Abstract
BACKGROUND
Multiple sites of metastasis and desmoplastic reactions in the stroma are key features of human pancreatic cancer (PC). There are currently no simple and reliable animal models that can mimic these features for accurate disease modeling.
AIM
To create a new xenograft animal model that can faithfully recapitulate the features of human PC.
METHODS
Interleukin 2 receptor subunit gamma (IL2RG) gene knockout Syrian hamster was created and characterized. A panel of human PC cell lines were transplanted into IL2RG knockout Syrian hamsters and severe immune-deficient mice subcutaneously or orthotopically. Tumor growth, local invasion, remote organ metastasis, histopathology, and molecular alterations of tumor cells and stroma were compared over time.
RESULTS
The Syrian hamster with IL2RG gene knockout (named ZZU001) demonstrated an immune-deficient phenotype and function. ZZU001 hamsters faithfully recapitulated most features of human PC, in particular, they developed metastasis at multiple sites. PC tissues derived from ZZU001 hamsters displayed desmoplastic reactions in the stroma and epithelial to mesenchymal transition phenotypes, whereas PC tissues derived from immune-deficient mice did not present such features.
CONCLUSION
ZZU001 hamsters engrafted with human PC cells are a superior animal model compared to immune-deficient mice. ZZU001 hamsters can be a valuable animal model for better understanding the molecular mechanism of tumorigenesis and metastasis and the evaluation of new drugs targeting human PC.
Keywords: Pancreatic cancer, Xenotrans plantation, Syrian hamster, IL-2 receptor gamma chain gene, Metastasis, Animal model
Core tip: Xenograft cell transplantation into immune-deficient mice has become the gold standard for assessing tumor progression and efficacy of cancer drugs. However, xenografting human pancreatic cancer (PC) models in nude mice rarely results in development of metastasis and thus does not accurately reflect tumor progression as seen in the human disease. Here, we created a new immune-deficient Syrian hamster with interleukin 2 receptor subunit gamma (IL2RG) gene knockout and demonstrated that the IL2RG-/- Syrian hamster is a promising animal model that can faithfully recapitulate most features of human PC, notably multiple sites of metastasis. Furthermore, this model can present other key features of human PC, such as stromal reaction and the communication between stromal cells and PC cells.
INTRODUCTION
Pancreatic cancer (PC) is one of the most deadly human diseases, with a 5-year survival rate of less than 7%. It is predicted to be the second leading cause of cancer-associated mortality within the next decade in developed countries[1]. Even when primary cancer can be removed by radical surgery, the recurrence rate of PC is as high as 85%[2,3]. Multiple-sites of metastasis from PC remains a significant hurdle in treating this disease. Thus, reliable animal models that can mimic the clinical features of PC are needed for better understanding the molecular mechanisms of tumorigenesis, development of practical approaches for early diagnosis, and evaluation of novel therapeutic agents.
In preclinical studies, there have been several models developed for human PC research, such as human PC cell lines, cell line-based xenografts, patient-derived tumor xenografts, transgenic mice models, as well as recently developed pancreatic ductal organoids, three-dimensional culture systems, and organoid-based xenografts[4]. Among these models, xenografting of human tumor cells into immune-deficient mice has become the gold standard for assessing tumor progression and the preclinical efficacy of cancer drugs. However, subcutaneous xenograft PC tumor models in nude mice rarely develop metastasis[5]. The orthotopic model of human PC in nude mice provides a better way to evaluate tumor growth, but metastasis to the lung or/and other organs occurs rarely from most human PC cell lines tested[6,7]. Furthermore, it has been shown that cell-line based xenografts in immune-deficient mice could not consistently predict therapeutic response of human PC[8-11]. The lack of predictive drug responsiveness using xenograft models is likely due to multiple factors[4]. The major reason is that xenografts of human tumors grow primarily in immune-deficient mice as homogenous masses of tumor cells with limited stromal infiltration. This may be particularly relevant for PC, since PC tumors are predominantly comprised of a stromal compartment consisting of an acellular extracellular matrix and a variety of non-neoplastic cell types, such as cancer-associated fibroblasts, immune cells, and vascular cells[12]. To overcome these challenges, more robust animal models are needed.
Accumulating evidence demonstrates that Syrian hamsters (Mesocricetus auratus) have advantages as models for various diseases due to the high similarities in anatomy, physiology, and pathology between Syrian hamsters and humans[13,14]. The Syrian hamster is the only rodent species that develops PC in an almost identical manner to the respective human disease regarding such features as clinical symptoms, tumor morphology, tumor biology, metabolic abnormality, and molecular genetic alterations[15]. In addition, we recently found that human interleukin (IL)-12 is effective in stimulating both human and hamster peripheral blood mononuclear cells. Human IL-12 effectively stimulates interferon-gamma and tumor necrosis factor-alpha expression in activated hamster splenocytes ex vivo and demonstrates toxicity in vivo in Syrian hamsters bearing PC[16]. However, human IL-12 does not function in mouse at all. Similarly, human granulocyte-macrophage colony-stimulating factor functions on hamster cells but not on mouse cells[17]. These observations suggest that human tumor cells may be able to communicate with the host cells of Syrian hamster through human tumor cell secreting molecules that function on Syrian hamster cells. We reasoned that immunocompromised Syrian hamsters might be the most appropriate hosts for human cancer xenografting to model the features of human PC. Recently, we made a technical breakthrough in establishing efficient gene targeting techniques in the Syrian hamster[18]. Here, we report the creation and characterization of an IL-2 receptor subunit gamma (IL2RG) gene knockout Syrian hamster model (named ZZU001) and its use as a host for human PC cell xenotransplantation. ZZU001 represents a convenient, cost-effective, and reliable model for recapitulating the progression and multiple-sites metastasis of human PC.
MATERIALS AND METHODS
Animals and ethics statement
Syrian hamsters (4- to 5-wk-old, 80 g in weight) were purchased from Beijing Vital River Laboratory Animal Technology Co. (Beijing, China). B-NDG (NOD-Prkdcscid IL2rgtm1/Bcgen mice deficient in mature T lymphocytes, B lymphocytes, and natural killer cells) mice (4- to 5-wk-old, male, 20 g in weight) were purchased from Beijing Biocytogen Co., Ltd. (Beijing, China). The animals were maintained under specific pathogen-free, 14 h light/10 h dark cycle (room temperature 23 ± 0.5°C, humidity 40%-60%) conditions, and the animals had free access to irradiated chow and water. To ameliorate the suffering of animals observed throughout experimental studies, animals were euthanized by CO2 inhalation. All surgery was performed under intraperitoneal injection of Avertin (Sigma-Aldrich, St Louis, MO, United States) anesthesia, and all efforts were made to minimize animal suffering. Avertin Stock solution was prepared as follows: 25 g avertin (2, 2, 2-Tribromoethanol) and 15.5 mL tert-Amyl Alcohol (2-methyl-2-butanol) mixed approximately 12 h in a dark bottle at room temperature. Working solution was prepared as follows: 0.5 mL Avertin stock and 39.5 mL 0.9% saline (NaCl) mixed in dark container, filter sterilized, and stored at 4°C. All animal care and experiments were approved by the Ethical Committee of the Zhengzhou University and were in accordance with the Provision and General Recommendation of Chinese Experimental Animals Administration Legislation.
