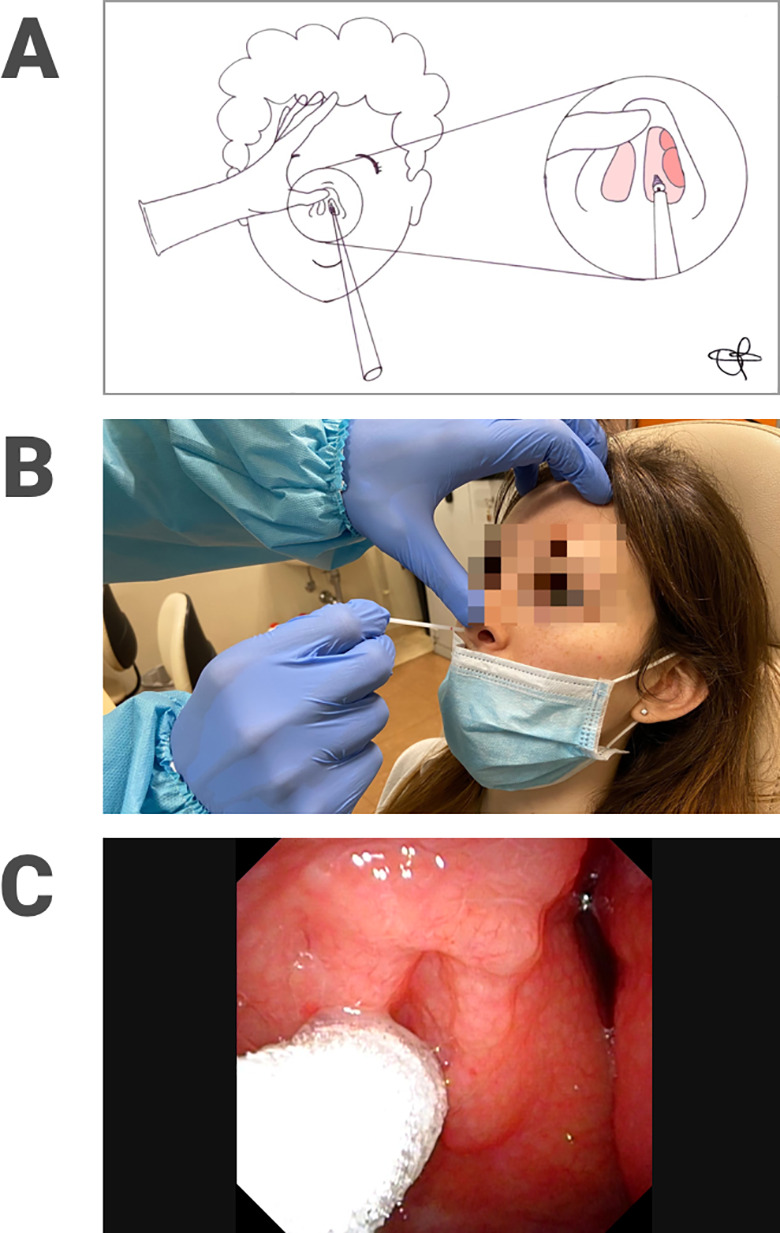
Nasopharyngeal swab execution represented in a drawing (A) and performed in a patient (B); correct placement of the tip of the swab into the nasopharynx documented by means of transnasal videoendoscopy (C).
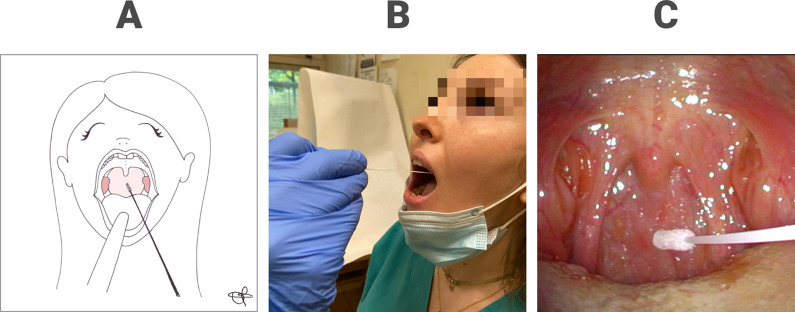
Figure 3.
Oropharyngeal swab execution represented in a drawing (A) and performed in a patient (B); correct placement of the tip of the swab at the posterior oropharyngeal wall documented by means of transnasal videoendoscopy (C).
