Abstract
Background
Sleep problems are common in eating disorders (EDs).
Purpose
We evaluated whether sleep-phasing regularity associates with the regularity of daily eating events.
Methods
ED patients (n = 29) completed hourly charts of mood and eating occasions for 2 weeks. Locomotor activity was recorded continuously by wrist actigraphy for a minimum of 10 days, and sleep was calculated based on periods of inactivity. We computed the center of daily inactivity (CenDI) as a measure of sleep phasing and consolidation of the daily inactivity (ConDI) as a measure of daily sleep rhythm strength. We assessed interday irregularities in the temporal structure of food intake using the standard deviation (SD) of frequency (IFRQ), timing (ITIM), and interval (IINT) of food intake. A self-evaluation of other characteristics included mood, anxiety, and early trauma.
Results
A later phasing of sleep associated with a lower frequency of eating (eating frequency with the CenDI rho = −0.49, p = .007). The phasing and rhythmic strength of sleep correlated with the degree of eating irregularity (CenDI with ITIM rho = 0.48, p = .008 and with IINT rho = 0.56, p = .002; SD of CenDI with ITIM rho = 0.47, p = .010, and SD of ConDI with IINT rho = 0.37, p = .048). Childhood Trauma Questionnaire showed associations with variation of sleep onset (rho = −0.51, p = .005) and with IFRQ (rho = 0.43, p = .023).
Conclusions
Late and variable phasing of sleep associated robustly with irregular pattern of eating. Larger data sets are warranted to enable the analysis of diagnostic subgroups, current medication, and current symptomatology and to confirm the likely bidirectional association between eating pattern stability and the timing of sleep.
Keywords: Eating disorder, Rest:activity rhythm, Circadian rhythm, Eating pattern, Actigraphy
In eating disorders, timing of sleep was late and irregular. Later sleep associated with a lower frequency and later and variable sleep with more irregular eating.
Introduction
The effects of chronic starvation in anorexia nervosa (AN) and of fluctuating eating patterns in bulimia nervosa (BN) on the sleep regulating processes were recognized in polysomnographic studies (PSG) more than two decades ago [1]. Robust data confirm a high prevalence of sleep problems in eating disorders (EDs). For instance, in a longitudinal study among 400 female patients diagnosed with an ED, approximately half of the patients reported sleep problems [2]. The association between sleep and eating patterns is likely bidirectional: while eating rhythms affect sleep [3], patients deprived of adequate sleep experience difficulty in controlling appetite and binge impulses [4]. This is supported by experimental human and animal studies as reviewed previously [3, 5]. Furthermore, patients with poorer sleep quality in self-report show a worse outcome of ED [6, 7].
Most of the reported sleep problems in EDs have to do with the desired and actual timing of sleep [4]. The immediate consequence of such impairment is insomnia, a subjective complaint dependent on subjective needs. A mismatch between the desired and actual timing of sleep and wakefulness, as well as an inability to maintain sleep, can have various causes, including starvation, stress, or trauma [8–10]. Such insults may act on the neural systems that regulate sleep itself, or their input systems, most importantly, the circadian clock [5]. Regardless of the cause and pathways involved, aberrations in the daily timing of sleep and wake, as well as the timing of eating, can have severe consequences on health [5, 11–13].
A promising methodological advance in psychiatric research is measuring the activity and sleep parameters objectively by actigraphy [14–16]. Wrist-worn accelerometric devices are increasingly used to determine phases of rest and activity in field studies across multiple days [16]. As compared to some other psychiatric illnesses [17, 18], knowledge on the rest:activity patterns in ED is very limited. The few existing studies involved recordings limited to few days and/or very small data sets [19, 20], or the actigraphy device was used to measure activity instead of sleep [21]. Thus, the previous studies did not inform about characteristics or correlates of sleep patterns among patients with an ED.
Data on daily eating has been collected to assess frequency, spacing, regularity, skipping, and timing of eating [22]. The temporal distribution of eating events typically associates with sleep timing: we eat while we are awake. Sleep:wake timing, on the other hand, is believed to be strongly influenced by the light:dark cycle in conjunction with the circadian clock. Generally, the 24 hr rhythmic biological processes, including sleep timing are phase coupled to the circadian clock oscillator, which, in turn, is phase coupled or entrained by the day:night oscillations of the solar day. On the other hand, exogenous factors other than light, such as social cues and, most importantly, eating timing, can affect circadian phasing and rhythmicity [3, 5, 23, 24]. Notably, eating timing is particularly dysregulated in patients with an ED, exhibiting binge and/or restrictive eating behaviors [25–27]. However, while monitoring the temporal aspects of feeding is feasible at high resolution in animals and has been shown to affect rest:activity rhythmicity [11, 28], human research on eating patterns in ED has mainly focused on retrospective self-report and content of food consumed.
In this study, we wanted to correlate eating patterns with sleep based on objective, actigraphy-derived data in a representative patient sample from a tertiary care ED program. We quantitatively assessed eating patterns employing a method we introduced recently [29]. As the accelerometry data from the device we used has been validated against PSG [30, 31], the data obtained could be translated into sleep scores. Furthermore, we used self-evaluation of other characteristics that have an impact on eating and sleep, including mood, anxiety, and early trauma. Our hypothesis was that sleep-phasing instability would associate with eating pattern irregularity in ED patients. This would be consistent with the view that the daily shaping of sleep and eating patterns relies at least in part on a common regulatory system or that the control systems of eating and sleep strongly interact.
Methods
Inclusion and Exclusion
Patients were eligible for participation if they had been diagnosed with an ED according to DSM-5 criteria [32], treated as outpatients, capable of consenting, and between the ages of 18 and 65 inclusively. Patients were excluded if their current level of suicidality or symptoms required hospitalization. The protocol for this study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and was approved by the local Ethics Committee. All participants provided written informed consent at the first in-person visit.