Cell lines and adenovirus
All human pancreatic cell lines listed in Table 1 and A549 cell line were purchased from American Type Culture Collection (ATCC, Manassas, VA, United States). All the cell lines had been short tandem repeat genotyped and confirmed identical to the published deoxyribonucleic acid (DNA) profiles of the American Type Culture Collection. All cells were grown in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, United States) supplemented with 10% fetal bovine serum (PAN, Germany), 50 μg/mL streptomycin, and 50 μg/mL penicillin (Sigma-Aldrich). Cells were maintained at 37°C and 5% CO2. Human Adenovirus type 5 (Ad5) was made and titrated as previously described[14].
Table 1.
Sites and frequency [n/n (%)] of distant metastasis and local tumor infiltration of MIA-PaCa-2 cells
|
MIAPaCa-2 (SC) |
MIAPaCa-2 (OrT) |
|||
| B-NDG | ZZU001 | B-NDG | ZZU001 | |
| Distant metastasis | ||||
| Liver | - | - | 3/5 (60) | 5/5 (100) |
| Lung | - | 5/5 (100) | - | 5/5 (100) |
| Retroperitoneum | - | - | 3/5 (60) | 5/5 (100) |
| Mesentery | - | - | 3/5 (60) | 5/5 (100) |
| Diaphragm | - | - | 2/5 (40) | 2/5 (40) |
| Spleen | - | - | 2/5 (40) | - |
| Stomach | - | - | - | 1/5 (20) |
| Kidney | - | 2/5 (40) | - | 5/5 (100) |
| Adrenal gland | - | 1/5 (20) | - | 2/5 (40) |
| Local infiltration | ||||
| Spleen | - | - | 3/5(60) | 4/5 (80) |
| Stomach | - | - | 1/5 (20) | 3/5 (60) |
| Liver (hilus) | - | - | 3/5 (60) | 5/5 (100) |
| Kidney (hilus) | - | - | - | 1/5 (20) |
| Retroperitoneum | - | - | 3/5 (60) | 5/5 (100) |
| Bowel | - | - | 3/5 (60) | 5/5 (100) |
| Mesentery (adjacent to pancreas) | - | 4/5 (80) | 5/5 (100) | |
| Signs of tumor burden | ||||
| Ascites | - | - | 3/5 (60) | 3/5 (60) |
| Jaundice | - | - | 2/5 (40) | 3/5 (60) |
| Ileus | - | - | - | 2/5 (40) |
| Cachexia | - | - | 1/5 (20) | 3/5 (60) |
OrT: Orthotopic; SC: Subcutaneous.
Generation of IL2RG knockout (ZZU001) Syrian hamsters by CRISPR/Cas9
Single-guide ribonucleic acid (sgRNA) design, synthesis, embryo manipulation, and embryo transfer were performed for establishment of IL2RG-/- Syrian hamster, as described previously[18]. In brief, sgRNA was designed to target a site-specific sequence within the first exon of IL2RG gene (NW_004801714.1). Microinjection was performed under red light, and HECM-9 medium covered by mineral oil was used as injection medium. For cytoplasmic injection, both Cas9 mRNA (100 ng/μL) and sgRNA (50 ng/μL) were co-injected into the cytoplasm of the fertilized eggs. The injected embryos were cultured in HECM-9 medium covered by mineral oil at 37.5˚C under 10% CO2, 5% O2, and 85% N2 for 0.5 h before use. Viable embryos after injection were transferred to each oviduct (15 embryos per oviduct) of pseudo-pregnant females. Genotyping analysis of pups produced from microinjected embryos was performed by Sanger sequence with genomic DNA isolated from toes collected from 2-wk-old pups. Genomic regions flanking the CRISPR targeted sites were amplified by polymerase chain reaction (PCR) using primers; Forward: GAGAGTGGTTCAGGGTTCTGACA, Reverse: TGGGCTGGAGCTCAGAACTG. The PCR products were directly sequenced. Potential off-target effect was analyzed based on the rule that sequences matching the final 12 nt of the target sequence and protospacer adjacent motif sequence might cause the off-target effect[19]. The off-target fragments were amplified from the founder's genomic DNA and identified by Sanger sequence. The primer sequences are shown in Table S1.
Reverse transcriptase-quantitative PCR (RT-qPCR) and western blotting
Total RNA from spleens or thymus or other organs of 5-wk-old Syrian hamsters was extracted using the TRIzol Reagent (Invitrogen, Carlsbad, CA, United States). First strand cDNA was synthesized from 1 μg of total RNA using PrimeScript RT Master Mix (Takara, Kyoto, Japan). SYBR-green based-qPCR reactions were performed in a StepOnePlus system (Applied Biosystems, Republic of Singapore) thermal cycler. Quantitative PCR reactions were carried out in triplicate, and the specific primers of different immune cell markers were previously described[13]. The mRNA expression was quantitated using the 2-(ΔCt sample–ΔCt control) method. Western blot analyses were performed as previously described[13]. Whole cell protein was isolated from spleens of 5-wk-old Syrian hamsters. Western blotting was performed for detection of the IL2RG protein (A-10 antibody, sc-271060, Santa Cruz Biotechnology, Dallas, TX, United States) while glyceraldehyde 3-phosphate dehydrogenase (60004-1-Ig, Proteintech, Rosemont, IL, United States) expression was used as a loading control.
Infection of hamsters with Ad5 and determination of anti-Ad5 neutralizing antibody (Nab) titers in the serum
The IL2RG homozygous Syrian hamsters and wild type (WT) control hamsters were anesthetized with 1.2% working solution avertin (45 mg/kg) and injected with 1 × 1010 plaque forming units /kg of Ad5 by intramuscular injection. Day 14 after prime immunization, the hamsters were boosted using the same dose and route, and the hamsters were sacrificed on day 17. The Nab titer of the samples was determined as described previously[20]. In brief, serum samples were collected and incubated for 30 min at 56°C to inactivate complement. Ad5 was incubated with serially diluted serum samples, and then then the mix (virus-serum) was added to A549 cells and incubated at 37°C. After 10 d, inhibition of cytopathic effects was measured. Nab titer in serum was determined as described previously[14].
