Abstract
Objective
To determine the clinical manifestations, risk factors, and maternal and perinatal outcomes in pregnant and recently pregnant women with suspected or confirmed coronavirus disease 2019 (covid-19).
Design
Living systematic review and meta-analysis.
Data sources
Medline, Embase, Cochrane database, WHO COVID-19 database, China National Knowledge Infrastructure (CNKI), and Wanfang databases from 1 December 2019 to 27 April 2021, along with preprint servers, social media, and reference lists.
Study selection
Cohort studies reporting the rates, clinical manifestations (symptoms, laboratory and radiological findings), risk factors, and maternal and perinatal outcomes in pregnant and recently pregnant women with suspected or confirmed covid-19.
Data extraction
At least two researchers independently extracted the data and assessed study quality. Random effects meta-analysis was performed, with estimates pooled as odds ratios or risk difference and proportions with 95% confidence intervals. All analyses are updated regularly.
Results
435 studies were included. Overall, 9% (95% confidence interval 7% to 10%; 149 studies, 926 232 women) of pregnant and recently pregnant women attending or admitted to hospital for any reason were diagnosed as having suspected or confirmed covid-19. The most common clinical manifestations of covid-19 in pregnancy were fever and cough (both 36%). Compared with non-pregnant women of reproductive age, pregnant and recently pregnant women with covid-19 were less likely to report symptoms of fever, dyspnoea, cough, and myalgia. The odds of admission to an intensive care unit (odds ratio 2.61, 95% confidence interval 1.84 to 3.71; I2=85.6%), and invasive ventilation (2.41, 2.13 to 2.71; I2=0%) were higher in pregnant and recently pregnant than non-pregnant women of reproductive age. Overall, 970 pregnant women (0.2%, 123 studies, 179 981 women) with confirmed covid-19 died from any cause. In pregnant women with covid-19, non-white ethnicity, increased maternal age, high body mass index, any pre-existing maternal comorbidity including chronic hypertension and diabetes, and pregnancy specific complications such as gestational diabetes and pre-eclampsia, were associated with serious complications (severe covid-19, admission to an intensive care unit, invasive ventilation, and maternal death). Compared to pregnant women without covid-19, those with the disease had increased odds of maternal death (odds ratio 6.09, 95% confidence interval 1.82 to 20.38; I2=76.6%), of admission to the intensive care unit (5.41, 3.59 to 8.14; I2=57.0%), caesarean section (1.17, 1.01 to 1.36; I2=80.3%), and of preterm birth (1.57, 1.36 to 1.81; I2=49.3%). The odds of stillbirth (1.81, 1.38 to 2.37, I2=0%), and admission to the neonatal intensive care unit (2.18, 1.46 to 3.26, I2=85.4%) were higher in babies born to women with covid-19 versus those without covid-19.
Conclusion
Pregnant and recently pregnant women with covid-19 attending or admitted to the hospitals for any reason are less likely to manifest symptoms such as fever, cough, dyspnoea, and myalgia, but are more likely to be admitted to the intensive care unit or needing invasive ventilation than non-pregnant women of reproductive age. Pre-existing comorbidities, non-white ethnicity, chronic hypertension, pre-existing diabetes, high maternal age, and high body mass index are risk factors for severe covid-19 outcomes in pregnancy. Pregnant women with covid-19 versus without covid-19 are more likely to deliver preterm and have an increased risk of maternal death and of being admitted to the intensive care unit. Their babies are more likely to be admitted to the neonatal intensive care unit.
Systematic review registration
PROSPERO CRD42020178076.
Readers’ note
This article is a living systematic review that will be updated to reflect emerging evidence. Updates may occur for up to two years from the date of original publication. This version is update 2 of the original article published on 1 September 2020 (BMJ 2020;370:m3320), and previous updates can be found as data supplements (https://www.bmj.com/content/370/bmj.m3320/related#datasupp). When citing this paper please consider adding the update number and date of access for clarity.
Introduction
Since the first report (December 2019) of the novel coronavirus disease 2019 (covid-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the number of confirmed cases and associated mortality and morbidity have increased rapidly.1 2 Pregnant women are considered a high risk group because of concerns about the effect of covid-19 on them during and after pregnancy, and on their neonates.3 Quantification of the rates of covid-19, its risk factors, clinical manifestations, and outcomes is key to planning clinical maternal care and management in an evolving pandemic scenario.4
Publications on covid-19 in pregnancy have risen steeply through individual case reports, case series, observational studies, and systematic reviews. Since the publication of our original living systematic review on covid-19 in pregnancy,5 over 150 reviews have been published in this area,6 7 8 9 10 11 with many more registered in PROSPERO.9 12 Early reviews mostly included case reports and case series that were often inappropriately meta-analysed providing biased estimates.13 Subsequent reviews differed little from each other, often including similar primary studies, many with duplicate data. These reviews became quickly outdated as new evidence emerged. Moreover, the sampling frames in primary studies have varied, ranging from universal SARS-CoV-2 testing for all pregnant women admitted to hospital14 15 to symptom based testing.16 17 Testing strategies have also differed within and between countries, with diagnosis in many early studies based on epidemiological risk assessment and clinical features without confirmed SARS-CoV-2 infection, which need to be considered in the analysis.18 Limitations in the external and internal validity of studies make it challenging for guideline developers and policy makers to make evidence based recommendations for the management of pregnant and recently pregnant women with covid-19.
We started this living systematic review in April 2020 to determine the clinical manifestations of covid-19 in pregnant and recently pregnant women, identify the risk factors for complications, and quantify maternal and perinatal outcomes. The systematic review is being updated on a regular basis.
Methods
Our systematic review is based on a prospectively registered protocol (PROSPERO CRD42020178076; registered 22 April 2020)19 to evaluate a series of research questions on covid-19 during and after pregnancy. We report our findings on the rates, clinical manifestations, risk factors, and maternal and perinatal outcomes in women with covid-19 in line with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) recommendations (see appendix 1). As more relevant data become available, we shall answer other research questions in our published protocol.20 Each cycle of our living systematic review involves weekly search updates (rounds), with analysis performed every 4-6 months for reporting through a dedicated website, with early analysis if new definitive evidence emerges. We are regularly reviewing the planned frequency of updates.
Literature search
Our weekly search update included a systematic search of major databases: Medline, Embase, Cochrane database, WHO (World Health Organization) COVID-19 database, China National Knowledge Infrastructure (CNKI), and Wanfang databases for relevant studies on covid-19 in pregnant and recently pregnant women.5 We also coordinated our search efforts with the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), WHO Library, and Cochrane Gynaecology and Fertility group. Additional searches were conducted of preprint servers, blogs, websites that serve as repositories for covid-19 studies such as the LOVE platform (Living Overview of the Evidence),21 social media, guidelines, and reference lists of included studies. For this second update of the review, we included studies from searches up to 27 April 2021. We contacted established groups that were coordinating or conducting surveillance and studies in pregnant women with covid-19, such as the WHO Maternal, Newborn, Child and Adolescent health (MNCAH) covid-19 research network, the International Network of Obstetric Survey Systems (INOSS), the United States Centers for Disease Control and Prevention (CDC), and the European Centre for Disease Prevention and Control for information on published and upcoming data. No language restrictions were applied. Appendix 2 provides details of the search strategies and databases searched.
Study selection
Two reviewers independently selected studies using a two stage process: they first screened the titles and abstracts of studies and then assessed the full text of the selected studies in detail for eligibility. A total of 28 reviewers contributed to study selection. Disagreements were resolved through discussion with a third reviewer (ST or JA). We excluded studies if the duplicated data for all outcomes of interest were published elsewhere, as reported by the study authors, or when the characteristics of the women or neonates matched the setting, characteristics, and duration of another study from the same geographical location. If there was an overlap of data or suspicion of duplicates of participants in studies, we included studies based on their study design (prioritising comparative cohorts), and sample size (larger study prioritised).
We defined women as having confirmed covid-19 if they had laboratory confirmation of SARS-CoV-2 infection irrespective of clinical signs and symptoms.22 Women with a diagnosis based only on clinical or radiological findings were defined as having suspected covid-19. The recently pregnant group comprised women in the postpartum and post-abortion period. We included studies that compared covid-19 rates, clinical manifestations (symptoms, laboratory and radiological results), risk factors, and associated mortality and morbidity between pregnant and recently pregnant and non-pregnant women of reproductive age, and those that compared maternal and perinatal outcomes in pregnant women with and without covid-19. In studies comparing maternal and perinatal outcomes of pregnant women with covid-19 to those without, we classified the comparative controls as being historical if the cohort of pregnant women without covid-19 gave birth before December 2019. Studies on non-comparative cohorts with a minimum of 10 participants were included if they reported on the rates and clinical manifestations of covid-19 and relevant outcomes in pregnant and recently pregnant women. We defined cohort studies as those that sampled participants on the basis of exposure, followed-up participants over time, and ascertained the outcomes.23 The PROSPERO protocol provides a full list of the risk factors, clinical features, and outcomes evaluated.19
The sampling frames for detecting covid-19 included universal screening and testing, when all women were assessed for covid-19 using reverse transcriptase polymerase chain reaction (RT-PCR) for SARS-CoV-2, chest computed tomography or antigen-detection lateral flow or rapid diagnostic tests (Ag-RDTs); risk based testing on the basis of epidemiological history and clinical manifestations by National Health Commission of China (NHCC) guidelines18; and symptom based testing when testing was only performed on women with symptoms and those with a history of contact with affected individuals. We defined the population as being selected when only specific groups of women were included, such as those undergoing caesarean section or in the third trimester. We categorised studies as a high risk group if only women with any pre-existing medical or obstetric risk factors were included, low risk if women did not have any risk factors, and any risk if all women were included.
Study quality assessment and data extraction
The quality of the comparative cohort studies was assessed for selection, comparability, and outcome ascertainment bias using the Newcastle Ottawa scale.24 Studies achieving four stars for selection, two for comparability, and three for ascertainment of the outcome were considered to have a low risk of bias. Studies achieving two or three stars for selection, one for comparability, and two for outcome ascertainment were considered to have a medium risk of bias, and any study achieving one star for selection or outcome ascertainment, or zero for any of the three domains, was regarded as having a high risk of bias. We assessed the quality of studies reporting on the prevalence of clinical manifestations or outcomes for internal and external validity using an existing tool.25 The following were considered as low risk of bias for external validity: representative of national population for relevant variables (population), representative of target population (sampling frame), random selection (selection bias), and more than 75% response rate in individuals with and without the outcome (non-response bias).25 Two independent reviewers extracted data using a pre-piloted form.
Statistical analysis
We pooled the comparative dichotomous data using random effects meta-analysis and summarised the findings as odds ratios and risk differences with 95% confidence intervals. To combine comparative continuous data with dichotomous data, we transformed standardised mean differences to logarithm odds ratios, assuming a normal underlying distribution.26 We pooled the dichotomous non-comparative data for rates of clinical manifestations and maternal and perinatal outcomes as proportions with 95% confidence intervals using Dersimonian and Laird random effects meta-analysis after transforming data using Freeman-Tukey double arcsin transformation. Heterogeneity was reported as I2 statistics. We undertook subgroup analysis by country status (high income v low and middle income), sampling frame (universal, risk based, and symptom based testing, including not reported), and risk status of women in the studies (high, low, any). Sensitivity analysis was performed by restricting the analysis to women with confirmed covid-19, study quality (high, low), and population (unselected, selected). All analyses were done with Stata (version 16).