Some procedural details can be adopted to minimize nosocomial transmission: as a fact, Won et al 23 proposed a laboratory-safe and low-cost protocol for COVID-19 detection. According to this protocol, collecting procedure is performed by the patient itself placed under a well-ventilated fume hood with a constant air inflow in a designated location with spatial separation between the OPS station and the laboratory for RNA extraction to prevent any possible contact between patients and health care personnel. Despite high biosafety and cost-effectiveness are definitely a big plus, some questions may arise about adequacy of collecting procedure through OPS when it is performed by the patient itself. Potential preanalytical vulnerability can be related to inadequate or inappropriate material collection (both in terms of volume and quality), which may result in instance from inadvertent swab contact with tongue and consequent oral cavity contamination. 3 As a fact, the authors 23 concluded with caution about the potential use of this protocol in clinics and suggested to limit its adoption to identify the negative people, rather than those with obvious COVID-19 symptoms who should be addressed to certified health facilities.
In addition, health care personnel training and education is desirable to make diagnostic procedure more accurate and, especially in the case of NPS, less unpleasant for the patient. It is worth remembering that bleeding after collection on nasopharyngeal secretions by means of NPS may also occur. Therefore, NPS should be avoided in patients treated with anticoagulants or with epistaxis. Advantages, limitations, and diagnostic accuracy of NPS and OPS are reported in Table 2.
Saliva Specimen
Self-collection of saliva specimens has been proposed as a safe, cheap, and noninvasive diagnostic mean to confirm SARS-CoV-2 infection. 25 -27 This procedure can be easily performed with minimal equipment required and no significant discomfort, as patients can be instructed to autonomously cough out saliva into a sterile container, possibly by using a throat-clearing maneuver in order to enhance collection of both descending secretions from the nasopharynx and lower fluids moved up from the tracheobronchial tree. 9 Secretions from the salivary glands, another possible target of SARS-CoV-2, 34 may be collected too.
In addition, Saito et al 28 performed diagnosis of SARS-CoV-2 infection in a 55-year-old febrile patient without respiratory symptoms by means of positive RT-PCR findings on OPS and gargle lavage obtained on days 8 and 9 from onset, with a slightly increased amount of viral genome isolated in the latter one.
The high concordance between nasopharyngeal and saliva samples in detection rate of some respiratory viruses, including coronavirus, 32,30,31 and the sole detection of coronavirus in saliva but not in nasopharyngeal aspirates previously reported in some patients pushed a Chinese scientific team 26,27 to test diagnostic reliability of saliva collection, as alternative diagnostic mean. This effort actualized in 2 studies: the first one, conducted in 12 patients with COVID-19, demonstrated that SARS-CoV-2 RNA could be detected in the saliva of all but 1 (91.7%) patients, and that serial collection of saliva specimens could be used to monitor viral load given the found decline in salivary SARS-CoV-2 RNA levels at repeated testing. 26 These encouraging results have been confirmed by a second study evaluating temporal profiles of viral load in deep saliva samples collected from the posterior oropharynx by means of a throat-clearing maneuver in a cohort of 23 adult patients with laboratory confirmed SARS-CoV-2 infection. 27 They found that, unlike severe acute respiratory syndromes of different pathogenesis, salivary viral load was maximal during the first week from symptoms onset, then progressively decreased during time, with persistence detection of viral RNA ≥20 days after symptom onset in one-third of cases. Higher viral load correlated with older age, but not with disease severity. The authors also documented a correlation between serum antibodies detection and virus neutralization titer.
Under these circumstances, this diagnostic tool seems to be very attractive, and it should be further studied by means of larger comparative trials specifically designed to test its diagnostic power over traditional NPS/OPS. Advantages, limitations, and diagnostic accuracy of self-collection of saliva specimens sampling are reported in Table 1.
Conclusions
Consistent diagnosis of SARS-CoV-2 infection on the grounds of traditional NPS and OPS has some limitations related to suboptimal diagnostic accuracy, due to inadequate sampling techniques and the non-negligible risk of nosocomial transmission. There is an urgent need for comparative trials specifically dedicated to define the diagnostic modality of choice.
Diagnostic reliability of RT-PCR analysis performed on saliva samples collected by different methods deserves further studies. Collection of deep saliva obtained through throat-clearing maneuver could be of particular interest as fluids moved from the posterior oropharynx are composed not only by saliva, but also by secretions both descending from the nasopharynx and coming up from the tracheobronchial district.
Clear and nonambiguous definition and diffusion of standardized procedures as well as adequate education and training of health care personnel are essential to make diagnostic procedures more effective and safer.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Sara Torretta  https://orcid.org/0000-0002-8461-6042
https://orcid.org/0000-0002-8461-6042
References
- 1. World Health Organization. Coronavirus Disease 2019 (COVID-19). Situation Report-120. Updated June 10, 2020. Accessed June 11, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200610-covid-19-sitrep-142.pdf?sfvrsn=180898cd_2.