Histopathological analysis
Tissues of the Syrian hamsters and immune-deficient mice were harvested, visually inspected, fixed in 10% neutral buffered formalin, and embedded in paraffin. The embedded tissues were stained with hematoxylin and eosin and Masson’s trichrome. Immunohistochemistry (IHC) analysis was conducted as described previously[16]. IHC of vimentin and smooth muscle actin (SMA) was performed using paraffin section of the tumors with a primary antibody against vimentin (ZM-0260, ZSGB-BIO, Beijing, China) and SMA antibody (ZM-0003, ZSGB-BIO). The histopathological results were evaluated by two independent pathologists.
Flow cytometry
Five-week-old hamsters were sacrificed, and single cells from the thymus and spleen were prepared for flow cytometric analyses. To obtain single-cell suspensions, tissues were cut to small pieces with scissors and passed through a 70-μm cell strainer (BD Biosciences, Franklin Lakes, NJ, United States) by pressing with plunger. The red blood cells were eliminated by using red blood cell lysis buffer. The prepared cells were stained with a 1:400 dilution of fluorescein isothiocyanate conjugated anti-mouse major histocompatibility complex (MHC) class II (I-Ek; clone 14-4-4S), a 1:300 dilution of antigen presenting cell conjugated anti-mouse CD4 (clone GK1.5) (all from E-Bioscience, San Diego, CA, United States), a 1:100 dilution of anti-CD3 (clone 4F11 developed by us[14]) was followed by incubation with a 1:50 dilution of a goat anti-mouse secondary antibody (ZF-0312, ZSGB-BIO). The samples were analyzed on a BD Accuri C6 (BD Biosciences). The data were acquired using Accuri CFlow software (BD Biosciences) on the LSR II and analyzed using the FlowJo VX software.
Tumor cell xenotransplantation
Subcutaneous injections of 5 × 106 MIA-PaCa2 cells or other cells in the right upper flank were performed on 5-wk-old male ZZU001 Syrian hamsters (n = 14) and same age-matched B-NDG mice (n = 14). Tumor size was measured using calipers twice weekly until tumors reached 2000 mm3 (for B-NDG mice) or 3500 mm3 (for ZZU001 Syrian hamster) or tumor ulceration occurred. Tumor volumes (V) were calculated using the formula V = (length × width2 × π)/6). Comparisons were analyzed by Student’s t-test. All data were expressed as the mean ± standard deviation.
Orthotopic tumor model establishment
ZZU001 hamsters (n = 5) and B-NDG mice (n = 5) were anesthetized with 1.2% working solution avertin (45 mg/kg). Animals were placed in the dorsal decubitus position, and left subcostal incision was made. The pancreas was carefully exposed. MIA-PaCa2 cells (1 × 106) were suspended in matrigel (Corning, Bedford, MA, United States) (final volume: 50 μL). The cell suspension was orthotopically injected into the tail of the pancreas with a 29-gauge needle. The pancreas was returned to the peritoneal cavity, and the abdominal wall was closed in two layers with 3-0 nylon sutures (Huato, Suzhou, China). The animals were monitored daily for their general condition and sacrificed by CO2 10 wk after the orthotopic cell injection. Animals had to be killed earlier if one of the following criteria occurred: Formation of ascites with visible abdominal distension; jaundice, cachexia, or both associated with a significant clinical deterioration of the animal.
Statistical analysis
Statistical analyses were conducted using a Student's t-test for the comparison between groups. Results were considered significant at P < 0.05. The results were analyzed with GraphPad software (La Jolla, CA, United States).
RESULTS
Generation of X-SCID Syrian hamsters by CRISPR/Cas9
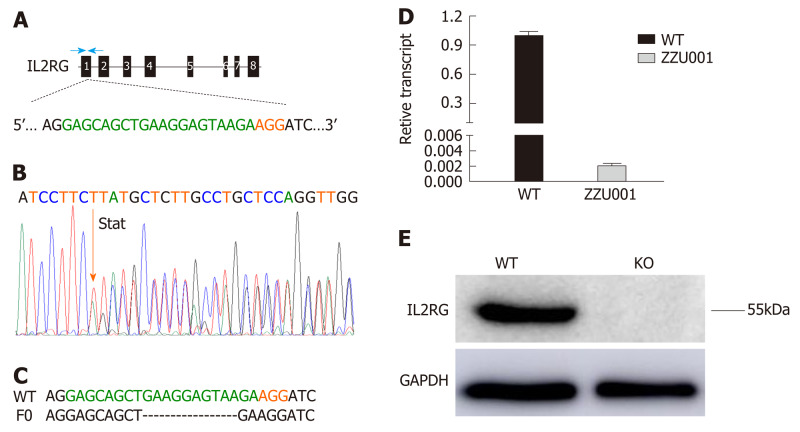
To produce IL2RG knock-out (KO) Syrian hamster, the CRISPR/Cas9 system with a single guide RNA designed to target the first exon of the hamster IL2RG gene (Figure 1A) was employed as described previously[18]. The validated sgRNA and Cas9 mRNA were delivered into Syrian hamster zygotes by cytoplasmic injection. Cytoplasmic injected fertilized eggs were then transferred to pseudopregnant female hamsters (Table S2). Screening of eight newborn pups revealed that three (37.5%) carried targeted mutations. One had a 10 bp deletion that resulted in a reading frameshift in the IL2RG gene, leading to a premature stop codon (at 16th amino acid, Figure 1B and C). We established a viable breeding colony from the F0 founder carrying this indel. Because IL2RG is located on the X chromosome, the term of “homozygous KO” used herein refers to either female with both of the X chromosome alleles targeted or males with its single X chromosome targeted. The homozygous IL2RG KO hamsters were named ZZU001. As shown in Figure 1D, ZZU001 Syrian hamsters with homozygous 10 bp deletion of IL2RG had very little mRNA expression of IL2RG compared to wild type hamsters in thymus, as revealed by RT-qPCR, and no IL2RG protein expression in ZZU001 spleen was detectable by Western blotting (Figure 1E). In order to determine whether the IL2RG-specific sgRNA only induced mutations in the IL2RG gene but not in any other genomic loci, we identified six potential off-target sites using Benchling software (Table S3). Off-target analysis showed that no off-target events occurred in the 10 bp deletion of IL2RG founder (Figure S1), indicating that the sgRNA used in this study is specific, and all features of ZZU001 are due to defects of the IL2RG gene and no other genetic changes.
Figure 1.