Patient and public involvement
The study was supported by Katie’s Team, a dedicated patients and public involvement group in Women’s Health. The team was involved in the conduct, interpretation, and reporting of this living systematic review through participation in virtual meetings, and creation of an animation is available at https://www.youtube.com/watch?v=Pr0L97k-22s.
Results
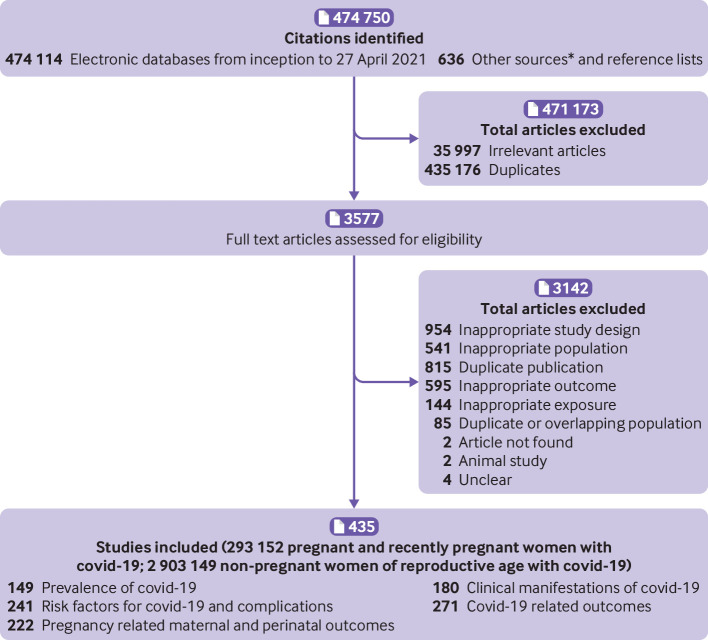
After removing duplicates from 474 750 citations, 39 574 unique citations were identified and 435 cohort studies (240 comparative, 195 non-comparative) were included in this second update of the systematic review (fig 1). All included studies were conducted before the emergence of SARS-CoV-2 variants of concern.
Fig 1.
Study selection process. *Twitter, national reports, blog by J Thornton, ObG Project, COVID-19 and Pregnancy Cases, https://ripe-tomato.org/2020/05/15/covid-19-in-pregnancy-101-onwards/; EPPI-Centre, COVID-19: a living systematic map of evidence, http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx; Norwegian Institute of Public Health, NIPH systematic and living map on COVID-19 evidence, www.nornesk.no/forskningskart/NIPH_mainMap.html; Johns Hopkins University Center for Humanitarian Health; COVID-19, Maternal and Child Health, Nutrition, http://hopkinshumanitarianhealth.org/empower/advocacy/covid-19/covid-19-children-and-nutrition/; ResearchGate, COVID-19 research community, www.researchgate.net/community/COVID-19; and Living Overview of the Evidence, Coronavirus disease (COVID-19), https://app.iloveevidence.com/loves/5e6fdb9669c00e4ac072701d?population=5d062d5fc80dd41e58ba8459
Characteristics of included studies
Of the 435 studies, the majority were from the United States (141, 32.4%), followed by China (8.5%, 37 studies), Spain (6.9%, 30 studies), Italy (6.2%, 27 studies), Turkey (5.1%, 22 studies), India (5.1%, 22 studies), Iran (3.4%, 15 studies) and the United Kingdom (3.0%, 13 studies). Seven were multinational studies. Most studies tested respiratory samples using RT-PCR (98%, 425/435); 15 studies tested for SARS-CoV-2 antibodies to confirm the presence of SARS-CoV-2; 56 studies additionally diagnosed covid-19 based only on clinical suspicion. Twenty seven studies (3 064 097 women) compared pregnant populations with non-pregnant populations,27 28 29 30 31 32 33 34 35 36 37 38 39 40 and 83 studies (653 470 women) compared pregnant women with covid-19 versus pregnant women without covid-19.41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 180 studies reported on clinical manifestations (173 964 pregnant women, 2 006 244 non-pregnant women), 271 studies reported on covid-19 related outcomes (237 288 pregnant, 2 706 610 non-pregnant women), and 222 studies reported on pregnancy related maternal (78 236 women) and perinatal outcomes (30 385 neonates; see appendix 3). The sampling frames included universal testing (207 studies), risk based NHCC guidelines (29 studies), and symptom based (53 studies) strategies. A total of 146 studies did not report the sampling strategy.
Quality of included studies
Overall, 71% (170/240) of the comparative cohort studies evaluated using the Newcastle Ottawa scale had an overall low risk of bias (see appendix 4a). Most (95%, 228/240) had a low risk of bias for study selection and 12 (5%) had a medium risk. The risk of bias for comparability of cohorts was low in 146 studies (61%), medium in 91 (38%), and high in three (1%). For outcome assessment of the cohorts, 100 (42%) studies had a low risk of bias, 138 (58%) a medium risk, and two (1%) a high risk. Quality assessment of the prevalence studies for external validity showed a low risk of bias for representativeness in 14% (61/435) of the studies, sampling in 27% (116/435), selection in 90% (390/435), and non-response in 99% (430/435). For internal validity, there was low risk of bias for data collection in 97% (424/435) of the studies, case definition in 61% (265/435), measurement in 99% (430/435), differential verification in 98% (427/435), adequate follow-up in 39% (170/435), and appropriate numerator and denominator in 89% (389/435;(see appendix 4b).
Rates of covid-19 in pregnant and recently pregnant women
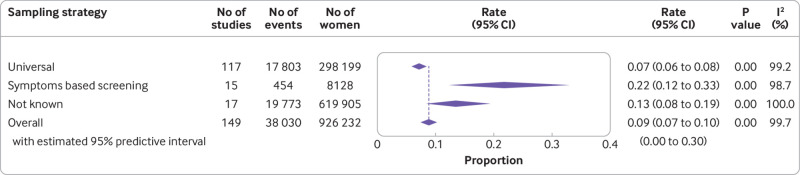
The overall rate of covid-19 diagnosis in pregnant and recently pregnant women attending or admitted to hospital for any reason was 9% (95% confidence interval 7% to 10%; 149 studies, 926 232 women) (fig 2). Rates varied by sampling strategy. Of the women tested as part of universal screening strategy, 7% (6% to 8%; 117 studies, 298 199 women) were diagnosed as having covid-19 compared with 22% (12% to 33%; 15 studies, 8128 women) in women tested on the basis of symptoms (see appendix 5a). About half of all studies with a prevalence rate for covid-19 greater than 15% were from the US (18/35),88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 except for two studies each from the UK, Chile, and India; and one each from Mexico, Turkey, France, Iran, Cameroon, Egypt, French Guiana, Kenya, Norway, Peru, and Spain.106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 About one in 30 asymptomatic women (3%, 2% to 5%; 39 studies) attending or admitted to hospital had SARS-CoV-2 infection (see appendix 5b). Two thirds (66%, 54% to 77%; 68 studies) of the 11 945 pregnant women with SARS-CoV-2 infection in the universal screening population were asymptomatic (see appendix 5c). Non-white ethnicity (odds ratio 2.41, 95% confidence interval 1.90 to 3.06; 35 studies; 616 668 women) and high body mass index (1.24, 1.13 to 1.37; 47 studies, 441 583 women), were associated with SARS-CoV-2 infection in pregnancy; none of the other maternal factors assessed was associated with SARS-CoV-2 infection in pregnant women (see appendix 6a).
Fig 2.
Prevalence of severe acute respiratory syndrome coronavirus 2 in pregnant and recently pregnant women identified by various sampling strategies. Meta-analysis includes one study (Liao 2020)44 screened using National Health Commission China criteria with no events
Clinical manifestations of covid-19 during pregnancy and after delivery
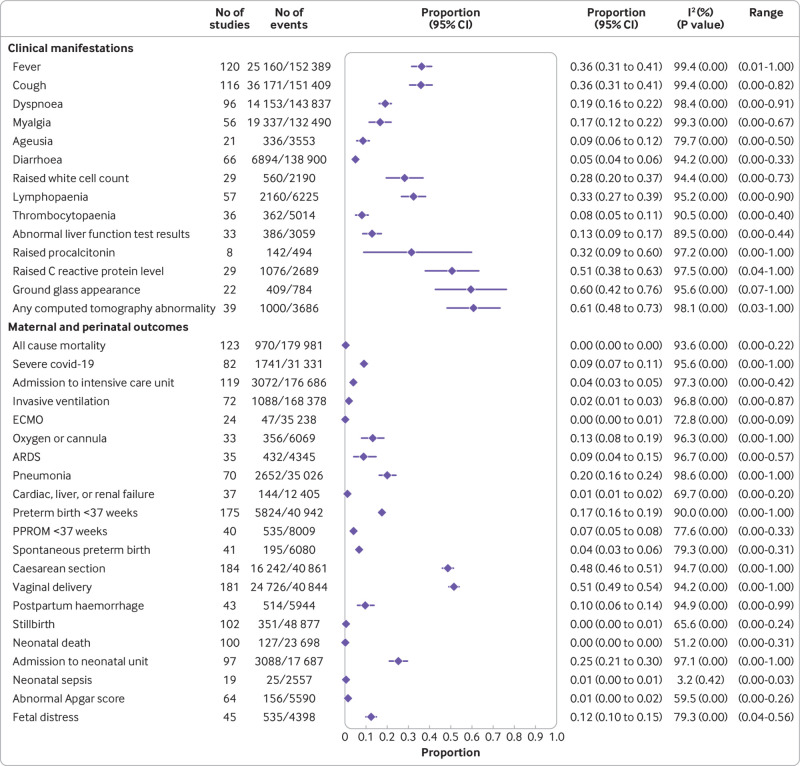
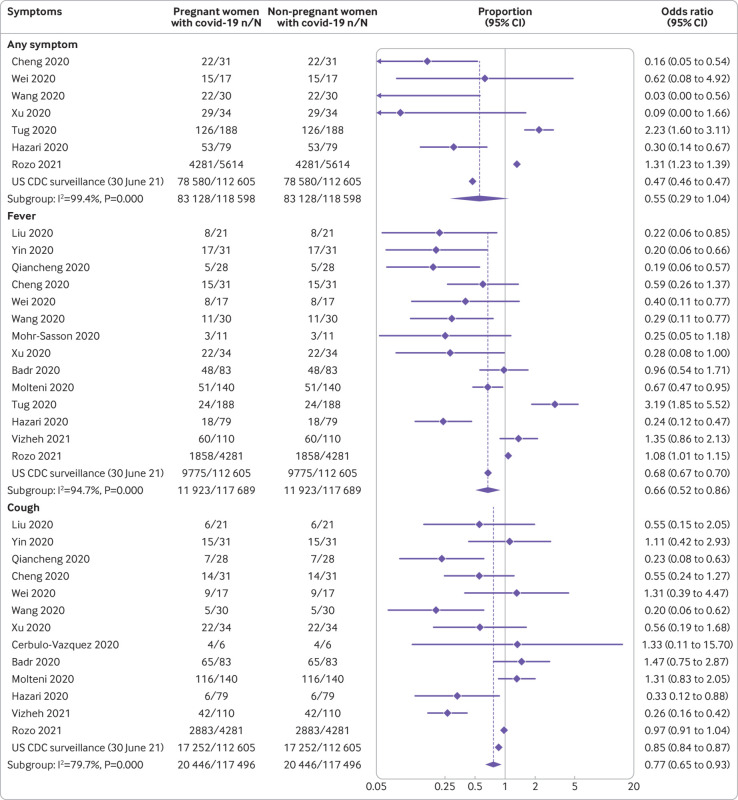
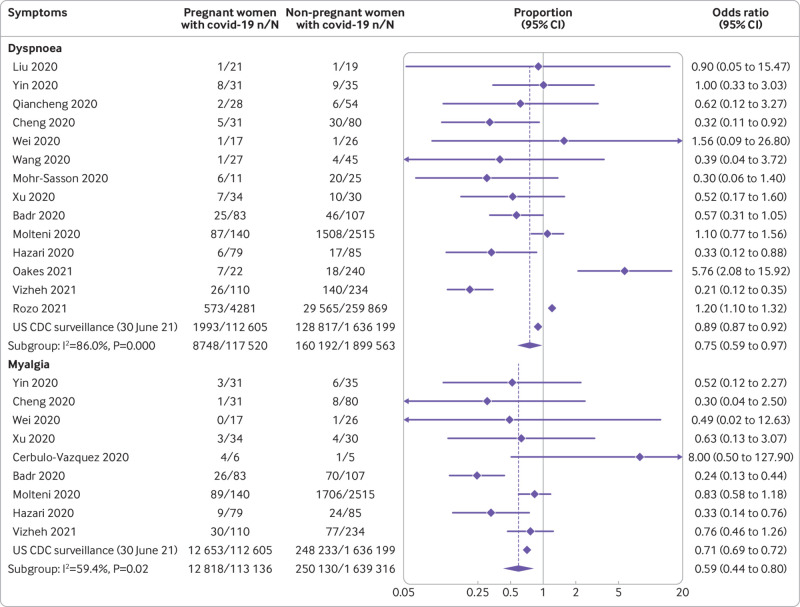
The most common symptoms reported by pregnant and recently pregnant women with suspected or confirmed covid-19 were fever (36%) and cough (36%); elevated C reactive protein levels (51%), elevated procalcitonin levels (32%), lymphopaenia (33%), and elevated white cell count (28%), were the most common laboratory findings (fig 3). Compared with non-pregnant women of reproductive age with covid-19, pregnant and recently pregnant women with the disease were less likely to report symptoms of fever (odds ratio 0.66, 95% confidence interval 0.52 to 0.86; 15 studies, 2 017 808 women), dyspnoea (0.75, 0.59 to 0.97; 15 studies; 2 017 083 women), cough (0.77, 0.65 to 0.91; 14 studies, 2 016 795 women) and myalgia (0.59, 0.44 to 0.80; 10 studies, 1 752 452 women) (fig 4 and fig 5). Pregnant women with covid-19 were more likely to have pre-existing comorbidities than non-pregnant women with the disease (1.98, 1.09 to 3.61; 7 studies, 2 139 363 women) and less likely to be of non-white ethnicity (0.78, 0.67 to 0.90; 3 studies, 1 398 642 women) or >35 years of age (0.41, 0.30 to 0.57; 11 studies, 2 302 581 women) (see appendix 6b). Sensitivity analysis restricted to various sampling frames showed lower estimates of reported symptoms in the universal screening population and higher estimates in the symptom based population (see appendix 7). The rates of clinical manifestations varied when the analysis was restricted to only women with RT-PCR confirmed covid-19, unselected populations, and women with any risk (see appendix 7).