Procedure
Study data were collected and managed using REDCap electronic data capture tools [33]. The patients were instructed to provide simultaneous recordings of eating and sleep. Patients documented hourly their mood and eating events, as well as onsets and offsets of rest in journals for 2 weeks (Supplementary Fig. 1) beginning at their inscription (V1). At the same time, participants were outfitted with accelerometric wrist-worn devices (GENEActiv, Activinsights) for measurement of (in)activity and were instructed to wear the devices continuously for 2 weeks. At V2, participants completed a survey of scales selected for the measurement of current mood and eating. Online questionnaires were completed in the clinic or online at the patient’s residence. Participants also participated in a semistructured clinical interview for mood by research assistants. Diagnoses and current medication were obtained by medical chart review.
Participation in the study was offered to 55 patients and 36 (65.5%) of those gave informed consent. For the current analysis, we consider the final data set as the 29 patients for whom continuous accelerometry data were available for a minimum of 10 days with simultaneous journal data [34, 35]. Data were excluded due to too short valid recording of sleep (n = 4) or missing report for eating (n = 3).
Questionnaire Measures
We used the Eating Disorder Examination Questionnaire (EDEQ) [36] to measure the current severity of EDs. Additionally, the scales to measure state-like symptoms included the Clinically Useful Anxiety Outcome Scale (CUXOS) [37], and the revised Reinforcement Sensitivity Theory Questionnaire (rRSTQ) [38] to describe personality features. The rRSTQ was recently used in ED patients and showed good psychometric properties [39]. We also asked about traumatic experiences using the first six items for experiences before the age of 17 from the Childhood Trauma Questionnaire (CTQ), as well as Item 7 “Have you had any other major upheavals, including as an adult, that you think may have shaped your life or personality significantly?” [40]. We used the total sum score of the seven items.
Interview
Mood symptoms were scored during the past week to measure the intensity of the symptoms using the Montgomery–Asberg Depression Scale (MADRS) [41]. While true manic symptoms were not present, the Young Mania Rating Scale (YMRS) [42] was considered as a measure for peak severity of arousal, including agitation, or irritability. We used the Social and Occupational Functioning Scale (SOFAS) [32] to measure functionality.
Descriptors of Eating
To assess the daily pattern of eating and mood, participants charted the time of any food intake (hereafter, the expression meal is used as a synonym for any eating occasion during waking hours over the 2 week period between two in-person visits (V1 and V2). Eating frequency refers, thus, to the daily number of meals or any eating occasions. To visualize daily eating patterns, we generated eatograms by entering eating occasions from the hourly charts into MATLAB (The MathWorks) based custom scripts. Eatograms were displayed in a double plot mode (x-axis = 48 hr, y-axis = days) in order to better discern patterns as is customary for actogram displays in the study of circadian rhythms.
To assess the temporal aspects of eating behavior (eating pattern) quantitatively, we computed three indices based on documented eating events across the 2 week recording period as previously described [29]. Briefly, IFRQ indicates the standard deviation (SD) of the daily number of meals (SD of meal frequency) and ITIM represents the SD of the daily timing of meals. We determined ITIM by calculating the average of the SDs of the timing of each daily meal (Meal 1 across days, Meal 2 across days, etc.). IINT represents the SD of the intermeal interval. For this index, we first calculated the SDs of the intervals between each consecutive daily meal and, then, calculated their average. These indices served as dimensional measures to assess regularity in the temporal pattern of food intake. A larger index value reflects a more irregular eating pattern, whereas 0 indicates no variation in meal frequency, times, or intermeal interval.
Descriptors of Sleep
Sleep was determined in accordance with an actigraphy-based method that has been previously validated against polysomnography using the accelerometry devices employed here [30, 31]. Raw accelerometry data were extracted from the GENEActiv devices as binary files containing x, y, and z acceleration values sampled at 10 Hz resolution. These values were median filtered using a rolling 5 s window, and an estimate of the arm angle was calculated as follows: angle = (where, ax, ay, and az are the median-filtered acceleration values). Next, the arm angles were averaged across 5 s epochs. Sleep was then defined as a 5 min period with arm angle change between 5 s epochs of less than 5° [30], which provided >90% sensitivity and >80% accuracy for sleep detection in reference to polysomnographic scoring [30]. Accordingly, each 5 s bin was assigned a score of 1 for sleep or 0 for no sleep, and the data were averaged to 1 min bins.
The actigraphy-based sleep score data were converted into double-plotted inactograms, which illustrate the sleep scores across an entire recording period (Fig. 1) using custom MATLAB (The MathWorks) scripts. Self-reported bedtime data from hourly charts were cross-checked for quality with inactograms. As the minimum continuous recording period was 10 days among patients, we only used the first 10 days of actigraphy data for analysis; days with more than 1 hr of nonwear were excluded from the calculation of values.
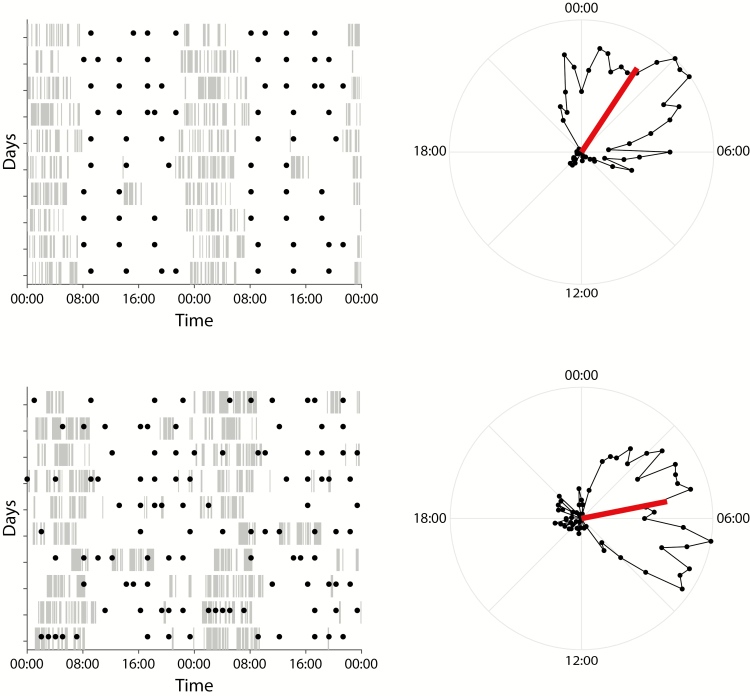
Fig. 1.