Interleukin 2 receptor subunit gamma knockout Syrian hamsters (ZZU001) generated by CRISPR/Cas9. A: Schematic representation of the Syrian hamster interleukin 2 receptor subunit gamma (IL2RG) gene. The single-guide RNA target sequence is shown in green with the protospacer adjacent motif shown in red; B: Polymerase chain reaction product sequencing confirming the founder is shown. “Stat” refers to the mutant site; C: Sequencing assay for single-guide RNA/Cas9-IL2RG-induced mutation in the target region in Syrian hamsters. Multiple deletions are depicted by dashes; D: Quantitative real-time polymerase chain reaction analysis of IL2RG mRNA expression in the thymus of wild type and ZZU001 Syrian hamsters. bP < 0.01. n = 3. Glyceraldehyde 3-phosphate dehydrogenase mRNA expression was used as an internal control; E: Western blotting assay to compare the expression of IL2RG protein in wild type and ZZU001 Syrian hamsters. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. KO: Knock-out; sgRNA: Single-guide ribonucleic acid; WT: Wild type.
Abnormal lymphoid cell development and impaired immune function in ZZU001 Syrian hamsters
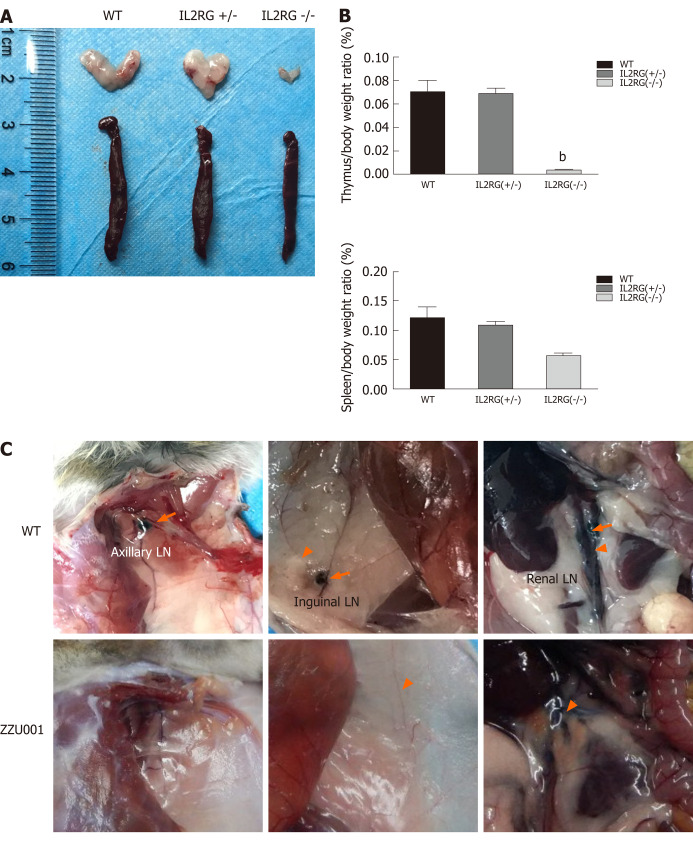
Gross necropsy and microscopic analyses of ZZU001 Syrian hamsters revealed impaired lymphoid development. The thymuses and spleens were examined at 5 wk of age in WT, heterozygous IL2RG KO, and homozygous IL2RG KO hamsters. The thymus of homozygous IL2RG KO hamsters was extremely hypoplastic and significantly reduced in size (Figure 2A), and consisted of an epithelial rudiment without any lymphocytes compared to WT (Figure 3A and B). The spleen of homozygous IL2RG KO hamsters was moderately decreased in size, as seen in Figure 2A. It was also much thinner and more loosely packed than those of heterozygous KO and WT hamsters, while the white pulp was almost completely devoid of lymphocytes compared to WT (Figure 3C and D). The thymus/body and spleen/body weight ratios in homozygous KO Syrian hamsters were lower than those in heterozygous KO and WT (Figure 2B). ZZU001 hamsters had no noticeable lymph nodes compared to WT hamsters (Figure 2C). In addition, IL2RG KO Syrian hamsters can survive over 72 wk (1.5 years), with a median survival time that exceeds 89 wk (Figure S2).
Figure 2.
ZZU001 Syrian hamsters demonstrate abnormal lymphoid cell development and impaired immune function. A: The thymus and spleen of interleukin 2 receptor subunit gamma (IL2RG) knock-out (KO) hamsters were both evidently smaller than those of age-matched interleukin 2 receptor subunit gamma +/- and wild type (WT) hamsters; B: Comparison of spleen-to-body weight ratio. Each value represents the mean ± standard deviation (n = 3/group), Student’s t-test. bP < 0.01; and C: Dye labeling of Syrian hamster lymph nodes (LNs) and lymphatic vessels. Efferent lymphatic vessel (orange arrowhead). Axillary LN, Inguinal LN, and Renal LN (orange arrow) are LNs.
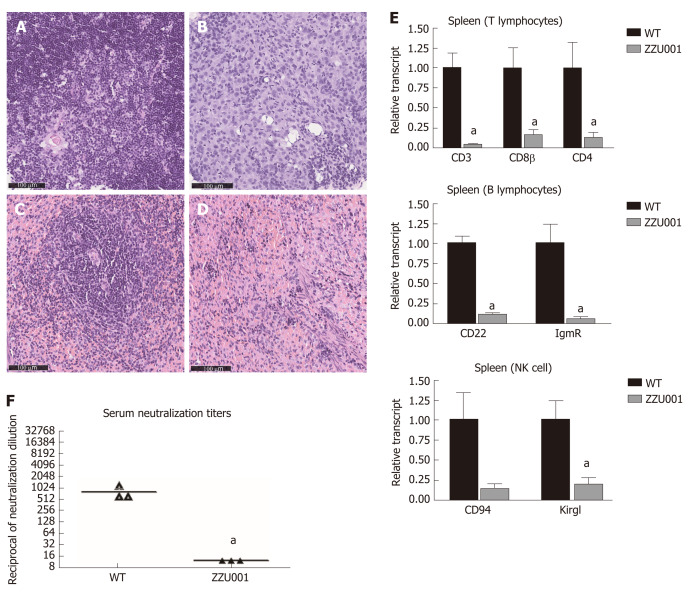
Figure 3.
ZZU001 Syrian hamsters demonstrate immune despair cellular abnormalities in lymphoid development. A and C: Representative histology of the wild type (WT) thymus and spleen. Bars = 100 µm; Representative histology of ZZU001 Syrian hamsters thymus B and spleen D and E: The expression of the indicated lymphocyte-specific genes in spleen homogenates was determined by reverse transcriptase quantitative polymerase chain reaction using the 2^ (-ΔΔCt) method. aP < 0.05. n = 3; F: Development of neutralizing antibodies against AdV-5. n = 3. aP < 0.05.