Fig 3.
Rates of clinical manifestations of coronavirus disease 2019 (covid-19) in pregnant women and recently pregnant women with suspected or confirmed covid-19 and associated maternal and perinatal outcomes. ECMO=extracorporeal membrane oxygenation; ARDS=acute respiratory distress syndrome; PPROM=preterm premature rupture of membranes
Fig 4.
Clinical manifestations of coronavirus disease 2019 (covid-19) in pregnant and recently pregnant women compared with non-pregnant women of reproductive age with covid-19 (part 1)
Fig 5.
Clinical manifestations of coronavirus disease 2019 (covid-19) in pregnant and recently pregnant women compared with non-pregnant women of reproductive age with covid-19 (part 2)
Outcomes related to covid-19 in pregnant and recently pregnant women
Overall, 970 pregnant women (123 studies, 179 981 women) with confirmed covid-19 died from any cause (0.2%, 95% confidence interval 0.01% to 0.39%). Severe covid-19 infection as defined by the authors, was diagnosed in 9% (7% to 11%; 82 studies, 31 331 women) of pregnant and recently pregnant women with suspected or confirmed covid-19; 4% (3% to 5%; 119 studies, 176 686 women) of pregnant women with covid-19 were admitted to an intensive care unit, 2% (1% to 3%; 72 studies, 168 378 women) required invasive ventilation, and 0.2% (0.0% to 0.6%; 24 studies, 35 238 women) required extracorporeal membrane oxygenation (fig 3). Appendix 8 provides the rates of outcomes by sampling strategy.
Compared with non-pregnant women of reproductive age with covid-19, the odds of admission to the intensive care unit (odds ratio 2.61, 95% confidence interval 1.84 to 3.71; 10 studies, 2 027 360 women) and need for invasive ventilation (2.41, 2.13 to 2.71; 8 studies, 1 889 174 women) were higher in pregnant and recently pregnant women (table 1). For every 100 women, two additional women were admitted to the intensive care unit (risk difference 2.0%, 95% confidence interval 0.5% to 3.1%; 10 studies, 2 027 360 women) and two more needed invasive ventilation (2.0%, 0.0% to 4.0%; eight studies, 1 889 174 women) when pregnant with covid-19 versus when not pregnant and of reproductive age with covid-19 (see appendix 9).
Table 1.
Outcomes in pregnant and recently pregnant women with coronavirus disease 2019 (covid-19)
| Outcomes | No of studies | Women (No with event/No in group (%)) | Odds ratio (95% CI) | I2 (%) | |
|---|---|---|---|---|---|
| Pregnant women with covid-19 | Comparison group | ||||
| Comparison group: non-pregnant women of reproductive age with covid-19 | |||||
| All cause mortality | 11 | 242/122 222 (0.2) | 5252/2 138 726 (0.2) | 1.48 (0.62 to 3.49) | 95.9 |
| ICU admission | 10 | 912/118 403 (0.8) | 11 513/1 908 957 (0.6) | 2.61 (1.84 to 3.71) | 85.6 |
| Invasive ventilation | 8 | 310/116 458 (0.3) | 3607/1 772 716 (0.2) | 2.41 (2.13 to 2.71) | 0 |
| ECMO | 5 | 19/30 694 (0.1) | 122/432 623 (0.0) | 3.71 (0.71 to 19.41) | 64.5 |
| Oxygen through nasal cannula | 2 | 8/48 (16.7) | 49/106 (46.2) | 0.21 (0.04 to 1.09) | 63.9 |
| ARDS | 4 | 22/197 (11.2) | 45/418 (10.8) | 1.19 (0.24 to 5.95) | 75.0 |
| Major organ failure | 4 | 5/197 (2.5) | 28/418 (6.7) | 0.39 (0.15 to 1.04) | 0 |
| Comparison group: pregnant women without covid-19 | |||||
| Maternal outcomes: | |||||
| All cause mortality | 21 | 47/11 362 (0.4) | 37/411 126 (0.0) | 6.09 (1.82 to 20.38) | 76.6 |
| ICU admission | 21 | 447/12 957 (3.4) | 1962/459 359 (0.4) | 5.41 (3.59 to 8.14) | 57.0 |
| Preterm birth <37 weeks | 48 | 1306/12 076 (10.8) | 26 068/436 964 (6.0) | 1.57 (1.36 to 1.81) | 49.3 |
| Caesarean section | 53 | 4165/12 385 (33.6) | 147 645/614 402 (24.0) | 1.17 (1.01 to 1.36) | 80.3 |
| Perinatal outcomes: | |||||
| Stillbirth | 25 | 76/9338 (0.8) | 1397/414 139 (0.3) | 1.81 (1.38 to 2.37) | 0 |
| Neonatal death | 21 | 16/3153 (0.5) | 28/9 263 (0.3) | 2.35 (1.16 to 4.76) | 0 |
| Admission to neonatal unit | 29 | 687/4072 (16.9) | 6968/193 124 (3.6) | 2.18 (1.46 to 3.26) | 85.4 |
| Abnormal Apgar score at 5 minutes | 16 | 41/1607 (2.6) | 7776/190 638 (4.1) | 1.31 (0.90 to 1.93) | 0 |
| Fetal distress | 6 | 131/1073 (12.2) | 246/3933 (6.3) | 2.22 (1.45 to 3.41) | 41.8 |
ICU=intensive care unit; ECMO=extracorporeal membrane oxygenation; ARDS=acute respiratory distress syndrome. Includes historical comparative cohorts from; Vousden et al 2021122 (694 women)—all cause mortality, admission to intensive care unit, caesarean section, preterm birth, stillbirth, neonatal death, and admission to neonatal unit; Li et al 2020123 (242 women)—preterm birth, caesarean section, and fetal distress; Gulersen et al 202058 (50 women)—caesarean section; Overtoom et al 2020124 (183 413 women)—caesarean section, admission to neonatal unit, and abnormal Apgar at 5 minutes; Janevic et al 202194 (3508 women)—preterm birth; Facchetti et al 2020125 (86 women)—stillbirth and abnormal Apgar at 5 minutes.