Examples of sleep and eating patterns in patients with an eating disorder. We present two cases. The first shows a relatively regular eating frequency and timing. The sleep is well consolidated around a midpoint of sleep as compared to a midpoint reported in the general population. The second shows very irregular eating, dispersed across the daily cycle, including nighttime, with fragmented sleep and a late midpoint of sleep. Left: daily sleep based on actigraphy-derived sleep scores is displayed as double-plotted inactograms with eating events indicated (filled circles). Tick marks indicate continuous sleep at 5 min resolution. Right: Circular plots representing the average distribution of sleep across the daily cycle. Vector (red) direction indicates the center of inactivity and, thus, the average phasing of the sleep:wake cycle, while vector length indicates the consolidation of daily inactivity as a measure of sleep fragmentation.
Center of daily inactivity (CenDI) is the mean direction of inactivity timing and describes the daily distribution of sleep in radian based on the sleep scores, providing information about the phasing of the sleep:wake cycle, which can be seen as a reflection of chronotype. Consolidation of daily inactivity (ConDI) represents the mean resultant vector length corrected for total duration of sleep, which describes the degree of daily sleep consolidation and, thus, informs on sleep continuity. Both values were calculated using the Circular Statistics Toolbox for MATLAB [43]. “Sleep onset” and “Sleep Offset” were calculated in hours and mark the beginning and end of the “Nighttime Sleep” period. “Nighttime Sleep” was determined by applying a rolling window with a length that is equal to 20% of daily total sleep time divided by ConDI, with total sleep time defined as the sum of all 1 min bins scored as sleep per each daily cycle. The window length had a 120 min cutoff, that is, a 120 min window length was applied to all those days where the calculated window length would exceed this value. To calculate “Nighttime Sleep,” the 24 hr time span defining one daily cycle was centered at mean CenDI, that is, a single cycle stretched ±12 hr from mean CenDI. The rolling window as defined above was then moved by 1 min increments across the 24 hr cycle as defined above and periods of sleep ended when the moving window started to encompass zero sleep scores. The longest sleep period per 24 hr cycle calculated in this manner was then defined as the “Nighttime Sleep” period. “Sleep onset” and “Sleep Offset” were defined as the beginning and end of this period and superimposed on inactograms for visual inspection of accuracy (data not shown). The calculated timing of sleep showed good correlation with the patient-reported timing (objective vs. subjective sleep midpoint (rho = 0.85, p < .001; Supplementary Fig. 2); this confirms the appropriateness of the actigraphy-derived variables. Notably, a similar approach has been recently reported to correlate well with PSG-determined sleep onsets and offsets [31]. Correlations between the dimensional sleep variables are presented in Supplementary Table 1 and correlations between the dimensional eating variables are presented in Supplementary Table 2.
Statistical Analyses
Data were analyzed employing SPSS version 25.0. Due to mostly nonnormally distributed values, we used Mann–Whitney U-Test in between-group comparisons and bivariate Spearman correlations with two-tailed significance. For descriptive purposes, significant findings were defined by p-values <.05.
Results
Description of the Cohort
All participants were female, and the median age at study onset was 26.0 years (Table 1). Seven patients had a principal diagnosis of BN, 11 AN (3 restricting and 8 bingeing/purging), 9 other specified feeding or ED (OSFED) (5 restricting, 3 purging, and 1 mixed), and 1 avoidant/restrictive food intake disorder (ARFID). The range of body mass index (BMI) was from 15.20 to 39.8 kg/m2, with three patients below the limit of mild AN (<17.5 kg/m2) and three above the obesity limit ≥30 kg/m2. One patient had sleep apnea and was using a continuous positive airway pressure machine, but no other patients had a primary sleep disorder. Most patients (21, 72.4%) were using psychoactive medication, including antidepressants (15, 51.7%), antipsychotics (6, 20.1%), hypnotics (8, 27.6%), and stimulants (2, 5.9%).
Table 1.
Sociodemographic and clinical descriptors of a sample of 29 eating disorder patients
| Characteristic | ||
|---|---|---|
| Median | IQR | |
| Age | 26.0 | 9.75 |
| BMI, kg/m2 | 20.9 | 6.25 |
| Depression (MADRS) | 20.0 | 12.0 |
| Manic symptoms (YMRS) | 4.0 | 4.0 |
| Functionality (SOFAS) | 58.0 | 12.5 |
| Anxiety (CUXOS) | 35.0 | 23.3 |
| EDEQ, global score | 4.0 | 1.60 |
| Trauma (CTQ) | 18.0 | 9.0 |
| Reward sensitivity (rRSTQ) | 55.0 | 8.75 |
Depression (MADRS) = Montgomery-Åsberg Depression Scale total score; Manic symptoms (YMRS) = Young Mania Rating Scale total score; SOFAS = Social and Occupational Functional Assessment Scale; Anxiety (CUXOS) = Clinically Useful Anxiety Outcome Scale self-rating scale total score; EDEQ = Eating disorder examination questionnaire mean global severity score; Trauma (CTQ) = Childhood Trauma Questionnaire total sum score of Items 1–7; Reward sensitivity = Revised Reinforcement Sensitivity Theory Questionnaire (rRSTQ) total score;
BMI, body mass index; IQR, interquartile range.