RT-qPCR analysis of splenocytes of ZZU001 Syrian hamsters demonstrated that the expression of T lymphocyte-specific genes (CD3γ and CD4), B lymphocyte-specific genes [CD22 and FCMR (immunoglobulin M receptor)], and natural killer cell-specific genes (CD94 and Klrg1) were significantly lower than those in WT hamsters (Figure 3E). Immune cell marker gene expression was also examined in the thymus and bone marrow (Figure S3), showing a similar pattern as in the spleen (Figure 3E). In order to confirm further the deficiency of immune cells in IL2RG KO hamsters, flow cytometry analysis of immune molecules in monocytes isolated from thymus and spleen were performed. As shown in Figure S4, there was a clear decrease of the lymphocytes in ZZU001 Syrian hamsters; CD3+ or CD4+ positive T cells were almost absent from the ZZU001 hamster thymus and spleen. MHC class II positive cells were markedly decreased in ZZU001 Syrian hamster spleen (Figure S4B). There were no MHC class II expressing cells in the thymus. Consistent with the histology, the number of splenocytes and thymocytes was dramatically reduced in ZZU001 Syrian hamster compared with WT hamsters. Analysis of B cell function demonstrated that ZZU001 Syrian hamsters could not produce neutralizing antibodies against Ad5 (Figure 3F) after infection with Ad5. Based on these findings, it is evident that ZZU001 hamsters have a severe immune deficiency in T cell, B cell, and possibly natural killer cells.
Subcutaneous xenotransplantation of human PC cells
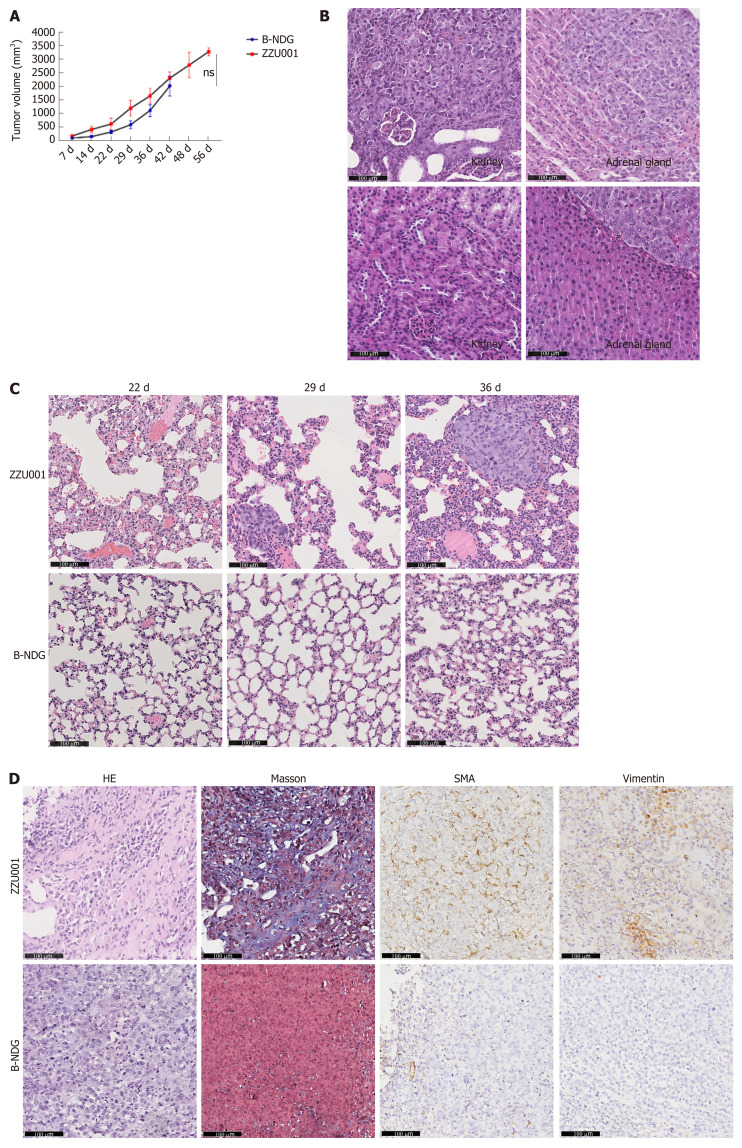
Immune-deficient mice can accept transplanted tissues from humans. Having made severely immune-deficient ZZU001 Syrian hamsters, subcutaneous tumors of the human PC cell line MIA-PaCa-2 were established in ZZU001 hamsters and immune-deficient B-NDG mice. It was found that xenografts tumors of the human PC cell line MIA-PaCa-2 developed well in both ZZU001 Syrian hamsters and B-NDG mice (Figure 4A). Consistent with previous reports, no distant metastasis in immune-deficient mice was observed (Table 1, Figure 4B and C). In contrast, all ZZU001 hamsters bearing subcutaneous tumors developed lung metastasis (occurring as early as 29 d after injection of the human PC cell line MIA-PaCa-2), coupled with metastasis in kidneys (40%) and adrenal glands (20%) (Table 1 and Figure 4B). Subcutaneous xenograft tumors from four other human PC cell lines, including Panc-1, SUIT-2, Patu8988T, and Capan-1, were established, and the tumor growth and remote organ metastasis were examined. As shown in Table 2, all four additional PC cell lines developed remote metastasis in lung, liver, and kidney although the remote metastatic frequency and sites varied between different PC cell lines.
Figure 4.
Xenotransplantation of human pancreatic cancer cells into ZZU001 Syrian hamsters recapitulates human pancreatic cancer metastasis. A: Growth curve of human MIA-PaCa-2 pancreatic cancer cells in ZZU01 Syrian hamsters and B-NDG mice. MIAPaCa-2 cells were subcutaneously inoculated into the flank of ZUU001 and B-NDG mice (n = 5/group). Tumors were measured twice weekly and the tumor volume estimated. Mean tumor size ± standard error of the mean is shown. ns: no significance; B: A representative hematoxylin and eosin (HE) stained section of a kidney and adrenal gland with metastatic tumor at the end of the experiment in ZZU001 hamsters (56 d) and B-NDG mice (42 d); C: Representative HE staining of a lung metastasis at 22 d, 29 d, 36 d after the injection of MIA-PaCa-2 cells in ZZU001 hamsters and B-NDG mice; D: Representative HE, Masson, smooth muscle actin (SMA) and vimentin staining of a subcutaneous tumor at 42 d after the injection of the MIA-PaCa-2 cells in ZZU001 hamsters and B-NDG mice. Scale bars = 100 µm.
Table 2.