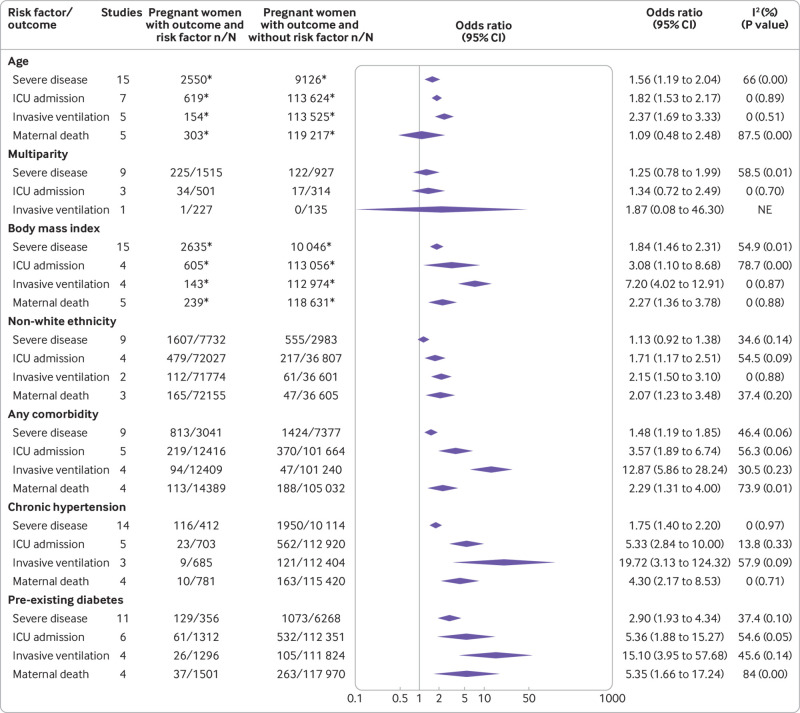
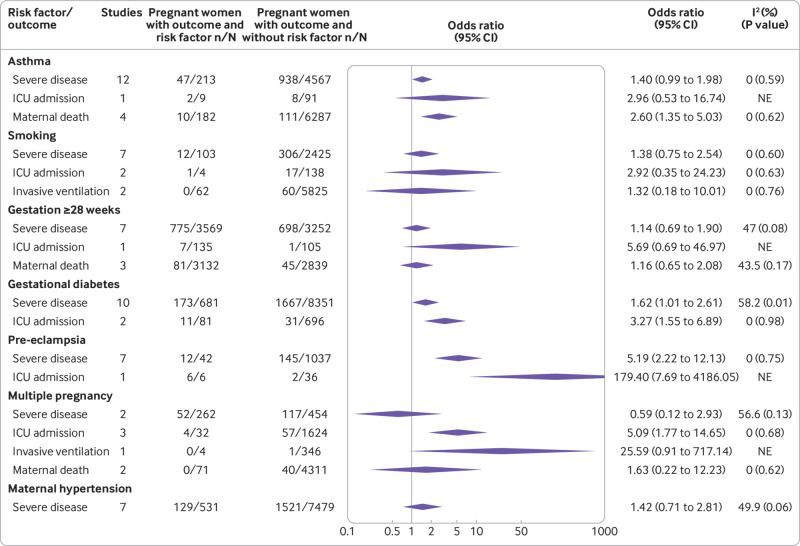
Maternal risk factors associated with severe covid-19 were increasing age (odds ratio 1.56, 95% confidence interval 1.19 to 2.04; 15 studies, 11 676 women), high body mass index (1.84, 1.46 to 2.31; 15 studies, 12 681 women), any pre-existing maternal comorbidity (1.48, 1.19 to 1.85; 9 studies; 10 418 women), chronic hypertension (1.75, 1.40 to 2.20; 14 studies, 10 526 women), pre-eclampsia (5.19, 2.22 to 12.13; 7 studies; 1079 women), gestational diabetes (1.62, 1.01 to 2.61; 10 studies; 9032 women), and pre-existing diabetes (2.90, 1.93 to 4.34; 11 studies, 6624 women) (fig 6 and fig 7). Increasing maternal age (1.82, 1.53 to 2.17; 7 studies, 114 243 women), high body mass index (3.08, 1.10 to 8.68; 4 studies, 113 661 women), non-white ethnicity (1.71, 1.17 to 2.51; 4 studies, 108 834 women), pre-existing maternal comorbidity (3.57, 1.89 to 6.74; 5 studies, 114 080 women), chronic hypertension (5.33, 2.84 to 10.0; 5 studies, 113 623 women), pre-existing diabetes (5.36, 1.88 to 15.27; 6 studies, 113 663 women), and gestational diabetes (3.27, 1.55 to 6.89; 2 studies, 777 women), were associated with admission to an intensive care unit. Risk factors associated with maternal death and the need for invasive ventilation included: non-white ethnicity (2.07, 1.23 to 3.48; 3 studies, 108 760 women; 2.15, 1.50 to 3.10; 2 studies, 108 375 women; respectively), and high body mass index (2.27, 1.36 to 3.78; 5 studies, 118 870 women; 7.2, 4.02 to 12.91; 4 studies, 113 117 women; respectively; fig 6 and fig 7).
Fig 6.
Risk factors associated with severe coronavirus disease 2019 (covid-19) and all cause maternal death in pregnant and recently pregnant women (part 1). ICU=intensive care unit; NE=not estimable. Cut-off threshold is ≥35 years for age and ≥30 for body mass index. *Includes one or more studies with continuous measurement of risk factor
Fig 7.
Risk factors associated with severe coronavirus disease 2019 (covid-19) and all cause maternal death in pregnant and recently pregnant women (part 2). ICU=intensive care unit; NE=not estimable
Maternal and perinatal outcomes in pregnant and recently pregnant women with covid-19
The odds of all cause mortality (odds ratio 6.09, 95% confidence interval 1.82 to 20.38; 21 studies, 422 488 women), admission to the intensive care unit (5.41, 95% confidence interval 3.59 to 8.14; 21 studies, 472 316 women) and caesarean section (1.17, 95% confidence interval 1.01 to 1.36; 53 studies, 626 787 women) were higher in pregnant and recently pregnant women with covid-19 than in pregnant and recently pregnant women without the disease (table 1). In pregnant and recently pregnant women with covid-19, the overall rate of preterm birth was 17% (95% confidence interval 16% to 19%; 175 studies, 40 942 women) and of spontaneous preterm birth was 7% (5% to 8%; 40 studies, 8009 women) (fig 3). Overall, 351 stillbirths (0.4%, 0.2% to 0.6%; 102 studies, 48 877 offspring) and 127 neonatal deaths (0.03%, 0.0% to 0.2%; 100 studies; 23 698 neonates) occurred among these women (fig 3). Compared with pregnant and recently pregnant women without the disease, pregnant women with covid-19 were at higher risk of any preterm birth (odds ratio 1.57, 95% confidence interval 1.36 to 1.81; 48 studies, 449 040 women), stillbirth (1.81, 1.38 to 2.37; 25 studies, 423 477 women), and neonatal death (2.35, 1.16 to 4.76; 21 studies, 12 416) although the overall number of neonatal death was small (only sixteen events in the covid-19 group) (table 1). Appendix 9 provides the absolute risk differences in maternal outcomes in pregnant and recently pregnant women with vs without covid-19.
Overall, 25% (95% confidence interval 21% to 30%; 97 studies, 17 687 women) of neonates born to women with covid-19 were admitted to the neonatal intensive care unit (NICU) (fig 3) and had a higher risk of NICU admission (odds ratio 2.18, 95% confidence interval 1.46 to 3.26; 29 studies, 197 196 neonates) than neonates born to women without covid-19 (table 1). The absolute risk differences for perinatal neonatal outcomes are provided in appendix 9, while appendix 10 provides the rates of covid-19 related and pregnancy related outcomes for the individual studies.
Discussion
Findings in this second update of our living systematic review remain consistent with our original review and our previous update for prevalence of covid-19, rates of clinical manifestations, and outcomes in pregnant and recently pregnant women, in studies published before the predominance of SARS-CoV-2 variants of concern. One in 10 pregnant or recently pregnant women who are attending or admitted to hospital for any reason were diagnosed as having suspected or confirmed covid-19, and the rates varied by sampling strategy. Pregnant and recently pregnant women were less likely to show covid-19 related symptoms of fever, dyspnoea, cough, and myalgia than non-pregnant women with covid-19. Although testing for SARS-CoV-2 in non-pregnant women is based on symptoms or contact history, testing in pregnant women is usually done when they are in hospital for reasons that might not be related to covid-19. Pregnant or recently pregnant women with covid-19 were at increased risk of requiring admission to an intensive care unit, and invasive ventilation compared to non-pregnant, reproductive aged women with covid-19. Increased maternal age, high body mass index, non-white ethnicity, pre-existing comorbidities, and pregnancy specific conditions such as pre-eclampsia and gestational diabetes are associated with severe disease. Compared to pregnant women without covid-19, pregnant women with covid-19 are at increased risk of death, admission to the intensive care unit, stillbirth, delivering preterm, and their babies being admitted to the neonatal unit. However, the overall rates of stillbirth and neonatal death remain low in women with suspected or confirmed covid-19. Absolute increases in maternal deaths, admissions to the intensive care unit, preterm births, caesarean sections, and admissions of babies to the neonatal unit were observed for pregnant women with covid-19 compared to pregnant women without the disease. Substantial heterogeneity was observed in the estimates for rates of clinical manifestations and outcomes, which varied by sampling frames, participant selection, and risk status of the participants.
This second update of the living systematic review includes more than double the number of studies included in the first update, and four times more pregnant women with covid-19. There continues to be increased precision in estimates for previously identified risk factors for severe disease in pregnant and recently pregnant women with covid-19, including stronger association between risk factors such as non-white ethnicity, and pregnancy specific conditions such as gestational diabetes and pre-eclampsia, and increased risk of adverse outcomes in pregnant women with covid-19 than without the disease.
Strengths and limitations of this review
In this unprecedented pandemic situation, where evidence is rapidly produced and published in various formats, our living systematic review underpinned by robust methods and continually updated at regular intervals is important for several reasons. Firstly, it addresses important research questions relevant to clinical decision making and policies. Secondly, uncertainties remain for key outcomes that require further evidence. Thirdly, the rapid turnover of evidence in various formats requires assessments of study quality and regular updating of the findings. Finally, our living systematic review is producing strong evidence base for living guidelines on covid-19 and pregnancy.
We undertook a comprehensive search and coordinated our efforts with key organisations and research groups, such as WHO, US CDC, Cochrane Centre, and EPPI-Centre. To minimise risk of bias we restricted our meta-analysis to cohort studies, and we reported the quality of the included studies. By contacting the authors and obtaining reports not published in PubMed, we minimised the risk of missing relevant studies. Our systematic review has a large sample size, and it is continuously increasing. Our living review framework will enable us to rapidly update the findings as new data emerge. We undertook extensive work to ensure that duplicate data are not included. Our various comparative analyses allowed us to comprehensively assess the association between pregnancy and covid-19 related outcomes, covid-19 and pregnancy outcomes, risk factors for SARS-CoV-2 infection, and complications. Our review helps to understand the variations in estimates through sensitivity analyses by sampling strategies, population characteristics, and risk factors, and it provides confidence in the rates of reported outcomes. The second update has allowed us to incorporate new evidence from 244 studies and more than two million women, published since our first update in February 2021.
Our systematic review also has limitations. Although our search spans the entire year 2020, none of the studies included was conducted by the time of emergence of any SARS-CoV-2 variant of concern. Despite every effort to quickly update the evidence, the number of new studies identified and the rigor required to remove duplicates meant that the review did not consider current changes in the pandemic such as variants of concern and vaccination, which could affect reported prevalence rates and presentation. The primary studies used varied sampling frames to identify women with covid-19, consisted of women with suspected and confirmed covid-19, and primarily reported on pregnant women who required visits to hospital, including for childbirth, thereby affecting the generalisability of the estimates. Although our sensitivity analyses aimed to tackle some of these problems, the numbers and sample sizes of the individual studies were too small to identify differences between the subgroups. The timing of assessment of the clinical manifestations of disease was generally not available. The definitions of symptoms, tests, and outcomes were heterogeneous. Furthermore, poor reporting of the criteria for caesarean section, admissions to the neonatal unit, and the causes of preterm birth, made it difficult to disentangle iatrogenic effects from direct pathophysiological effects of the disease. Studies comparing maternal and perinatal outcomes in pregnant women with covid-19 against historical cohorts of pregnant women, could be biased owing to differences in the environment in which births occur. During the pandemic, healthcare systems have faced increased pressure and strain on services, with resulting effects on service delivery and quality of care.126 127 Lockdown measures, physical distancing, and changes to livelihood have led to increased depression and anxiety, and reduction in physical activity and access or attendance to healthcare facilities, and could have also contributed to maternal and perinatal complications.128
Not many studies reported outcomes by trimester for symptom onset, making it difficult to assess the rates of miscarriage and postpartum complications. For some outcomes, the findings were influenced by a few large studies.
Comparison with existing evidence
Between the publication of the first update of the living systematic review and this second update, estimates for the prevalence of covid-19, and rates of clinical manifestations and outcomes of pregnant and recently pregnant women with covid-19 have remained similar with a slight reduction in rates, and improved precision in findings. We found a reduction in caesarean section rates and admission to the neonatal unit compared with the first update, which could be attributed to increasing knowledge of the disease and better healthcare practices. The rate of maternal pneumonia appears to also be lower. High heterogeneity remains in the estimates for rates of clinical manifestations and outcomes.