The description of mood, anxiety, and personality based on the diagnostic groups are presented in Table 1. Most patients had depressive symptoms to some extent, with 15 (51.7%) reporting moderate (MADRS 18–34), and 2 (6.9%) severe depressive symptoms (≥35) [44]. While none of them had a hypomanic episode, four patients were scored to the range of mild manic symptoms (YMRS scores ≥12 but ≤20 [45]). On average, the patients also scored high on CUXOS, with 11 (37.9%) scoring above severe anxiety (≥41). The median eating frequency in the sample was 3.75 (interquartile range [IQR] 1.84), median CenDI 3:40AM (IQR 1.57), median sleep onset 24:18 AM (IQR 3:03), and median sleep offset 7:46 AM (IQR 1:51).
Correlation Between the Descriptors of Sleep Pattern and Eating Pattern
A later timing of sleep associated with a lower frequency (FRQ) of eating as seen in a correlation of FRQ with CenDI and sleep onset, respectively (Table 2). Furthermore, ITIM and IINT, both describing irregular eating, were moderately/strongly correlated with all descriptors of the timing of sleep (CenDI, sleep onset, and sleep offset). ITIM also correlated with SD of CenDI, indicating that variability in the timing of sleep associated with the variability in meal timing.
Table 2.
Correlations between sleep and eating pattern in a sample of 29 eating disorder patients
| Frequency | IFRQ | ITIM | IINT | |||||
|---|---|---|---|---|---|---|---|---|
| Rho | p | Rho | p | Rho | p | Rho | p | |
| CenDI | −0.49 | 0.007 | −0.30 | 0.12 | 0.48 | 0.008 | 0.56 | 0.002 |
| SD | −0.32 | 0.091 | −0.96 | 0.62 | 0.47 | 0.010 | 0.37 | 0.048 |
| ConDI | −0.24 | 0.21 | −0.074 | 0.70 | 0.077 | 0.69 | 0.21 | 0.28 |
| SD | 0.010 | 0.96 | −0.037 | 0.85 | 0.025 | 0.90 | −0.17 | 0.39 |
| Onset | −0.42 | 0.024 | −0.22 | 0.26 | 0.41 | 0.028 | 0.53 | 0.003 |
| SD | −0.23 | 0.24 | 0.009 | 0.96 | 0.075 | 0.70 | 0.15 | 0.44 |
| Offset | −0.28 | 0.14 | −0.15 | 0.44 | 0.42 | 0.022 | 0.46 | 0.012 |
| SD | 0.22 | 0.26 | 0.15 | 0.43 | 0.00 | 1.0 | −0.070 | 0.72 |
| Nighttime sleep | 0.13 | 0.49 | 0.13 | 0.52 | −0.13 | 0.52 | −0.15 | 0.43 |
| SD | −0.34 | 0.075 | −0.20 | 0.30 | 0.13 | 0.52 | 0.12 | 0.54 |
CenDI = center of daily inactivity. This measure serves as approximate of the midpoint of sleep, which is known to reflect chronotype (ref needed); SD = standard deviation as a descriptor of variance in each measure; ConDI = vector length of the mean midpoint of sleep during 24 hr, normalized for the length of sleep; Onset = mean sleep onset in hours; Offset = mean sleep offset in hours; Nighttime sleep = mean total nighttime sleep; Frequency = the daily number of meals (any eating occasions during the day). IFRQ = the SD of the daily number of meals (SD of meal frequency). ITIM = the SD of the daily timing of meals. We determined ITIM by calculating the SDs of each daily meal and, then, by averaging these SDs. IINT is the SD of the intermeal interval. For this index, we first calculated the SDs of the intervals between each consecutive daily meal and, then, averaged these SDs. These indices served as dimensional measures to assess regularity in the temporal pattern of food intake. A larger index value reflects a more irregular eating pattern, whereas 0 indicates no variation in meal frequency, times, or intermeal interval. Rho = Spearman’s rank correlation coefficient.
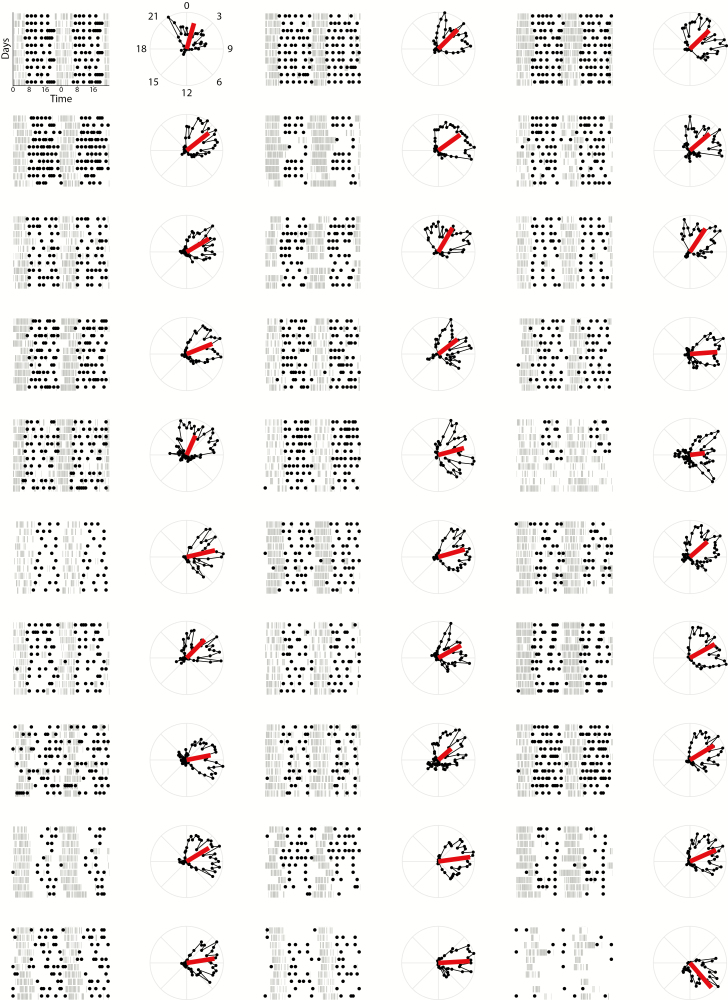
We ordered the individual patient data by increasing variability of the intervals between eating occasions (index IINT; Fig. 2), CenID (Supplementary Fig. 3), and ITIM (Supplementary Fig. 4). Observation of these panels provides further evidence for the association between patterns of sleep and eating.