Sites and frequency [n/n (%)] of distant metastasis of four different human pancreatic cancer cell lines
|
Panc-1 (SC) |
SUIT-2 (SC) |
Patu8988T (SC) |
Capan-1 (SC) |
|
| ZZU001 | ZZU001 | ZZU001 | ZZU001 | |
| Distant metastasis | ||||
| Liver | - | 1/3 (33) | 3/5 (60) | - |
| Lung | 5/5 (100) | 3/3 (100) | - | 5/5 (100) |
| Retroperitoneum | - | - | - | - |
| Mesentery | - | - | - | - |
| Diaphragm | - | - | - | - |
| Spleen | - | - | - | - |
| Stomach | - | - | - | - |
| Kidney | - | 1/3 (33) | 1/5 (20) | - |
| Adrenal gland | - | - | - | - |
SC: Subcutaneous.
Orthotopic transplantation of human PC cells
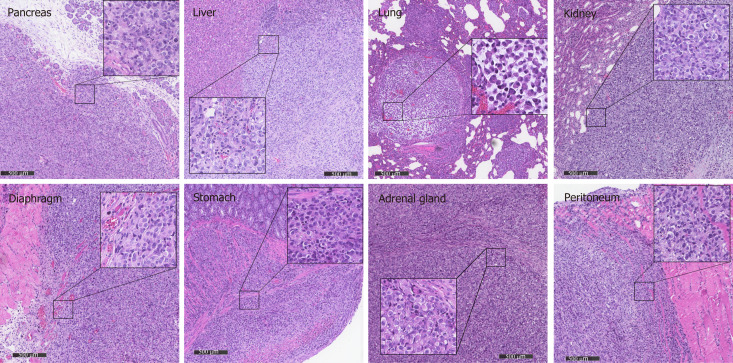
Orthotopic transplantation models can mimic the biological behavior of the primary tumor more closely compared with subcutaneous transplantation models, although orthotopic xenotransplantation procedures are technically difficult to perform for PC, with strong potential for complications. In order to assess the potential value of a PC orthotopic model in ZZU001 hamsters, MIA-PaCa-2 cells were injected into pancreas of ZZU001 hamsters and B-NDG mice. Tumor formation rate was 100% (5/5) in the ZZU001 Syrian hamsters that received orthotopic transplantation of Mia-PaCa2 cells (Table 1), and hamsters developed metastasis at multiple-sites after orthotopic implantation, including the liver (100%), lung (100%), retroperitoneum (100%), mesentery (100%), diaphragm (40%), stomach (20%), kidney (100%), and adrenal gland (40%). In comparison, no lung metastasis was detected in the B-NDG mouse orthotopic model, and the frequency of metastasis to other organs was lower (Table 1). The histopathological features of cancer cells in the metastatic nodules presented similar morphological changes as those found in the primary tumors of pancreas, showing epithelial cell morphology. Tumor cells are strikingly heterogeneous within tumors and presented solid growth with nuclear polymorphism and abnormal mitoses while the desmoplastic stromal reaction is less developed in the most of metastatic tumors (Figure 5). Furthermore, ZZU001 Syrian hamster orthotopic models presented similar clinical and pathological features as observed in human patients, such as local infiltration of cancer cells and signs of tumor burden, including ascites, jaundice, ileus, and cachexia (Table 1).
Figure 5.
Metastasis in different organs reflects the histology of the primary tumor in ZZZU001 orthotopic xenograft tumor models. The primary tumors were established by injection of 1 × 106 of MIA-PaCa-2 cells into the pancreatic tail. The representative tumor in the pancreas, liver, lung, kidney, diaphragm, stomach, adrenal gland, and peritoneum of ZZU001 Syrian hamsters are shown. Inset square shows tumor cells at a magnification 400 ×. Scale bars = 500 µm.
Interaction of PC cells and stroma in xenograft tumors of PC
In order to dissect the possible mechanisms for metastasis of human PC in ZZU001 hamsters, we investigated the alteration of stroma and tumor cells within the xenograft tumors derived from ZZU001 hamsters and B-NDG mice through biochemistry and IHC staining. Of note, desmoplastic reactions that consist of extracellular matrix proteins, including collagen and fibronectin (Figure 4D) and active fibroblasts [SMA-positive staining (Figure 4D)] were readily observed in the stroma of ZZU001 hamster xenograft tumors but not in the xenograft tumors derived from B-NDG mice. Most interestingly, the tumor cells growing in ZZU001 hamsters displayed strong vimentin positive staining, indicative of epithelial to mesenchymal transition (EMT) (Figure 4D), whereas no obviously vimentin-positive tumor cells were observed in B-NDG mice xenograft tumors (Figure 4D). These observations suggest human PC cells could communicate with host cells of ZZU001 hamsters, which results in the improved engraftments and development of human tumor cells in ZZU001 Syrian hamsters. Thus, the ZZU001 hamster bearing PC model can recapitulate more key features of human PC, including metastasis and stromal reaction, compared to severe immune-deficient mice models.
DISCUSSION
Implantation of human PC cells subcutaneously into immune-deficient mice has been widely used for the generation of in vivo human PC models, largely due to their comparatively low cost, ease and speed of establishment, and predictable tumor growth. However, these models do not faithfully recapitulate the features of human PC, in particular, as they fail to model the multiple-sites of metastasis observed during disease progression[5-7]. In the current study, we created an IL2RG KO immune-deficient Syrian hamster (ZZU001) and demonstrated that an array of human PC cell lines, when subcutaneously transplanted, can result in multiple-sites of metastasis, including the lung, liver, kidney, and adrenal glands, although the metastasis frequency in liver is still low compared to observed levels in human patients. In striking contrast, no metastasis from any of these cell lines was observed in severe immune-deficient B-NDG mice. Therefore, the ZZU001 Syrian hamster model provides a novel animal model to recapitulate more accurately metastasis of human PC.
Pancreatic metastases can arise in any organ site but are mostly detected in abdominal sites. The liver is the most common organ in which metastasis is detected, followed by the peritoneum, lung and pleura, bones, and adrenal glands[21]. The comparison of MIA-PaCa-2 orthotopic models in B-NDG and ZZU001 (Table 1) demonstrates that the ZZU001 orthotopic tumor model presents a more accurate representation of human PC than the mouse models, particularly with regards to distant metastasis and local infiltration of cancer cells. Interestingly, ZZU001 xenograft models show a high frequency of kidney metastasis (40% in the subcutaneous model and 100% in the orthotopic model), although kidney metastasis of PC in humans has not been extensively reported[22]. This warrants further investigation.