We found that the same risk factors for severe covid-19 identified in the original version and first update of the living systematic review remained associated with severe covid-19 in this second update, with increased precision. Our findings on the association of severe covid-19 with ethnic minority status in pregnant women are consistent with similar reports of disproportionately high rates of severe covid-19 in non-pregnant ethnic minority populations,129 and disparities in other areas of maternity care by ethnic minority status.130 131 The observed disparity could be attributed to associated comorbidities, socioeconomic characteristics, and factors related to access to and quality of care in the preconception, pregnancy, and postpartum periods.132 The multifaceted contributors to ethnic disparities need to be investigated to reduce mortality and morbidity related to both covid-19 and pregnancy. This latest version of our review update also identified an increased risk of caesarean section, neonatal death, and stillbirth in pregnant women with covid-19 compared to pregnant women without the disease.
Alongside the spread of the pandemic, a shift has occurred in the types of studies published, with initial studies involving pregnant women from epidemic regions in China, followed by reports of large regional and national datasets from the US, UK, Netherlands, Spain, and Latin American countries. However, few reports have come from African countries. The study design has also changed from initial small case series and case reports to large observational data, with recent studies also providing comparative data.
The prevalence of covid-19 varied widely between studies, particularly when sampling was done based on symptoms or history of contact, highlighting the variations in criteria for testing. The current update of our living systematic review includes twice as many studies as our first update on the overall prevalence of covid-19 in pregnancy. Despite the addition of more studies from diverse populations globally, the prevalence of covid-19 in pregnant and recently pregnant women remains unchanged. Unlike the general population who are mostly tested for SARS-CoV-2 based on symptoms or contact history, universal screening of all pregnant women attending the hospital for any reason could contribute to the consistency in the findings. However, the true prevalence of covid-19 in pregnancy is likely to be lower than the current estimate if all pregnant women, including those not attending the hospital are included.
Despite the potential higher possibility of universal screening to detect pregnant women with mild disease, we observed an increase in admissions to the intensive care unit and need for invasive ventilation compared with non-pregnant women of reproductive age with covid-19. These results were mainly influenced by the large US CDC report,40 the Canadian Surveillance of covid-19 in pregnancy report,133 and a report from the Mexican General Directorate of Epidemiology registry.39
By accessing the unpublished data from our collaborators, we were able to include women both with and without symptoms from the US CDC surveillance data, in addition to the women with symptoms only who were included in the published report.40 Pregnancy status was not ascertained in a large proportion of women of reproductive age in the CDC report, which could affect the estimates. Furthermore, the outcomes for which the data were missing from the report were considered to be absent in women, potentially leading to bias. The report from the Mexican General Directorate of Epidemiology registry, remains available only as a preprint, and included only women with symptoms who might be at higher risk of complications. Non-pregnant women in the Canadian Surveillance report included women ≤55 years of age compared to ≤45 years in the pregnant women group. We recommend that studies comparing covid-19 related outcomes in pregnant versus non-pregnant women report the relevant estimates for both women with and without symptoms to avoid overestimation of the risk of complications due to selective reporting. The pooled estimates for severe covid-19 and admission to an intensive care unit were, however, still relatively high in the non-comparative data, indicative of a potential increased risk in pregnancy. This is supported by an analysis in a Swedish study suggesting a high risk of admission to an intensive care unit and invasive ventilation in pregnant women compared to non-pregnant women,134 and similar results reported in a recent French national cohort study suggesting higher risk of mortality, admission to intensive care unit, and pregnancy related complications in pregnant women with covid-19 compared to pregnant women without the disease.135
Similar to the general population, high body mass index and pre-existing comorbidity seemed to be risk factors for severity of covid-19 in pregnancy, including admission to an intensive care unit and invasive ventilation.136 Complications related to covid-19 did not seem to be increased in women presenting in the third trimester versus earlier in pregnancy or in multiparous versus primiparous women—but existing sample sizes are not large. Both chronic hypertension and pre-existing diabetes were associated with maternal death in pregnant women with covid-19, which are known risk factors for death in the general population. But it is not known if covid-19 was the direct cause of death for these women, and the numbers of studies are small.
We observed an increase in rates of preterm birth in pregnant women with covid-19 compared with pregnant women without the disease. These preterm births could have been medically indicated owing to maternal disease and resulting fetal distress, as the overall rates of spontaneous preterm births in pregnant women with covid-19 was broadly similar to those observed in the pre-pandemic period. The overall rates of stillbirths and neonatal deaths, were still relatively low, with the absolute differences for stillbirth not significant between the groups. A recent report from the US CDC found that relative risk of stillbirth with covid-19 in pregnant women increased significantly after circulation of the delta variant in the US, from an adjusted relative risk of 1.47 before delta dominance to 4.04 during delta dominance.137 Rates of adverse outcomes should continue to be monitored as new SARS-CoV-2 variants continue to emerge. Local policies on observation and quarantine of infants with exposure to SARS-CoV-2 might have influenced admission rates to the neonatal unit, which was lower in this second update than in our first update.
Relevance for clinical practice and research
Based on existing data, healthcare professionals should be aware that pregnant and recently pregnant women with covid-19 might manifest fewer symptoms than the general population, with the overall pattern similar to that of the general population. Pregnant women should be informed of the increase in severity of covid-19 including admission to intensive care units, and invasive ventilation compared with non-pregnant women; and should be encouraged to undertake safety measures to reduce the risk of infection, including receiving the covid-19 vaccine to reduce risk of severe disease. Pregnant women with pre-existing comorbidities will need to be considered as a high risk group for covid-19, along with those who are obese and of older maternal age. Healthcare professionals need to be aware of the increased risk of severe disease in pregnant and recently pregnant women of non-white ethnic origin, to plan close monitoring and have a low threshold for escalation of care. Clinicians will need to balance the need for regular multidisciplinary antenatal care to manage women with pre-existing comorbidities against unnecessary exposure to the virus, through virtual clinic appointments when possible. Pregnant women with covid-19 before term gestation might need to be managed in a unit with facilities to care for preterm neonates.
Further data are still needed to robustly assess the association between pregnancy specific risk factors (such as pre-eclampsia and gestational diabetes) and covid-19 related outcomes.138 Robust collection of maternal data by trimester of exposure, including the periconception period, is required to determine the effects of covid-19 on early pregnancy outcomes, fetal growth, and risk of miscarriage or stillbirth. We need detailed reporting of outcomes by ethnicity to quantify the risk of severe covid-19 in women from different ethnicities. Qualitative studies on behaviour and attitude to the pandemic can help to disentangle the relative importance of factors behind the ethnic disparities observed in the severity of covid-19. Understanding and dealing with inequities in social determinants of health that put ethnic minority groups at higher risk of severe disease can help improve outcomes in this group.
Evidence syntheses on covid-19 faces unprecedented unique challenges, even for agile designs such as living systematic reviews. Firstly, there is rapid change in the nature of the virus with emerging variants of concern, with varied infectivity, presentation, and outcomes. Primary studies in non-pregnant and pregnant populations suggest increased risk of hospital admission and death with the alpha and delta variants of concern compared with the wildtype variant.139 140 141 142 However, the timeline of most of the published literature lags with regards to the emerging variants of concern. As we update our search beyond the pre-variant period, we are likely to encounter new studies reporting on pregnancies before and after the emergence of variants. In such instances, it will be inappropriate to update our meta-analysis by combining all studies.
Secondly, countries are rapidly rolling out covid-19 immunisation programmes, which affects the prevalence, presentation, and outcomes in general and pregnant populations. Similar to the methodological challenges with the variants, it is also inappropriate to update our meta-analysis without considering vaccination status.
Thirdly, our living systematic review aims to synthesise evidence rapidly as they emerge. However, many primary studies publish partially duplicated data published elsewhere or at different time points or report different categories of outcomes for the same participants in multiple journals. Furthermore, large numbers of non-peer reviewed scientific papers and reports are available in the public domain in multiple versions. Because primary studies do not often explicitly state if duplicate data have been included, we had to undertake resource intensive efforts to check each included study against all studies published since the beginning of the pandemic to avoid double counting of participants. This approach led to delays between completion of the search and finalisation of data for analysis.
With the establishment of several national and global prospective cohorts, we expect the sample size of our meta-analysis to increase further in the coming months. Future reviews will need to consider the effects of variants of concerns on the rates of coivd-19, manifestation of symptoms, and the risk of covid-19 and pregnancy related complications in pregnant and recently pregnant women. In doing so, we propose a shift in how the findings are reported. In order to capture the trends in prevalence, presentation, and outcomes of SARS-CoV-2 in pregnancy, in line with the changing environment in terms of variants and vaccination status, we plan to synthesise and present the data according to the time of recruitment of participants rather than the traditional update by timing of publication. Providing such data in half-yearly intervals alongside information on the trends in variants and vaccine status would provide accurate information reflecting the underlying conditions.
Including vaccination information within global systematic reviews of covid-19 and pregnancy will also allow collection of data on the range of covid-19 vaccines that are in use in different settings, which can inform international guidelines on vaccination in pregnancy. This information could lead to stronger recommendations on covid-19 vaccination for all pregnant women and help combat misinformation on vaccination in pregnancy.
What is already known on this topic
Pregnant women are considered to be a high risk group for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and the potential adverse effects of the virus on maternal and perinatal outcomes are of concern
In non-pregnant populations admitted to hospital with coronavirus disease 2019 (covid-19) the most common symptoms are fever, cough, and dyspnoea, reported in more than two thirds of individuals
Advancing age, high body mass index, non-white ethnicity, and pre-existing comorbidities are risk factors for severe covid-19 in the general population
What this study adds
Pregnant and recently pregnant women with covid-19 diagnosed in hospital are less likely to manifest symptoms of fever, cough, dyspnoea, and myalgia than non-pregnant women of reproductive age
Pregnant and recently pregnant women with covid-19 are at increased risk of admission to an intensive care unit, receiving invasive ventilation, or death compared with non-pregnant women of reproductive age with covid-19
Risk factors for severe disease in pregnancy include increasing maternal age, high body mass index, non-white ethnicity, pre-existing comorbidities, and pregnancy specific disorders such as gestational diabetes and pre-eclampsia
Pregnant women with covid-19 are more likely to experience preterm birth and their neonates are more likely to be stillborn or admitted to a neonatal unit
Pregnant women with covid-19 had an absolute risk increase for admission to intensive care unit, preterm birth, caesarean section, and admission of their neonates to neonatal unit compared with pregnant women without covid-19
Acknowledgments
Other members of the PregCOV-19 Living Systematic Review Consortium are Gianfranco Spiteri, Julien Beaute, Uma Ram, Ajith S Nair, Pura Rayco-Solon, Hector Pardo-Hernandez, Shaunak Chatterjee, Luke Debenham, Anna Clavé Llavall, Anushka Dixit, Rishab Balaji, Gurimaan Sandhu, Siang Ing Lee, Xiu Qiu, and Mingyang Yuan.
The Cochrane Gynaecology and Fertility Group thank Maxime Verschuuren, Marijke Strikwerda, and Bethany Clark for help with searches and data extraction. The PregCOV-19 Living Systematic Review Group would also like to thank Katie’s Team for its contribution towards the development and reporting of this work, James Thomas from the EPPI-Centre for helping with search updates, and the PregCOV-19 Living Systematic Review steering committee members, Pisake Lumbiganon, Carolina Carvalho Ribeiro do Valle, Samantha Lissauer, Clare Whitehead, David Lissauer, Joao Paulo Souza, and Marian Knight, who provided guidance throughout.
Web extra.