Fig. 2.
Inactograms of 29 eating disorder patients with eating events indicated. Plots are ordered by increasing variability of the intervals between eating occasions (index IINT). The vector in the adjacent circular plots indicates the center of daily inactivity and consolidation of the daily inactivity, that is, the phasing (vector direction) and consolidation (vector length) of the sleep–wake cycle, respectively.
Correlations of Sleep and Eating Patterns With Other Clinical Characteristics
Associations between sleep and eating patterns were not better explained by other clinical features (Table 3). However, a higher eating frequency correlated with less severe depressive symptoms (MADRS) and better functionality. A higher EDEQ score correlated with later offset of sleep, higher SD of nighttime sleep, and lower eating frequency. A higher CTQ total score correlated with later sleep onset and with IFRQ (Table 3).
Table 3.
Correlations between sleep and eating patterns with mood in a sample of 29 eating disorder patients
| MADRS | YMRS | SOFAS | CUXOS | EDEQ total | rRSTQ | CTQ total | |
|---|---|---|---|---|---|---|---|
| CenDI | −0.076 | 0.11 | 0.028 | −0.007 | 0.14 | −0.013 | −0.314 |
| SD | −0.17 | 0.24 | −0.29 | 0.092 | 0.096 | −0.11 | 0.019 |
| ConDI | 0.062 | −0.19 | −0.081 | −0.23 | 0.004 | −0.31 | −0.31 |
| SD | −0.10 | 0.21 | 0.02 | 0.079 | 0.13 | 0.14 | 0.091 |
| Onset | −0.13 | 0.004 | 0.035 | −0.063 | −0.12 | 0.025 | −0.51* |
| SD | 0.34 | 0.16 | −0.23 | 0.19 | 0.27 | 0.016 | 0.030 |
| Offset | 0.023 | 0.016 | −0.093 | 0.19 | 0.39* | 0.20 | 0.023 |
| SD | −0.015 | 0.27 | 0.082 | −0.12 | −0.077 | −0.006 | 0.26 |
| Nighttime sleep | 0.093 | −0.14 | −0.13 | 0.25 | 0.26 | 0.077 | 0.32 |
| SD | 0.30 | 0.25 | −0.19 | 0.22 | 0.53** | 0.18 | 0.13 |
| Frequency | −0.36* | −0.20 | 0.39* | −0.25 | −0.42* | −0.077 | 0.15 |
| IFRQ | −0.27 | 0.045 | 0.15 | −0.040 | −0.33 | 0.13 | 0.43* |
| ITIM | −0.18 | 0.15 | 0.012 | 0.13 | 0.25 | 0.18 | 0.098 |
| IINT | 0.006 | 0.002 | −0.16 | 0.034 | 0.20 | 0.14 | −0.036 |
CenDI = angle of the midpoint of sleep as reflecting chronotype; SD = standard deviation as a descriptor of variance in each measure; ConDI = vector length of the mean midpoint of sleep during 24 hr, normalized for the length of sleep; Onset = mean sleep onset in hours; Offset = mean sleep offset in hours; Nighttime sleep = mean total nighttime sleep in minutes; Frequency = the daily number of meals (any eating occasions during the day). IFRQ = the SD of the daily number of meals (SD of meal frequency). ITIM = the SD of the daily timing of meals. We determined ITIM by calculating the SDs of each daily meal and, then, by averaging these SDs. IINT is the SD of the intermeal interval. For this index, we first calculated the SDs of the intervals between each consecutive daily meal and, then, averaged these SDs.
MADRS, Montgomery–Åsberg Depression Scale total score; YMRS, Young Mania Rating Scale total score; SOFAS, Social and Occupational Functional Assessment Scale; EDEQ, Eating disorder examination questionnaire total score; CUXOS, Clinically Useful Anxiety Outcome Scale self-rating scale total score; rRSTQ, Revised Reinforcement Sensitivity Theory Questionnaire total score; CTQ, the Childhood Trauma Questionnaire total score of Items 1–7.
*p-value 0.01 to 0.05.
**p-value <0.01.
Discussion
This is the first study to describe rest:activity rhythms in individuals with EDs using an objective measure, wrist actigraphy. We found that late and variable phasing of sleep associated robustly with an irregular pattern of eating and eating frequency. The associations of current ED symptoms, mood, or anxiety with eating patterns were separate from the associations between sleep and eating patterns. However, an association of reported early trauma was seen with the sleep patterns. Notably, the association between eating and sleep was very robust considering the presence of several other characteristics that could affect both eating and sleep, most importantly, primary diagnosis, medication, phase of treatment, BMI, mood, or anxiety.
We are the first to present a detailed analysis of both sleep and eating patterns in a sample of ED patients. Our work provides the basis for tools for clinical interventions and research to further understand causal relations between eating and sleep patterns. Yet, this is a proof of concept study where the size of the sample is small, and the results await replication. However, the findings are statistically strong given the size of the data, and they repeat consistently with similar measures. A major strength is that we used sleep scores based on actigraphy data, which is well validated to objectively track a person’s rest:activity patterns [30, 46]. The wrist-accelerometry device used is affordable, noninvasive, waterproof, and transportable, enabling a month of continuous recording without a need for being recharged. It, thus, permits the patients to adhere to their daily routines as opposed to PSG, which is much more invasive [47]. The observed late and variable phasing of sleep and the associated irregular meal patterning could be due to internal timing system perturbations. Alternatively, it can result from external factors, such as social and workplace-related demands or medication. However, even external factors could exert their effect on sleep and eating via internal timer perturbation. In future studies, evaluating central pacemaker phasing more directly by measuring dim-light melatonin onset or core body temperature will likely shed more light on the true causes of the observed simultaneous aberrations in eating and sleep.