PC progression is complemented by a fibrotic stromal (desmoplastic) reaction characterized by extensive deposition of extracellular matrix components, recruitment and activation of cancer-associated fibroblasts, decreased vasculature patency, and altered immune-surveillance[23]. Stromal remodeling leads to altered interactions between tumor cells and stromal compartments, which can promote tumor progression[24]. Presence of a robust, reactive, and desmoplastic stroma and the crosstalk between cancer cells and stromal cells are critical for PC progression and metastasis[25,26]. An ideal animal model of PC should reflect these important features, but xenografted tumors in mice rarely develop stromal reactions nor the EMT phenotype (Figure 4D). Strikingly, ZZU001 hamster xenograft tumors present a considerable amount of extracellular matrix reaction, as evidenced by diffusive strong Masson staining (Figure 4D) and activation of fibroblasts measured by expression of SMA (Figure 4D). The activated fibroblast may be a key factor affecting distant metastasis in ZZU001 hamster model as cancer-associated fibroblasts promote tumor growth and metastasis in a variety of ways[27]. Importantly, some tumor cells in ZZU001 hamster xenograft tumor over-express vimentin, whereas no vimentin positive tumor cells were observed in mouse xenograft tumor. These observations suggest that the EMT of cancer cells in the ZZU001 hamster may be influenced by the stroma, which is highly associated with PC metastasis. The ZZU001 Syrian hamsters described in this study can be a valuable animal model for better understanding of the molecular mechanism of tumorigenesis, in particular for the metastasis of PC and testing novel therapeutics for this most aggressive disease.
ARTICLE HIGHLIGHTS
Research background
Pancreatic cancer (PC) is an extremely aggressive cancer with a poor prognosis. Multiple sites of metastasis from PC remain a significant hurdle in treating this disease. Reliable animal models that can mimic the clinical features of the disease are needed to study disease progression and develop effective therapies. Xenograft cancer cell transplantation animal models are cost-effective and easily established methods by which to assess tumor progression, metastasis, and pre-clinical efficacy of cancer drugs. However, current xenograft tumor models of human PC in immune-deficient mice rarely develop metastasis. The development of a model that can reflect more accurately human PC progression is therefore required.
Research motivation
Syrian hamsters (Mesocricetus auratus) have advantages as models for various diseases due to the high similarities in anatomy, physiology, and pathology between Syrian hamsters and humans. The Syrian hamster is the only rodent species that develop PC in an almost identical manner to the respective human disease regarding such features as clinical symptoms, tumor morphology, tumor biology, metabolic abnormality, and molecular genetic alterations. We reasoned that the immune-deficient Syrian hamster might be a better animal species for establishing xenograft models of human PC and more faithfully recapitulate the features of PC, in particular the multiple sites of metastasis seen during progression of human PC.
Research objectives
This study aimed to create an immune-deficient Syrian hamster by knockout of interleukin 2 (IL-2) receptor subunit gamma (IL2RG), characterize the phenotypes of IL-2RG knockout (KO) Syrian hamsters, and evaluate whether this animal can present the distinguishing features of human PC.
Research methods
CRISPR/Cas9-mediated genetic editing and cytoplasmic injection into hamster zygotes were employed to create an IL2RG KO Syrian hamster. The phenotypes and immune functions of the IL2RG KO Syrian hamster were characterized. A panel of human PC cell lines were subcutaneously or orthotopically transplanted into IL2RG KO Syrian hamsters or immune-deficient mice. The tumor growth, local invasion of the tumor cells, and remote organ metastasis were compared over time. The histopathology of tumor xenografts, the molecular alterations of tumor cells, and the stroma within the xenograft tumors were investigated by hematoxylin and eosin and immunohistochemistry staining.
Research results
A new immune-deficient Syrian hamster with IL2RG gene knockout was created and named ZZU001. We demonstrated that ZZU001 Syrian hamsters have a lymphoid compartment that is greatly reduced in size and diversity and are impaired in their immune function. The comparison studies on xenografting tumors in ZZU001 and severely immune-deficient mice demonstrated that ZZU001 Syrian hamsters engrafted with human tumor cells are a promising animal model, which can recapitulate most of the features of human PC, in particular, the multiple-sites of metastasis. PC tissues derived from ZZU001 hamsters also displayed other key features of human PC, such as desmoplastic reactions in the stroma and epithelial to mesenchymal transition phenotype, whereas PC tissues derived from immune-deficient mice did not present such features.
Research conclusions
This work demonstrates that ZZU001 Syrian hamster can be an extremely valuable animal model for better understanding the molecular mechanisms of tumorigenesis, in particular the metastasis of human PC, and maybe more appropriate in comparison to xenograft mouse models for robust testing of the anti-tumor potential of novel therapeutics.
Research perspectives
Current findings provide a promising xenotransplantation animal model for human PC research. Its wider application requires further evaluation, but the strong similarities between progression of human and Syrian hamster PC may suggest a similarly useful application of this model for more accurately modeling the progression of other human tumors. The model characterized in this study may also provide a useful platform for identification of novel molecules and pathways that control the metastasis of human PC.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Academy of Medical Sciences, Zhengzhou University Institutional Review Board.
Institutional animal care and use committee statement: All animal experiments conformed to the Provision and General Recommendation of Chinese Experimental Animals Administration Legislation accepted principles for the care and use of laboratory animals (IACUC-ZZU-2016/Wang, The Ethics Committee on Animal Experiment of Zhengzhou University, Zhengzhou, Henan, China).
Conflict-of-interest statement: The authors disclose that they have no competing interests.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Peer-review started: April 14, 2020
First decision: June 18, 2020
Article in press: August 4, 2020
P-Reviewer: Sun XT, Wang XB, Zhang XB S-Editor: Zhang L L-Editor: Filipodia P-Editor: Zhang YL
Contributor Information
Jin-Xin Miao, Sino-British Research Centre for Molecular Oncology, National Centre for International Research in Cell and Gene Therapy, Academy of Medical Sciences, Zhengzhou University, Zhengzhou 450000, Henan Province, China; Academy of Chinese Medical Sciences, Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China.
Jian-Yao Wang, Sino-British Research Centre for Molecular Oncology, National Centre for International Research in Cell and Gene Therapy, Academy of Medical Sciences, Zhengzhou University, Zhengzhou 450000, Henan Province, China.
Hao-Ze Li, Sino-British Research Centre for Molecular Oncology, National Centre for International Research in Cell and Gene Therapy, Academy of Medical Sciences, Zhengzhou University, Zhengzhou 450000, Henan Province, China.
Hao-Ran Guo, Sino-British Research Centre for Molecular Oncology, National Centre for International Research in Cell and Gene Therapy, Academy of Medical Sciences, Zhengzhou University, Zhengzhou 450000, Henan Province, China.
Louisa S Chard Dunmall, Centre for Biomarkers and Biotherapeutics, Barts Cancer Institute, Queen Mary University of London, London EC1M6BQ, United Kingdom.