Extra material supplied by authors
Supplementary information: Appendices 1-10
Contributors: ST, MB, and JA conceptualised the study. MY, SC, LD, TK, ACL, AD, DZ, RB, SL, XQ, MYuan, JS, HL, and KA selected the studies. JA, ES, MY, LD, DZ, XQ, and MYuan extracted the data. JZ conducted the analyses. All coauthors contributed to the writing of the manuscript and approved the final version. ST, JA, ES, and JZ are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The project was partially supported by the German Federal Ministry of Health (BMG) COVID-19 Research and development support to the World Health Organization (WHO) and UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), a co-sponsored programme executed by WHO. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: partial funding by WHO and HRP for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The corresponding author (ST) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been disclosed.
Dissemination to participants and related patient and public communities: The PregCov-19 LSR Group will disseminate the findings through a dedicated website (www.birmingham.ac.uk/research/who-collaborating-centre/pregcov/index.aspx) and social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
No additional data available.
References
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Coronavirus disease. (COVID-19) Pandemic, https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed 7 May 2020)
- 3.Cabinet Office. Guidance. Staying alert and safe (social distancing). Coronavirus (COVID-19) Guidance and support. Updated 2022. http://www.gov.uk/government/publications/staying-alert-and-safe-social-distancing.
- 4.RCOG. (COVID-19) Infection in Pregnancy, https://www.rcog.org.uk/guidance/coronavirus-covid-19-pregnancy-and-women-s-health/
- 5. Allotey J, Stallings E, Bonet M, et al. for PregCOV-19 Living Systematic Review Consortium . Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID-19: A systematic review of 108 pregnancies. Acta Obstet Gynecol Scand 2020;99:823-9. 10.1111/aogs.13867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parazzini F, Bortolus R, Mauri PA, Favilli A, Gerli S, Ferrazzi E. Delivery in pregnant women infected with SARS-CoV-2: A fast review. Int J Gynaecol Obstet 2020;150:41-6. 10.1002/ijgo.13166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elshafeey F, Magdi R, Hindi N, et al. A systematic scoping review of COVID-19 during pregnancy and childbirth. Int J Gynaecol Obstet 2020;150:47-52. 10.1002/ijgo.13182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2020;2:100107. 10.1016/j.ajogmf.2020.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Della Gatta AN, Rizzo R, Pilu G, Simonazzi G. Coronavirus disease 2019 during pregnancy: a systematic review of reported cases. Am J Obstet Gynecol 2020;223:36-41. 10.1016/j.ajog.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheruiyot I, Henry BM, Lippi G. Is there evidence of intra-uterine vertical transmission potential of COVID-19 infection in samples tested by quantitative RT-PCR? Eur J Obstet Gynecol Reprod Biol 2020;249:100-1. 10.1016/j.ejogrb.2020.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gajbhiye R, Modi D, Mahale S. Pregnancy outcomes, Newborn complications and Maternal-Fetal Transmission of SARS-CoV-2 in women with COVID-19: A systematic review of 441 cases. medRxiv 2020. 10.1101/2020.04.11.20062356 [DOI]
- 13. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018;23:60-3. 10.1136/bmjebm-2017-110853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breslin N, Baptiste C, Gyamfi-Bannerman C, et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM 2020;2:100118. 10.1016/j.ajogmf.2020.100118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vintzileos WS, Muscat J, Hoffmann E, et al. Screening all pregnant women admitted to labor and delivery for the virus responsible for coronavirus disease 2019. Am J Obstet Gynecol 2020;223:284-6. 10.1016/j.ajog.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu L, Yang Q, Shi H, et al. Clinical presentations and outcomes of SARS-CoV-2 infected pneumonia in pregnant women and health status of their neonates. Sci Bull (Beijing) 2020;65:1537-42. 10.1016/j.scib.2020.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blitz MJ, Grünebaum A, Tekbali A, et al. Intensive care unit admissions for pregnant and nonpregnant women with coronavirus disease 2019. Am J Obstet Gynecol 2020;223:290-1. 10.1016/j.ajog.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei PF, ed. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chin Med J (Engl) 2020;133:1087-95. 10.1097/CM9.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allotey J, Bonet M, Zamora J, et al. COVID-19 in pregnant women: a Living Systematic Review on prevalence, presentation, prognosis and treatment. PROSPERO 2020 CRD42020178076. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020178076.
- 20. Yap M, Debenham L, Kew T, et al. PregCOV-19 Consortium . Clinical manifestations, prevalence, risk factors, outcomes, transmission, diagnosis and treatment of COVID-19 in pregnancy and postpartum: a living systematic review protocol. BMJ Open 2020;10:e041868. 10.1136/bmjopen-2020-041868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Living Overview of the Evidence . (LOVE) Platform. https://app.iloveevidence.com.
- 22.World Health Organization. Global surveillance for COVID-19 caused by human infection with COVID-19 virus: interim guidance, 20 March 2020. World Health Organization. https://apps.who.int/iris/handle/10665/331506. 2020.
- 23. Dekkers OM, Egger M, Altman DG, Vandenbroucke JP. Distinguishing case series from cohort studies. Ann Intern Med 2012;156:37-40. 10.7326/0003-4819-156-1-201201030-00006 [DOI] [PubMed] [Google Scholar]
- 24.Wells G. Proceedings or the Third Symposium on Systematic Reviews beyond the Basics. SBOD. Improving Quality and Impact; The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of non-randomised Studies in Meta-analysis. July 3-5 2000, Oxford. [Google Scholar]
- 25. Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012;65:934-9. 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 26. Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med 2000;19:3127-31. [DOI] [PubMed] [Google Scholar]
- 27. Cheng B, Jiang T, Zhang L, et al. Clinical Characteristics of Pregnant Women With Coronavirus Disease 2019 in Wuhan, China. Open Forum Infect Dis 2020;7:a294. 10.1093/ofid/ofaa294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu F, Liu H, Hou L, et al. Clinico-Radiological Features and Outcomes in Pregnant Women with COVID-19 Pneumonia Compared with Age-Matched Non-Pregnant Women. Infect Drug Resist 2020;13:2845-54. 10.2147/IDR.S264541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mohr-Sasson A, Chayo J, Bart Y, et al. Laboratory characteristics of pregnant compared to non-pregnant women infected with SARS-CoV-2. Arch Gynecol Obstet 2020; published online 22 June. 10.1007/s00404-020-05655-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qiancheng X, Jian S, Lingling P, et al. sixth batch of Anhui medical team aiding Wuhan for COVID-19 . Coronavirus disease 2019 in pregnancy. Int J Infect Dis 2020;95:376-83. 10.1016/j.ijid.2020.04.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Z, Wang Z, Xiong G. Clinical characteristics and laboratory results of pregnant women with COVID-19 in Wuhan, China. Int J Gynaecol Obstet 2020. 10.1002/ijgo.13265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei L, Gao X, Chen S, et al. Clinical Characteristics and Outcomes between Pregnant and Non-Pregnant Women with Coronavirus Disease 2019: A Retrospective Cohort Study. SSRN 2020. https://ssrn.com/abstract=3569858 10.2139/ssrn.3569858 [DOI]
- 33. Yin M, Zhang L, Deng G, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection During Pregnancy In China: A Retrospective Cohort Study. medRxiv 2020. 10.1101/2020.04.07.20053744 [DOI]
- 34. Blitz MJ, Grünebaum A, Tekbali A, et al. Intensive care unit admissions for pregnant and nonpregnant women with coronavirus disease 2019. Am J Obstet Gynecol 2020;223:290-1. 10.1016/j.ajog.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu S, Shao F, Bao B, et al. Clinical Manifestation and Neonatal Outcomes of Pregnant Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. Open Forum Infect Dis 2020;7:a283. 10.1093/ofid/ofaa283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cérbulo-Vázquez A, Zavala-Barrios B, Briones-Garduño JC, et al. Serological Cytokine and chemokine profile in pregnant women with COVID19 in Mexico City. medRxiv 2020. 10.1101/2020.07.14.20153585. [DOI]
- 37. Badr DA, Mattern J, Carlin A, et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching. Am J Obstet Gynecol 2020;223:764-8. 10.1016/j.ajog.2020.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Molteni E, Astley CM, Ma W, et al. SARS-CoV-2 (COVID-19) infection in pregnant women: characterization of symptoms and syndromes predictive of disease and severity through real-time, remote participatory epidemiology. medRxiv 2020. 10.1101/2020.08.17.20161760. [DOI]
- 39. Leon-Abarca JA, Pena-Gallardo MT, Soliz J, Accinelli RA. Clinical evolution of COVID-19 during pregnancy at different altitudes: a population-based study. medRxiv 2020. 10.1101/2020.09.14.20193177. [DOI]
- 40. Zambrano LD, Ellington S, Strid P, et al. CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team . Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1641-7. 10.15585/mmwr.mm6944e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Campbell KH, Tornatore JM, Lawrence KE, et al. Prevalence of SARS-CoV-2 Among Patients Admitted for Childbirth in Southern Connecticut. JAMA 2020. 10.1001/jama.2020.8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Knight M, Bunch K, Vousden N, et al. Characteristics and outcomes of pregnant women hospitalised with confirmed SARS-CoV-2 infection in the UK: a national cohort study using the UK Obstetric Surveillance System (UKOSS). 2020. 10.1101/2020.05.08.20089268. [DOI]
- 43. Li N, Han L, Peng M, et al. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis 2020;ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liao J, He X, Gong Q, Yang L, Zhou C, Li J. Analysis of vaginal delivery outcomes among pregnant women in Wuhan, China during the COVID-19 pandemic. Int J Gynaecol Obstet 2020;150:53-7. 10.1002/ijgo.13188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ahlberg M, Neovius M, Saltvedt S, et al. Association of SARS-CoV-2 Test Status and Pregnancy Outcomes. JAMA 2020;324:1782. 10.1001/jama.2020.19124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bender WR, Srinivas S, Coutifaris P, Acker A, Hirshberg A. The Psychological Experience of Obstetric Patients and Health Care Workers after Implementation of Universal SARS-CoV-2 Testing. Am J Perinatol 2020;37:1271-9. 10.1055/s-0040-1715505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bianco A, Buckley AB, Overbey J, et al. Testing of patients and support persons for coronavirus disease 2019 (COVID-19) infection before scheduled deliveries. Obstet Gynecol 2020;136:283-7. 10.1097/AOG.0000000000003985 [DOI] [PubMed] [Google Scholar]
- 48. Brandt JS, Hill J, Reddy A, et al. Epidemiology of coronavirus disease 2019 in pregnancy: risk factors and associations with adverse maternal and neonatal outcomes. Am J Obstet Gynecol 2020;S0002-9378(20)31134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buckley A, Bianco A, Stone J. Universal testing of patients and their support persons for severe acute respiratory syndrome coronavirus 2 when presenting for admission to labor and delivery at Mount Sinai Health System. Am J Obstet Gynecol MFM 2020;2:100147. 10.1016/j.ajogmf.2020.100147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cosma S, Borella F, Carosso A, et al. The “scar” of a pandemic: Cumulative incidence of COVID-19 during the first trimester of pregnancy. J Med Virol 2021;93:537-40. 10.1002/jmv.26267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Egerup P, Fich Olsen L, Christiansen AH, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibodies at Delivery in Women, Partners, and Newborns. Obstet Gynecol 2021;137:49-55. 10.1097/AOG.0000000000004199. [DOI] [PubMed] [Google Scholar]