- 2. Centers for Disease Control and Prevention. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19). Updated July 8, 2020. Accessed April 16, 2020. https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html
- 3. Lippi G, Simundic AM, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19). Clin Chem Lab Med. 2020;58(7):1070–1076. [DOI] [PubMed] [Google Scholar]
- 4. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Food and Drug Administration. New-York SARS-CoV-2. Real-Time RT-PCR Diagnostic Panel-February 29. Updated March 15, 2020. Accessed March 9, 2020. https://www.fda.gov/media/135662/download
- 6. Winichakoon P, Chaiwarith R, Liwsrisakun C, et al. Negative nasopharyngeal and oropharyngeal swab does not rule out COVID-19. J Clin Microbiol. 2020;58(5):00297–00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. a first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel. Updated July 13, 2020. Accessed July 13, 2020 https://www.fda.goc/media/134922/download.
- 9. Torretta S, Zuccotti G, Cristofaro V, et al. Nonserologic test for COVID-19: how to manage? Head Neck. 2020;42(7):1552–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pascarella G, Strumia A, Piliego C, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Interim Guidelines for COVID-19 Antibody Testing. Updated August 1, 2020. Accessed July 15, 2020 https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html#anchor_1590280017822.
- 12. Torretta S, Gaini LM, Pignataro L. Why Italian ENT physicians should be aware of SARS-CoV-2. Acta Otorhinolaryngol Ital. 2020;40(2):152–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Istituto Superiore di Sanità (Roma). Epidemia COVID-19. Sorveglianza Integrata COVID-19 in Italia. Aggiornamento del 10 Giugno; 2020. Updated June 10, 2020. Accessed June 11, 2020 https://www.epicentro.iss.it/coronavirus/bollettino/Infografica_10giugno%20ITA.pdf.
- 14. Federazione Nazionale degli Ordini dei medici Chirurghi e degli odontoiatri. Elenco dei medici caduti nel corso dell’epidemia COVID-19 ; Updated August 18, 2020. Accessed June 11, 2020. https://portale.fnomceo.it/elenco-dei-medici-caduti-nel-corso-dellepidemia-di-covid-19/
- 15. Loeffelholz MJ, Tang YW. Laboratory diagnosis of emerging human coronavirus infections - the state of the art. Emerg Microbes Infect. 2020;9(1):747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hase R, Kurita T, Muranaka E, Sasazawa H, Mito H, Yano Y. A case of imported COVID-19 diagnosed by PCR-positive lower respiratory specimen but with PCR-negative throat swabs. Infect Dis (Lond). 2020;52(6):423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen W, Lan Y, Yuan X, et al. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9(1):469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest Ct for typical 2019-ncov pneumonia: relationship to negative rt-pcr testing. Radiology. 2020;296(2):E41–E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020;581(7809):465–469. [DOI] [PubMed] [Google Scholar]
- 20. Ling Y, Xu SB, Lin YX, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl). 2020;133(9):1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xie C, Jiang L, Huang G, et al. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int J Infect Dis. 2020;93:264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Won J, Lee S, Park M, et al. Development of a Laboratory-safe and Low-cost Detection Protocol for SARS-CoV-2 of the Coronavirus Disease 2019 (COVID-19). Exp Neurobiol. 2020; 29(2):107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lo IL, Lio CF, Cheong HH, et al. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int J Biol Sci. 2020;16(10):1698–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khurshid Z, Asiri FYI, Al Wadaani H. Human saliva: non-Invasive Fluid for Detecting Novel Coronavirus (2019-nCoV). Int J Environ Res Public Health. 2020;17(7):E2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. To KK, Tsang OT, Yip CCY, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71(15):841–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saito M, Adachi E, Yamayoshi S, et al. Gargle lavage as a safe and sensitive alternative to swab samples to diagnose COVID-19: a case report in Japan. Clin Infect Dis. 2020;71(15):893–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Torretta S, Capaccio P, Gaffuri M, et al. ENT management of children with adenotonsillar disease during COVID-19 pandemic. Ready to start again? Int J Pediatr Otorhinolaryngol. 2020;28:110145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. To KK, Lu L, Yip CC, et al. Additional molecular testing of saliva specimens improves the detection of respiratory viruses. Emerg Microbes Infect. 2017;6(6):e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. To KKW, Yip CCY, Lai CYW, et al. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect. 2019;25(3):372–378. [DOI] [PubMed] [Google Scholar]
- 32. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (covid-19) in china: a report of 1014 cases. Radiology. 2020; 296(2):E32–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Istituto Superiore di Sanità (Roma). Rapporto ISS COVID-19. N 11\2020. Raccomandazioni per il corretto prelievo, conservazione e analisi sul tampone oro/nasofaringeo per la diagnosi di COVID-19. Gruppo di lavoro ISS Diagnostica e sorveglianza microbiologica COVID-19: aspetti di analisi molecolare e sierologica. Updated May 29, 2020. Accessed April 16, 2020. https://www.iss.it/rapporti-covid-19/-/asset_publisher/btw1J82wtYzH/content/id/5329985.
- 34. Capaccio P, Pignataro L, Corbellino M, et al. Acute Parotitis: a possible precocious clinical manifestation of SARS-COV-2 infection. Otolaryngol Head Neck Surg. 2020;163(1):182–183. [DOI] [PubMed] [Google Scholar]