Zhong-Xian Zhang, Sino-British Research Centre for Molecular Oncology, National Centre for International Research in Cell and Gene Therapy, Academy of Medical Sciences, Zhengzhou University, Zhengzhou 450000, Henan Province, China.
Zhen-Guo Cheng, Sino-British Research Centre for Molecular Oncology, National Centre for International Research in Cell and Gene Therapy, Academy of Medical Sciences, Zhengzhou University, Zhengzhou 450000, Henan Province, China.
Dong-Ling Gao, Sino-British Research Centre for Molecular Oncology, National Centre for International Research in Cell and Gene Therapy, Academy of Medical Sciences, Zhengzhou University, Zhengzhou 450000, Henan Province, China.
Jian-Zeng Dong, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing 100029, China.
Zhong-De Wang, Department of Animal Dairy, and Veterinary Sciences, Utah State University, Logan UT 84341, United States.
Yao-He Wang, Sino-British Research Centre for Molecular Oncology, National Centre for International Research in Cell and Gene Therapy, Academy of Medical Sciences, Zhengzhou University, Zhengzhou 450000, Henan Province, China; Centre for Biomarkers and Biotherapeutics, Barts Cancer Institute, Queen Mary University of London, London EC1M6BQ, United Kingdom. yaohe.wang@qmul.ac.uk.
Data sharing statement
No additional data are available.
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 3.Schnelldorfer T, Ware AL, Sarr MG, Smyrk TC, Zhang L, Qin R, Gullerud RE, Donohue JH, Nagorney DM, Farnell MB. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247:456–462. doi: 10.1097/SLA.0b013e3181613142. [DOI] [PubMed] [Google Scholar]
- 4.Hwang CI, Boj SF, Clevers H, Tuveson DA. Preclinical models of pancreatic ductal adenocarcinoma. J Pathol. 2016;238:197–204. doi: 10.1002/path.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu W, Su GH. Challenges and advances in mouse modeling for human pancreatic tumorigenesis and metastasis. Cancer Metastasis Rev. 2013;32:83–107. doi: 10.1007/s10555-012-9408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotz HG, Reber HA, Hotz B, Yu T, Foitzik T, Buhr HJ, Cortina G, Hines OJ. An orthotopic nude mouse model for evaluating pathophysiology and therapy of pancreatic cancer. Pancreas. 2003;26:e89–e98. doi: 10.1097/00006676-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Loukopoulos P, Kanetaka K, Takamura M, Shibata T, Sakamoto M, Hirohashi S. Orthotopic transplantation models of pancreatic adenocarcinoma derived from cell lines and primary tumors and displaying varying metastatic activity. Pancreas. 2004;29:193–203. doi: 10.1097/00006676-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Hilaris B. Iodine 125 source in interstitial tumor therapy. Clinical and biological considerations. Am J Roentgenol Radium Ther Nucl Med. 1975;123:163–169. doi: 10.2214/ajr.123.1.163. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M, Arbuck S, Hollingshead M, Sausville EA. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype C, Neumann H, Safran H, Humblet Y, Perez Ruixo J, Ma Y, Von Hoff D. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430–1438. doi: 10.1200/JCO.2004.10.112. [DOI] [PubMed] [Google Scholar]
- 11.Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227–4239. [PubMed] [Google Scholar]
- 12.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao J, Ying B, Li R, Tollefson AE, Spencer JF, Wold WSM, Song SH, Kong IK, Toth K, Wang Y, Wang Z. Characterization of an N-Terminal Non-Core Domain of RAG1 Gene Disrupted Syrian Hamster Model Generated by CRISPR Cas9. Viruses. 2018;10 doi: 10.3390/v10050243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tysome JR, Li X, Wang S, Wang P, Gao D, Du P, Chen D, Gangeswaran R, Chard LS, Yuan M, Alusi G, Lemoine NR, Wang Y. A novel therapeutic regimen to eradicate established solid tumors with an effective induction of tumor-specific immunity. Clin Cancer Res. 2012;18:6679–6689. doi: 10.1158/1078-0432.CCR-12-0979. [DOI] [PubMed] [Google Scholar]
- 15.Pour PM. Why the Hamster Pancreatic Cancer Model is Still the Most Useful Tool for Clinical Studies? JOP. 2016;17:566–569. [Google Scholar]
- 16.Wang P, Li X, Wang J, Gao D, Li Y, Li H, Chu Y, Zhang Z, Liu H, Jiang G, Cheng Z, Wang S, Dong J, Feng B, Chard LS, Lemoine NR, Wang Y. Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat Commun. 2017;8:1395. doi: 10.1038/s41467-017-01385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho SA, Park JH, Seok SH, Juhn JH, Kim SJ, Ji HJ, Choo YS, Park JH. Effect of granulocyte macrophage-colony stimulating factor (GM-CSF) on 5-FU-induced ulcerative mucositis in hamster buccal pouches. Exp Toxicol Pathol. 2006;57:321–328. doi: 10.1016/j.etp.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Li R, Miao J, Fan Z, Song S, Kong IK, Wang Y, Wang Z. Production of Genetically Engineered Golden Syrian Hamsters by Pronuclear Injection of the CRISPR/Cas9 Complex. J Vis Exp. 2018 doi: 10.3791/56263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toth K, Lee SR, Ying B, Spencer JF, Tollefson AE, Sagartz JE, Kong IK, Wang Z, Wold WS. STAT2 Knockout Syrian Hamsters Support Enhanced Replication and Pathogenicity of Human Adenovirus, Revealing an Important Role of Type I Interferon Response in Viral Control. PLoS Pathog. 2015;11:e1005084. doi: 10.1371/journal.ppat.1005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133:413–422. doi: 10.5858/133.3.413. [DOI] [PubMed] [Google Scholar]
- 22.Malkoç E, Aktaş Z, Kara K, Haholu A. Renal metastasis from pancreatic adenocarcinoma: a rare case along with literature review. Turk J Urol. 2015;41:93–95. doi: 10.5152/tud.2015.54280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinha S, Leach SD. New insights in the development of pancreatic cancer. Curr Opin Gastroenterol. 2016;32:394–400. doi: 10.1097/MOG.0000000000000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crawford HC, Pasca di Magliano M, Banerjee S. Signaling Networks That Control Cellular Plasticity in Pancreatic Tumorigenesis, Progression, and Metastasis. Gastroenterology. 2019;156:2073–2084. doi: 10.1053/j.gastro.2018.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas D, Radhakrishnan P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol Cancer. 2019;18:14. doi: 10.1186/s12943-018-0927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47–60. doi: 10.1016/j.semcancer.2014.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.