- 52. Emeruwa UN, Ona S, Shaman JL, et al. Associations Between Built Environment, Neighborhood Socioeconomic Status, and SARS-CoV-2 Infection Among Pregnant Women in New York City. JAMA 2020;324:390-2. 10.1001/jama.2020.11370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Facchetti F, Bugatti M, Drera E, et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine 2020;59:102951. 10.1016/j.ebiom.2020.102951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Farghaly MAA, Kupferman F, Castillo F, Kim RM. Characteristics of Newborns Born to SARS-CoV-2-Positive Mothers: A Retrospective Cohort Study. Am J Perinatol 2020;37:1310-6. 10.1055/s-0040-1715862 [DOI] [PubMed] [Google Scholar]
- 55. Fassett MJ, Lurvey LD, Yasumura L, et al. Universal SARS-Cov-2 Screening in Women Admitted for Delivery in a Large Managed Care Organization. Am J Perinatol 2020;37:1110-4. 10.1055/s-0040-1714060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Flaherman VJ, Afshar Y, Boscardin J, et al. Infant Outcomes Following Maternal Infection with SARS-CoV-2: First Report from the PRIORITY Study. Clin Infect Dis 2020;ciaa1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Goldfarb IT, Clapp MA, Soffer MD, et al. Prevalence and Severity of Coronavirus Disease 2019 (COVID-19) Illness in Symptomatic Pregnant and Postpartum Women Stratified by Hispanic Ethnicity. Obstet Gynecol 2020;136:300-2. 10.1097/AOG.0000000000004005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gulersen M, Prasannan L, Tam Tam H, et al. Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection. Am J Obstet Gynecol MFM 2020;2:100211. 10.1016/j.ajogmf.2020.100211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kalafat E, Yassa M, Koc A, Tug N, TULIP collaboration . Utility of lung ultrasound assessment for probable SARS-CoV-2 infection during pregnancy and universal screening of asymptomatic individuals. Ultrasound Obstet Gynecol 2020;56:624-6. 10.1002/uog.23099 [DOI] [PubMed] [Google Scholar]
- 60. Khalil A, Hill R, Ladhani S, Pattisson K, O’Brien P. Severe acute respiratory syndrome coronavirus 2 in pregnancy: symptomatic pregnant women are only the tip of the iceberg. Am J Obstet Gynecol 2020;223:296-7. 10.1016/j.ajog.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. LaCourse SM, Kachikis A, Blain M, et al. Low prevalence of SARS-CoV-2 among pregnant and postpartum patients with universal screening in Seattle, Washington. Clin Infect Dis 2020;ciaa675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li M, Yin H, Jin Z, et al. Impact of Wuhan lockdown on the indications of cesarean delivery and newborn weights during the epidemic period of COVID-19. PLoS One 2020;15:e0237420. 10.1371/journal.pone.0237420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu W, Cheng H, Wang J, et al. Clinical Analysis of Neonates Born to Mothers with or without COVID-19: A Retrospective Analysis of 48 Cases from Two Neonatal Intensive Care Units in Hubei Province. Am J Perinatol 2020;37:1317-23. 10.1055/s-0040-1716505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Martinez-Perez O, Prats Rodriguez P, Muner Hernandez M, et al. Spanish Obstetric Emergency Group . The association between SARS-CoV-2 infection and preterm delivery: a prospective study with a multivariable analysis. BMC Pregnancy Childbirth 2021;21:273. 10.1186/s12884-021-03742-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maru S, Patil U, Carroll-Bennett R, et al. Universal screening for SARS-CoV-2 infection among pregnant women at Elmhurst Hospital Center, Queens, New York. PLoS One 2020;15:e0238409. 10.1371/journal.pone.0238409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Miller ES, Grobman WA, Sakowicz A, Rosati J, Peaceman AM. Clinical Implications of Universal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Testing in Pregnancy. Obstet Gynecol 2020;136:232-4. 10.1097/AOG.0000000000003983 [DOI] [PubMed] [Google Scholar]
- 67. Naqvi M, Burwick RM, Ozimek JA, Greene NH, Kilpatrick SJ, Wong MS. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Universal Testing Experience on a Los Angeles Labor and Delivery Unit. Obstet Gynecol 2020;136:235-6. 10.1097/AOG.0000000000003987 [DOI] [PubMed] [Google Scholar]
- 68. Nayak AH, Kapote DS, Fonseca M, et al. Impact of the Coronavirus Infection in Pregnancy: A Preliminary Study of 141 Patients. J Obstet Gynaecol India 2020;70:256-61. 10.1007/s13224-020-01335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Norooznezhad AH, Eskandarion S, Akbari R, et al. Changes of leukocytes, neutrophils, and lymphocytes count and dependent variables in pregnant women with coronavirus disease 2019 before and after cesarean delivery. J Med Virol 2021;93:664-6. 10.1002/jmv.26525 [DOI] [PubMed] [Google Scholar]
- 70. Ochiai D, Kasuga Y, Iida M, Ikenoue S, Tanaka M. Universal screening for SARS-CoV-2 in asymptomatic obstetric patients in Tokyo, Japan. Int J Gynaecol Obstet 2020;150:268-9. 10.1002/ijgo.13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Peng S, Zhu H, Yang L, et al. A study of breastfeeding practices, SARS-CoV-2 and its antibodies in the breast milk of mothers confirmed with COVID-19. Lancet Reg Health West Pac 2020;4:100045. 10.1016/j.lanwpc.2020.100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pineles BL, Alamo IC, Farooq N, et al. Racial-ethnic disparities and pregnancy outcomes in SARS-CoV-2 infection in a universally-tested cohort in Houston, Texas. Eur J Obstet Gynecol Reprod Biol 2020;254:329-30. 10.1016/j.ejogrb.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pirjani R, Hosseini R, Soori T, et al. Maternal and neonatal outcomes in COVID-19 infected pregnancies: a prospective cohort study. J Travel Med 2020;27:taaa158. 10.1093/jtm/taaa158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG 2020;127:1548-56. 10.1111/1471-0528.16403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ruggiero M, Somigliana E, Tassis B, et al. Clinical relevance of SARS-CoV-2 infection in late pregnancy. BMC Pregnancy Childbirth 2021;21:505. 10.1186/s12884-021-03985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sakowicz A, Ayala AE, Ukeje CC, Witting CS, Grobman WA, Miller ES. Risk factors for severe acute respiratory syndrome coronavirus 2 infection in pregnant women. Am J Obstet Gynecol MFM 2020;2:100198. 10.1016/j.ajogmf.2020.100198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Smithgall MC, Liu-Jarin X, Hamele-Bena D, et al. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology 2020;77:994-9. 10.1111/his.14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sutton D, Fuchs K, D’Alton M, Goffman D. Universal Screening for SARS-CoV-2 in Women Admitted for Delivery. N Engl J Med 2020;382:2163-4. 10.1056/NEJMc2009316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. van Keulen BJ, Romijn M, Bondt A, et al. Breastmilk; a source of SARS-CoV-2 specific IgA antibodies. medRxiv 2020. 10.1101/2020.08.18.20176743 [DOI]
- 80. Vintzileos WS, Muscat J, Hoffmann E, et al. Screening all pregnant women admitted to labor and delivery for the virus responsible for coronavirus disease 2019. Am J Obstet Gynecol 2020;223:284-6. 10.1016/j.ajog.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang P, Salafia C, Heyman T, Salafia C, Lederman S, Dygulska B. Detection of severe acute respiratory syndrome coronavirus 2 in placentas with pathology and vertical transmission. Am J Obstet Gynecol MFM 2020;2:100197. 10.1016/j.ajogmf.2020.100197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Crovetto F, Crispi F, Llurba E, Figueras F, Gomez-Roig MD, Gratacos E. Seroprevalence and presentation of SARS-Cov-2 in pregnancy. Lancet 2020;396:530-31. 10.1016/S0140-6736(20)31714-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yang H, Xie F, Cheng Y, et al. Research on Pregnant Women Suspected of Having COVID-19 in the Epidemic Outbreak Area. SSRN 2020.
- 84. Wang MJ, Schapero M, Iverson R, Yarrington CD. Obstetric Hemorrhage Risk Associated with Novel COVID-19 Diagnosis from a Single-Institution Cohort in the United States. Am J Perinatol 2020;37:1411-6. 10.1055/s-0040-1718403 [DOI] [PubMed] [Google Scholar]
- 85. Mattern J, Vauloup-Fellous C, Zakaria H, et al. Post lockdown COVID-19 seroprevalence and circulation at the time of delivery, France. PLoS One 2020;15:e0240782. 10.1371/journal.pone.0240782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Díaz-Corvillón P, Mönckeberg M, Barros A, et al. Routine screening for SARS CoV-2 in unselected pregnant women at delivery. PLoS One 2020;15:e0239887. 10.1371/journal.pone.0239887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Afshar Y, Gaw SL, Flaherman VJ, et al. Pregnancy CoRonavIrus Outcomes RegIsTrY (PRIORITY) Study . Clinical Presentation of Coronavirus Disease 2019 (COVID-19) in Pregnant and Recently Pregnant People. Obstet Gynecol 2020;136:1117-25. 10.1097/AOG.0000000000004178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vintzileos WS, Muscat J, Hoffmann E, et al. Screening all pregnant women admitted to labor and delivery for the virus responsible for coronavirus disease 2019. Am J Obstet Gynecol 2020;223:284-6. 10.1016/j.ajog.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Blitz MJ, Rochelson B, Rausch AC, et al. Universal testing for coronavirus disease 2019 in pregnant women admitted for delivery: prevalence of peripartum infection and rate of asymptomatic carriers at four New York hospitals within an integrated healthcare system. Am J Obstet Gynecol MFM 2020;2:100169. 10.1016/j.ajogmf.2020.100169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dotters-Katz SK, Harris H, Wheeler SM, Swamy GK, Hughes BL. Specialized prenatal care delivery for coronavirus disease 2019-exposed or -infected pregnant women. Am J Obstet Gynecol 2021;224:325-7. 10.1016/j.ajog.2020.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Duffy CR, Hart JM, Modest AM, et al. Lymphopenia and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection Among Hospitalized Obstetric Patients. Obstet Gynecol 2020;136:229-31. 10.1097/AOG.0000000000003984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Farghaly MAA, Kupferman F, Castillo F, Kim RM. Characteristics of Newborns Born to SARS-CoV-2-Positive Mothers: A Retrospective Cohort Study. Am J Perinatol 2020;37:1310-6. 10.1055/s-0040-1715862 [DOI] [PubMed] [Google Scholar]