Definitions for the pattern of eating are used inconsistently in research on EDs and this has to be taken into account when comparing our work to previous literature about meal patterns. One of the commonly used measures for patterns is the Eating Disorder Examination (EDE), which is based on the subjective and retrospective frequency of predefined eating events, that is, the average occurrence of breakfast, lunch, and an evening meal, as well as snacks during the 28 days immediately prior to assessment. Using this approach, in a sample of ED patients, dinner was the most and breakfast the least commonly consumed meal, snacking was most often reported during the evening time, and nocturnal eating was infrequent [26].
In treatment, regular eating patterns are defined as three meals and two snacks per day and refer to the frequency and content of the meals. Psychoeducation toward regular eating is a routine focus of therapy to treat ED [48]. A regular eating frequency has shown beneficial effects on symptom severity, especially, the frequency of bingeing in BN [25]. Accumulating evidence also supports the idea that manipulating eating patterns can have an impact on circadian rhythm and sleep, while optimal eating pattern for health remains open [49]. For instance, a shift of three evenly spaced daily meals toward later hours appeared to cause a corresponding delay in the phasing of the central circadian pacemaker [50]; this seems to suggest that clock phasing is sensitive to feeding cues. A circadian misalignment as seen, for example, in shift work results in dysbalanced energy homeostasis [51], which can partly be compensated for by maintaining an eating rhythm that is aligned with the day:dark cycle.
The size of our data set was not sufficient to reliably exclude differences between diagnostic subgroups in sleep or eating patterns. People with AN report an increased prevalence of sleep disturbances, especially, delayed sleep onset, early morning awakening, and poorer sleep quality [1, 4, 52]. While the previously published data on sleep is very limited in binge-purge AN and BN patients, results remain inconsistent [1, 4, 52]. A recent PSG study with 23 BN and AN patients reported disrupted sleep in both groups when compared to controls [53]. Patients with AN also typically follow rigid dietary behaviors, such as fixed meal times [26, 54, 55], while BN and binge eating tend to be associated with more chaotic and inconsistent dietary behaviors, as well as greater intraindividual variability as compared to AN [56–58].
We selected a transdiagnostic approach to ED. This theory suggests that common core psychopathology is the central component across a range of EDs and has been used for phenotypes, such as restraint model of binge eating [59]. Future studies should replicate our findings in larger data sets to show whether specific features of the sleep pattern represent a transdiagnostic finding reflecting core psychopathology or are specific to subgroups or certain phenotypes, such as purging.
Limitations of the study include the lack of a structured evaluation of sleep disorders. Most importantly, we did not differentiate current and chronic problems with the timing of sleep and, thus, cannot exclude primary circadian sleep–wake disorders. We also did not have an age-matched control group. However, large data sets with noncomparable methods have described characteristics of rest:activity rhythm in the general population; for sleep, see, for example, Mitchell et al. [60] and, for eating patterns, see Kant and Uzhova et al. [61, 62]. Our approach to eating occasion recording does not make any assumptions on the type of food intake at a given eating occasion. Patients with an ED have a tendency for restriction most of the time, and occasional extreme energy content, often followed by purging. Thus, the energy content of eating occasions is highly variable and not comparable to the general population. We cannot exclude the enrichment of patients with sleep problems. The patients received psychoeducation as part of the treatment, including advice to eat frequently. Thus, the findings about the characteristics of sleep and eating pattern might not be epidemiologically generalizable to all ED patients. However, these factors can be expected to rather weaken the association between sleep and eating patterns.
In conclusion, irregular eating was strongly associated with later and variable sleep phasing in our sample of ED patients. Our results suggest that, in addition to cognitive and/or social influences, dysregulated eating pattern in EDs could be partly explained by internal dysregulation of rest:activity rhythms.
Supplementary Material
Supplementary material is available at Annals of Behavioral Medicine online.
Figure S1. Hourly journal for eating and sleep used to define eating occasions and subjective onset and offset of sleep.
Figure S2. Correlation between calculated (based on actigraphy records) and patient reported sleep on- (left) and offsets (right) for the cohort of 29 patients with an ED. Shown are individual daily on- and offsets (blue) and their averages (red) for a given patient.
Figure S3. Inactograms of 29 ED patients with eating events indicated. Plots are ordered by increasing variability of the timing of sleep midpoint (CenDI).
Figure S4. Inactograms of 29 ED patients with eating events indicated. Plots are ordered by increasing variability of the timing of eating occasions (index ITIM).
Acknowledgments
The authors would like to thank the patients for their participation in this study and for helping us advance the knowledge in eating disorders. Their kindness and availability is highly appreciated. We would also like to thank the entire staff at the Eating Disorder Program of the Douglas Institute for their support throughout the conduct of this study.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors report no conflicts of interest.
Funding This work was supported by The Fonds de Recherche du Québec – Santé (FRQS) #8400863 and #252872 (OL); The Canadian Institutes of Health Research (CIHR) #366858 and The Natural Sciences and Engineering Research Council of Canada (NSERC): #RGPIN-2015-04034 (K-FS).
Authors’ Contributions O.L., H.S., and K.F.S. planned the study protocol; A.B. and D.S. participated in planning the data collection methods; O.L., H.S., O.C., and D.S. were in response of data collection and processing clinical data; C.B., K.F.S., and O.L. planned the methods for dimensional measurement of sleep and eating patterns; O.L. and O.C. conducted the statistical analysis; O.L. and K.F.S. wrote the first draft; all authors approved the final manuscript.