- 93. Griffin I, Benarba F, Peters C, et al. The Impact of COVID-19 Infection on Labor and Delivery, Newborn Nursery, and Neonatal Intensive Care Unit: Prospective Observational Data from a Single Hospital System. Am J Perinatol 2020;37:1022-30. 10.1055/s-0040-1713416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Janevic T, Glazer KB, Vieira L, et al. Racial/Ethnic Disparities in Very Preterm Birth and Preterm Birth Before and During the COVID-19 Pandemic. JAMA Netw Open 2021;4:e211816. 10.1001/jamanetworkopen.2021.1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. LaCourse SM, Kachikis A, Blain M, et al. Low Prevalence of Severe Acute Respiratory Syndrome Coronavirus 2 Among Pregnant and Postpartum Patients With Universal Screening in Seattle, Washington. Clin Infect Dis 2021;72:869-72. 10.1093/cid/ciaa675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Liu C, Andrusier M, Silver M, Applewhite L, Clare CA. Effect of SARS-CoV-2 Infection on Pregnancy Outcomes in an Inner-City Black Patient Population. J Community Health 2021;46:1029-35. 10.1007/s10900-021-00988-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. London V, McLaren R, Jr, Atallah F, et al. The Relationship between Status at Presentation and Outcomes among Pregnant Women with COVID-19. Am J Perinatol 2020;37:991-4. 10.1055/s-0040-1712164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Malhotra Y, Knight C, Patil UP, et al. Impact of evolving practices on SARS-CoV-2 positive mothers and their newborns in the largest public healthcare system in America. J Perinatol 2021;41:970-80. 10.1038/s41372-021-01023-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Malhotra Y, Miller R, Bajaj K, Sloma A, Wieland D, Wilcox W. No change in cesarean section rate during COVID-19 pandemic in New York City. Eur J Obstet Gynecol Reprod Biol 2020;253:328-9. 10.1016/j.ejogrb.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Maru S, Patil U, Carroll-Bennett R, et al. Universal screening for SARS-CoV-2 infection among pregnant women at Elmhurst Hospital Center, Queens, New York. PLoS One 2020;15:e0238409. 10.1371/journal.pone.0238409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. McLaren R, Jr, London V, Narayanamoorthy S, Atallah F, Silver M, Minkoff H. Cesarean Birth Morbidity among Women with SARS-CoV-2. Am J Perinatol 2021. 10.1055/s-0041-1739430 [DOI] [PubMed] [Google Scholar]
- 102. Ndubizu C, Colon-Aponte C, Galli J, et al. Are socioeconomically disadvantaged pregnant patients disproportionally infected by SARS-CoV-2 virus? Am J Obstet Gynecol 2021;224:S641. 10.1016/j.ajog.2020.12.1059. [DOI] [Google Scholar]
- 103. Reforma LG, Duffy C, Collier AY, et al. A multidisciplinary telemedicine model for management of coronavirus disease 2019 (COVID-19) in obstetrical patients. Am J Obstet Gynecol MFM 2020;2:100180. 10.1016/j.ajogmf.2020.100180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wilcox W, Bajaj K, Rossberg MC, Knight C, Wieland D, Malhotra Y. Lessons learnt in transitioning from universal screening to universal testing of pregnant patients for SARS-CoV-2 at the largest municipal health system in America. J Perinatol 2021;41:1174-6. 10.1038/s41372-020-00889-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang P, Salafia C, Heyman T, Salafia C, Lederman S, Dygulska B. Detection of severe acute respiratory syndrome coronavirus 2 in placentas with pathology and vertical transmission. Am J Obstet Gynecol MFM 2020;2:100197. 10.1016/j.ajogmf.2020.100197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Antoun L, Taweel NE, Ahmed I, Patni S, Honest H. Maternal COVID-19 infection, clinical characteristics, pregnancy, and neonatal outcome: A prospective cohort study. Eur J Obstet Gynecol Reprod Biol 2020;252:559-62. 10.1016/j.ejogrb.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ahmed I, Quinn L, Tan BK. COVID-19 and the ABO blood group in pregnancy: A tale of two multiethnic cities. Int J Lab Hematol 2021;43:e45-7. 10.1111/ijlh.13355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sahin D, Tanacan A, Erol SA, et al. A pandemic center’s experience of managing pregnant women with COVID-19 infection in Turkey: A prospective cohort study. Int J Gynaecol Obstet 2020;151:74-82. 10.1002/ijgo.13318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Farhat AS, Sayedi SJ, Akhlaghi F, Hamedi A, Ghodsi A. Coronavirus (COVID-19) Infection in Newborns. Int J Pediatr 2020;8:11513-7. [Google Scholar]
- 110. Vivanti AJ, Mattern J, Vauloup-Fellous C, et al. Retrospective Description of Pregnant Women Infected with Severe Acute Respiratory Syndrome Coronavirus 2, France. Emerg Infect Dis 2020;26:2069-76. 10.3201/eid2609.202144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Secretaría de Salud. Informes epidemiológicos de embarazadas y puérperas estudiadas, ante sospecha de COVID 19 Dirección General de Epidemiología. Semana Epidemiológica 28 https://www.gob.mx/cms/uploads/attachment/file/594825/sem28.pdf.
- 112. Bachani S, Arora R, Dabral A, et al. Clinical Profile, Viral Load, Maternal-Fetal Outcomes of Pregnancy With COVID-19: 4-Week Retrospective, Tertiary Care Single-Centre Descriptive Study. J Obstet Gynaecol Can 2021;43:474-82. 10.1016/j.jogc.2020.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Crovetto F, Crispi F, Llurba E, et al. KidsCorona Pregnancy COVID-19 Group . Impact of Severe Acute Respiratory Syndrome Coronavirus 2 Infection on Pregnancy Outcomes: A Population-based Study. Clin Infect Dis 2021;73:1768-75. 10.1093/cid/ciab104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Haye MT, Cartes G, Gutiérrez J, et al. Maternal and perinatal outcomes in pregnant women with confirmed severe and mild COVID-19 at one large maternity hospital in Chile. J Matern Fetal Neonatal Med 2021;1-6. 10.1080/14767058.2021.1902498 [DOI] [PubMed] [Google Scholar]
- 115. Hcini N, Maamri F, Picone O, et al. Maternal, fetal and neonatal outcomes of large series of SARS-CoV-2 positive pregnancies in peripartum period: A single-center prospective comparative study. Eur J Obstet Gynecol Reprod Biol 2021;257:11-8. 10.1016/j.ejogrb.2020.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lucinde R, Mugo D, Bottomley C, et al. Sero-surveillance for IgG to SARS-CoV-2 at antenatal care clinics in two Kenyan referral hospitals. medRxiv 2021. 10.1101/2021.02.05.21250735. [DOI] [PMC free article] [PubMed]
- 117. Magnus MC, Oakley L, Gjessing HK, et al. Pregnancy and risk of COVID-19: a Norwegian registry-linkage study. BJOG 2022;129:101-9. 10.1111/1471-0528.16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Morales MN, González TF, Cartallier O, et al. Pandemia SARS-CoV-2 y embarazo en el Hospital el Pino: un estudio descriptivo. Rev Chil Obstet Ginecol 2020;85:S50-8. 10.4067/S0717-75262020000700008. [DOI] [Google Scholar]
- 119. Mostafa A, Kandil S, El-Sayed MH, et al. SARS-CoV-2 seroconversion among 4040 Egyptian healthcare workers in 12 resource-limited healthcare facilities: A prospective cohort study. Int J Infect Dis 2021;104:534-42. 10.1016/j.ijid.2021.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ngalame AN, Neng HT, Inna R, et al. Materno-Fetal Outcomes of COVID-19 Infected Pregnant Women Managed at the Douala Gyneco-Obstetric and Pediatric Hospital—Cameroon. Open J Obstet Gynecol 2020;10:1279-94 10.4236/ojog.2020.1090118. [DOI] [Google Scholar]
- 121. Zumalave Grados I, Lacunza Paredes R, Benavides Zavala G, et al. Características de la infección en gestantes y puérperas por SARS-CoV-2, en el hospital nacional del Callao, Perú. Rev Peru Ginecol Obstet 2020;66 10.31403/rpgo.v66i2271. [DOI] [Google Scholar]
- 122. Vousden N, Bunch K, Morris E, et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: A national cohort study using the UK Obstetric Surveillance System (UKOSS). PLoS One 2021;16:e0251123. 10.1371/journal.pone.0251123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Li N, Han L, Peng M, et al. Maternal and Neonatal Outcomes of Pregnant Women With Coronavirus Disease 2019 (COVID-19) Pneumonia: A Case-Control Study. Clin Infect Dis 2020;71:2035-41. 10.1093/cid/ciaa352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Overtoom EM, Rosman AN, Zwart JJ, et al. SARS-CoV-2 infection in pregnancy during the first wave of COVID-19 in the Netherlands: a prospective nationwide population-based cohort study (NethOSS). BJOG 2022;129:91-100. 10.1111/1471-0528.16903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Facchetti F, Bugatti M, Drera E, et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine 2020;59:102951. 10.1016/j.ebiom.2020.102951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Charlesworth A. Shock to the system: COVID-19’s long-term impact on the NHS. 2020. https://www.health.org.uk/news-and-comment/blogs/shock-to-the-system-covid-19s-long-term-impact-on-the-nhs
- 127. Papoutsi E, Giannakoulis VG, Ntella V, Pappa S, Katsaounou P. Global burden of COVID-19 pandemic on healthcare workers. ERJ Open Res 2020;6:00195-2020. 10.1183/23120541.00195-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Davenport MH, Meyer S, Meah VL, Strynadka MC, Khurana R. Moms Are Not OK: COVID-19 and Maternal Mental Health. Frontiers in Global Women's Health 2020. https://www.frontiersin.org/articles/10.3389/fgwh.2020.00001/full [DOI] [PMC free article] [PubMed]
- 129.Public Health England. Disparities in the risk and outcomes of COVID-19. 2020 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/908434/Disparities_in_the_risk_and_outcomes_of_COVID_August_2020_update.pdf.
- 130. Petersen EE, Davis NL, Goodman D, et al. Racial/Ethnic Disparities in Pregnancy-Related Deaths - United States, 2007-2016. MMWR Morb Mortal Wkly Rep 2019;68:762-5. 10.15585/mmwr.mm6835a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.MBRRACE-UK. Saving lives, improving mothers’ care. Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2015-17. 2019 https://www.npeu.ox.ac.uk/assets/downloads/mbrrace-uk/reports/MBRRACE-UK%20Maternal%20Report%202019%20-%20WEB%20VERSION.pdf.
- 132. Pareek M, Bangash MN, Pareek N, et al. Ethnicity and COVID-19: an urgent public health research priority. Lancet 2020;395:1421-2. 10.1016/S0140-6736(20)30922-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Money D. Canadian Surveillance of COVID-19 in Pregnancy: Epidemiology. Maternal and Infant Outcomes, 2021. [Google Scholar]
- 134. Collin J, Byström E, Carnahan A, Ahrne M. Public Health Agency of Sweden’s Brief Report: Pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand 2020;99:819-22. 10.1111/aogs.13901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Epelboin S, Labrosse J, De Mouzon J, et al. Obstetrical outcomes and maternal morbidities associated with COVID-19 in pregnant women in France: A national retrospective cohort study. PLoS Med 2021;18:e1003857. 10.1371/journal.pmed.1003857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Docherty AB, Harrison EM, Green CA, et al. ISARIC4C investigators . Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020;369:m1985. 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. DeSisto CL, Wallace B, Simeone RM, et al. Risk for Stillbirth Among Women With and Without COVID-19 at Delivery Hospitalization - United States, March 2020-September 2021. MMWR Morb Mortal Wkly Rep 2021;70:1640-5. 10.15585/mmwr.mm7047e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mendoza M, Garcia-Ruiz I, Maiz N, et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG 2020;127:1374-80. 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Cevik M, Mishra S. SARS-CoV-2 variants and considerations of inferring causality on disease severity. Lancet Infect Dis 2021;21:1472-4. 10.1016/S1473-3099(21)00338-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Mahajan NN, Pophalkar M, Patil S, et al. Pregnancy Outcomes and Maternal Complications During the Second Wave of Coronavirus Disease 2019 (COVID-19) in India. Obstet Gynecol 2021;138:660-2. 10.1097/AOG.0000000000004529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Takemoto MLS, Nakamura-Pereira M, Menezes MO, et al. Higher case fatality rate among obstetric patients with COVID-19 in the second year of pandemic in Brazil: do new genetic variants play a role? medRxiv 2021. 10.1101/2021.05.06.21256651. [DOI]
- 142. Vousden N, Ramakrishnan R, Bunch K, et al. Severity of maternal infection and perinatal outcomes during periods of SARS-CoV-2 wildtype, alpha, and delta variant dominance in the UK: prospective cohort study. BMJ Med 2022;1:e000053. 10.1136/bmjmed-2021-000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Appendices 1-10
Data Availability Statement
No additional data available.