Ethical Approval The protocol was accepted by the Douglas Mental Health University Institute ethical committee (protocol 15/47).
Informed Consent All participants provided an informed consent.
References
- 1. Lauer CJ, Krieg JC. Sleep in eating disorders. Sleep Med Rev. 2004;8:109–118. [DOI] [PubMed] [Google Scholar]
- 2. Kim KR, Jung YC, Shin MY, Namkoong K, Kim JK, Lee JH. Sleep disturbance in women with eating disorder: Prevalence and clinical characteristics. Psychiatry Res. 2010;176:88–90. [DOI] [PubMed] [Google Scholar]
- 3. Potter GD, Cade JE, Grant PJ, Hardie LJ. Nutrition and the circadian system. Br J Nutr. 2016;116:434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allison KC, Spaeth A, Hopkins CM. Sleep and eating disorders. Curr Psychiatry Rep. 2016;18:92. [DOI] [PubMed] [Google Scholar]
- 5. Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry. 2014;26:139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lombardo C, Battagliese G, Venezia C, Salvemini V. Persistence of poor sleep predicts the severity of the clinical condition after 6 months of standard treatment in patients with eating disorders. Eat Behav. 2015;18:16–19. [DOI] [PubMed] [Google Scholar]
- 7. Sauchelli S, Jiménez-Murcia S, Sánchez I, et al. Orexin and sleep quality in anorexia nervosa: Clinical relevance and influence on treatment outcome. Psychoneuroendocrinology. 2016;65:102–108. [DOI] [PubMed] [Google Scholar]
- 8. Murray JM, Phillips AJK, Magee M, et al. ; Delayed Sleep on Melatonin (DelSoM) Study Group Sleep regularity is associated with sleep-wake and circadian timing, and mediates daytime function in delayed sleep-wake phase disorder. Sleep Med. 2019;58:93–101. [DOI] [PubMed] [Google Scholar]
- 9. Murray JM, Sletten TL, Magee M, et al. Prevalence of circadian misalignment and its association with depressive symptoms in Delayed Sleep Phase Disorder. Sleep. 2017;40(1):1–10. [DOI] [PubMed] [Google Scholar]
- 10. Beattie L, Espie CA, Kyle SD, Biello SM. How are normal sleeping controls selected? A systematic review of cross-sectional insomnia studies and a standardized method to select healthy controls for sleep research. Sleep Med. 2015;16:669–677. [DOI] [PubMed] [Google Scholar]
- 11. Zarrinpar A, Chaix A, Panda S. Daily eating patterns and their impact on health and disease. Trends Endocrinol Metab. 2016;27:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qian J, Scheer FAJL. Circadian system and glucose metabolism: Implications for physiology and disease. Trends Endocrinol Metab. 2016;27:282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grandner MA. Addressing sleep disturbances: An opportunity to prevent cardiometabolic disease? Int Rev Psychiatry. 2014;26:155–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grierson AB, Hickie IB, Naismith SL, Hermens DF, Scott EM, Scott J. Circadian rhythmicity in emerging mood disorders: State or trait marker? Int J Bipolar Disord. 2016;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kapczinski F, Frey BN, Vieta E. Sleep and circadian rhythm disturbances in bipolar disorder: An urgent need for objective assessment and systematic follow-up. J Clin Psychiatry. 2011;72:724. [DOI] [PubMed] [Google Scholar]
- 16. Roebuck A, Monasterio V, Gederi E, et al. A review of signals used in sleep analysis. Physiol Meas. 2014;35:R1–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones SG, Benca RM. Circadian disruption in psychiatric disorders. Sleep Med Clin. 2015;10:481–493. [DOI] [PubMed] [Google Scholar]
- 18. McClung CA. How might circadian rhythms control mood? Let me count the ways. Biol Psychiatry. 2013;74:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lehmann CS, Hofmann T, Elbelt U, et al. The role of objectively measured, altered physical activity patterns for body mass index change during inpatient treatment in female patients with anorexia nervosa. J Clin Med. 2018;7(9):1–12. pii: E289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roveda E, Montaruli A, Galasso L, et al. Rest-activity circadian rhythm and sleep quality in patients with binge eating disorder. Chronobiol Int. 2018;35:198–207. [DOI] [PubMed] [Google Scholar]
- 21. Sauchelli S, Arcelus J, Sánchez I, et al. Physical activity in anorexia nervosa: How relevant is it to therapy response? Eur Psychiatry. 2015;30:924–931. [DOI] [PubMed] [Google Scholar]
- 22. Leech RM, Worsley A, Timperio A, McNaughton SA. Understanding meal patterns: Definitions, methodology and impact on nutrient intake and diet quality. Nutr Res Rev. 2015;28:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gerhart-Hines Z, Lazar MA. Circadian metabolism in the light of evolution. Endocr Rev. 2015;36:289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim MJ, Lee JH, Duffy JF. Circadian rhythm sleep disorders. J Clin Outcomes Manag. 2013;20:513–528. [PMC free article] [PubMed] [Google Scholar]
- 25. Ellison JM, Simonich HK, Wonderlich SA, et al. Meal patterning in the treatment of bulimia nervosa. Eat Behav. 2016;20:39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harvey K, Rosselli F, Wilson GT, Debar LL, Striegel-Moore RH. Eating patterns in patients with spectrum binge-eating disorder. Int J Eat Disord. 2011;44:447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matheson BE, Tanofsky-Kraff M, Shafer-Berger S, et al. Eating patterns in youth with and without loss of control eating. Int J Eat Disord. 2012;45:957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hatori M, Panda S. Response of peripheral rhythms to the timing of food intake. Methods Enzymol. 2015;552:145–161. [DOI] [PubMed] [Google Scholar]
- 29. Buyukkurt A, Bourguignon C, Antinora C, et al. Irregular eating patterns associate with hypomanic symptoms in bipolar disorders. [published online ahead of print Mar 15, 2019] Nutr Neurosci. 2019:1–12. doi: 10.1080/1028415X.2019.1587136 [DOI] [PubMed] [Google Scholar]
- 30. van Hees VT, Sabia S, Anderson KN, et al. A novel, open access method to assess sleep duration using a wrist-worn accelerometer. PLoS One. 2015;10:e0142533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Hees VT, Sabia S, Jones SE, et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci Rep. 2018;8:12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. APA. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 33. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aili K, Åström-Paulsson S, Stoetzer U, Svartengren M, Hillert L. Reliability of actigraphy and subjective sleep measurements in adults: The design of sleep assessments. J Clin Sleep Med. 2017;13:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: An American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14:1209–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luce KH, Crowther JH: The reliability of the eating disorder examination—Self-report questionnaire version (EDE-Q). Int J Eating Disord. 1999, 25:349–351. [DOI] [PubMed] [Google Scholar]
- 37. Zimmerman M, Chelminski I, Young D, Dalrymple K. A clinically useful anxiety outcome scale. J Clin Psychiatry. 2010;71:534–542. [DOI] [PubMed] [Google Scholar]
- 38. Reuter M, Cooper AJ, Smillie LD, Markett S, Montag C. A new measure for the revised reinforcement sensitivity theory: Psychometric criteria and genetic validation. Front Syst Neurosci. 2015;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilson DR, Loxton NJ, O’Shannessy D, Sheeran N, Morgan A. Similarities and differences in revised reinforcement sensitivities across eating disorder subtypes. Appetite. 2019;133:70–76. [DOI] [PubMed] [Google Scholar]
- 40. Pennebaker JW, Susman JR. Childhood Trauma Questionnaire Measurement Instrument Database for the Social Science 2013. Available at https://www.midss.org/sites/default/files/trauma.pdf. [DOI] [PubMed]
- 41. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 42. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 43. Berens P: CircStat: A MATLAB toolbox for circular statistics. J Stat Softw. 2009;31(10):1–21. [Google Scholar]
- 44. Müller MJ, Szegedi A, Wetzel H, Benkert O. Moderate and severe depression. Gradations for the Montgomery-Asberg Depression Rating Scale. J Affect Disord. 2000;60:137–140. [DOI] [PubMed] [Google Scholar]
- 45. Lukasiewicz M, Gerard S, Besnard A, et al. ; Emblem Study Group Young Mania Rating Scale: How to interpret the numbers? Determination of a severity threshold and of the minimal clinically significant difference in the EMBLEM cohort. Int J Methods Psychiatr Res. 2013;22:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marino M, Li Y, Rueschman MN, et al. Measuring sleep: Accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36:1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McCall C, McCall WV. Comparison of actigraphy with polysomnography and sleep logs in depressed insomniacs. J Sleep Res. 2012;21:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hilbert A, Hoek HW, Schmidt R. Evidence-based clinical guidelines for eating disorders: International comparison. Curr Opin Psychiatry. 2017;30:423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mattson MP, Allison DB, Fontana L, et al. Meal frequency and timing in health and disease. Proc Natl Acad Sci USA. 2014;111:16647–16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wehrens SMT, Christou S, Isherwood C, et al. Meal timing regulates the human circadian system. Curr Biol. 2017, 27:1768–1775.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Paschos GK. Circadian clocks, feeding time, and metabolic homeostasis. Front Pharmacol. 2015;6:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baglioni C, Nanovska S, Regen W, et al. Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychol Bull. 2016;142:969–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Asaad Abdou T, Esawy HI, Abdel Razek Mohamed G, et al. Sleep profile in anorexia and bulimia nervosa female patients. Sleep Med. 2018;48:113–116. [DOI] [PubMed] [Google Scholar]
- 54. Burd C, Mitchell JE, Crosby RD, et al. An assessment of daily food intake in participants with anorexia nervosa in the natural environment. Int J Eat Disord. 2009;42:371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van der Ster Wallin G, Norring C, Lennernäs MA, Holmgren S. Food selection in anorectics and bulimics: Food items, nutrient content and nutrient density. J Am Coll Nutr. 1995;14:271–277. [DOI] [PubMed] [Google Scholar]
- 56. Masheb RM, Grilo CM, White MA. An examination of eating patterns in community women with bulimia nervosa and binge eating disorder. Int J Eat Disord. 2011;44:618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hetherington MM, Altemus M, Nelson ML, Bernat AS, Gold PW. Eating behavior in bulimia nervosa: Multiple meal analyses. Am J Clin Nutr. 1994;60:864–873. [DOI] [PubMed] [Google Scholar]
- 58. Goldfein JA, Walsh BT, LaChaussée JL, Kissileff HR, Devlin MJ. Eating behavior in binge eating disorder. Int J Eat Disord. 1993;14:427–431. [DOI] [PubMed] [Google Scholar]
- 59. Elran-Barak R, Sztainer M, Goldschmidt AB, et al. Dietary restriction behaviors and binge eating in anorexia nervosa, bulimia nervosa and binge eating disorder: Trans-diagnostic examination of the restraint model. Eat Behav. 2015;18:192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mitchell JA, Quante M, Godbole S, et al. Variation in actigraphy-estimated rest-activity patterns by demographic factors. Chronobiol Int. 2017;34:1042–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kant AK. Eating patterns of US adults: Meals, snacks, and time of eating. Physiol Behav. 2018;193:270–278. [DOI] [PubMed] [Google Scholar]
- 62. Uzhova I, Woolhead C, Timon CM, et al. : Generic meal patterns identified by latent class analysis: Insights from NANS (National Adult Nutrition Survey). Nutrients. 2018;10(310):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.